Abstract
Basils of the genus Ocimum are aromatic plants grown widely throughout the tropical and temperate regions. The essential oils obtained from their aerial parts are enriched with volatile organic compounds with high market demand for food and pharmaceutical industries. The volatile organic compounds have been shown to exhibit biological activities. Therefore, their novel applications have been extensively explored in the last few decades. The most widely available basils in the tropical areas include white holy basil (O. sanctum var. Shyama), red holy basil (O. sanctum var. Rama), Thai basil (O. basilicum var. thyrsiflorum), lemon basil (O. citriodorum), and tree basil (O. gratissimum). Over 60 volatiles of different classes have been exclusively described, and some of them could be useful as biomarkers for genotype specification. The major volatile ingredient is the phenylpropanoids, such as methyl eugenol, which has the potential as a natural product for mitigating Oriental fruit fly (Bactrocera dorsalis) during tropical fruit production. Moreover, basil essential oils are also used to control diseases of the fruits during post-harvest storage. As a result, the application of basil essential oils as a sustainable defect control strategy for tropical fruit value chains seems intriguing. This review provides comprehensive information on plant taxonomy and volatile compositions of the essential oil fractions from different basil species. Their biological activities and applications are also discussed, mainly during the pre- and post-production of tropical fruits. Additionally, the available techniques to enhance the efficacy of the volatile active compounds are also described.
1. Introduction
Ocimum is one of the important genera within the wealthiest essential oil-bearing plant family, the Lamiaceae. It is represented by more than 150 species cultivated and distributed throughout the tropical and temperate regions [1]. They are collectively known as the “basils” that retain the commercial demand for their nutritional, aromatic, ornamental, culinary, religious, and medicinal importance [2]. Different basil types are commonly used, including holy basil (O. sanctum), sweet or Thai basil (O. basilicum), lemon basil (O. citriodorum), and tree basil (O. gratissimum) [3,4]. It is well established that different basil cultivars have the genetic potential to create and maintain distinct sets of volatile components, resulting in a wide variety of chemotypes within the same basil species [5]. The essential oils of these basils are predominantly constituted of phenylpropanoids such as estragole, eugenol, and methyl eugenol; however, they also contain common monoterpenes such as geranial, neral, and α-ocimene, as well as sesquiterpenes such as β-caryophyllene, α-cubebene, and γ-muurolene [6]. Most of which are biologically active on living organisms, especially the antimicrobial and antioxidant properties for food and medicinal uses [7,8]. It was discovered that eugenol has antimicrobial and analgesic effects on humans [9]. Additionally, the essential oils also possess a wide range of biological functions that theoretically minimise post-harvest deteriorations. Volatile organic compounds have been shown to inhibit the growth of microorganisms, especially those responsible for post-harvest diseases such as Aspergillus spp. [10,11,12], Colletotrichum acutatum [13], Botrytis cinerea [14], and Penicillium italicum [15]. They have also been extensively used in insect pest management to control rice weevil (Sitophilus oryzae) [16], bean weevil (Acanthoscelides obtectus) [17], and cotton bollworm (Helicoverpa armigera) [18]. Prominently, methyl eugenol has been claimed for its ability to attract Oriental fruit flies (Bactrocera dorsalis) [19], the most important tropical fruit pest [20,21]. The estimated annual loss from this pest alone is roughly over US$ 100 million, and mangoes have been the most susceptible crops [20,21]. Aside from the infestation of the Oriental fruit flies that cause physiological damage to fresh fruits, biological stress could encourage post-harvest biochemical mechanisms such as browning and physiological decay [22]. A study of fresh apple has also proven that spraying the sweet basil essential oil on the fruit skin illustrated the preservative effect, thereby extending its shelf life [23]. With all these advantages, it is interesting to use basil essential oils as biological controls during the production of tropical fruits. However, the instability of essential oils at ambient conditions, as well as harsh environmental exposure, are the limitations. Moreover, volatile organic compounds decompose quickly with the presence of light, heat, humidity, and oxygen [24].
This review aims to serve as a guide to using the volatile components obtained from commercially available Ocimum species in the development of functional products for the sustainable production of tropical fruits. It attempts to provide the relevant data, both taxonomical and chemotypes, with particular attention to the biological activities and applications. The typical constraints of these applied uses are discussed, along with the recent approaches to improve efficiency.
2. Taxonomy
The genus Ocimum is known as one of the most prominent genera in the Lamiaceae family and currently comprises more than 150 species [1,25]. The distribution is mainly in the tropical and template regions and is likely to have originated (mainly the holy basil) in India [26]. Recently, they have been cultivated worldwide as culinary herbs and for essential oil extraction [27]. Taxonomical identification within the genus and between the varieties can be made by the morphological characteristics such as leaf shape and its colour, flower, and seed morphology [4,28]. Numerous polymorphisms resulting from extended cultivation and inter- and intra-specific cross hybridisation result in a vast range of subspecies, each with its own chemical makeup and biological activity [25,29,30].
The commonly available Ocimum plants were studied in the previous work for their distinct morphological characteristics [6]. The leaf is generally simple, petiolate and the leaf blade is ovate with a rounded base, oblique, and the apex is acute. O. gratissimum has a large leaf size (~45 cm2), whereas O. citriodorum has a leaf size of around 3.5 cm2 (Figure 1c,d). The O. sanctum of var. Rama and Shyama can be distinguished by having the aerials of red and white (Figure 1a). Similarly, different leaf and stem colours were noticed, ranging from red, purple-green, and green among the different varieties of the O. basilicum L. used in Iran [31]. Singh [32] used the number of leaf veins to show that O. americanum was described to have seven distinct veins, and the mid-vein reached the apex, while O. tenuiflorum has nine distinct veins, and the mid-vein does not reach the apex. The typical inflorescence of Ocimum spp. is a thyrse composed of opposite 1–3-flowered cymes (Figure 1b) [33]. The calyx is generally a short tube or funnel-shaped; it is straight or slightly curved. The corolla is formed forward (sometimes bent downwards), larger upper lip and a smaller lower one and declinate stamens [34]. The posterior lip of the corolla comprises four lobes. There are always four stamens, an anterior pair that attaches near the corolla mouth and a posterior pair that connects close to the corolla base. The size of basil seeds varies depending on the phenotype, cultivating location, and moisture content [35]. Its colour can occasionally be used to differentiate between varieties [36]. The complex polysaccharide structure gives the seed a unique mucilaginous characteristic after soaking in water, which is prominent in O. citriodorum and O. basilicum var. thyrsiflorum. Table 1 illustrates the taxonomical characteristics of different basil species.

Figure 1.
Morphological characteristics of some Ocimum species; aerial part (a) inflorescence (b) upper (c) and lower (d) leaf surface of lemon basil (O. citriodorum), Thai basil (O. basilicum var. thyrsiflorum), red holy basil (O. sanctum var. Rama), white holy basil (O. sanctum var. Shyama) and tree basil (O. gratissimum).

Table 1.
Comparison of morphological characteristics of studied Ocimum species.
