Effect of Elevated CO2 during Low Temperature Storage on the Quality Attributes of Cut Spearmint
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Quality Assessments
2.2.1. Weight Loss and Colour Measurement
2.2.2. Texture Analysis
2.2.3. Total Phenolic Content and Antioxidant Activity
2.2.4. Ascorbic Acid
2.3. Volatile Organic Compositions
2.3.1. Ammonia and Ethanol-Acetaldehyde Contents
2.3.2. Solid Phase Microextraction (SPME)
2.4. Sensory Evaluation
2.5. Statistical Analyses
3. Results and Discussion
3.1. Effect of High CO2 in CA on Physiological Attributes
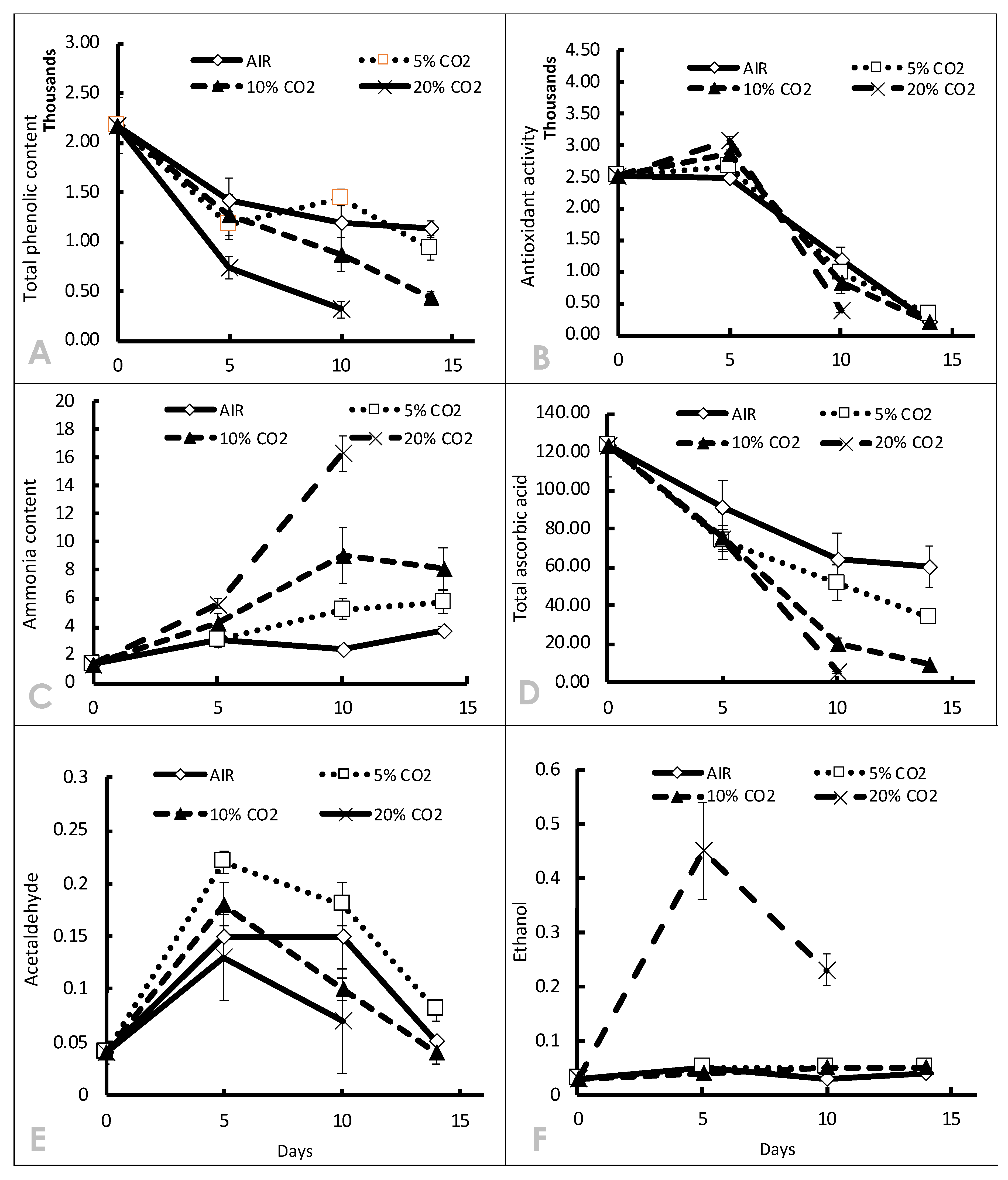
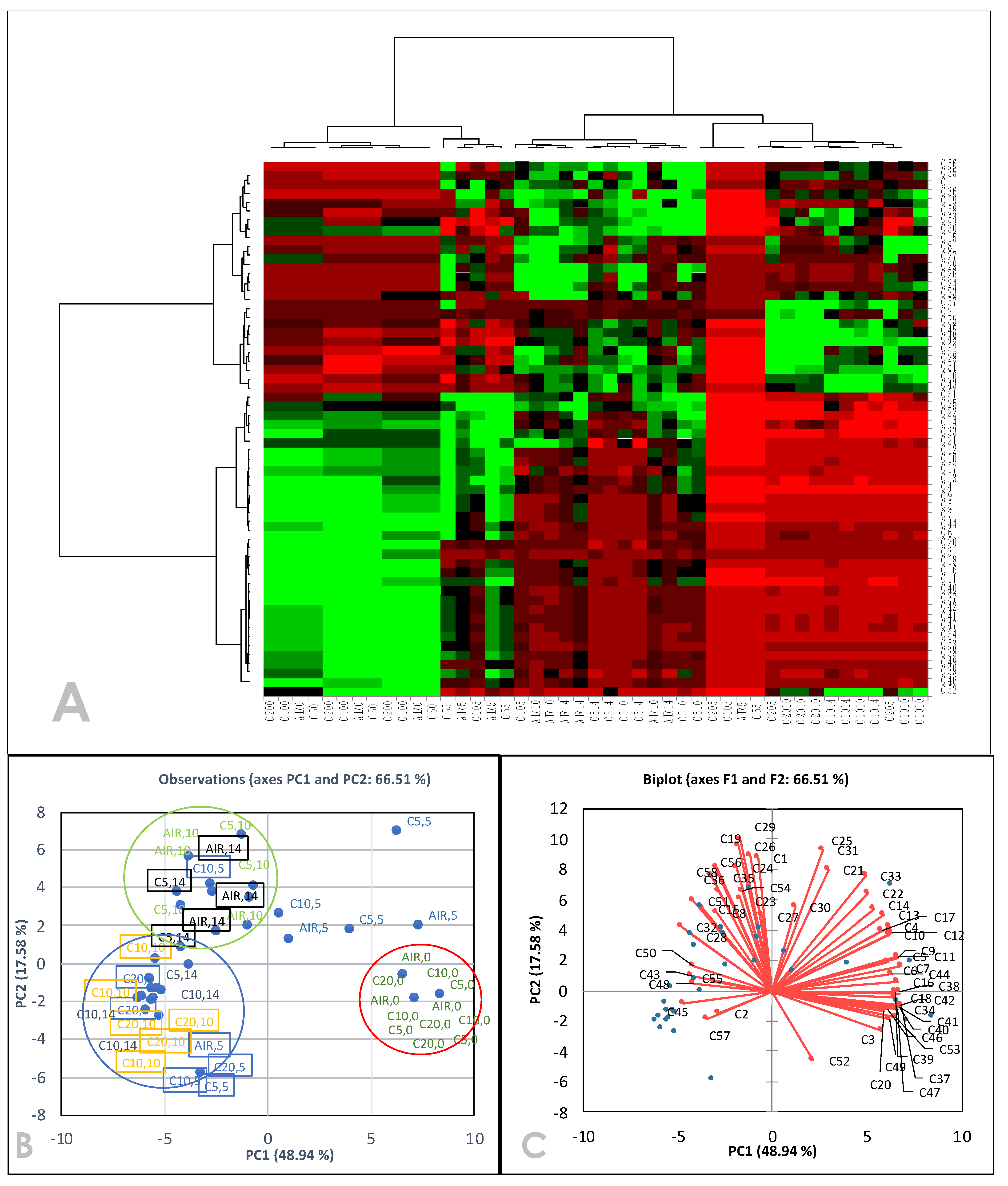
3.2. Effect of High CO2 in CA on Phytochemical Attributes
3.3. Volatile Organic Compositions
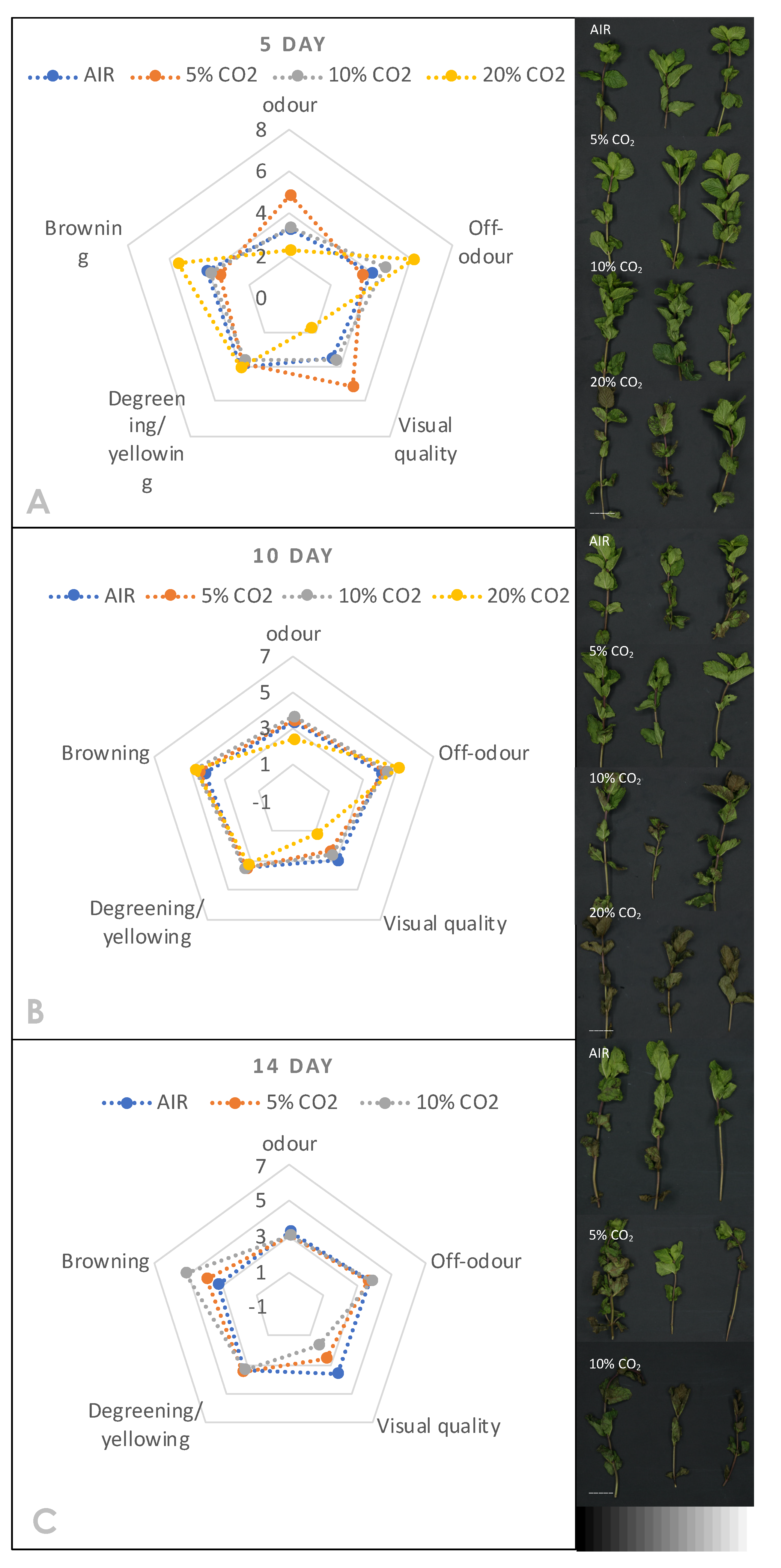
