The Differential Response of Intracellular Water Metabolism Derived from Intrinsic Electrophysiological Information in Morus alba L. and Broussonetia papyrifera (L.) Vent. Subjected to Water Shortage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Measurement of Electrophysiological Parameters of Plant Leaves under Different Clamping Forces
2.3. Calculation of Intrinsically Electrophysiological Parameters of Plant Leaves
2.4. Definition of the Intracellular Water Utilization Parameters
2.5. Determination of Leaf Photosynthetic Parameters, Soil Moisture Content, Leaf Water Content
2.6. Determination of Leaf Water Potential
2.7. Data Analyses
3. Results
3.1. Soil Moisture Content of M. alba and B. papyrifera in Two Habitats
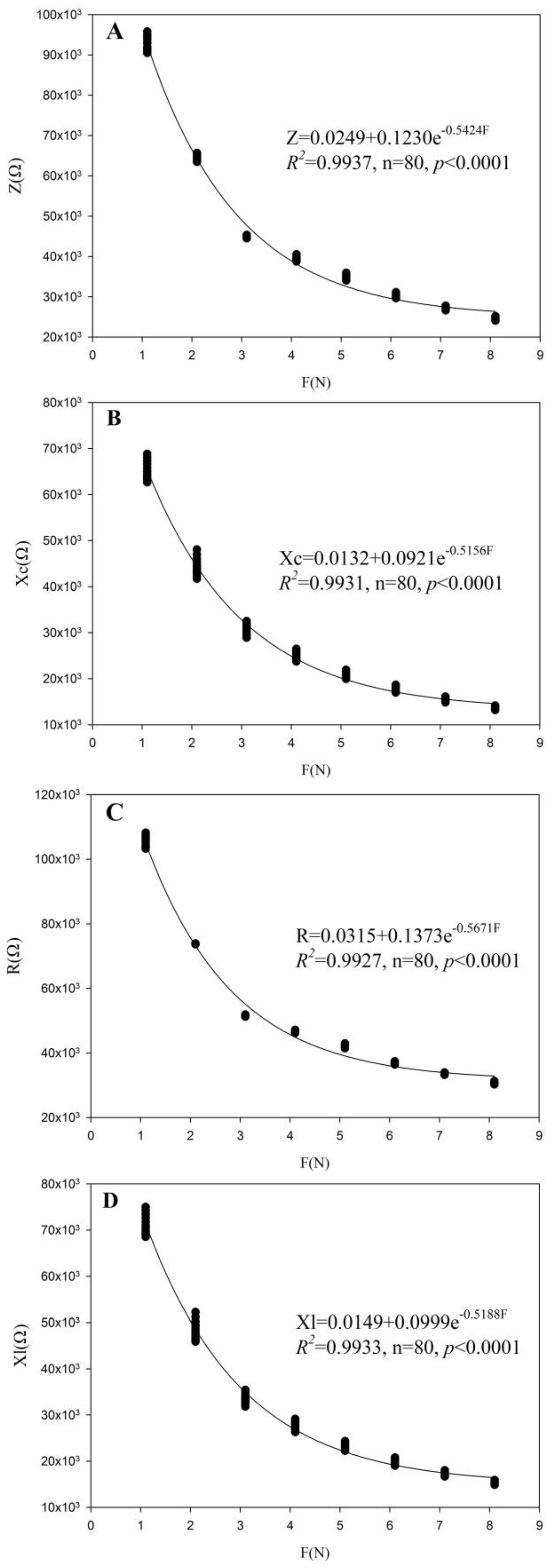
3.2. Fitting Equation Parameters of M. alba and B. papyrifera in Two Habitats
3.3. Electrophysiological Information of M. alba and B. papyrifera in Two Habitats
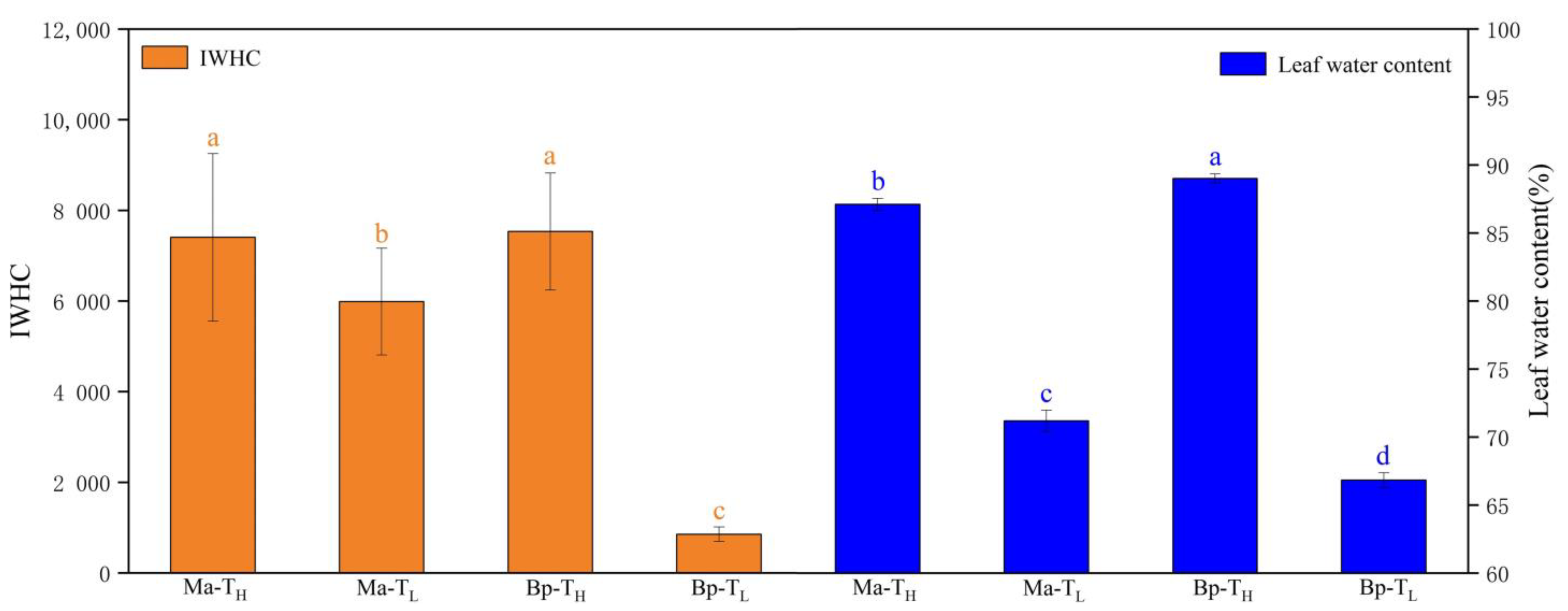
3.4. Intracellular Water Utilization of M. alba and B. papyrifera in Two Habitats
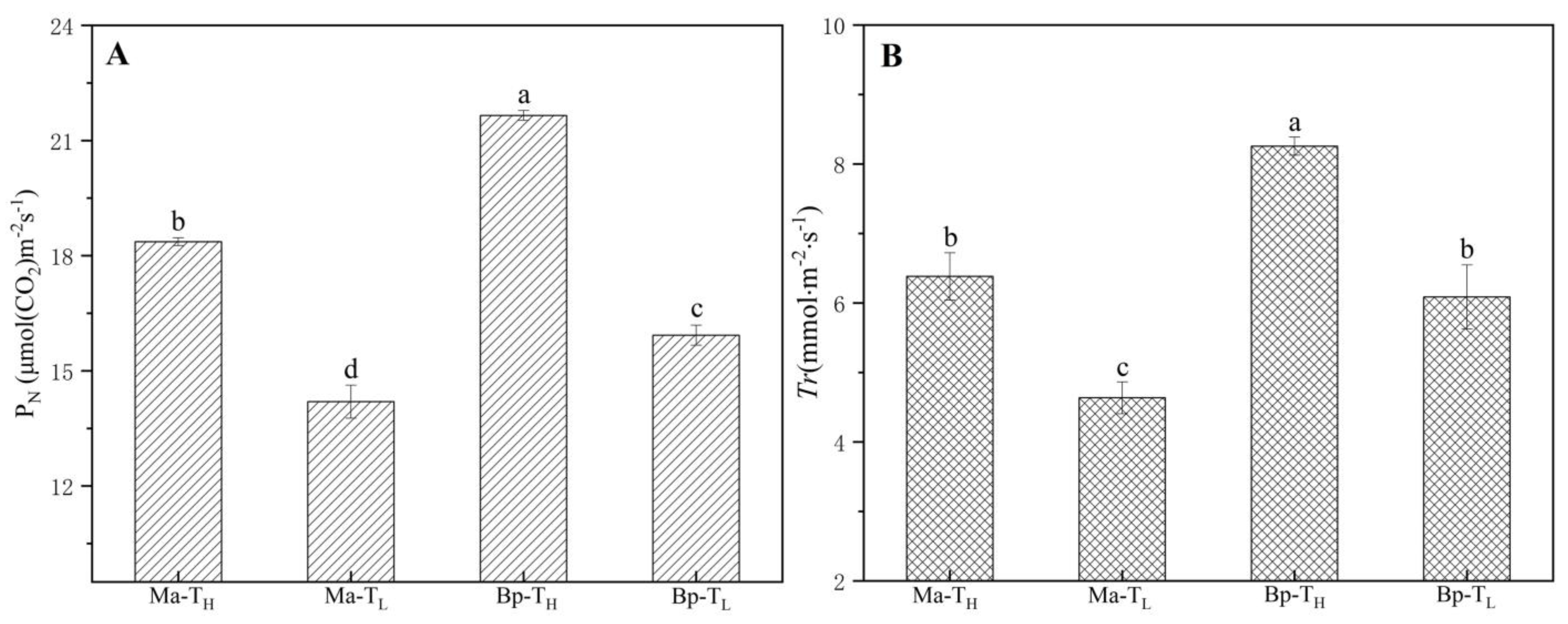
3.5. Photosynthetic Parameters of M. alba and B. papyrifera in Two Habitats
3.6. Leaf Water Potential of M. alba and B. papyrifera in Two Habitats
3.7. Correlation of Different Physiological Information and Intracellular Water Utilization Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wu, Y.Y.; Liu, C.Q.; Li, P.P.; Wang, J.Z.; Xing, D.; Wang, B.L. Photosynthetic characteristics involved in adaptability to Karst soil and alien invasion of paper mulberry (Broussonetia papyrifera (L.) Vent.) in comparison with mulberry (Morus alba L.). Photosynthetica 2009, 47, 155–160. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhang, T.; Wang, X.; Hamann, M.T.; Kang, J.; Yu, D.Q.; Chen, R.Y. A chemical investigation of the leaves of Morus alba L. Molecules 2018, 23, 1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayan, K.; Raju, P.J.; Tikader, A.; Saratchnadra, B.; Alkhayri, J.; Subramaniam, S. Biotechnology of mulberry (Morus L.)—A review. Emir. J. Food Agric. 2014, 26, 472–496. [Google Scholar] [CrossRef]
- Ye, J.; Chen, W.F.; Feng, L.W.; Liu, G.Z.; Wang, Y.; Li, H.X.; Ye, Z.B.; Zhang, Y.Y. The chaperonin 60 protein SlCpn60a1 modulates photosynthesis and photorespiration in tomato. J. Exp. Bot. 2020, 71, 7224–7240. [Google Scholar] [CrossRef] [PubMed]
- Samarah, N.H.; AL-Quraan, N.A.; Massad, R.S.; Welbaum, G.E. Treatment of bell pepper (Capsicum annuum L.) seeds with chitosan increases chitinase and glucanase activities and enhances emergence in a standard cold test. Sci. Hortic. 2020, 269, 109393. [Google Scholar] [CrossRef]