3. Volatile Chemical Compositions of Basil Essential Oils
A number of unique epidermal structures known as trichomes are developed on the surface of the aerial part, which may or may not be secretory [45]. These include the glandular trichomes where the essential oil is localised and the non-glandular trichomes for pest defence [46]. Essential oils are refined lipophilic mixes derived as liquids that possess aromatic properties due to the volatile aroma-active components (i.e., molecules that elicit a distinctive taste and smell) [47]. According to the French Agency for Normalization (AFNOR), the essential oil is defined as follows (NF T 75-006): “The essential oil is the product obtained from a vegetable raw material, either by steam distillation or mechanical processes, from the epicarp of citrus, or dry.” [48]. The conventional essential oil extractions are steam distillation [49,50] and hydro-distillation [51,52]. However, a few techniques have been used to enhance the efficiency of the extraction process, including microwave-assisted extraction [52,53] and ultrasonication [51]. The extraction techniques and processes used to influence the quality and quantity of the extract result in a range of bioactive levels, for example, biopesticide activity against stored-grain pests [51]. Basil plants contain up to 1% of the essential oil, depending on genotypes, cultivation, growing location, and post-harvest management [54,55,56,57]. The essential oils are more concentrated in leaves and flowers and much less in the stems [58]. In the study of different basils used as culinary herbs, the essential oil yield of white holy basil (O. sanctum var. Rama) and Thai basil (O. basilicum var. thyrsiflorum) was ~0.4%, followed by lemon basil (O. citriodorum) and red holy basil (O. sanctum var. Shyama) ~0.3%, and tree basil O. gratissimum) was the least (<~0.2%) [6]. Variation of essential oil colours also depends on the genotypes, harvesting stages as well as different extraction techniques [58,59]. Under the visible light, the essential oil of O. gratissimum, O. citriodorum, O. sanctum, and O. basilicum var. thyrsiflorum colour are orange, yellow, and colourless, respectively. However, the colour difference is not noticed within the same species, such as those of white and red holy basils (O. sanctum var. Rama and Shyama) [6,58,60]. According to this, the volatile chemical compositions of essential oils may play a crucial role in the colour characteristic of the essential oils [61]. Other factors include thermal degradation, oxidation, isomerisation, dehydrogenation, and polymerisation [62,63,64].
Essential oils are a complex mixture of various classes of volatile organic components such as alcohols, aldehydes, esters, ketones, phenylpropanoids, and terpenoids [65]. Table 2 illustrates the different volatile classes in the essential oils with the representative descriptors of the Ocimum plants. The essential oil profiles are displayed by the heat map of mass spectrums of the different volatile components from Thai basil plants (Figure 2). It is apparent that there is the closest relationship between the volatile organic compounds of plants within the same species (white and red holy basils). The phenylpropanoids (estragole, eugenol, and methyl eugenol) are dominant with a proportion of up to 30%–50% of analysed compounds, followed by the sesquiterpenes (i.e., trans-caryophyllene, trans-α-bergamotene, τ-cadinol, cis-α-bisabolene, β-elemene, and germacrene) and monoterpenes (i.e., trans-ocimene, linalool, 1,8-cineole, and camphor) [57,65,66]. The principal constituents of O. citriodorum essential oil are estragole, citral, and neral, which serve as crucial fingerprints representing its distinctive citrus scent [6]. Holy basil oil comprises a mixture of 17 volatile compounds with methyl eugenol, trans-caryophyllene, eugenol representing clove-like aroma being dominant [6,66,67]. In the essential oil of O. basilicum, estragole is the key volatile element. At the same time, others, such as those of alcohols (i.e., linalool), ketones (i.e., camphor), and esters, are variable among different varieties [68]. It also illustrates that O. gratissimum essential oil is enriched with eugenol, trans-ocimene, trans-α-bergamotene, and linalool as the significant components [6,66] projected away from the other basil species. In another study, thymol, eugenol, and geraniol were used as volatile markers to distinguish sub-varieties grown in the USA [69].

Table 2.
Chemical classes of the volatile organic compounds in the essential oils of the Ocimum spp.
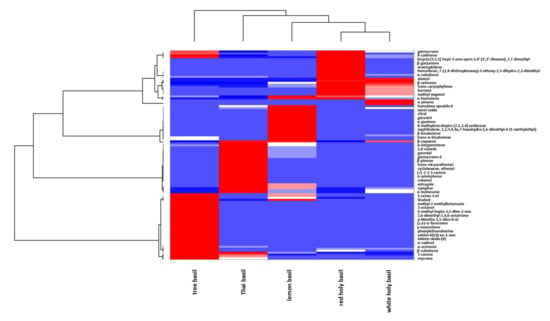
Figure 2.
Heat map on volatile organic components in the essential oil of different basil species. The volatile components of different basil species as from the previous studies [6,66]. The heat map was generated using XLSTAT version 2020 (Addinsoft Inc., New York, NY, USA).
4. The Applications of the Basil Essential Oils in the Production of the Tropical Fruits
Plants synthesise various volatile organic compounds in the essential oils to defend themselves from environmental stresses, both biotic and abiotic [76]. These compounds’ beneficial or adverse effects on the living matter are known as “biological activity”. Consequently, essential oils have been used in many industrial applications and mainly replace synthetic chemicals [77,78,79]. During the pre- and post-harvest productions of the horticultural crops, essential oils were tested for their antipathogenic and pest control properties, such as insecticidal agents [80], repellents, attractants [81], and microbial disease controlling agents. In the production of most tropical fruits, the Oriental fruit flies attack the soft-skin fruits by laying eggs and feeding the larvae inside the fruits [66]. At post-harvest, Colletotrichum spp. is a major fungus causing anthracnose disease, accelerating the fruit deterioration process [82].
4.1. Pre-Harvest Applications
Several studies have investigated the control potential of essential oils from basil plants against pests during pre-harvesting (Table 3). The volatile organic constituents in the essential oils of some Ocimum species influence the behaviour of insects; for example, the ability to attract the Oriental fruit flies [66] and Ceraeochrysa cubana (herbivore predator) [83] as well as the repellent effect on Allacophora foveicollis, a serious pest that causes severe damage to pumpkin [84]. In addition, essential oils from O. basilicum and O. gratissimum were shown to have the ability to prevent egg hatching and adult emergence in Callosobruchus maculatus, the cowpea seed beetle [85]. Therefore, basil plants have been used as an intercrop in integrated pest management that has proven to reduce the total pest infestation in the cotton field [86] and greenhouse tomato production [87]. Methyl eugenol has been found in almost all types of basil essential oils, and it is the most active attractant for the Oriental fruit flies [66], while essential oil of the sweet basil is attractive to green lacewings Ceraeochrysa cubana Hagen (Neuroptera: Chrysopidae) [83]. The toxicity of methyl eugenol against larvae of the tobacco armyworms, Spodoptera litura has also been well defined [88]. Furthermore, the toxicity of Ocimum essential oils to fruit flies have been investigated [89]. Chang et al. [89] tested the toxicity of the three main components detected in the essential oil of O. basilicum L. viz., trans-anethole, estragole, and linalool. It successfully eliminated the flies, especially the estragole was the most effective.

Table 3.
Uses of volatile organic compounds from the studied Ocimum species against pests of horticulture crops.
The essential oil of sweet basil also illustrated the promising effect in controlling symptoms of wilt or root rot disease of cumin caused by Fusarium spp. [90]. It was also found that the mycelial growth of Botrytis fabae was significantly reduced by the basil oil types that were rich in methyl chavicol (or estragole) and linalool, while methyl chavicol, linalool, eugenol, and eucalyptol significantly reduced the overall growth of the fungus [10].
