Contact Toxicity and Ovideterrent Activity of Three Essential Oil-Based Nano-Emulsions against the Olive Fruit Fly Bactrocera oleae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Bactrocera oleae Mass-Rearing
2.3. GC-MS Analysis of EOs
2.4. Nano-Emulsion Formulation and Physical Characterization
2.5. Residual Contact Toxicity
2.6. Oviposition Deterrence
2.6.1. No-Choice Bioassays
2.6.2. Choice Bioassays
2.7. Data Analysis
3. Results
3.1. Characterization of Essential Oils and Nano-Emulsions
3.2. Residual Contact Toxicity
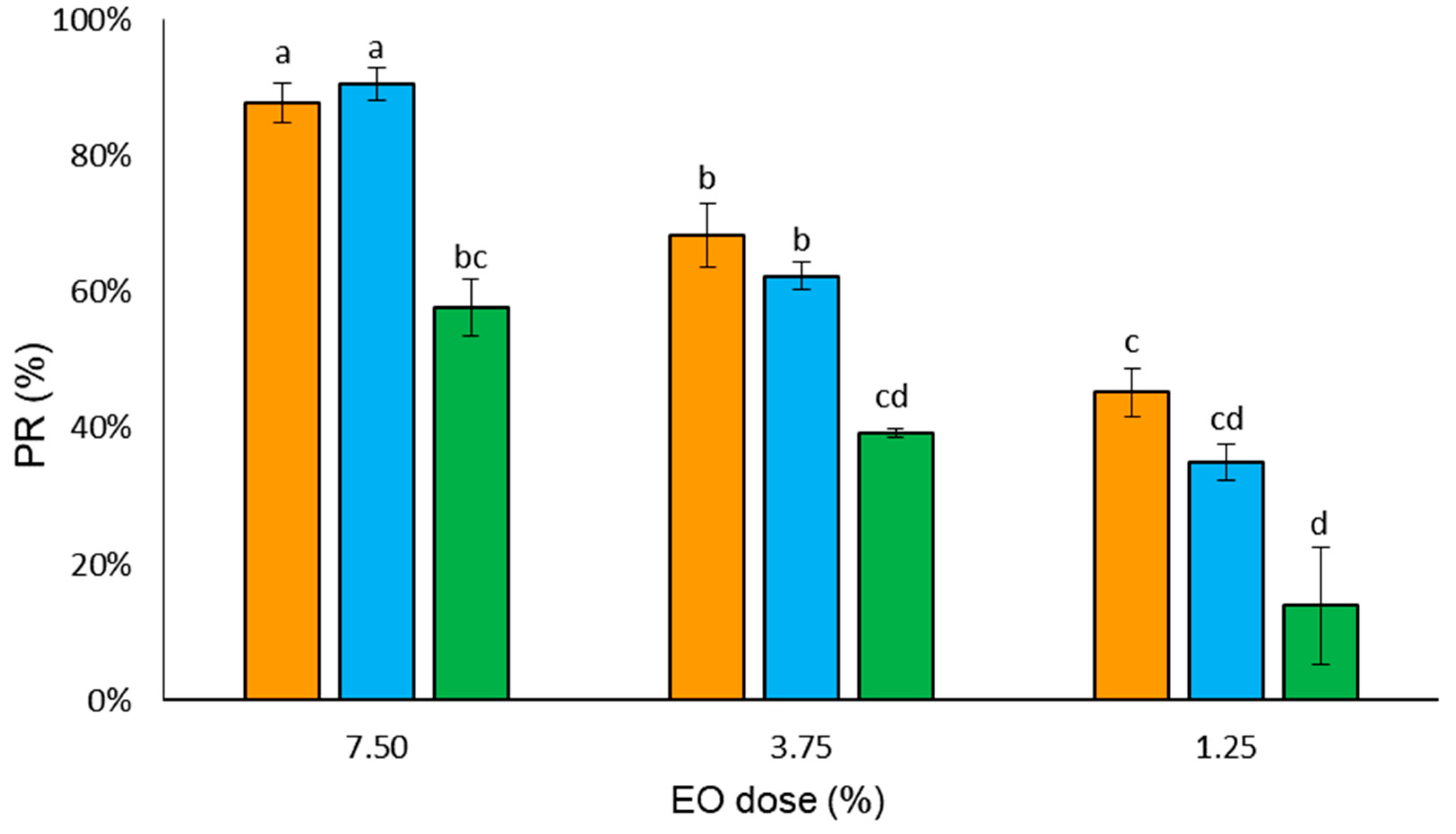
3.3. Oviposition Deterrence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mojdehi, M.R.A.; Keyhanian, A.A.; Rafiei, B. Application of oviposition deterrent compounds for the control of olive fruit fly, Bactrocera oleae Rossi. (Dip. Tephritidae) control. Int. J. Trop. Insect Sci. 2021, 42, 63–70. [Google Scholar] [CrossRef]
- Tzanakakis, M. Seasonal development and dormancy of insects and mites feeding on olive: A review. Neth. J. Zool. 2003, 52, 87–224. [Google Scholar] [CrossRef] [Green Version]
- Daane, K.M.; Johnson, M.W. Olive fruit fly: Managing an ancient pest in modern times. Annu. Rev. Entomol. 2010, 55, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.I.; Nasr, F.N.; Stefanos, S.S.; Elkhair, S.S.A.; Shehata, W.A.; Agamy, E.; Herz, A.; Hassan, S.A. The side-effects of plant protection products used in olive cultivation on the hymenopterous egg parasitoid Trichogramma cacoeciae Marchal. J. Appl. Entomol. 2004, 128, 593–599. [Google Scholar] [CrossRef]
- Malheiro, R.; Casal, S.; Baptista, P.; Pereira, J.A. Physico-chemical characteristics of olive leaves and fruits and their relation with Bactrocera oleae (Rossi) cultivar oviposition preference. Sci. Hortic. 2015, 194, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Malheiro, R.; Casal, S.; Pinheiro, L.; Baptista, P.; Pereira, J.A. Olive cultivar and maturation process on the oviposition preference of Bactrocera oleae (Rossi) (Diptera: Tephritidae). Bull. Entomol. Res. 2019, 109, 43–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Núñez, M.; Pascual, S.; Cobo, A.; Seris, E.; Cobos, G.; Fernández, C.E.; Sánchez-Ramos, I. Copper and kaolin sprays as tools for controlling the olive fruit fly. Entomol. Gen. 2021, 41, 97–110. