Effects of Light Intensity and Temperature on the Photosynthesis Characteristics and Yield of Lettuce
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Light–Response Curves Measurements
2.3. Yield Measurements
2.4. Statistical Analysis
3. Results
3.1. Light–Response Curves
3.2. Light–Response Curves Parameters
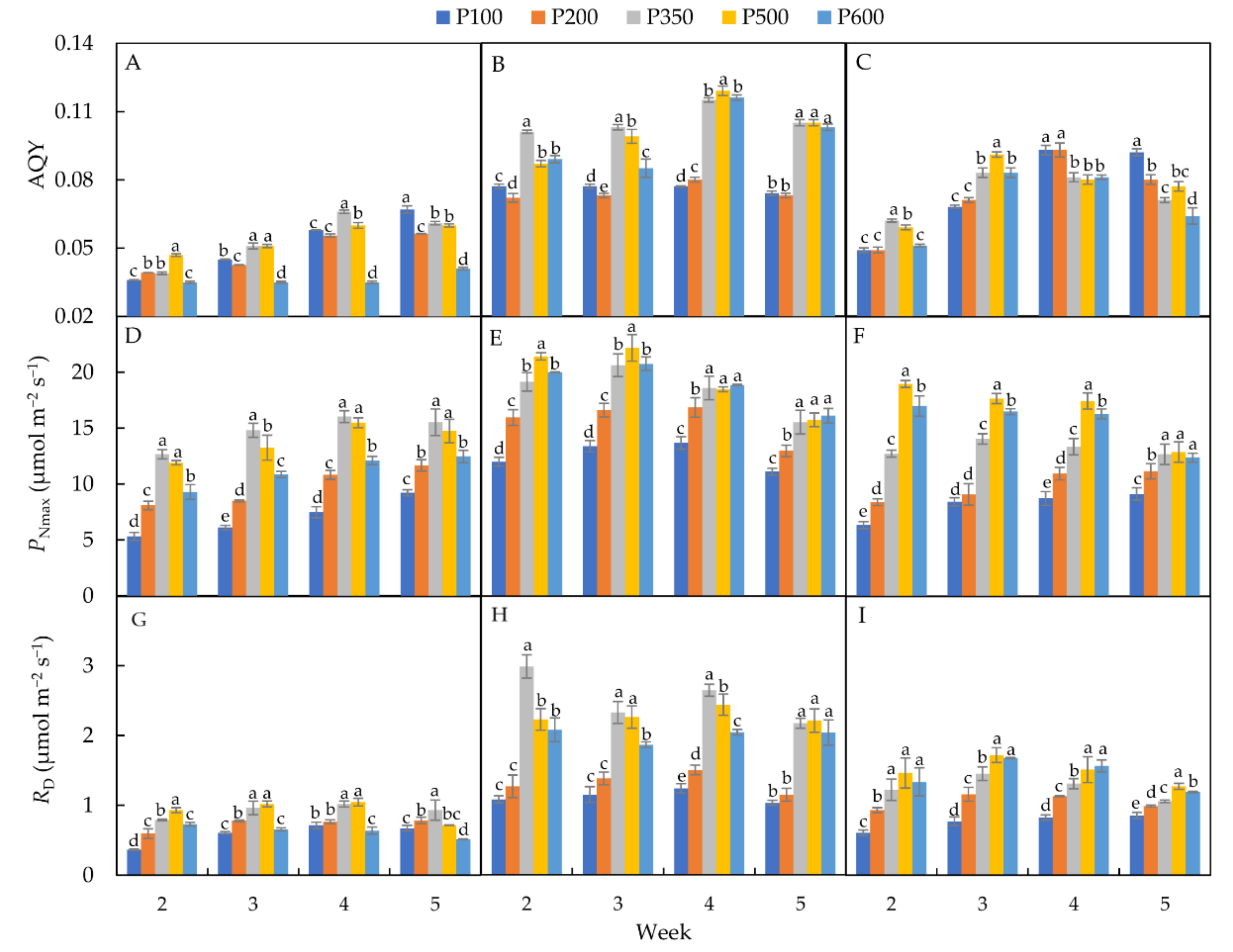
3.2.1. Apparent Quantum Efficiency (AQY)
3.2.2. Maximum Photosynthetic Rate (PNmax)
3.2.3. Dark Respiration Rate (RD)
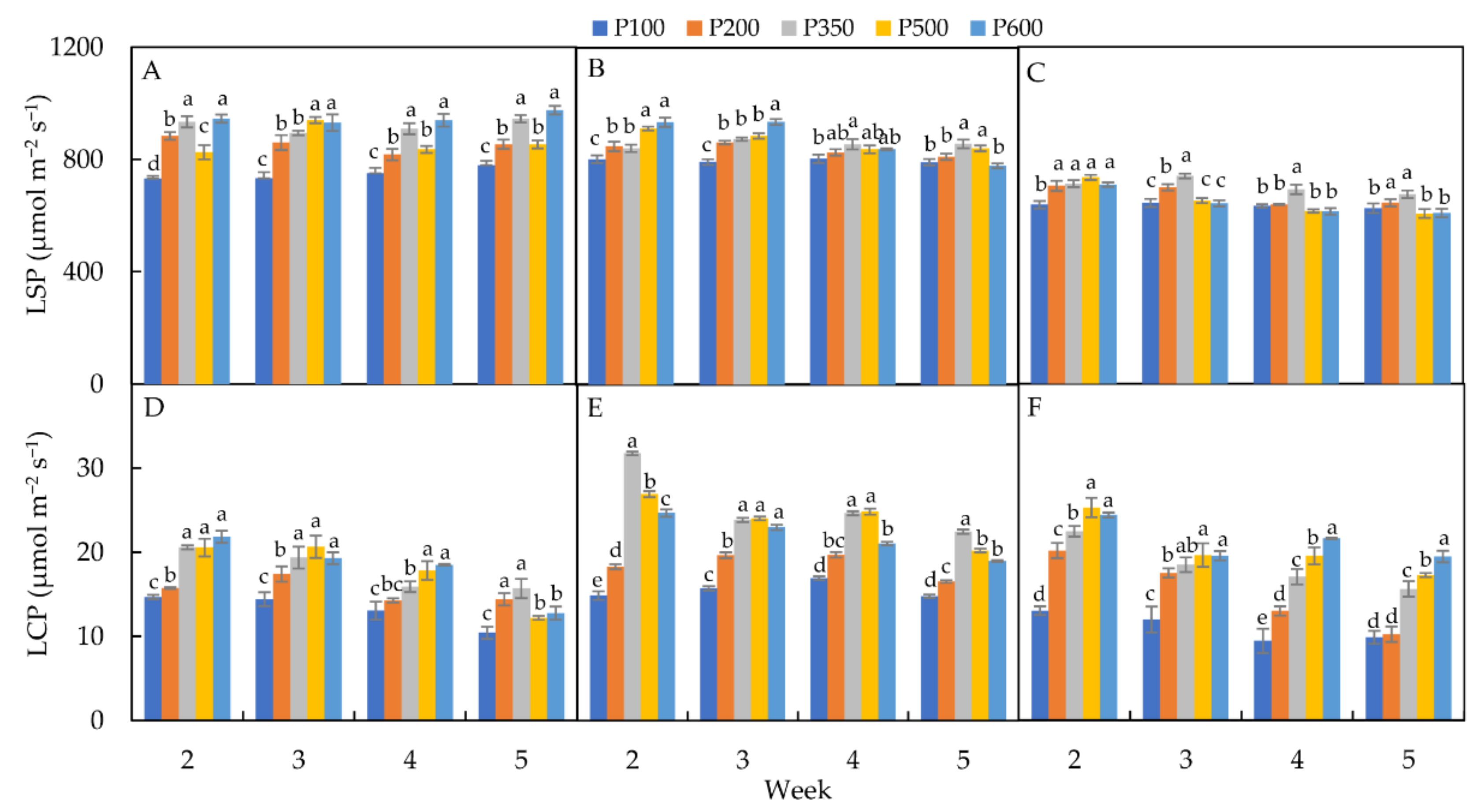
3.2.4. Light-Saturation Point (LSP)
3.2.5. Light-Compensation Point (LCP)
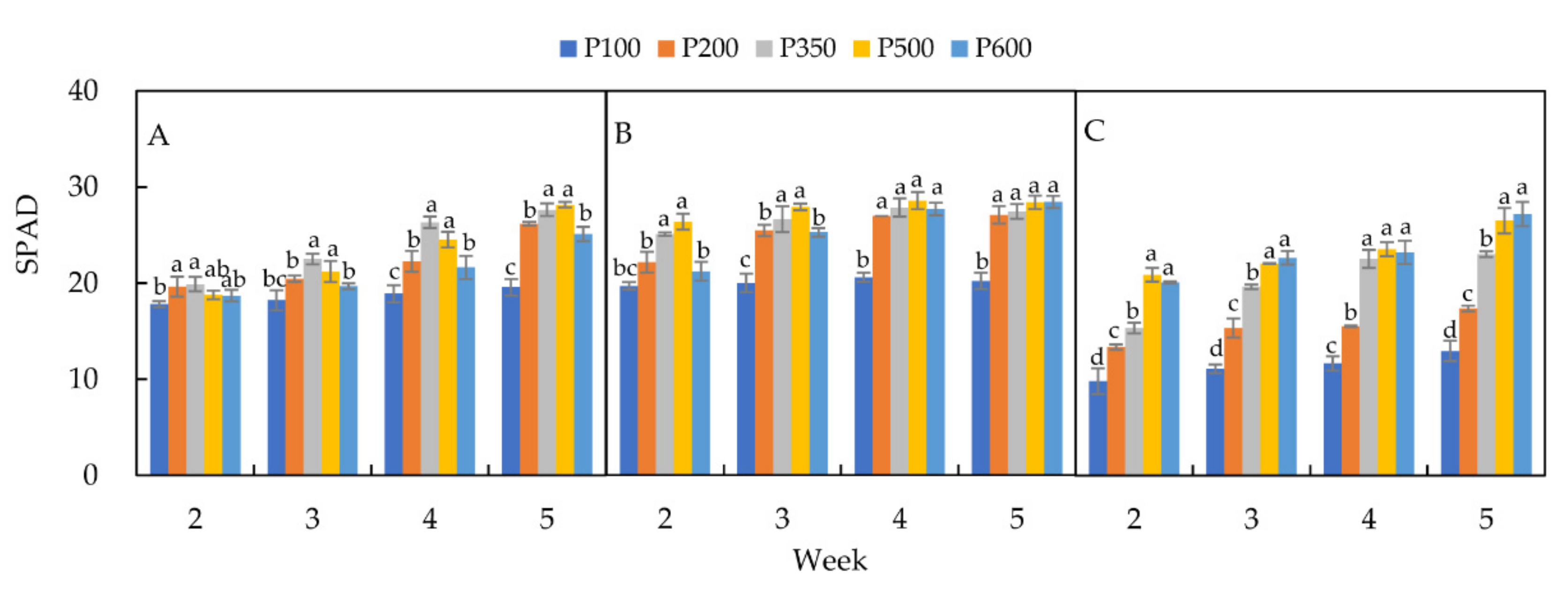
3.3. Chlorophyll Content (SPAD)
3.4. Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed Hesham, A.; Tong, Y.; Yang, Q. Optimal Control of Environmental Conditions Affecting Lettuce Plant Growth in a Controlled Environment with Artificial Lighting: A Review. S. Afr. J. Bot. 2020, 130, 75–89. [Google Scholar] [CrossRef]
- Chen, J.-W.; Kuang, S.-B.; Long, G.-Q.; Yang, S.-C.; Meng, Z.-G.; Li, L.-G.; Chen, Z.-J.; Zhang, G.-H. Photosynthesis, light energy partitioning, and photoprotection in the shade-demanding species Panax notoginseng under high and low level of growth irradiance. Funct. Plant Biol. 2016, 43, 479. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Li, P.; Wu, Y.; Tang, J. Effects of Different Light Intensities on Anti-Oxidative Enzyme Activity, Quality and Biomass in Lettuce. Hortic. Sci. 2012, 39, 129–134. [Google Scholar]
- Allen, D.J.; Ort, D.R. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 2001, 6, 36–42. [Google Scholar] [CrossRef]
- Dufault, R.J.; Ward, B.; Hassell, R.L. Dynamic relationships between field temperatures and romaine lettuce yield and head quality. Sci. Hortic. 2009, 120, 452–459. [Google Scholar] [CrossRef]
- Ou, L.J.; Wei, G.; Zhang, Z.Q.; Dai, X.Z.; Zou, X.X. Effects of low temperature and low irradiance on the physiological characteristics and related gene expression of different pepper species. Photosynthetica 2015, 53, 85–94. [Google Scholar] [CrossRef]