4.2. Post-Harvest Applications
In addition to their potential to control pre-harvest insect pests, the influence of extracts from Omimum spp. plants on the control of post-harvest insect pests was also investigated (Table 4). It was discovered that the essential oils of basils (O. basilicum and O. tenuiflorum) had volatile toxicity against stored-grain pests such as Sitophilus oryzae, Rhyzopertha dominica, Cryptolestes pusillus, Sitophilus zeamais, Tribolium castaneum, and Acanthoscelides obtectus [16,17,91,92,93] as well as the stored dates pests (Ectomyelois ceratoniae and Ephestia kuehniella) [94]. The powder form of the dried sweet basil plant has been used to repel Sitophilus zeamais Motschulsky, a post-harvest pest causing considerable damage to maise grain in most stores in Africa [95]. In addition to its ability to control insects, the role of essential oils as a natural post-harvest fungicide was also well recognised. From previous studies, the vapour of essential oils has the potential to inhibit post-harvest microorganisms [96,97], such as the harvested avocado fruit disease fungus (Cercospora purpurea) [98] and the peach and nectarine disease fungus (Monilinia laxa) [99]. The substances with low molecular weight and low polarity of essential oil play a role in the loss of cell membrane integrity of the pathogen by altering the pH in the cell, thereby inhibiting the growth as well as inducing programmed cell death [100]. The essential oils from Ocimum spp. were able to inhibit the fungi causing the post-harvest diseases of the tropical fruits [10,13,14,101]. This also includes Colletotrichum spp., the fungus that causes anthracnose disease in common tropical fruits. Linalool is the most active substance in the O. basilicum essential oil that could inhibit the diseases of the stored seeds of lettuce and tomatoes caused by F. oxysporum, Penicillium spp., and C. gloeosporioides [102]. The crown rot pathogens that infected cut bananas during farm-level handling and packhouses were positively controlled by the combination of aluminium sulphate and basil oil in the modified atmosphere packaging during cold storage (12–14 °C) [103].

Table 4.
Uses of volatile organic compounds from the studied Ocimum species during post-harvest management of horticultural produce.
5. Techniques for Enhancing the Essential Oil Efficiency
Encapsulation is a widely used process for generating an external membrane or coating material that protects or preserves sensitive bioactive, volatile, and quickly degradable substances from biochemical and thermal degradation [104]. Encapsulation is a technique that is commonly used in the flavour and fragrance industries to enhance both taste and scent. This technology also increases the efficacy of pesticides, fertilisers, and other toxic agrochemicals in agriculture, thereby improving productivity and food security. The active substances are encapsulated to regulate the release under accurate conditions (e.g., humidity, temperature, pH, and time) and to be active for a specific object (e.g., organisms or parts of the organisms). Moreover, encapsulation in agriculture can minimise harmful chemicals [105,106] and increase the efficiency of the natural extracts’ action [66]. The encapsulation can be performed by coating with the material, creating core materials, filing in the internal phase or payload, and the substance’s characteristics can be pure or mixed.
The coating materials are packing material, capsule, wall material, film, membrane, carrier, or outer shell [107]. They are usually made of natural or modified polysaccharides, gums, proteins, lipids, and synthetic polymers [108]. The organic flavour and the aroma of interest are low molecular weight compounds that are relatively volatile and very sensitive to open conditions (air, heat, light, and moisture). Depending on the applied encapsulation technique, the encapsulated essential oil products can be in powder, paste, or liquid forms [109,110]. Numerous techniques are available for encapsulating essential oils for agricultural uses, depending on the nature of the environment in which the products are applied.
5.1. Emulsification
To encapsulate the essential oil by the emulsion technique, the oil, including those of low polar molecules, has to be dissolved with emulsifiers such as gum Arabic and converted to droplets in water before further processes [111]. The droplets of basil oil are highly needed in food, perfumery, oral, and dental products. Emulsifiers such as proteins, phospholipids, and polysaccharides are used to maintain the stability of the essential oil emulsion. In addition, surfactants such as sugar esters and polyoxyethylene are also used to reduce the interfacial tension of the emulsion solution by electrostatic/steric stabilisation [112]. This technique has been successfully proven to maintain the efficacy of the essential oil over harsh environmental conditions such as high temperature and provide the slow-release rate of the essential oil [113,114,115].
5.2. Complex Coacervation
Complex coacervation is an encapsulation method that links and forms two differently charged biopolymers in a solution with the appropriate pH value. The most commonly used biopolymers are gum Arabic, gelatine, carrageenan, chitosan, carboxymethyl cellulose, and pectin [116]. This technique is claimed to be suitable for application at high temperatures and humidity exposure [117,118].
5.3. Spray Drying
Spray drying is a method of forming a liquid essential oil into a powdery form. First, the essential oils are mixed in a solution containing wall materials such as maltodextrin, modified starch, gum, and the combination. Adding emulsifiers and homogenising agents is required to obtain smaller oil globules. Subsequently, the well-mixed solution is sprayed into hot air under high pressure, creating a mist that spreads in the drying chamber [119]. This results in a physical guard of the core matrix that protects the viability of essential oil during processing, storage, and transport [120].
5.4. Complexation
Encapsulation by complexation usually refers to the applied use of oligosaccharides such as cyclodextrins, specifically β-cyclodextrin, which are often used to encapsulate low polar substances such as essential oils. β-cyclodextrin, a cone-shaped molecule, comprises a network of compounds with 7 D-glucose α-1,4 glycosidic bonds. This structure allows essential oils to dissolve well in water and aids in fixing low-polarity substances and controlling evaporation [121]. The inclusion complex is said to increase the stability of the essential oils, particularly when exposed to sunlight [122].
5.5. Ionic Gelation
Essential oil encapsulation using the ionic gelation technique uses charged polymers with essential oils to form the solution. It is then moulded by dripping it into a crosslinking solution. Sodium alginate is a low-cost polymer often used to encapsulate essential oils due to its biocompatibility and biodegradability [123]. This alginate microsphere provides a protective structure from environmental factors such as volatilisation or oxidation. As for food, it facilitates the mobility of the essential oil into the animal digestive system [124].
5.6. Nanoprecipitation
The process of hydrophobic component encapsulation using the nanoprecipitation (solvent displacement or interfacial deposition) technique involves first dissolving the essential oil in an organic solvent together with the polymers. The solution is then added to the water that is being stirred at the proper speed. The solution is then supersaturated, nucleated, and then it expands and coagulates [125]. This technique is suitable during post-harvest to increase the insecticidal efficiency against stored-grain insect pests [126]. There is, however, limited study on insect pest control during post-harvest and handling of tropical fruits.
Tangpao et al. [66] studied the Oriental fruit fly-attracting ability of methyl eugenol plant-based essential oils and encapsulated the oil using an adapted complexation with the paste method of different wall materials. The holy basil essential oil was found to have the ability to attract the Oriental fruit flies, and encapsulation with maltodextrin and gum Arabic at a ratio of 75:25 could enhance its effectiveness in attracting the flies in mango orchard. For this purpose, the paste method is the economical and straightforward technique to encapsulate the essential oil using the chemical and mechanical reaction between the core and wall materials [127]. In addition, the encapsulation of basil essential oil by freeze-drying technique is encouraged to prevent the loss of such heat-sensitive active volatiles that are unstable in aqueous solution [128].
The antimicrobial properties of essential oils have led to research interest in their applications during the post-harvest and storage of fruits. However, the downside is that the strong scents from essential oils could interfere with the true aroma of the produce. This is generally mitigated by the nanoemulsion technique with sodium alginate or pectin-based edible coating and the high-pressure homogenisation technique [112]. All in all, several considerations should be taken into account when applying the essential oil in agricultural productions, such as its volatilisation nature [129] and the activity losses due to the exposure to ultraviolet light, temperature, humidity, and oxygen [130]. More importantly, the release-control rate of the products needs to be investigated [131,132]. Scheme 1 illustrates the possible encapsulation approaches to increase the efficiency of the essential oil during tropical fruit production.