- Tosin, R.; Pôças, I.; Novo, H.; Teixeira, J.; Fontes, N.; Graça, A.; Cunha, M. Assessing predawn leaf water potential based on hyperspectral data and pigment’s concentration of Vitis vinifera L. in the Douro Wine Region. Sci. Hortic. 2021, 278, 109860. [Google Scholar] [CrossRef]
- Bhusal, N.; Han, S.G.; Yoon, T.M. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Arif, S.W.; Aqil, A.; Shamsul, H.; Qazi, F. Salt-induced modulation in growth, photosynthesis and antioxidant system in two varieties of Brassica juncea. Saudi J. Biol. Sci. 2013, 20, 183–193. [Google Scholar]
- Vos, J.; Groenwold, J. Genetic differences in water-use efficiency, stomatal conductance and carbon isotope fractionation in potato. Potato Res. 1989, 32, 113–121. [Google Scholar] [CrossRef]
- Horike, H.; Kinoshita, T.; Kume, A.; Hanba, Y. Responses of leaf photosynthetic traits, water use efficiency, and water relations in five urban shrub tree species under drought stress and recovery. Trees 2021, 35, 1–5. [Google Scholar] [CrossRef]
- Hammoud, M.; Alturk, S.M.; Sebaaly, Z.E.; Sassine, Y.N. Drip vs. mini-sprinkler irrigation system on leaf water potential and various vegetative attributes of Annona squamosa under Lebanese conditions. J. Agr. Sci. 2021, 43, 338–346. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Huner, N.P.A. Introduction to Plant Physiology, 3rd ed.; John Wiley & Sons Inc.: New York, NY, USA, 2004; p. 27. [Google Scholar]
- Choi, W.G.; Hilleary, R.; Swanson, S.J.; Kim, S.H.; Gilroy, S. Rapid, long-distance electrical and calcium signaling in plants. Annu. Rev. Plant Biol. 2016, 67, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Hedrich, R.; Salvador-Recatala, V.; Dreyer, I. Electrical wiring and long-distance plant communication. Trends Plant Sci. 2016, 21, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Kurenda, A.; Stolz, S.; Chetelat, A.; Farmer, E.E. Identification of cell populations necessary for leaf-to-leaf electrical signaling in a wounded plant. Proc. Natl. Acad. Sci. USA 2018, 115, 10178–10183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahlberg, R.; Cleland, R.E.; Volkenburgh, E.V. Slow wave potentials—A propagating electrical signal unique to higher plants. In Communication in Plants; Springer: Berlin/Heidelberg, Germany, 2006; pp. 291–308. [Google Scholar]
- Sorkin, M.L.; Nusinow, D.A. Time will tell: Intercellular communication in the plant clock. Trends Plant Sci. 2021, 26, 706–719. [Google Scholar] [CrossRef]