- Branco, M.C.D.S.; de Almeida, A.-A.F.; Dalmolin, A.; Ahnert, D.; Baligar, V.C. Influence of low light intensity and soil flooding on cacao physiology. Sci. Hortic. 2017, 217, 243–257. [Google Scholar] [CrossRef]
- Zheng, Y.; Mai, B.; Wu, R.; Feng, Y.; Sofo, A.; Ni, Y.; Sun, J.; Li, J.; Xu, J. Acclimation of Winter Wheat (Triticum Aestivum, Cv. Yangmai 13) to Low Levels of Solar Irradiance. Photosynthetica 2011, 49, 3. [Google Scholar] [CrossRef]
- Ruelland, E.; Zachowski, A. How plants sense temperature. Environ. Exp. Bot. 2010, 69, 225–232. [Google Scholar] [CrossRef]
- Pan, J.; Guo, B. Effects of Light Intensity on the Growth, Photosynthetic Characteristics, and Flavonoid Content of Epimedium pseudowushanense B.L. Guo. Molecules 2016, 21, 1475. [Google Scholar] [CrossRef] [Green Version]
- Percival, G. The Use Of Chlorophyll Fluorescence To Identify Chemical And Environmental Stress In Leaf Tissue Of Three Oak (Quercus) Species. Arboric. Urban For. 2005, 31, 215–227. [Google Scholar] [CrossRef]
- Yan, N.; Xu, X.-F.; Wang, Z.-D.; Huang, J.-Z.; Guo, D.-P. Interactive effects of temperature and light intensity on photosynthesis and antioxidant enzyme activity in Zizania latifolia Turcz. plants. Photosynthetica 2013, 51, 127–138. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Hang, T.; Lu, N.; Takagaki, M.; Mao, H. Leaf area model based on thermal effectiveness and photosynthetically active radiation in lettuce grown in mini-plant factories under different light cycles. Sci. Hortic. 2019, 252, 113–120. [Google Scholar] [CrossRef]
- Wang, H.; Sánchez-Molina, J.A.; Li, M.; Berenguel, M.; Yang, X.T.; Bienvenido, J.F. Leaf Area Index Estimation for a Greenhouse Transpiration Model Using External Climate Conditions Based on Genetics Algorithms, Back-Propagation Neural Networks and Nonlinear Autoregressive Exogenous Models. Agric. Water Manag. 2017, 183, 107–115. [Google Scholar] [CrossRef]