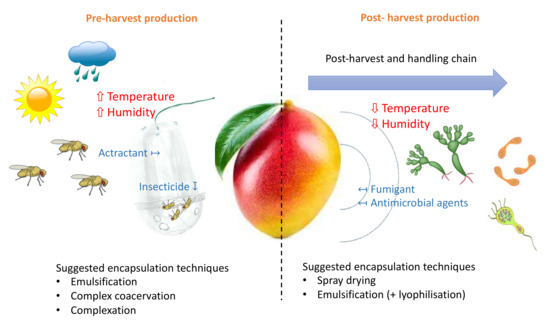
Scheme 1.
Enhancing the efficiency of basil volatile organic compounds during pre- and post-production of the tropical fruits.
6. Conclusions
This review provides essential information for understanding the usefulness of volatile organic compounds from diverse types of basil essential oils and their biological activities. Moreover, as far as sustainable food production in the tropical region is concerned, it is interesting to value-add the natural products from the commonly available resources. Several studies validated the bioassays of these beneficial components during the pre- and post-harvest stages of food crop development. However, the limitation is that essential oils generally decompose fast when exposed to the environment. Consequently, encapsulation techniques are recommended to improve its stability and control its release rate. The option of choice depends on the targeted applications and better-controlled release performance of the essential oils.
Author Contributions
Conceptualization, S.R.S. and T.T.; software, T.T.; validation, S.R.S. and T.T.; formal analysis, T.T., N.C. and P.T.; resources, T.T., N.C. and P.T.; data curation, T.T.; writing—original draft preparation, T.T.; writing—review and editing, S.R.S.; visualisation, T.T.; supervision, S.R.S., R.C.; project administration, T.T.; funding acquisition, N.L., K.J., P.R., P.S., Y.P., T.C., W.R., P.J. and H.V.D. All authors have read and agreed to the published version of the manuscript.
Funding
The research project is funded by the National Research Council of Thailand (NRCT): (contact no: N41A640354). This research project was partially supported by Chiang Mai University.
Acknowledgments
We would like to acknowledge the Teaching Assistant and Research Assistant (TA/RA) scholarship from the Graduate School, Chiang Mai University.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Pandey, A.K.; Singh, P.; Tripathi, N.N. Chemistry and bioactivities of essential oils of some Ocimum species: An overview. Asian Pac. J. Trop. Biomed. 2014, 4, 682–694. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.P.; Singh, R.; Rao, B.R.R.; Singh, R.R.; Srivastava, A.; Lal, R.K. Differential response of genotype×environment on phenology, essential oil yield and quality of natural aroma chemicals of five Ocimum species. Ind. Crops Prod. 2016, 87, 210–217. [Google Scholar] [CrossRef]
- Juntachote, T.; Berghofer, E.; Siebenhandl, S.; Bauer, F. The antioxidative properties of Holy basil and Galangal in cooked ground pork. Meat Sci. 2006, 72, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, N.; Rawal, S.; Verma, M.; Poddar, M.; Alok, S. A phytopharmacological overview on Ocimum species with special emphasis on Ocimum sanctum. Biomed. Prev. Nutr. 2013, 3, 185–192. [Google Scholar] [CrossRef]
- Avetisyan, A.; Markosian, A.; Petrosyan, M.; Sahakyan, N.; Babayan, A.; Aloyan, S.; Trchounian, A. Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Complement. Altern. Med. 2017, 17, 60. [Google Scholar] [CrossRef] [Green Version]
- Tangpao, T.; Chung, H.-H.; Sommano, S.R. Aromatic Profiles of Essential Oils from Five Commonly Used Thai Basils. Foods 2018, 7, 175. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.H.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Ademiluyi, A.O.; Oyeleye, S.I.; Oboh, G. Biological activities, antioxidant properties and phytoconstituents of essential oil from sweet basil (Ocimum basilicum L.) leaves. Comp. Clin. Pathol. 2016, 25, 169–176. [Google Scholar] [CrossRef]
- Zabka, M.; Pavela, R.; Prokinova, E. Antifungal activity and chemical composition of twenty essential oils against significant indoor and outdoor toxigenic and aeroallergenic fungi. Chemosphere 2014, 112, 443–448. [Google Scholar] [CrossRef]
- Oxenham, S.; Svoboda, K.; Walters, D. Antifungal Activity of the Essential Oil of Basil (Ocimum basilicum). J. Phytopathol. 2005, 153, 174–180. [Google Scholar] [CrossRef]
- Onaebi, C.; Onyeke, C.; Osibe, D.; Ugwuja, F.; Okoro, A.; Onyegirim, P. Antimicrobial activity of Ocimum gratissimum L. and Carica papaya L. against postharvest pathogens of avocado pear (Persea americana Mill.). J. Plant Pathol. 2020, 102, 319–325. [Google Scholar] [CrossRef]
- Kumar, A.; Shukla, R.; Singh, P.; Prakash, B.; Dubey, N.K. Chemical composition of Ocimum basilicum L. essential oil and its efficacy as a preservative against fungal and aflatoxin contamination of dry fruits. Int. J. Food Sci. Technol. 2011, 46, 1840–1846. [Google Scholar] [CrossRef]
- Danh, L.T.; Giao, B.T.; Duong, C.T.; Nga, N.T.T.; Tien, D.T.K.; Tuan, N.T.; Huong, B.T.C.; Nhan, T.C.; Trang, D.T.X. Use of Essential Oils for the Control of Anthracnose Disease Caused by Colletotrichum acutatum on Post-Harvest Mangoes of Cat Hoa Loc Variety. Membranes 2021, 11, 719. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Reyes, J.G.; Spadaro, D.; Prelle, A.; Garibaldi, A.; Gullino, M.L. Efficacy of Plant Essential Oils on Postharvest Control of Rots Caused by Fungi on Different Stone Fruits In Vivo. J. Food Prot. 2013, 76, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Dubey, N.K.; Banerji, R.; Chansouria, J.P.N. Evaluation of some essential oils as botanical fungitoxicants in management of post-harvest rotting of citrus fruits. World J. Microbiol. Biotechnol. 2004, 20, 317–321. [Google Scholar] [CrossRef]
- Bhavya, M.L.; Chandu, A.G.S.; Devi, S.S. Ocimum tenuiflorum oil, a potential insecticide against rice weevil with anti-acetylcholinesterase activity. Ind. Crops Prod. 2018, 126, 434–439. [Google Scholar] [CrossRef]
- Rodríguez-González, Á.; Álvarez-García, S.; González-López, Ó.; Silva, F.D.; Casquero, P.A. Insecticidal Properties of Ocimum basilicum and Cymbopogon winterianus against Acanthoscelides obtectus, Insect Pest of the Common Bean (Phaseolus vulgaris L.). Insects 2019, 10, 151. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Jayaramaiah, R.H.; Sarate, P.; Thulasiram, H.V.; Kulkarni, M.J.; Giri, A.P. Insecticidal Potential of Defense Metabolites from Ocimum kilimandscharicum against Helicoverpa armigera. PLoS ONE 2014, 9, e104377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, K.H.; Nishida, R. Methyl eugenol: Its occurrence, distribution, and role in nature, especially in relation to insect behavior and pollination. J. Insect Sci. 2012, 12, 56. [Google Scholar] [CrossRef] [Green Version]