- Fromm, J.; Lautner, S. Electrical signals and their physiological significance in plants. Plant Cell Environ. 2010, 30, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Lautner, S.; Grams, T.; Matyssek, R.; Fromm, J. Characteristics of electrical sifnals in poplar and responses in photosynthesis. Plant Physiol. 2005, 138, 2200–2209. [Google Scholar] [CrossRef] [Green Version]
- Seo, P.J.; Mas, P. Stressing the role of the plant circadian clock. Trends Plant Sci. 2015, 20, 230–237. [Google Scholar] [CrossRef]
- Asner, G.P.; Brodrick, P.G.; Anderson, C.B.; Vaughn, N.; Martin, R.E. Progressive forest canopy water loss during the 2012–2015 California drought. Proc. Natl. Acad. Sci. USA 2016, 113, E249. [Google Scholar] [CrossRef] [Green Version]
- Gallé, A.; Lautner, S.; Flexas, J.; Fromm, J. Environmental stimuli and physiological responses: The current view on electrical signaling. Environ. Exp. Bot. 2015, 114, 15–21. [Google Scholar] [CrossRef]
- Buckley, D.J.; Lefebvre, M.; Meijer, E.G.M.; Brown, D.C.W. A signal generator for electrofusion of plant protoplasts. Comput. Electron. Agr. 1990, 5, 179–185. [Google Scholar] [CrossRef]
- Sanan-Mishra, N. Electric signaling and long-distance communication in plants. In Sensory Biology of Plants; Springer Nature: Singapore, 2019; pp. 509–535. [Google Scholar]
- Zhang, C.; Wu, Y.Y.; Su, Y.; Xing, D.K.; Dai, Y.; Wu, Y.S.; Fang, L. A plant’s electrical parameters indicate its physiological state: A study of intracellular water metabolism. Plants 2020, 9, 1256. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot.-Lond. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- David, T.S.; Henriques, M.O.; Kurz-Besson, C.; Nunes, J.; Valente, F.; Vaz, M.; Pereira, J.S.; Siegwolf, R.; Chaves, M.M.; Gazarini, L.C. Water-use strategies in two co-occurring Mediterranean evergreen oaks: Surviving the summer drought. Tree Physiol. 2007, 27, 793–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tranmotini, S.; Leeuwen, C.V.; Domec, J.C.; Destrac-Irvine, A.; Basteau, C.; Vitali, M.; Mosbach-Schulz, O.; Lovisolo, C. Impact of soil texture and water availability on the hydraulic control of plant and grape-berry development. Plant Soil 2013, 368, 215–230. [Google Scholar] [CrossRef]
- Sun, Z.J.; Livingston, N.J.; Guy, R.D.; Ethier, G.J. Stable carbon isotope as indicators of increased water use efficiency and productivity in white spruce (Picea glauca (Moench) Voss) seedlings. Plant Cell Environ. 1996, 19, 887–894. [Google Scholar] [CrossRef]