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical Insecticides in the Twenty-First Century—Fulfilling Their Promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isman, M.B.; Grieneisen, M.L. Botanical insecticide research: Many publications, limited useful data. Trends Plant Sci. 2014, 19, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B.; Miresmailli, S. Plant essential oils as repellents and deterrents to agricultural pests. In Proceedings of the ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2011; Volume 1090, pp. 67–77. [Google Scholar]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Campolo, O.; Giunti, G.; Laigle, M.; Michel, T.; Palmeri, V. Essential oil-based nano-emulsions: Effect of different surfactants, sonication and plant species on physicochemical characteristics. Ind. Crops Prod. 2020, 157, 112935. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R. Beyond mosquitoes—Essential oil toxicity and repellency against bloodsucking insects. Ind. Crops Prod. 2018, 117, 382–392. [Google Scholar] [CrossRef]
- Zeni, V.; Benelli, G.; Campolo, O.; Giunti, G.; Palmeri, V.; Maggi, F.; Rizzo, R.; Lo Verde, G.; Lucchi, A.; Canale, A. Toxics or lures? Biological and behavioral effects of plant essential oils on tephritidae fruit flies. Molecules 2021, 26, 5898. [Google Scholar] [CrossRef] [PubMed]
- Pavoni, L.; Pavela, R.; Cespi, M.; Bonacucina, G.; Maggi, F.; Zeni, V.; Canale, A.; Lucchi, A.; Bruschi, F.; Benelli, G. Green micro- and nanoemulsions for managing parasites, vectors and pests. Nanomaterials 2019, 9, 1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, R.; Pistillo, M.; Germinara, G.S.; Lo Verde, G.; Sinacori, M.; Maggi, F.; Petrelli, R.; Spinozzi, E.; Cappellacci, L.; Zeni, V.; et al. Bioactivity of Carlina acaulis essential oil and its main component towards the olive fruit fly, Bactrocera oleae: Ingestion toxicity, electrophysiological and behavioral insights. Insects 2021, 12, 880. [Google Scholar] [CrossRef] [PubMed]
- Canale, A.; Benelli, G.; Conti, B.; Lenzi, G.; Flamini, G.; Francini, A.; Cioni, P.L. Ingestion toxicity of three Lamiaceae essential oils incorporated in protein baits against the olive fruit fly, Bactrocera oleae (Rossi) (Diptera Tephritidae). Nat. Prod. Res. 2013, 27, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, R.; Lo Verde, G.; Sinacori, M.; Maggi, F.; Cappellacci, L.; Petrelli, R.; Vittori, S.; Morshedloo, M.R.; Fofie, N.G.B.Y.; Benelli, G. Developing green insecticides to manage olive fruit flies? Ingestion toxicity of four essential oils in protein baits on Bactrocera oleae. Ind. Crops Prod. 2020, 143, 111884. [Google Scholar] [CrossRef]
- Siskos, E.P.; Konstantopoulou, M.A.; Mazomenos, B.E. Insecticidal activity of Citrus aurantium peel extract against Bactrocera oleae and Ceratitis capitata adults (Diptera: Tephritidae). J. Appl. Entomol. 2009, 133, 108–116. [Google Scholar] [CrossRef]
- Lo Scalzo, R.; Scarpati, M.L.; Verzegnassi, B.; Vita, G. Olea europaea chemicals repellent to Dacus oleae females. J. Chem. Ecol. 1994, 20, 1813–1823. [Google Scholar] [CrossRef]