3.4. Effect of High CO2 in CA on the Sensory and Flavour Attributes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cantwell, M.I.; Reid, M.S. Postharvest Physiology and Handling of Fresh Culinary Herbs. J. Herbs Spices Med. Plants 1993, 1, 93–127. [Google Scholar] [CrossRef]
- Kenigsbuch, D.; Chalupowicz, D.; Aharon, Z.; Maurer, D.; Aharoni, N. The effect of CO2 and 1-methylcyclopropene on the regulation of postharvest senescence of mint, Mentha longifolia L. Postharvest Biol. Technol. 2007, 43, 165–173. [Google Scholar] [CrossRef]
- Watada, A.E.; Ko, N.P.; Minott, D.A. Factors affecting quality of fresh-cut horticultural products. Postharvest Biol. Technol. 1996, 9, 115–125. [Google Scholar] [CrossRef]
- Lin, Q.; Lu, Y.; Zhang, J.; Liu, W.; Guan, W.; Wang, Z. Effects of high CO2 in-package treatment on flavor, quality and antioxidant activity of button mushroom (Agaricus bisporus) during postharvest storage. Postharvest Biol. Technol. 2017, 123, 112–118. [Google Scholar] [CrossRef]
- Villanueva, M.J.; Tenorio, M.D.; Sagardoy, M.; Redondo, A.; Saco, M.D. Physical, chemical, histological and microbiological changes in fresh green asparagus (Asparagus officinalis L.) stored in modified atmosphere packaging. Food Chem. 2005, 91, 609–619. [Google Scholar] [CrossRef]
- Cantín, C.M.; Minas, I.S.; Goulas, V.; Jiménez, M.; Manganaris, G.A.; Michailides, T.J.; Crisosto, C.H. Sulfur dioxide fumigation alone or in combination with CO2-enriched atmosphere extends the market life of highbush blueberry fruit. Postharvest Biol. Technol. 2012, 67, 84–91. [Google Scholar] [CrossRef]
- Thompson, A.K.; Prange, R.K.; Bancroft, R.; Puttongsiri, T. Controlled Atmosphere Storage of Fruit and Vegetables; CABI: Oxfordshire, UK, 2018. [Google Scholar]
- Kader, A.A. Biochemical and physiological basic for effects of controlled and modified atmospheres on fruits and vegetables. Food Technol. 1986, 40, 99–104. [Google Scholar]
- Kader, A.A.; Zagory, D.; Kerbel, E.L.; Wang, C.Y. Modified atmosphere packaging of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 1989, 28, 1–30. [Google Scholar] [CrossRef]
- Philosoph-Hadas, S.; Jacob, D.; Meir, S.; Aharoni, N. Mode of action of CO2 in delaying senescence of chervil leaves. Physiol. Basis Postharvest Technol. 1992, 343, 117–122. [Google Scholar] [CrossRef]
- Riudavets, J.; Alonso, M.; Gabarra, R.; Arnó, J.; Jaques, J.A.; Palou, L. The effects of postharvest carbon dioxide and a cold storage treatment on Tuta absoluta mortality and tomato fruit quality. Postharvest Biol. Technol. 2016, 120, 213–221. [Google Scholar] [CrossRef]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.; Barclay, G. Mints in Ethnic Cuisines. Available online: http://www.theherbaltouch.com/iha/mint98.html (accessed on 21 May 2021).