- González-Fernández, A.B.; Rodríguez-Pérez, J.R.; Marcelo, V.; Valenciano, J.B. Using field spectrometry and a plant probe accessory to determine leaf water content in commercial vineyards. Agr. Water Manag. 2015, 156, 43–50. [Google Scholar] [CrossRef]
- Yu, R.; Wu, Y.Y.; Xing, D.K. Can Electrophysiological parameters substitute for growth, and photosynthetic parameters to characterize the response of Mulberry and paper Mulberry to drought? Plants 2021, 10, 1772. [Google Scholar] [CrossRef]
- Martínez, J.P.; Silva, H.; Ledent, J.F.; Pinto, M. Effect of drought stress on the osmotic adjustment, cell wall elasticity and cell volume of six cultivars of common beans (Phaseolus vulgaris L.). Eur. J. Agron. 2007, 26, 30–38. [Google Scholar] [CrossRef]
- Xing, D.K.; Chen, X.L.; Wu, Y.Y.; Chen, Q.; Li, L.; Fu, W.G.; Shu, Y. Leaf stiffness of two Moraceae species based on leaf tensity determined by compressing different external gripping forces under dehydration stress. J. Plant Interact. 2019, 14, 610–616. [Google Scholar] [CrossRef] [Green Version]
- Xing, D.K.; Xu, X.J.; Wu, Y.Y.; Liu, Y.J.; Wu, Y.S.; Ni, J.H.; Azeem, A. Leaf tensity: A method for rapid determination of water requirement information in Brassica napus L. J. Plant Interact. 2018, 13, 380–387. [Google Scholar] [CrossRef] [Green Version]
- Xing, D.K.; Chen, X.; Wu, Y.Y.; Zwiazek, J.J. Leaf physiological impedance and elasticity modulus in Orychophragmus violaceus seedlings subjected to repeated osmotic stress. Sci. Hortic. 2021, 276, 109763. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Y.Y.; Su, Y.; Li, H.; Fang, L.; Xing, D.K. Plant’s electrical information manifests the composition and nutrient transport characteristics of membrane proteins. Plant Signal. Behav. 2021, 16, 1918867. [Google Scholar] [CrossRef]
- Chen, D.Q.; Wang, S.W.; Cao, B.B.; Cao, D.; Leng, G.H.; Li, H.B.; Yin, L.N.; Shan, L.; Deng, X.P. Genotypic variation in growth and physiological response to drought stress and re-watering reveals the critical role of recovery in drought adaptation in maize seedlings. Front. Plant Sci. 2016, 6, 1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacramento, B.L.D.; Azevedo, A.D.D.; Alves, A.T.; Moura, S.C.; Ribas, R.F. Photosynthetic parameters as physiological indicators of tolerance to cadmium stress in sunflower genotypes. Rev. Caatinga 2018, 31, 907–916. [Google Scholar] [CrossRef] [Green Version]
- Dghim, F.; Abdellaoui, R.; Boukhris, M.; Neffati, M.; Chaieb, M. Physiological and biochemical changes in Periploca angustifolia plants under withholding irrigation and rewatering conditions. S. Afr. J. Bot. 2018, 114, 241–249. [Google Scholar] [CrossRef]