- Malheiro, R.; Casal, S.; Cunha, S.C.; Baptista, P.; Pereira, J.A. Olive volatiles from Portuguese cultivars Cobrançosa, Madural and Verdeal Transmontana: Role in oviposition preference of Bactrocera oleae (Rossi) (Diptera: Tephritidae). PLoS ONE 2015, 10, e0125070. [Google Scholar] [CrossRef] [PubMed]
- Giunti, G.; Campolo, O.; Laudani, F.; Algeri, G.M.; Palmeri, V. Olive fruit volatiles route intraspecific interactions and chemotaxis in Bactrocera oleae (Rossi) (Diptera: Tephritidae) females. Sci. Rep. 2020, 10, 1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltrán, G.; Uceda, M.; Hermoso, M.; Frias, L. Maduración. In El Cultivo De Olivo; Barranco, D., Fernández-Escobar, R., Rallo, L., Eds.; Mundi-Prensa: Madrid, Spain, 2004; pp. 159–183. [Google Scholar]
- Gonçalves, M.F.; Malheiro, R.; Casal, S.; Torres, L.; Pereira, J.A. Influence of fruit traits on oviposition preference of the olive fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae), on three Portuguese olive varieties (Cobrançosa, Madural and Verdeal Transmontana). Sci. Hortic. (Amst.) 2012, 145, 127–135. [Google Scholar] [CrossRef]
- Canale, A.; Germinara, S.G.; Carpita, A.; Benelli, G.; Bonsignori, G.; Stefanini, C.; Raspi, A.; Rotundo, G. Behavioural and electrophysiological responses of the olive fruit fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae), to male- and female-borne sex attractants. Chemoecology 2013, 23, 155–164. [Google Scholar] [CrossRef]
- Giunti, G.; Palermo, D.; Laudani, F.; Algeri, G.M.; Campolo, O.; Palmeri, V. Repellence and acute toxicity of a nano-emulsion of sweet orange essential oil toward two major stored grain insect pests. Ind. Crops Prod. 2019, 142, 111869. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Steam, IL, USA, 1995; ISBN 978-1-932633-21-4. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Jennings, W. Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography; Acad. Press: New York, NY, USA, 1980; ISBN 0323141056. [Google Scholar]
- Masada, Y. Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry; John Wiley and Sons, Inc.: New York, NY, USA, 1976. [Google Scholar]
- Stenhagen, E.; Abrahamsson, S.; McLafferty, F.W. Registry of Mass Spectral Data; John Wiley & Sons, Ltd.: New York, NY, USA, 1974. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Am. Mosq. Control Assoc. 1925, 3, 302–303. [Google Scholar] [CrossRef]
- Campolo, O.; Giunti, G.; Russo, A.; Palmeri, V.; Zappalà, L. Essential Oils in Stored Product Insect Pest Control. J. Food Qual. 2018, 2018, 6906105. [Google Scholar] [CrossRef] [Green Version]