- Canhanga, L.; de Meyer, M.; Cugala, D.; Massimiliano, V.; Maulid, M. Economic injury level of the Oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae), on commercial mango farms in Manica Province, Mozambique. Afr. Entomol. 2020, 28, 278–289. [Google Scholar] [CrossRef]
- Orankanok, W.; Chinvinijkul, S.; Thanaphum, S.; Sitilob, P.; Enkerlin, W. Area-Wide Integrated Control of Oriental Fruit Fly Bactrocera Dorsalis and Guava Fruit Fly Bactrocera Correcta in Thailand; Springer: Dordrecht, The Netherlands, 2007; pp. 517–526. [Google Scholar]
- Alam, S.K.; Rahman, M.; Reza, M.; Amin, M.N.; Hussen, M.A. Postharvest loss assessment of mango at different stages of supply chain through traditional and improved handling practices. Adv. Plants Agric. Res. 2019, 9, 384–388. [Google Scholar] [CrossRef]
- Amin, A. Determination of Some Essential Oils Effects on the Quality Traits of the Egyptian Anna Apple Fruit During its Shelf Life. J. Hortic. Sci. Ornam. Plants 2016, 8, 35–45. [Google Scholar] [CrossRef]
- Dharanivasan, G.; Sithanantham, S.; Kannan, M.; Chitra, S.; Kathiravan, K.; Janarthanan, S. Metal Oxide Nanoparticles Assisted Controlled Release of Synthetic Insect Attractant for Effective and Sustainable Trapping of Fruit Flies. J. Clust. Sci. 2017, 28, 2167–2183. [Google Scholar] [CrossRef]
- Chowdhury, T.; Mandal, A.; Roy, S.C.; de Sarker, D. Diversity of the genus Ocimum (Lamiaceae) through morpho-molecular (RAPD) and chemical (GC–MS) analysis. J. Genet. Eng. Biotechnol. 2017, 15, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Shasany, A.K. The Holy basil (Ocimum sanctum L.) and its genome. Indian J. Hist. Sci. 2016, 51, 343–350. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Cantrell, C.L.; Tekwani, B.; Khan, S.I. Content, composition, and bioactivity of the essential oils of three basil genotypes as a function of harvesting. J. Agric. Food Chem. 2008, 56, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Vani, S.R.; Cheng, S.; Chuah, C. Comparative study of volatile compounds from genus Ocimum. Am. J. Appl. Sci. 2009, 6, 523. [Google Scholar] [CrossRef]
- Khalid, K.A.; Hendawy, S.; El-Gezawy, E. Ocimum basilicum L. production under organic farming. Res. J. Agric. Biol. Sci. 2006, 2, 25–32. [Google Scholar]
- Simon, J.E.; Morales, M.R.; Phippen, W.B.; Vieira, R.F.; Hao, Z. Basil: A source of aroma compounds and a popular culinary and ornamental herb. Perspect. New Crops New Uses 1999, 16, 499–505. [Google Scholar]
- Javanmardi, J.; Khalighi, A.; Kashi, A.; Bais, H.P.; Vivanco, J.M. Chemical Characterization of Basil (Ocimum basilicum L.) Found in Local Accessions and Used in Traditional Medicines in Iran. J. Agric. Food Chem. 2002, 50, 5878–5883. [Google Scholar] [CrossRef]
- Singh, A.K. Seedling morphology of four species of Ocimum L.(Lamiaceae) and its taxonomic significance. Bangladesh J. Plant Taxon. 2012, 19, 89–92. [Google Scholar] [CrossRef] [Green Version]
- Nahak, G.; Mishra, R.; Sahu, R. Taxonomic distribution, medicinal properties and drug development potentiality of Ocimum (Tulsi). Drug Invent. Today 2011, 3, 95–113. [Google Scholar]
- Prinsi, B.; Morgutti, S.; Negrini, N.; Faoro, F.; Espen, L. Insight into Composition of Bioactive Phenolic Compounds in Leaves and Flowers of Green and Purple Basil. Plants 2020, 9, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderón Bravo, H.; Vera Céspedes, N.; Zura-Bravo, L.; Muñoz, L.A. Basil Seeds as a Novel Food, Source of Nutrients and Functional Ingredients with Beneficial Properties: A Review. Foods 2021, 10, 1467. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-Y.; Heo, S.; Bae, S.; Kim, J.; Moon, K.-D. Discriminating the origin of basil seeds (Ocimum basilicum L.) using hyperspectral imaging analysis. LWT 2020, 118, 108715. [Google Scholar] [CrossRef]
- Kumar, A.; Lal, R.K. The consequence of genotype × environment interaction on high essential oil yield and its composition in clove basil (Ocimum gratissimum L.). Acta Ecol. Sin. 2021. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, P.; Rodrigues, V.; Baskaran, K.; Verma, R.S.; Padalia, R.C.; Sundaresan, V. Delineation of Ocimum gratissimum L. complex combining morphological, molecular and essential oils analysis. Ind. Crops Prod. 2019, 139, 111536. [Google Scholar] [CrossRef]
- Pattanayak, P.; Behera, P.; Das, D.; Panda, S.K. Ocimum sanctum Linn. A reservoir plant for therapeutic applications: An overview. Pharm. Rev. 2010, 4, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Khosla, M. Study of inter-relationship, phylogeny and evolutionary tendencies in genus Ocimum. Ind. J. Genet. 1995, 55, 71–83. [Google Scholar]
- Helen, H.D. Investigation of the Cultivars of the Basils (Ocimum). Econ. Bot. 1974, 28, 63–67. [Google Scholar]
- Obeng-Ofori, D.e.; Reichmuth, C. Bioactivity of eugenol, a major component of essential oil of Ocimum suave (Wild.) against four species of stored-product Coleoptera. Int. J. Pest Manag. 1997, 43, 89–94. [Google Scholar] [CrossRef]
- Asase, A.; Hesse, D.N.; Simmonds, M.S.J. Uses of multiple plants prescriptions for treatment of malaria by some communities in southern Ghana. J. Ethnopharmacol. 2012, 144, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Raina, A.P.; Gupta, V. Chemotypic characterization of diversity in essential oil composition of Ocimum species and varieties from India. J. Essent. Oil Res. 2018, 30, 444–456. [Google Scholar] [CrossRef]
- Werker, E.; Putievsky, E.; Ravid, U.; Dudai, N.; Katzir, I. Glandular Hairs and Essential Oil in Developing Leaves of Ocimum basilicum L. (Lamiaceae). Ann. Bot. 1993, 71, 43–50. [Google Scholar] [CrossRef]
- Oksanen, E. Trichomes form an important first line of defence against adverse environment—New evidence for ozone stress mitigation. Plant Cell Environ. 2018, 41, 1497–1499. [Google Scholar] [CrossRef] [PubMed]
- Ameh, S.J.; Obodozie-Ofoegbu, O. Chapter 11—Essential Oils as Flavors in Carbonated Cola and Citrus Soft Drinks. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 111–121. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mindaryani, A.; Rahayu, S. Essential Oil from Extraction and Steam Distillation of Ocimum basillicum. Lect. Notes Eng. Comput. Sci. 2007, 2167, 24–26. [Google Scholar]
- Cassel, E.; Vargas, R.M.F.; Martinez, N.; Lorenzo, D.; Dellacassa, E. Steam distillation modeling for essential oil extraction process. Ind. Crops Prod. 2009, 29, 171–176. [Google Scholar] [CrossRef]