- Guo, W.H.; Li, B.; Huang, Y.M.; Zhao, H.X.; Zhang, X.S. Effects of different water stresses on eco-physiological characteristics of Hippophae rhamnoides seedlings. Acta Bot. Sin. 2003, 45, 1238–1244. [Google Scholar]
- Djanaguiraman, M.; Nair, R.; Giraldo, J.P.; Prasad, P.V.V. Cerium oxide nanoparticles decrease drought-induced oxidative damage in sorghum leading to higher photosynthesis and grain yield. ACS Omega 2018, 3, 14406–14416. [Google Scholar] [CrossRef]
- Sourour, A. A review: Morphological, physiological, biochemical and molecular plant responses to water deficit stress. Int. J. Eng. Sci. 2017, 6, 1–4. [Google Scholar] [CrossRef]
- Buckley, T.N. The control of stomata by water balance. New Phytol. 2005, 168, 275–292. [Google Scholar] [CrossRef]
- Yang, X.H.; Chen, X.Y.; Ge, Q.Y.; Li, B.; Tong, Y.P.; Zhang, A.M.; Li, Z.S.; Kuang, T.Y.; Lu, C.M. Tolerance of photosynthesis to photoinhibition, high temperature and drought stress in flag leaves of wheat: A comparison between a hybridization line and its parents grown under field conditions. Plant Sci. 2006, 171, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Sterratt, D. Nernst Equation. In Encyclopedia of Computational Neuroscience; Springer Science & Business Media: New York, NY, USA, 2014; pp. 1–3. [Google Scholar]
- Reitner, J. Encyclopedia of Earth Sciences Series; Springer Science & Business: Berlin/Heidelberg, Germany, 2011; pp. 227–238. [Google Scholar]
- Debono, M.W. Dynamic protoneural networks in plants: A new approach of spontaneous extracellular potential variations. Plant Signal. Behav. 2013, 8, e24207. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, C.; Gopal, R.; Dube, B.K. Impact of iron stress on biomass, yield, metabolism and quality of potato (Solanum tuberosum L.). Sci. Hortic. 2006, 108, 1–6. [Google Scholar] [CrossRef]
- Qin, J.; He, N.J.; Wang, Y.; Xiang, Z.H. Ecological issues of Mulberry and sustainable development. J. Resour. Ecol. 2012, 3, 330–339. [Google Scholar]
- Javed, Q.; Wu, Y.Y.; Xing, D.K.; Azeem, A.; Ullah, I.; Zaman, M. Re-watering: An effective measure to recover growth and photosynthetic characteristics in salt-stressed Brassica napus L. Chil. J. Agric. Res. 2017, 77, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Kertész, Á.; Hlaváčová, Z.; Vozáry, E.; Staroňová, L. Relationship between moisture content and electrical impedance of carrot slices during drying. Int. Agrophys. 2015, 29, 61–66. [Google Scholar] [CrossRef]