- Stejskal, V.; Vendl, T.; Aulicky, R.; Athanassiou, C. Synthetic and natural insecticides: Gas, liquid, gel and solid formulations for stored-product and food-industry pest control. Insects 2021, 12, 590. [Google Scholar] [CrossRef]
- Levchenko, M.A.; Silivanova, E.A.; Khodakov, P.E.; Gholizadeh, S. Insecticidal efficacy of some essential oils against adults of Musca domestica L. (Diptera: Muscidae). Int. J. Trop. Insect Sci. 2021, 41, 2669–2677. [Google Scholar] [CrossRef]
- Tarelli, G.; Zerba, E.N.; Alzogaray, R.A. Toxicity to vapor exposure and topical application of essential oils and monoterpenes on Musca domestica (Diptera: Muscidae). J. Econ. Entomol. 2009, 102, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Konstantopoulou, M.A.; Mazomenos, B.E. Evaluation of Beauveria bassiana and B. brongniartii strains and four wild-type fungal species against adults of Bactrocera oleae and Ceratitis capitata. BioControl 2005, 50, 293–305. [Google Scholar] [CrossRef]
- Renkema, J.M.; Wright, D.; Buitenhuis, R.; Hallett, R.H. Plant essential oils and potassium metabisulfite as repellents for Drosophila suzukii (Diptera: Drosophilidae). Sci. Rep. 2016, 6, 21432. [Google Scholar] [CrossRef] [PubMed]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils: A review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An overview of micro- and nanoemulsions as vehicles for essential oils: Formulation, preparation and stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maruno, M.; da Rocha-Filho, P.A. O/W Nanoemulsion after 15 years of preparation: A suitable vehicle for pharmaceutical and cosmetic applications. J. Dispers. Sci. Technol. 2009, 31, 17–22. [Google Scholar] [CrossRef]
- Mohammadi, R.; Khoobdel, M.; Talebi, A.A.; Negahban, M.; Norani, M.; Moradi, M.; Dehghan, O. In vivo evaluation of the repellency effects of nanoemulsion of Mentha piperita and Eucalyptus globulus essential oils against mosquitoes. Open Biotechnol. J. 2021, 14, 145–152. [Google Scholar] [CrossRef]
- Sabbour, M.M.; Shadia, E.; El-Aziz, A. Bioefficacy of some essential oils and nano gel chitosan on two insect species of stored pea seeds. J. Entomol. Res. 2021, 45, 647–652. [Google Scholar] [CrossRef]
- Palermo, D.; Giunti, G.; Laudani, F.; Palmeri, V.; Campolo, O. Essential oil-based nano-biopesticides: Formulation and bioactivity against the confused flour beetle Tribolium confusum. Sustainability 2021, 13, 9746. [Google Scholar] [CrossRef]
- Giunti, G.; Campolo, O.; Laudani, F.; Zappalà, L.; Palmeri, V. Bioactivity of essential oil-based nano-biopesticides toward Rhyzopertha dominica (Coleoptera: Bostrichidae). Ind. Crops Prod. 2021, 162, 113257. [Google Scholar] [CrossRef]