- Da Silva Moura, E.d.S.; D’Antonino Faroni, L.R.; Fernandes Heleno, F.F.; Aparecida Zinato Rodrigues, A.A.Z.; Figueiredo Prates, L.H.; Lopes Ribeiro de Queiroz, M.E. Optimal Extraction of Ocimum basilicum Essential Oil by Association of Ultrasound and Hydrodistillation and Its Potential as a Biopesticide against a Major Stored Grains Pest. Molecules 2020, 25, 2781. [Google Scholar] [CrossRef] [PubMed]
- Chenni, M.; El Abed, D.; Rakotomanomana, N.; Fernandez, X.; Chemat, F. Comparative Study of Essential Oils Extracted from Egyptian Basil Leaves (Ocimum basilicum L.) Using Hydro-Distillation and Solvent-Free Microwave Extraction. Molecules 2016, 21, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, T.H.; Nguyen, H.H.H.; Nguyen, D.C.; Nguyen, T.Q.; Tan, H.; Nhan, L.T.H.; Nguyen, D.H.; Tran, L.D.; Do, S.T.; Nguyen, T.D. Optimization of Microwave-Assisted Extraction of Essential Oil from Vietnamese Basil (Ocimum basilicum L.) Using Response Surface Methodology. Processes 2018, 6, 206. [Google Scholar] [CrossRef] [Green Version]
- Kothari, S.K.; Bhattacharya, A.K.; Ramesh, S. Essential oil yield and quality of methyl eugenol rich Ocimum tenuiflorum L.f. (syn. O. sanctum L.) grown in south India as influenced by method of harvest. J. Chromatogr. A 2004, 1054, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Zheljazkov, V.D.; Callahan, A.; Cantrell, C.L. Yield and Oil Composition of 38 Basil ( Ocimum basilicum L.) Accessions Grown in Mississippi. J. Agric. Food Chem. 2008, 56, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Khalid, K.A. Influence of water stress on growth, essential oil, and chemical composition of herbs (Ocimum sp.). Int. Agrophys. 2006, 20, 289–296. [Google Scholar]
- Singh, P.; Kalunke, R.M.; Giri, A.P. Towards comprehension of complex chemical evolution and diversification of terpene and phenylpropanoid pathways in Ocimum species. RSC Adv. 2015, 5, 106886–106904. [Google Scholar] [CrossRef]
- Chalchat, J.-C.; Özcan, M.M. Comparative essential oil composition of flowers, leavesand stems of basil (Ocimum basilicum L.) used as herb. Food Chem. 2008, 110, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.; Chalchat, J.-C. Essential oil composition of Ocimum basilicum L. Czech J. Food Sci 2002, 20, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Kathirvel, P.; Ravi, S. Chemical composition of the essential oil from basil (Ocimum basilicum Linn.) and its in vitro cytotoxicity against HeLa and HEp-2 human cancer cell lines and NIH 3T3 mouse embryonic fibroblasts. Nat. Prod. Res. 2012, 26, 1112–1118. [Google Scholar] [CrossRef]
- Siddique, A.; Rahman, S.M.; Hossain, M.A. Chemical composition of essential oil by different extraction methods and fatty acid analysis of the leaves of Stevia Rebaudiana Bertoni. Arab. J. Chem. 2016, 9, S1185–S1189. [Google Scholar] [CrossRef] [Green Version]
- Taraj, K.; Delibashi, A.; Andoni, A.; Lazo, P.; Kokalari, E.; Lame, A.; Xhaxhiu, K. Extraction of chamomile essential oil by subcritical CO2 and its analysis by UV-VIS spectrophotometer. Asian J. Chem. 2013, 25, 7361. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Hădărugă, D.I.; Hădărugă, N.G.; Costescu, C.I.; David, I.; Gruia, A.T. Thermal and oxidative stability of the Ocimum basilicum L. essential oil/β-cyclodextrin supramolecular system. Beilstein J. Org. Chem. 2014, 10, 2809–2820. [Google Scholar] [CrossRef] [Green Version]
- Carson, C.F.; Hammer, K.A. Chemistry and bioactivity of essential oils. Lipids Essent Oils Antimicrob Agents 2011, 25, 203–238. [Google Scholar]
- Tangpao, T.; Krutmuang, P.; Kumpoun, W.; Jantrawut, P.; Pusadee, T.; Cheewangkoon, R.; Sommano, S.R.; Chuttong, B. Encapsulation of Basil Essential Oil by Paste Method and Combined Application with Mechanical Trap for Oriental Fruit Fly Control. Insects 2021, 12, 633. [Google Scholar] [CrossRef] [PubMed]
- Sutaphanit, P.; Chitprasert, P. Optimisation of microencapsulation of holy basil essential oil in gelatin by response surface methodology. Food Chem. 2014, 150, 313–320. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Tajima, K.; Toi, N.; Sugimura, Y. Characteristic Components Found in the Essential Oil of Ocimum basilicum L. Flavour Fragr. J. 1997, 12, 195–200. [Google Scholar] [CrossRef]
- Vieira, R.F.; Grayer, R.J.; Paton, A.; Simon, J.E. Genetic diversity of Ocimum gratissimum L. based on volatile oil constituents, flavonoids and RAPD markers. Biochem. Syst. Ecol. 2001, 29, 287–304. [Google Scholar] [CrossRef]
- The Good Scents Company Information System. Available online: http://www.thegoodscentscompany.com/index.html (accessed on 3 December 2021).
- Zeller, A.; Rychlik, M. Character impact odorants of fennel fruits and fennel tea. J. Agric. Food Chem. 2006, 54, 3686–3692. [Google Scholar] [CrossRef] [PubMed]
- Pripdeevech, P.; Khummueng, W.; Park, S.-K. Identification of odor-active components of agarwood essential oils from Thailand by solid phase microextraction-GC/MS and GC-O. J. Essent. Oil Res. 2011, 23, 46–53. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Leone, T.; Paduano, A.; Mena, C.; Perez-Jimenez, M.A.; Sacchi, R. Use of odorant series for extra virgin olive oil aroma characterisation. J. Sci. Food Agric. 2019, 99, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Nomura, M.; Marumoto, S.; Mori, K. Characteristic odor components of essential oil from Scutellaria laeteviolacea. J. Oleo Sci. 2013, 62, 51–56. [Google Scholar] [CrossRef]
- Jiang, L.; Kubota, K. Differences in the volatile components and their odor characteristics of green and ripe fruits and dried pericarp of Japanese pepper (Xanthoxylum piperitum DC.). J. Agric. Food Chem. 2004, 52, 4197–4203. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Mittal, R.; Rana, A.; Jaitak, V. Essential Oils: An Impending Substitute of Synthetic Antimicrobial Agents to Overcome Antimicrobial Resistance. Curr. Drug Targets 2019, 20, 605–624. [Google Scholar] [CrossRef]
- Chávez-González, M.L.; Rodríguez-Herrera, R.; Aguilar, C.N. Chapter 11—Essential Oils: A Natural Alternative to Combat Antibiotics Resistance. In Antibiotic Resistance; Kon, K., Rai, M., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 227–237. [Google Scholar] [CrossRef]
- Valgimigli, L. (Ed.) Essential Oils as Natural Food Additives: Composition, Applications, Antioxidant and Antimicrobial Properties; Nova Science Publishing: New York, NY, USA, 2012; ISBN 978-1-62100-241-3. [Google Scholar]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Bedini, S.; Farina, P.; Conti, B. Repellence and Attractiveness: The Double Effect of Essential Oils on Insect Pests. 2020. Available online: www.researchgate.net/publication/343761132 (accessed on 18 December 2021).