- Zaidi, M.; Murase, H.; Honami, N. Neural Network Model for the Evaluation of Lettuce Plant Growth. J. Agric. Eng. Res. 1999, 74, 237–242. [Google Scholar] [CrossRef]
- Křístková, E.; Doležalová, I.; Lebeda, A.; Vinter, V.; Novotná, A. Description of Morphological Characters of Lettuce (Lactuca sativa L.) Genetic Resources. Sci. Hortic. 2008, 35, 113–129. [Google Scholar] [CrossRef] [Green Version]
- Mou, B. Lettuce. In Vegetables I: Asteraceae, Brassicaceae, Chenopodicaceae, and Cucurbitaceae; Prohens, J., Nuez, F., Eds.; Springer: New York, NY, USA, 2008; pp. 75–116. [Google Scholar]
- Fu, W.; Li, P.; Wu, Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Fallovo, C.; Rouphael, Y.; Cardarelli, M.; Colla, G.; Rea, E.; Battistelli, A.; Colla, G. Yield and Quality of Leafy Lettuce in Response to Nutrient Solution Composition and Growing Season. J. Food Agric. Environ. 2009, 7, 456–462. [Google Scholar]
- Ghorbanzadeh, P.; Aliniaeifard, S.; Esmaeili, M.; Mashal, M.; Azadegan, B.; Seif, M. Dependency of Growth, Water Use Efficiency, Chlorophyll Fluorescence, and Stomatal Characteristics of Lettuce Plants to Light Intensity. J. Plant Growth Regul. 2021, 40, 2191–2207. [Google Scholar] [CrossRef]
- Kang, J.H.; KrishnaKumar, S.; Atulba, S.L.S.; Jeong, B.R.; Hwang, S.J. Light intensity and photoperiod influence the growth and development of hydroponically grown leaf lettuce in a closed-type plant factory system. Hortic. Environ. Biotechnol. 2013, 54, 501–509. [Google Scholar] [CrossRef]
- Wheeler, T.; Hadley, P.; Ellis, R.; Morison, J. Changes in growth and radiation use by lettuce crops in relation to temperature and ontogeny. Agric. For. Meteorol. 1993, 66, 173–186. [Google Scholar] [CrossRef]
- Yan, Z.; He, D.; Niu, G.; Zhai, H. Evaluation of growth and quality of hydroponic lettuce at harvest as affected by the light intensity, photoperiod and light quality at seedling stage. Sci. Hortic. 2019, 248, 138–144. [Google Scholar] [CrossRef]