- Gil, P.M.; Gurovich, L.; Schaffer, B.; Alcayaga, J.; Rey, S.; Iturriaga, R. Root to leaf electrical signaling in avocado in response to light and soil water content. J. Plant Physiol. 2008, 165, 1070–1078. [Google Scholar] [CrossRef]

| Plants | Treatment | ξS (H2O) (%) |
|---|---|---|
| M. alba | TH | 21.54 ± 0.89 a |
| TL | 7.90 ± 0.54 b | |
| B. papyrifera | TH | 21.26 ± 0.64 a |
| TL | 7.77 ± 0.50 b |
| Plants | Treatment | Z-F | Xc-F | R-F | Xl-F |
|---|---|---|---|---|---|
| y0/k1/b1 R2/p< | p0/k2/b2 R2/p< | g0/k3/b3 R2/p< | q0/k4/b4 R2/p< | ||
| M. alba | TH-1 | 0.0249/0.1230/0.5424 0.9937/0.0001 | 0.0132/0.0921/0.5156 0.9931/0.0001 | 0.0315/0.1373/0.5671 0.9927/0.0001 | 0.0149/0.0999/0.5188 0.9933/0.0001 |
| TH-2 | 0.0288/0.2364/0.5862 0.9925/0.0001 | 0.0173/0.2489/0.7858 0.9674/0.0001 | 0.0365/0.2418/0.5473 0.9960/0.0001 | 0.0194/0.2611/0.7665 0.9700/0.0001 | |
| TH-3 | 0.0178/0.1831/0.5822 0.9779/0.0001 | 0.0072/0.0874/0.4052 0.9795/0.0001 | 0.0230/0.2495/0.6632 0.9764/0.0001 | 0.0088/0.0998/0.4278 0.9792/0.0001 | |
| TH-4 | 0.0255/0.2368/0.5748 0.9878/0.0001 | 0.0177/0.2925/0.6980 0.9845/0.0001 | 0.0296/0.2419/0.5606 0.9879/0.0001 | 0.0195/0.3059/0.6904 0.9847/0.0001 | |
| TL-1 | 0.0337/0.1395/0.5515 0.9922/0.0001 | 0.0236/0.1842/0.7192 0.9832/0.0001 | 0.0393/0.1409/0.5402 0.9914/0.0001 | 0.0258/0.1915/0.7078 0.9842/0.0001 | |
| TL-2 | 0.0371/0.0561/0.3261 0.9918/0.0001 | 0.0290/0.1020/0.3708 0.9908/0.0001 | 0.0413/0.0547/0.3380 0.9910/0.0001 | 0.0314/0.1052/0.3696 0.9909/0.0001 | |
| TL-3 | 0.0537/0.2467/0.9774 0.9733/0.0001 | 0.0337/0.1832/0.6666 0.9673/0.0001 | 0.0622/0.2995/1.1238 0.9783/0.0001 | 0.0373/0.1972/0.6847 0.9676/0.0001 | |
| TL-4 | 0.0277/0.1153/0.8068 0.9792/0.0001 | 0.0167/0.1170/0.7476 0.9797/0.0001 | 0.0332/0.1279/0.8939 0.9789/0.0001 | 0.0186/0.1240/0.7530 0.9797/0.0001 | |
| B. papyrifera | TH-1 | 0.0298/0.2235/0.6178 0.9889/0.0001 | 0.0145/0.1875/0.6226 0.9844/0.0001 | 0.0403/0.2442/0.6525 0.9889/0.0001 | 0.0167/0.2014/0.6237 0.9850/0.0001 |
| TH-2 | 0.0252/0.1182/0.4428 0.9933/0.0001 | 0.0129/0.1109/0.5128 0.9899/0.0001 | 0.0343/0.1210/0.4478 0.9910/0.0001 | 0.0148/0.1179/0.5084 0.9905/0.0001 | |
| TH-3 | 0.0277/0.2136/0.5703 0.9914/0.0001 | 0.0138/0.1335/0.5496 0.9853/0.0001 | 0.0369/0.2539/0.5912 0.9932/0.0001 | 0.0158/0.1477/0.5528 0.9863/0.0001 | |
| TH-4 | 0.0349/0.1283/0.3730 0.9938/0.0001 | 0.0194/0.0936/0.4646 0.9887/0.0001 | 0.0438/0.1399/0.3266 0.9940/0.0001 | 0.0219/0.1013/0.4534 0.9894/0.0001 | |
| TL-1 | 0.0980/0.5444/0.5290 0.9793/0.0001 | 0.0513/0.3909/0.4726 0.9949/0.0001 | 0.1225/0.6150/0.5545 0.9844/0.0001 | 0.0582/0.4256/0.4785 0.9943/0.0001 | |