- Zakaria, L. Diversity of Colletotrichum Species Associated with Anthracnose Disease in Tropical Fruit Crops—A Review. Agriculture 2021, 11, 297. [Google Scholar] [CrossRef]
- Batista, M.C.; Fonseca, M.C.M.; Teodoro, A.V.; Martins, E.F.; Pallini, A.; Venzon, M. Basil (Ocimum basilicum L.) attracts and benefits the green lacewing Ceraeochrysa cubana Hagen. Biol. Control 2017, 110, 98–106. [Google Scholar] [CrossRef]
- Dube, S.; Upadhyay, P.; Tripathi, S. Antifungal, physicochemical, and insect-repelling activity of the essential oil of Ocimum basilicum. Can. J. Bot. 1989, 67, 2085–2087. [Google Scholar] [CrossRef]
- Kéita, S.M.; Vincent, C.; Schmit, J.-P.; Arnason, J.T.; Bélanger, A. Efficacy of essential oil of Ocimum basilicum L. and O. gratissimum L. applied as an insecticidal fumigant and powder to control Callosobruchus maculatus (Fab.)[Coleoptera: Bruchidae]. J. Stored Prod. Res. 2001, 37, 339–349. [Google Scholar] [CrossRef]
- Schader, C.; Zaller, J.G.; Köpke, U. Cotton-Basil Intercropping: Effects on Pests, Yields and Economical Parameters in an Organic Field in Fayoum, Egypt. Biol. Agric. Hortic. 2005, 23, 59–72. [Google Scholar] [CrossRef]
- Parolin, P.; Bresch, C.; Poncet, C.; Suay-Cortez, R.; van Oudenhove, L. Testing basil as banker plant in IPM greenhouse tomato crops. Int. J. Pest Manag. 2015, 61, 235–242. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Kumar Tewary, D.; Kumar, R.; Kumar, V.; Kumar Sinha, A.; Shanker, A. Larvicidal and structure–activity studies of natural phenylpropanoids and their semisynthetic derivatives against the tobacco armyworm Spodoptera litura (Fab.)(Lepidoptera: Noctuidae). Chem. Biodivers. 2010, 7, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Cho, I.K.; Li, Q.X. Insecticidal activity of basil oil, trans-anethole, estragole, and linalool to adult fruit flies of Ceratitis capitata, Bactrocera dorsalis, and Bactrocera cucurbitae. J. Econ. Entomol. 2009, 102, 203–209. [Google Scholar] [CrossRef]
- Hashem, M.; Moharam, A.M.; Zaied, A.A.; Saleh, F.E.M. Efficacy of essential oils in the control of cumin root rot disease caused by Fusarium spp. Crop Prot. 2010, 29, 1111–1117. [Google Scholar] [CrossRef]
- Popović, Z.; Kostić, M.; Popović, S.; Skorić, S. Bioactivities of essential oils from basil and sage to Sitophilus oryzae L. Biotechnol. Biotechnol. Equip. 2006, 20, 36–40. [Google Scholar] [CrossRef]
- Kim, S.-I.; Lee, D.-W. Toxicity of basil and orange essential oils and their components against two coleopteran stored products insect pests. J. Asia-Pac. Entomol. 2014, 17, 13–17. [Google Scholar] [CrossRef]
- López, M.D.; Jordán, M.J.; Pascual-Villalobos, M.J. Toxic compounds in essential oils of coriander, caraway and basil active against stored rice pests. J. Stored Prod. Res. 2008, 44, 273–278. [Google Scholar] [CrossRef]
- Chaaban, S.B.; Hamdi, S.H.; Mahjoubi, K.; Jemâa, J.M.B. Composition and insecticidal activity of essential oil from Ruta graveolens, Mentha pulegium and Ocimum basilicum against Ectomyelois ceratoniae Zeller and Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). J. Plant Dis. Prot. 2019, 126, 237–246. [Google Scholar] [CrossRef]
- Mwangangi, B.M.; Mutisya, D.L. Performance of basil powder as insecticide against maize weevil, Sitopillus zeamais (Coleoptera: Curculionidae). Discourse J. Agric. Food Sci. 2013, 1, 196–201. [Google Scholar]
- D’Agostino, M.; Tesse, N.; Frippiat, J.P.; Machouart, M.; Debourgogne, A. Essential Oils and Their Natural Active Compounds Presenting Antifungal Properties. Molecules 2019, 24, 3713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plotto, A.; Roberts, D.D.; Roberts, R.G. Evaluation of plant essential oils as natural postharvest disease control of tomato (Lycopersicon esculentum). Acta Hortic. 2003, 628, 737–745. [Google Scholar] [CrossRef]
- Ogbo, E.M.; Oyibo, A. Effects of three plant extracts (Ocimum gratissimum, Acalypha wilkesiana and Acalypha macrostachya) on post harvest pathogen of Persea americana. J. Med. Plants Res. 2008, 2, 311–314. [Google Scholar]
- Carović-Stanko, K.; Fruk, G.; Satovic, Z.; Ivić, D.; Politeo, O.; Sever, Z.; Grdiša, M.; Strikić, F.; Jemrić, T. Effects of Ocimum spp. essential oil on Monilinia laxa in vitro. J. Essent. Oil Res. 2013, 25, 143–148. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, L.; Tanaka, F.; Tanaka, F. Preservation of strawberry fruit with an Aloe vera gel and basil (Ocimum basilicum) essential oil coating at ambient temperature. J. Food Processing Preserv. 2021, 45, e15836. [Google Scholar] [CrossRef]
- Torre, R.; Pereira, E.A.D.; Nascimento, R.V.; Guedes, T.F.; Faria, P.R.d.S.; Alves, M.d.S.; Souza, M.A.A.d. Agroecological approach to seed protection using basil essential oil. Ind. Crops Prod. 2021, 171, 113932. [Google Scholar] [CrossRef]
- Siriwardana, H.; Abeywickrama, K.; Kannangara, S.; Jayawardena, B.; Attanayake, S. Basil oil plus aluminium sulfate and modified atmosphere packaging controls Crown rot disease in Embul banana (Musa acuminata, AAB) during cold storage. Sci. Hortic. 2017, 217, 84–91. [Google Scholar] [CrossRef]
- Saifullah, M.; Shishir, M.R.I.; Ferdowsi, R.; Tanver Rahman, M.R.; van Vuong, Q. Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: A critical review. Trends Food Sci. Technol. 2019, 86, 230–251. [Google Scholar] [CrossRef]
- Han, X.; Chen, S. Controlled-release fertilizer encapsulated by starch/polyvinyl alcohol coating. Desalination 2009, 240, 21–26. [Google Scholar] [CrossRef]
- Maes, C.; Bouquillon, S.; Fauconnier, M.-L. Encapsulation of Essential Oils for the Development of Biosourced Pesticides with Controlled Release: A Review. Molecules 2019, 24, 2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- De Souza Simões, L.; Madalena, D.A.; Pinheiro, A.C.; Teixeira, J.A.; Vicente, A.A.; Ramos, Ó.L. Micro- and nano bio-based delivery systems for food applications: In vitro behavior. Adv. Colloid Interface Sci. 2017, 243, 23–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of oils: A comprehensive review of benefits, techniques, and applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef] [PubMed]