- Knight, S.L.; Mitchell, C.A. Stimulation of lettuce productivity by manipulation of diurnal temperature and light. Sci. Hortic. 1983, 18, 462–463. [Google Scholar]
- Galieni, A.; Stagnari, F.; Speca, S.; Pisante, M. Leaf traits as indicators of limiting growing conditions for lettuce (Lactuca sativa). Ann. Appl. Biol. 2016, 169, 342–356. [Google Scholar] [CrossRef]
- Chen, Z.; Jahan, M.S.; Mao, P.; Wang, M.; Liu, X.; Guo, S. Functional growth, photosynthesis and nutritional property analyses of lettuce grown under different temperature and light intensity. J. Hortic. Sci. Biotechnol. 2021, 96, 53–61. [Google Scholar] [CrossRef]
- Noda, H.; Muraoka, H.; Washitani, I. Morphological and Physiological Acclimation Responses to Contrasting Light and Water Regimes in Primula sieboldii. Ecol. Res. 2004, 19, 331–340. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, Q.; Fan, D.; Lu, C. Photosynthetic light and CO2 utilization and C4 traits of two novel super-rice hybrids. J. Plant Physiol. 2006, 163, 529–537. [Google Scholar] [CrossRef]
- Avola, G.; Cavallaro, V.; Patanè, C.; Riggi, E. Gas exchange and photosynthetic water use efficiency in response to light, CO2 concentration and temperature in Vicia faba. J. Plant Physiol. 2008, 165, 796–804. [Google Scholar] [CrossRef]
- Xu, W.Z.; Deng, X.P.; Xu, B.C. Effects of water stress and fertilization on leaf gas exchange and photosynthetic light-response curves of Bothriochloa ischaemum L. Photosynthetica 2013, 51, 603–612. [Google Scholar] [CrossRef]
- Ye, Z.P.; Yu, Q. A coupled model of stomatal conductance and photosynthesis for winter wheat. Photosynthetica 2008, 46, 637–640. [Google Scholar] [CrossRef]
- Mihaljević, I.; Lepeduš, H.; Šimić, D.; Vuletić, M.V.; Tomaš, V.; Vuković, D.; Dugalić, K.; Teklić, T.; Babojelić, M.S.; Zdunić, Z. Photochemical efficiency of photosystem II in two apple cultivars affected by elevated temperature and excess light in vivo. S. Afr. J. Bot. 2020, 130, 316–326. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, T.-J.; Chow, W.S.; Xie, X.; Chen, Y.