- De Carvalho, C.C.C.R.; da Fonseca, M.M.R. Carvone: Why and how should one bother to produce this terpene. Food Chem. 2006, 95, 413–422. [Google Scholar] [CrossRef]
- Kedia, A.; Prakash, B.; Mishra, P.K.; Chanotiya, C.S.; Dubey, N.K. Antifungal, antiaflatoxigenic, and insecticidal efficacy of spearmint (Mentha spicata L.) essential oil. Int. Biodeterior. Biodegrad. 2014, 89, 29–36. [Google Scholar] [CrossRef]
- Wu, Z.; Tan, B.; Liu, Y.; Dunn, J.; Martorell Guerola, P.; Tortajada, M.; Cao, Z.; Ji, P. Chemical Composition and Antioxidant Properties of Essential Oils from Peppermint, Native Spearmint and Scotch Spearmint. Molecules 2019, 24, 2825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koukounaras, A.; Siomos, A.S.; Sfakiotakis, E. Postharvest CO2 and ethylene production and quality of rocket (Eruca sativa Mill.) leaves as affected by leaf age and storage temperature. Postharvest Biol. Technol. 2007, 46, 167–173. [Google Scholar] [CrossRef]
- Cozzolino, R.; Pace, B.; Cefola, M.; Martignetti, A.; Stocchero, M.; Fratianni, F.; Nazzaro, F.; De Giulio, B. Assessment of volatile profile as potential marker of chilling injury of basil leaves during postharvest storage. Food Chem. 2016, 213, 361–368. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, A.; Ros-Chumillas, M.; Antolinos, V.; Buendía-Moreno, L.; Navarro-Segura, L.; Sánchez-Martínez, M.J.; Martínez-Hernández, G.B.; Soto-Jover, S. Fresh culinary herbs decontamination with essential oil vapours applied under vacuum conditions. Postharvest Biol. Technol. 2019, 156, 110942. [Google Scholar] [CrossRef]
- Curutchet, A.; Dellacassa, E.; Ringuelet, J.A.; Chaves, A.R.; Viña, S.Z. Nutritional and sensory quality during refrigerated storage of fresh-cut mints (Mentha × piperita and M. spicata). Food Chem. 2014, 143, 231–238. [Google Scholar] [CrossRef]
- Santos, J.; Herrero, M.; Mendiola, J.A.; Oliva-Teles, M.T.; Ibáñez, E.; Delerue-Matos, C.; Oliveira, M.B.P.P. Fresh-cut aromatic herbs: Nutritional quality stability during shelf-life. LWT—Food Sci. Technol. 2014, 59, 101–107. [Google Scholar] [CrossRef]
- Brecht, J.K.; Chau, K.V.; Fonseca, S.C.; Oliveira, F.A.R.; Silva, F.M.; Nunes, M.C.N.; Bender, R.J. Maintaining optimal atmosphere conditions for fruits and vegetables throughout the postharvest handling chain. Postharvest Biol. Technol. 2003, 27, 87–101. [Google Scholar] [CrossRef]
- Zudaire, L.; Viñas, I.; Abadias, M.; Simó, J.; Echeverria, G.; Plaza, L.; Aguiló-Aguayo, I. Quality and bioaccessibility of total phenols and antioxidant activity of calçots (Allium cepa L.) stored under controlled atmosphere conditions. Postharvest Biol. Technol. 2017, 129, 118–128. [Google Scholar] [CrossRef] [Green Version]
- Saltveit, M.E. Is it possible to find an optimal controlled atmosphere? Postharvest Biol. Technol. 2003, 27, 3–13. [Google Scholar] [CrossRef]
- D’Amato, D.; Droste, N.; Allen, B.; Kettunen, M.; Lähtinen, K.; Korhonen, J.; Leskinen, P.; Matthies, B.D.; Toppinen, A. Green, circular, bio economy: A comparative analysis of sustainability avenues. J. Clean. Prod. 2017, 168, 716–734. [Google Scholar] [CrossRef]
- Lopresti, J.; Tomkins, B. Postharvest Handling and Packaging of Fresh Herbs; Rural Industries Research and Development Corporation: Kingston, Australia, 1997. [Google Scholar]