- Prost, C.; Poinot, P.; Rannou, C.; Arvisenet, G. 21—Bread aroma. In Breadmaking, 2nd ed.; Cauvain, S.P., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 523–561. [Google Scholar] [CrossRef]
- Garcia, L.; Tonon, R.; Hubinger, M. Effect of Homogenization Pressure and Oil Load on the Emulsion Properties and the Oil Retention of Microencapsulated Basil Essential Oil (Ocimum basilicum L.). Dry. Technol. 2012, 30, 1413. [Google Scholar] [CrossRef]
- Tripathi, A.D.; Sharma, R.; Agarwal, A.; Haleem, D.R. Nanoemulsions based edible coatings with potential food applications. Int. J. Biobased Plast. 2021, 3, 112–125. [Google Scholar] [CrossRef]
- Moretti, M.D.L.; Sanna-Passino, G.; Demontis, S.; Bazzoni, E. Essential oil formulations useful as a new tool for insect pest control. AAPS PharmSciTech 2002, 3, 64–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovanović, J.; Krnjajić, S.; Ćirković, J.; Radojković, A.; Popović, T.; Branković, G.; Branković, Z. Effect of encapsulated lemongrass (Cymbopogon citratus L.) essential oil against potato tuber moth Phthorimaea operculella. Crop Prot. 2020, 132, 105109. [Google Scholar] [CrossRef]
- Lucia, A.; Girard, C.; Fanucce, M.; Coviella, C.; Rubio, R.G.; Ortega, F.; Guzmán, E. Development of an Environmentally Friendly Larvicidal Formulation Based on Essential Oil Compound Blend to Control Aedes aegypti Larvae: Correlations between Physicochemical Properties and Insecticidal Activity. ACS Sustain. Chem. Eng. 2020, 8, 10995–11006. [Google Scholar] [CrossRef]
- Hernández-Nava, R.; López-Malo, A.; Palou, E.; Ramírez-Corona, N.; Jiménez-Munguía, M.T. Encapsulation of oregano essential oil (Origanum vulgare) by complex coacervation between gelatin and chia mucilage and its properties after spray drying. Food Hydrocoll. 2020, 109, 106077. [Google Scholar] [CrossRef]
- Muhoza, B.; Xia, S.; Wang, X.; Zhang, X.; Li, Y.; Zhang, S. Microencapsulation of essential oils by complex coacervation method: Preparation, thermal stability, release properties and applications. Crit. Rev. Food Sci. Nutr. 2020, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhao, S.-Q.; Zhang, J.; Huang, G.-Y.; Chen, L.-Y.; Zhao, F.-Y. Chemical composition, antimicrobial property and microencapsulation of Mustard (Sinapis alba) seed essential oil by complex coacervation. Food Chem. 2014, 165, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Le, T.V.A.; Dang, N.N.; Nguyen, D.C.; Nguyen, P.T.N.; Tran, T.T.; Nguyen, Q.V.; Bach, L.G.; Thuy Nguyen Pham, D. Microencapsulation of Essential Oils by Spray-Drying and Influencing Factors. J. Food Qual. 2021, 2021, 5525879. [Google Scholar] [CrossRef]
- Veiga, R.D.S.D.; Aparecida da Silva-Buzanello, R.; Corso, M.P.; Canan, C. Essential oils microencapsulated obtained by spray drying: A review. J. Essent. Oil Res. 2019, 31, 457–473. [Google Scholar] [CrossRef]
- Marques, C.S.; Carvalho, S.G.; Bertoli, L.D.; Villanova, J.C.O.; Pinheiro, P.F.; dos Santos, D.C.M.; Yoshida, M.I.; de Freitas, J.C.C.; Cipriano, D.F.; Bernardes, P.C. β-Cyclodextrin inclusion complexes with essential oils: Obtention, characterization, antimicrobial activity and potential application for food preservative sachets. Food Res. Int. 2019, 119, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Rakmai, J.; Cheirsilp, B.; Mejuto, J.C.; Torrado-Agrasar, A.; Simal-Gándara, J. Physico-chemical characterization and evaluation of bio-efficacies of black pepper essential oil encapsulated in hydroxypropyl-beta-cyclodextrin. Food Hydrocoll. 2017, 65, 157–164. [Google Scholar] [CrossRef]
- Caceres, L.; Velasco, G.; Dagnino, E.; Chamorro, E. MICROENCAPSULATION of grapefruit oil with sodium alginate by gelation and ionic extrusion: Optimization and modeling of crosslinking and study of controlled release kinetics. Rev. Tecnol. Y Cienc. 2020, 41–61. [Google Scholar] [CrossRef]
- Wan, L.Q.; Jiang, J.; Arnold, D.E.; Guo, X.E.; Lu, H.H.; Mow, V.C. Calcium concentration effects on the mechanical and biochemical properties of chondrocyte-alginate constructs. Cell. Mol. Bioeng. 2008, 1, 93–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lammari, N.; Louaer, O.; Meniai, A.H.; Elaissari, A. Encapsulation of Essential Oils via Nanoprecipitation Process: Overview, Progress, Challenges and Prospects. Pharmaceutics 2020, 12, 431. [Google Scholar] [CrossRef]
- Rajkumar, V.; Gunasekaran, C.; Paul, C.A.; Dharmaraj, J. Development of encapsulated peppermint essential oil in chitosan nanoparticles: Characterization and biological efficacy against stored-grain pest control. Pestic. Biochem. Physiol. 2020, 170, 104679. [Google Scholar] [CrossRef]
- Bhandari, B.R.; D’Arc, B.R.; Padukka, I. Encapsulation of Lemon Oil by Paste Method Using β-Cyclodextrin: Encapsulation Efficiency and Profile of Oil Volatiles. J. Agric. Food Chem. 1999, 47, 5194–5197. [Google Scholar] [CrossRef]
- Chenni, M.; El Abed, D.; Neggaz, S.; Rakotomanomana, N.; Fernandez, X.; Chemat, F. Solvent free microwave extraction followed by encapsulation of O. basilicum L. essential oil for insecticide purpose. J. Stored Prod. Res. 2020, 86, 101575. [Google Scholar] [CrossRef]
- Beyki, M.; Zhaveh, S.; Khalili, S.T.; Rahmani-Cherati, T.; Abollahi, A.; Bayat, M.; Tabatabaei, M.; Mohsenifar, A. Encapsulation of Mentha piperita essential oils in chitosan–cinnamic acid nanogel with enhanced antimicrobial activity against Aspergillus flavus. Ind. Crops Prod. 2014, 54, 310–319. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Hosseini, H.; Mohammadifar, M.A.; Mortazavian, A.M.; Mohammadi, A.; Khosravi-Darani, K.; Shojaee-Aliabadi, S.; Dehghan, S.; Khaksar, R. Incorporation of essential oil in alginate microparticles by multiple emulsion/ionic gelation process. Int. J. Biol. Macromol. 2013, 62, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Chattopadhyay, P.; Ghosh, A.; Goyary, D.; Karmakar, S.; Veer, V. Influence of process variables on essential oil microcapsule properties by carbohydrate polymer–protein blends. Carbohydr. Polym. 2013, 93, 691–697. [Google Scholar] [CrossRef]
- Benavides, S.; Cortés, P.; Parada, J.; Franco, W. Development of alginate microspheres containing thyme essential oil using ionic gelation. Food Chem. 2016, 204, 77–83. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).