| TL-2 | 0.1448/0.6665/0.6820 0.9840/0.0001 | 0.0880/0.5156/0.5623 0.9858/0.0001 | 0.1700/0.7525/0.7302 0.9804/0.0001 | 0.0975/0.5559/0.5698 0.9851/0.0001 | |
| TL-3 | 0.1104/0.7424/0.4926 0.9807/0.0001 | 0.0794/0.6497/0.5687 0.9861/0.0001 | 0.1461/0.9212/0.6337 0.9944/0.0001 | 0.0937/0.7578/0.6509 0.9844/0.0001 | |
| TL-4 | 0.1176/0.9636/0.8204 0.9709/0.0001 | 0.0635/0.6419/0.7601 0.9769/0.0001 | 0.1480/1.1231/0.8556 0.9620/0.0001 | 0.0717/0.7038/0.7656 0.9770/0.0001 |
| Plants | Treatment | IZ (MΩ) | IR (MΩ) | IXl (MΩ) | IXc (MΩ) | IC (pF) |
|---|---|---|---|---|---|---|
| M. alba | TH | 0.22 ± 0.03 b | 0.25 ± 0.03 b | 0.21 ± 0.06 b | 0.19 ± 0.06 b | 358.55 ± 44.96 a |
| TL | 0.18 ± 0.04 b | 0.20 ± 0.06 b | 0.18 ± 0.03 b | 0.17 ± 0.02 b | 325.33 ± 43.61 a | |
| B. papyrifera | TH | 0.20 ± 0.03 b | 0.23 ± 0.03 b | 0.16 ± 0.02 b | 0.15 ± 0.02 b | 380.16 ± 45.24 a |
| TL | 0.85 ± 0.09 a | 1.00 ± 0.11 a | 0.69 ± 0.08 a | 0.62 ± 0.07 a | 88.96 ± 10.86 b |
| Plants | Treatment | d | IWUE | IWHT | WTR |
|---|---|---|---|---|---|
| M. alba | TH | 547.54 ± 58.45 a | 0.12 ± 0.04 a | 71.18 ± 5.14 a | 93.18 ± 25.03 a |
| TL | 279.29 ± 61.00 b | 0.05 ± 0.01 a | 53.03 ± 7.88 a | 124.90 ± 35.21 a | |
| B. papyrifera | TH | 468.86 ± 55.19 a | 0.07 ± 0.02 a | 72.88 ± 5.65 a | 104.93 ± 19.79 a |
| TL | 102.62 ± 12.17 c | 0.13 ± 0.02 a | 72.94 ± 4.17 a | 11.66 ± 1.94 b |
| IR | IXl | IXc | IC | d | IWHC | IWUE | IWHT | WTR | Ψ | ξL | PN | Tr | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IZ | 0.961 ** | 0.741 * | 0.713 * | −0.622 | 0.131 | −0.583 | 0.624 | 0.184 | −0.940 ** | 0.289 | 0.260 | 0.261 | 0.123 |
| IR | 0.537 | 0.501 | −0.404 | 0.215 | −0.364 | 0.432 | 0.426 | −0.857 ** | 0.277 | 0.245 | 0.247 | 0.069 | |
| IXl | 0.999 ** | −0.951 ** | −0.151 | −0.927 ** | 0.869 ** | −0.477 | −0.806 * | 0.176 | 0.152 | 0.157 | 0.082 | ||
| IXc | −0.957 ** | −0.169 | −0.935 ** | 0.868 ** | −0.512 | −0.785 * | 0.163 | 0.140 | 0.146 | 0.080 | |||
| IC | 0.397 | 0.998 ** | −0.714 * | 0.642 | 0.705 | 0.119 | 0.131 | 0.073 | 0.026 | ||||
| d | 0.449 | 0.295 | 0.649 | −0.189 | 0.761 * | 0.799 * | 0.530 | 0.319 | |||||
| IWHC | −0.671 | 0.676 | 0.669 | 0.183 | 0.193 | 0.129 | 0.057 | ||||||
| IWUE | −0.302 | −0.720 * | 0.549 | 0.550 | 0.406 | 0.259 | |||||||
| IWHT | −0.061 | 0.373 | 0.372 | 0.307 | 0.115 | ||||||||
| WTR | −0.271 | −0.252 | −0.213 | −0.122 | |||||||||
| Ψ | 0.977 ** | 0.839 ** | 0.573 | ||||||||||
| ξL | 0.883 ** | 0.667 | |||||||||||
| PN | 0.861 ** |