- Rodoni, L.; Vicente, A.; Azevedo, S.; Concellón, A.; Cunha, L.M. Quality retention of fresh-cut pepper as affected by atmosphere gas composition and ripening stage. LWT—Food Sci. Technol. 2015, 60, 109–114. [Google Scholar] [CrossRef]
- Sadowska, U.; Żabiński, A.; Szumny, A.; Dziadek, K. An effect of peppermint herb (Mentha piperita L.) pressing on physico-chemical parameters of the resulting product. Ind. Crops Prod. 2016, 94, 909–919. [Google Scholar] [CrossRef]
- Piazzolla, F.; Pati, S.; Amodio, M.L.; Colelli, G. Effect of harvest time on table grape quality during on-vine storage. J. Sci. Food Agric. 2016, 96, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Ricci, I.; Amodio, M.L.; Colelli, G. Influence of pre-cutting operations on quality of fresh-cut artichokes (Cynara scolymus L.): Effect of storage time and temperature before cutting. Postharvest Biol. Technol. 2013, 85, 124–131. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Cefola, M.; Amodio, M.L.; Cornacchia, R.; Rinaldi, R.; Vanadia, S.; Colelli, G. Effect of atmosphere composition on the quality of ready-to-use broccoli raab (Brassica rapa L.). J. Sci. Food Agric. 2010, 90, 789–797. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Cantwell, M.; Hong, G.; Nie, X. Using tissue ammonia and fermentative volatile concentrations as indicators of beneficial and stressful modified atmospheres for leafy and floral vegetables. Acta Horticuturea 2010, 876, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Wongkaew, M.; Sangta, J.; Chansakaow, S.; Jantanasakulwong, K.; Rachtanapun, P.; Sommano, S.R. Volatile profiles from over-ripe purée of Thai mango varieties and their physiochemical properties during heat processing. PLoS ONE 2021, 16, e0248657. [Google Scholar] [CrossRef] [PubMed]
- Urban, L.; Six, S.; Barthélémy, L.; Bearez, P. Effect of elevated CO2 on leaf water relations, water balance and senescence of cut roses. J. Plant Physiol. 2002, 159, 717–723. [Google Scholar] [CrossRef]
- Murray, D.R. Carbon Dioxide and Plant Responses; CABI: Oxfordshire, UK; Wiley: Taunton, UK; New York, NY, USA, 1997; p. xv. 255p. [Google Scholar]
- Fonseca, S.C.; Oliveira, F.A.R.; Brecht, J.K. Modelling respiration rate of fresh fruits and vegetables for modified atmosphere packages: A review. J. Food Eng. 2002, 52, 99–119. [Google Scholar] [CrossRef]
- Maguire, K.M.; Sabarez, H.T.; Tanner, D.J. Postharvest preservation and storage. In Handbook of Vegetable Preservation and Processing; Online-Ressource; Hui, Y.H., Ed.; Dekker: New York, NY, USA, 2004. [Google Scholar]
- Waghmare, R.B.; Annapure, U.S. Integrated effect of sodium hypochlorite and modified atmosphere packaging on quality and shelf life of fresh-cut cilantro. Food Packag. Shelf Life 2015, 3, 62–69. [Google Scholar] [CrossRef]
- Belay, Z.A.; Caleb, O.J.; Opara, U.L. Impacts of low and super-atmospheric oxygen concentrations on quality attributes, phytonutrient content and volatile compounds of minimally processed pomegranate arils (cv. Wonderful). Postharvest Biol. Technol. 2017, 124, 119–127. [Google Scholar] [CrossRef]
- Ben-Yehoshua, S. Transpiration, water stress and gas exchange. In Postharvest Physiology of Vegetables; Weichmann, J., Ed.; M. Dekker: New York, NY, USA, 1987; pp. 113–170. [Google Scholar]