-J.; Peng, C.-L. Photosynthetic characteristics and light energy conversions under different light environments in five tree species occupying dominant status at different stages of subtropical forest succession. Funct. Plant Biol. 2015, 42, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. PLANT COLD ACCLIMATION: Freezing Tolerance Genes and Regulatory Mechanisms. Annu. Rev. Plant Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hikosaka, K.; Ishikawa, K.; Borjigidai, A.; Muller, O.; Onoda, Y. Temperature acclimation of photosynthesis: Mechanisms involved in the changes in temperature dependence of photosynthetic rate. J. Exp. Bot. 2006, 57, 291–302. [Google Scholar] [CrossRef] [Green Version]
- Loh, F.C.W.; Grabosky, J.; Bassuk, N.L. Using the SPAD 502 Meter to Assess Chlorophyll and Nitrogen Content of Benjamin Fig and Cottonwood Leaves. HortTechnology 2002, 12, 682–686. [Google Scholar] [CrossRef] [Green Version]
- Ling, Q.; Huang, W.; Jarvis, P. Use of a SPAD-502 meter to measure leaf chlorophyll concentration in Arabidopsis thaliana. Photosynth. Res. 2011, 107, 209–214. [Google Scholar] [CrossRef] [Green Version]
- Sharma, L.; Priya, M.; Bindumadhava, H.; Nair, R.; Nayyar, H. Influence of high temperature stress on growth, phenology and yield performance of mungbean [Vigna radiata (L.) Wilczek] under managed growth conditions. Sci. Hortic. 2016, 213, 379–391. [Google Scholar] [CrossRef]
- Patriyawaty, N.R.; Rachaputi, R.C.; George, D. Physiological mechanisms underpinning tolerance to high temperature stress during reproductive phase in mungbean (Vigna radiata (L.) Wilczek). Environ. Exp. Bot. 2018, 150, 188–197. [Google Scholar] [CrossRef] [Green Version]
- Pires, M.V.; Almeida, A.-A.F.; Figueiredo, A.L.; Gomes, F.P.; Souza, M.M. Photosynthetic characteristics of ornamental passion flowers grown under different light intensities. Photosynthetica 2011, 49, 593–602. [Google Scholar] [CrossRef]