- Kubeczka, K.-H. History and sources of essential oil research. In Handbook of Essential Oils; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 3–39. ISBN 9781351246460. [Google Scholar]
- Isman, M.B. Bioinsecticides based on plant essential oils: A short overview. Z. Nat. C 2020, 75, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2020, 19, 235–241. [Google Scholar] [CrossRef]


| EO 1 | Storage Time | Dimension (nm) | PDI 2 | Zeta Potential (mV) | Conductivity (mS/cm) |
|---|---|---|---|---|---|
| Anise | 24 h | 133.7 ± 0.9 ab | 0.193 ± 0.003 a | −23.8 ± 0.1 ab | 0.008 ± 0.000 b |
| 1 year | 141.7 ± 0.3 ab | 0.132 ± 0.008 b | −29.3 ± 2.9 a | 0.010 ± 0.004 ab | |
| Fennell | 24 h | 116.5 ± 0.5 b | 0.156 ± 0.003 ab | −14.4 ± 0.3 b | 0.012 ± 0.005 ab |
| 1 year | 151.9 ± 0.6 a | 0.187 ± 0.006 a | −13.2 ± 0.9 b | 0.020 ±0.002 ab | |
| Mint | 24 h | 122.4 ± 0.5 ab | 0.183 ± 0.006 ab | −20.3 ± 0.5 ab | 0.025 ± 0.000 a |
| 1 year | 130.3 ± 0.3 ab | 0.150 ± 0.002 ab | −15.4 ± 0.2 ab | 0.019 ± 0.000 ab |
| EO 1 Dose (%) | Mortality (%) ± SE 2 | ||
|---|---|---|---|
| Anise | Fennel | Mint | |
| 7.50 | 1.67 ± 0.33 a | 5.00 ± 0.58 a | 3.33 ± 0.33 a |
| 5.00 | 1.67 ± 0.33 a | 3.33 ± 0.33 b | 3.33 ± 0.67 ab |
| 3.75 | 0.00 ± 0.00 b | 0.00 ± 0.00 c | 1.67 ± 0.33 b |
| 2.50 | 0.00 ± 0.00 b | 0.00 ± 0.00 c | 0.00 ± 0.00 c |
| 1.25 | 0.00 ± 0.00 b | 0.00 ± 0.00 c | 0.00 ± 0.00 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giunti, G.; Laudani, F.; Lo Presti, E.; Bacchi, M.; Palmeri, V.; Campolo, O. Contact Toxicity and Ovideterrent Activity of Three Essential Oil-Based Nano-Emulsions against the Olive Fruit Fly Bactrocera oleae. Horticulturae 2022, 8, 240. https://doi.org/10.3390/horticulturae8030240
Giunti G, Laudani F, Lo Presti E, Bacchi M, Palmeri V, Campolo O. Contact Toxicity and Ovideterrent Activity of Three Essential Oil-Based Nano-Emulsions against the Olive Fruit Fly Bactrocera oleae. Horticulturae. 2022; 8(3):240. https://doi.org/10.3390/horticulturae8030240
Chicago/Turabian StyleGiunti, Giulia, Francesca Laudani, Emilio Lo Presti, Monica Bacchi, Vincenzo Palmeri, and Orlando Campolo. 2022. "Contact Toxicity and Ovideterrent Activity of Three Essential Oil-Based Nano-Emulsions against the Olive Fruit Fly Bactrocera oleae" Horticulturae 8, no. 3: 240. https://doi.org/10.3390/horticulturae8030240
APA StyleGiunti, G., Laudani, F., Lo Presti, E., Bacchi, M., Palmeri, V., & Campolo, O. (2022). Contact Toxicity and Ovideterrent Activity of Three Essential Oil-Based Nano-Emulsions against the Olive Fruit Fly Bactrocera oleae. Horticulturae, 8(3), 240. https://doi.org/10.3390/horticulturae8030240