- Bahadori, M.B.; Zengin, G.; Bahadori, S.; Dinparast, L.; Movahhedin, N. Phenolic composition and functional properties of wild mint (Mentha longifolia var. calliantha (Stapf) Briq.). Int. J. Food Prop. 2018, 21, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Mirondo, R.; Barringer, S. Deodorization of garlic breath by foods, and the role of polyphenol oxidase and phenolic compounds. J. Food Sci. 2016, 81, C2425–C2430. [Google Scholar] [CrossRef]
- Neocleous, D.; Ntatsi, G. Seasonal variations of antioxidants and other agronomic features in soilless production of selected fresh aromatic herbs. Sci. Hortic. 2018, 234, 290–299. [Google Scholar] [CrossRef]
- Vina, S.Z.; Chaves, A.R. Antioxidant responses in minimally processed celery during refrigerated storage. Food Chem. 2006, 94, 68–74. [Google Scholar] [CrossRef]
- MacLean, D.; Murr, D.; DeEll, J. A modified total oxyradical scavenging capacity assay for antioxidants in plant tissues. Postharvest Biol. Technol. 2003, 29, 183–194. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Mahfouz, S.A. Effect of 1-methylcyclopropene (1-MCP) on the postharvest senescence of coriander leaves during storage and its relation to antioxidant enzyme activity. Sci. Hortic. 2012, 141, 69–75. [Google Scholar] [CrossRef]
- la Zazzera, M.; Rinaldi, R.; Amodio, M.L.; Colelli, G. Influence of high CO2 atmosphere composition on fresh-cut artichoke quality attributes. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium, Lisboa, Portugal, 22–27 August 2010; pp. 633–640. [Google Scholar]
- Tudela, J.A.; Marín, A.; Garrido, Y.; Cantwell, M.; Medina-Martínez, M.S.; Gil, M.I. Off-odour development in modified atmosphere packaged baby spinach is an unresolved problem. Postharvest Biol. Technol. 2013, 75, 75–85. [Google Scholar] [CrossRef]
- Ramos, B.; Miller, F.A.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Fresh fruits and vegetables—An overview on applied methodologies to improve its quality and safety. Innov. Food Sci. Emerg. Technol. 2013, 20, 1–15. [Google Scholar] [CrossRef]
- Mehmet Ali, K.; Tuba Dİ, L. Determination of Vitamin C and Organic Acid Changes in Strawberry by HPLC during Cold Storage. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 95–98. [Google Scholar] [CrossRef]
- Sommano, S.; Caffin, N.; McDonald, J.; Kerven, G. Measurement of ascorbic acid in Australian native plants. Int. Food Res. J. 2011, 18, 1017–1020. [Google Scholar]
- Ranjitha, K.; Shivashankara, K.S.; Sudhakar Rao, D.V.; Oberoi, H.S.; Roy, T.K.; Bharathamma, H. Improvement in shelf life of minimally processed cilantro leaves through integration of kinetin pretreatment and packaging interventions: Studies on microbial population dynamics, biochemical characteristics and flavour retention. Food Chem. 2017, 221, 844–854. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, R.S.; Kaul, M.K.; Shahi, A.K.; Kumar, A.; Ram, G.; Tawa, A. Chemical composition of essential oils in Mentha spicata L. accession [IIIM(J)26] from North-West Himalayan region, India. Ind. Crops Prod. 2009, 29, 654–656. [Google Scholar] [CrossRef]