- Noguchi, K. Effects of Light Intensity and Carbohydrate Status on Leaf and Root Respiration. In Plant Respiration: From Cell to Ecosystem; Lambers, H., Ribas-Carbo, M., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 63–83. [Google Scholar]
- Kaiser, E.; Morales, A.; Harbinson, J.; Heuvelink, E.; Prinzenberg, A.E.; Marcelis, L. Metabolic and diffusional limitations of photosynthesis in fluctuating irradiance in Arabidopsis thaliana. Sci. Rep. 2016, 6, 31252. [Google Scholar] [CrossRef] [PubMed]


| Effect | Week | df | AQY | PNmax | RD | LSP | LCP | SPAD |
|---|---|---|---|---|---|---|---|---|
| Light | 2 | 4 | ** | ** | ** | ** | ** | ** |
| 3 | 4 | ** | ** | ** | ** | ** | ** | |
| 4 | 4 | ** | ** | ** | ** | ** | ** | |
| 5 | 4 | ** | ** | ** | ** | ** | ** | |
| Temperature | 2 | 2 | ** | ** | ** | ** | ** | ** |
| 3 | 2 | ** | ** | ** | ** | ** | ** | |
| 4 | 2 | ** | ** | ** | ** | ** | ** | |
| 5 | 2 | ** | ** | ** | ** | ** | ** | |
| Light × Temperature | 2 | 8 | ** | ** | ** | ** | ** | ** |
| 3 | 8 | ** | ** | ** | ** | * | ** | |
| 4 | 8 | ** | ** | ** | ** | ** | ** | |
| 5 | 8 | ** | ** | ** | ** | ** | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Li, P.; Wang, J. Effects of Light Intensity and Temperature on the Photosynthesis Characteristics and Yield of Lettuce. Horticulturae 2022, 8, 178. https://doi.org/10.3390/horticulturae8020178
Zhou J, Li P, Wang J. Effects of Light Intensity and Temperature on the Photosynthesis Characteristics and Yield of Lettuce. Horticulturae. 2022; 8(2):178. https://doi.org/10.3390/horticulturae8020178
Chicago/Turabian StyleZhou, Jing, Pingping Li, and Jizhang Wang. 2022. "Effects of Light Intensity and Temperature on the Photosynthesis Characteristics and Yield of Lettuce" Horticulturae 8, no. 2: 178. https://doi.org/10.3390/horticulturae8020178
APA StyleZhou, J., Li, P., & Wang, J. (2022). Effects of Light Intensity and Temperature on the Photosynthesis Characteristics and Yield of Lettuce. Horticulturae, 8(2), 178. https://doi.org/10.3390/horticulturae8020178