| IR | IXl | IXc | IC | d | IWHC | IWUE | IWHT | WTR | Ψ | ξL | PN | Tr | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IZ | 0.998 ** | 0.970 ** | 0.979 ** | −0.930 ** | −0.858 ** | −0.901 ** | 0.783 * | 0.082 | −0.893 ** | −0.938 ** | −0.931 ** | −0.979 ** | −0.885 ** |
| IR | 0.979 ** | 0.985 ** | −0.927 ** | −0.854 ** | −0.897 ** | 0.792 * | 0.066 | −0.891 ** | −0.935 ** | −0.931 ** | −0.977 ** | −0.894 ** | |
| IXl | 0.998 ** | −0.931 ** | −0.860 ** | −0.899 ** | 0.756 * | −0.098 | −0.880 ** | −0.941 ** | −0.937 ** | −0.951 ** | −0.917 ** | ||
| IXc | −0.939 ** | −0.866 ** | −0.908 ** | 0.758 * | −0.077 | −0.889 ** | −0.948 ** | −0.942 ** | −0.960 ** | −0.917 ** | |||
| IC | 0.786 * | 0.996 ** | −0.769 * | 0.038 | 0.977 ** | 0.947 ** | 0.921 ** | 0.866 ** | 0.782 * | ||||
| d | 0.738 * | −0.400 | −0.033 | 0.748 * | 0.918 ** | 0.931 ** | 0.922 ** | 0.694 | |||||
| IWHC | −0.773 * | 0.030 | 0.981 ** | 0.922 ** | 0.891 ** | 0.823 * | 0.745 * | ||||||
| IWUE | 0.004 | −0.746 * | −0.617 | −0.586 | −0.657 | −0.734 * | |||||||
| IWHT | −0.126 | 0.032 | 0.015 | −0.111 | 0.169 | ||||||||
| WTR | 0.901 ** | 0.875 ** | 0.826 * | 0.710 * | |||||||||
| Ψ | 0.996 ** | 0.933 ** | 0.765 * | ||||||||||
| ξL | 0.939 ** | 0.759 * | |||||||||||
| PN | 0.869 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, R.; Wu, Y.; Xing, D. The Differential Response of Intracellular Water Metabolism Derived from Intrinsic Electrophysiological Information in Morus alba L. and Broussonetia papyrifera (L.) Vent. Subjected to Water Shortage. Horticulturae 2022, 8, 182. https://doi.org/10.3390/horticulturae8020182
Yu R, Wu Y, Xing D. The Differential Response of Intracellular Water Metabolism Derived from Intrinsic Electrophysiological Information in Morus alba L. and Broussonetia papyrifera (L.) Vent. Subjected to Water Shortage. Horticulturae. 2022; 8(2):182. https://doi.org/10.3390/horticulturae8020182
Chicago/Turabian StyleYu, Rui, Yanyou Wu, and Deke Xing. 2022. "The Differential Response of Intracellular Water Metabolism Derived from Intrinsic Electrophysiological Information in Morus alba L. and Broussonetia papyrifera (L.) Vent. Subjected to Water Shortage" Horticulturae 8, no. 2: 182. https://doi.org/10.3390/horticulturae8020182
APA StyleYu, R., Wu, Y., & Xing, D. (2022). The Differential Response of Intracellular Water Metabolism Derived from Intrinsic Electrophysiological Information in Morus alba L. and Broussonetia papyrifera (L.) Vent. Subjected to Water Shortage. Horticulturae, 8(2), 182. https://doi.org/10.3390/horticulturae8020182