- Dumont, M.-J.; Orsat, V.; Raghavan, V. 7-Reducing Postharvest Losses. In Emerging Technologies for Promoting Food Security; Madramootoo, C., Ed.; Woodhead Publishing: Oxford, UK, 2016; pp. 135–156. [Google Scholar]
- Ragaert, P.; Devlieghere, F.; Debevere, J. Role of microbiological and physiological spoilage mechanisms during storage of minimally processed vegetables. Postharvest Biol. Technol. 2007, 44, 185–194. [Google Scholar] [CrossRef]
- Lopez-Galvez, G.; Peiser, G.; Nie, X.; Cantwell, M. Quality changes in packaged salad products during storage. Z. Lebensm. Und-Forsch. A 1997, 205, 64–72. [Google Scholar] [CrossRef]
- Jacxsens, L.; Devlieghere, F.; Ragaert, P.; Vanneste, E.; Debevere, J. Relation between microbiological quality, metabolite production and sensory quality of equilibrium modified atmosphere packaged fresh-cut produce. Int. J. Food Microbiol. 2003, 83, 263–280. [Google Scholar] [CrossRef]
- Ragaert, P.; Devlieghere, F.; Devuyst, E.; Dewulf, J.; Van Langenhove, H.; Debevere, J. Volatile metabolite production of spoilage micro-organisms on a mixed-lettuce agar during storage at 7 °C in air and low oxygen atmosphere. Int. J. Food Microbiol. 2006, 112, 162–170. [Google Scholar] [CrossRef] [PubMed]

| Quality Attributes | Factor Effects | ||
|---|---|---|---|
| Treatment | Storage Time | Treatment * Storage Time | |
| Physiological attribute | |||
| Weight loss (%) | ** | *** | NS |
| Firmness (N) | *** | *** | *** |
| Colour | |||
| Lightness (L) | *** | *** | *** |
| a * | *** | *** | *** |
| b * | *** | *** | *** |
| Delta E | *** | *** | *** |
| Phytochemical attributes | |||
| Total phenolic content (mg gallic acid equivalent 100 g FW−1) | ** | *** | NS |
| Antioxidant capacity (DPPH) (mg trolox equivalent 100 g FW−1) | NS | *** | * |
| Total ascorbic acid (mg 100 g FW−1) | ** | *** | NS |
| Off flavour attributes | |||
| Ammonia content (µg g−1) | *** | *** | *** |
| Ethanol (µmoL g−1) | ** | *** | NS |
| Acetaldehyde (µmoL g−1) | *** | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommano, S.R.; Khamsaw, P.; Van Doan, H.; Cheewangkoon, R.; Amodio, M.L.; De Chiara, M.L.V.; Mastrandrea, L.; Pati, S.; Colelli, G. Effect of Elevated CO2 during Low Temperature Storage on the Quality Attributes of Cut Spearmint. Horticulturae 2022, 8, 126. https://doi.org/10.3390/horticulturae8020126
Sommano SR, Khamsaw P, Van Doan H, Cheewangkoon R, Amodio ML, De Chiara MLV, Mastrandrea L, Pati S, Colelli G. Effect of Elevated CO2 during Low Temperature Storage on the Quality Attributes of Cut Spearmint. Horticulturae. 2022; 8(2):126. https://doi.org/10.3390/horticulturae8020126
Chicago/Turabian StyleSommano, Sarana Rose, Pattarapol Khamsaw, Hien Van Doan, Ratchadawan Cheewangkoon, Maria Luisa Amodio, Maria Lucia Valeria De Chiara, Leonarda Mastrandrea, Sandra Pati, and Giancarlo Colelli. 2022. "Effect of Elevated CO2 during Low Temperature Storage on the Quality Attributes of Cut Spearmint" Horticulturae 8, no. 2: 126. https://doi.org/10.3390/horticulturae8020126
APA StyleSommano, S. R., Khamsaw, P., Van Doan, H., Cheewangkoon, R., Amodio, M. L., De Chiara, M. L. V., Mastrandrea, L., Pati, S., & Colelli, G. (2022). Effect of Elevated CO2 during Low Temperature Storage on the Quality Attributes of Cut Spearmint. Horticulturae, 8(2), 126. https://doi.org/10.3390/horticulturae8020126









