Abstract
Solar ultraviolet (UV) radiation mainly includes UVA (320–400 nm). UVA intensity varies depending on the season and geographic location, while it is projected to rise owing to climate change. Since it elicits secondary metabolism, additional knowledge on the UVA dependence of phytochemical production is required for both farmers and processors, particularly under natural settings. In this field study, the pheno-morphological traits and essential oil composition responses to UVA intensity were addressed in three Thymus species [T. daenensis (endemic to Iran), T. fedtschenkoi (semi-endemic), T. vulgaris (common thyme)]. During growth, three UVA levels (ambient, enriched, excluded) were realized in combination with spraying protectants [water (control), melatonin, glutathione, iron-zinc nanofertilizer]. In T. daenensis, enriched UVA caused early flowering. The height of T. daenensis was the longest under enriched UVA, and the shortest under excluded UVA. In control plants, enriched and excluded UVA stimulated the accumulation of oxygenated metabolites in T. daenensis and T. fedtschenkoi. Altogether, under enriched UVA some phenolic compounds (e.g., thymol, carvacrol, γ-terpinene) increased in the essential oil of all three species, but others decreased. In all taxa, glutathione caused a significant essential oil content reduction. Iron-zinc nanofertilizer increased essential oil accumulation in T. daenensis and T. vulgaris. Treatments also induced an alteration of the essential oil composition. In conclusion, cultivation regime effects on the essential oil quality (composition) and quantity were strongly species dependent. T. deanensis underwent the most consistent enhancement under UVA, making the species more adaptable to climate change, whereas T. fedtschenkoi the least.
1. Introduction
Although most chemical substances are currently synthetic, plants remain an important source of pharmaceuticals and other compounds employed in a wide range of industries [1,2]. For instance, as much as 25% of prescription medicines are sourced directly or indirectly from herbal products [3]. Notably, the demand for plant material employed for medicines, perfumery, cosmetics and food additives currently shows an upward trend [4,5]. Among plant-derived compounds, essential oils have received considerable attention owing to their unique properties [6,7]. The endogenous production and composition of essential oil are mediated by the secondary metabolism, which is considerably influenced by environmental conditions during cultivation [8,9].
The solar ultraviolet (UV) spectrum mostly (up to 95%) includes UVA (320–400 nm). UVA levels strongly vary depending on the season and geographic location. They are projected to rise globally owing to anthropogenic climate change. UVA radiation exerts a large impact on agricultural ecosystems [10,11]. Similar to other abiotic stressors, UVA has both direct and indirect implications on metabolism, modifies biochemical composition, and thus activates the synthesis and accumulation of secondary metabolites, including phenolic compounds [12,13,14]. In several instances, the UVA activation of secondary metabolites has been associated with improved product quality [15]. In medicinal plants, for instance, up-regulation of secondary metabolite biosynthesis has been related to increased value [12,16,17]. In this context, knowledge regarding secondary metabolite biosynthesis in response to UVA radiation, besides having an intriguing ecological role, is particularly relevant for both farmers and processors [4,6,17]. In instances where UVA exceeds a threshold level, adverse effects on plants are rapidly evident [18,19]. Exogenous application of various bioactive compounds (e.g., melatonin, glutathione, various nanofertilizers) has been documented to enhance tolerance against a series of abiotic stressors, including UV [20,21,22,23,24]. Among others, the enhanced tolerance following application of these compounds has been related to oxidative stress alleviation through reactive oxygen species scavenging [24,25,26,27]. Therefore, exploring their amelioration effect in the context of targeted manipulation of secondary metabolism is of significant interest to the related industry.
The UVA effect on secondary metabolite biosynthesis has mostly been addressed in indoor environments (growth chambers or greenhouses), while relevant work in outdoor settings is scant. In controlled environments, however, the temporal and spatial variability in environmental conditions is much less pronounced than in natural environments. For instance, light intensity is often lower than outdoors, while spectral composition generally differs considerably compared to solar light. Therefore, field studies with experimentally modulated UVA levels are valuable in gaining a better insight into the UVA effects on secondary metabolite biosynthesis [28,29].
Owing to its unique aromatic and medicinal properties, the genus Thymus spp. (Lamiaceae) has gained global popularity and economic importance. It naturally grows in rangelands and rocky-gravelly grounds of high altitudes, with the Mediterranean region being the center of diversity [30,31]. In this region, prevailing conditions allow a great potential of receiving UV radiation [28,31,32].
The present study aimed to investigate the response to UVA radiation and to the application of protectants. The generated baseline information on the specific effects of UVA on essential oil composition may be employed to identify the most suitable conditions correlating with desired metabolite biosynthesis. The obtained knowledge is also of significant interest in predicting product quality and pharmaceutical properties in a changing climate perspective.
2. Materials and Methods
2.1. Plant Material and Growth Conditions
In an outdoor setting, the pheno-morphological traits and essential oil composition responses to UVA radiation were investigated in three Thymus species. T. daenensis Celak (Figure 1) is endemic to Iran, and its essential oil mainly contains the phenolic compound thymol (78.1%) [33]; T. fedtschenkoi Ronniger (Figure 1) is semi endemic to Iran, and its major (83.1%) essential oil component is the acyclic monoterpene alcohol linalool [34]; T. vulgaris L. (common thyme) is widely cultivated and studied, and it was considered for comparison. It is native to the southern European and the Mediterranean region [35]. Although differences among T. vulgaris chemotypes have been documented, the major essential oil components generally consist of thymol, p-Cymene, γ-terpinene, and carvacrol [36,37]. During cultivation, three UVA levels (ambient, enriched, excluded) were realized in combination with exogenous application of different protectants (melatonin, glutathione, iron-zinc nanofertilizer).

Figure 1.
Flowering plants of Thymus daenensis (left panel) and T. fedtschenkoi (middle panel). The rosette form of the latter species is also provided (right panel).
According to national guidelines and under the auspices of Lorestan University (Khorramabad, Iran), T. daenensis and T. fedtschenkoi propagules were collected from their natural habitats situated at the mountains of Malayer (34°15′ N, 48°35′ E, 1840–1850 m) and Soobashi (34°11′ N, 48°15′ E, 2430–2440 m), respectively. Species identification was performed by an expert by using Voucher specimens (herbarium numbers 36614 and 36622, respectively), which are deposited at the Herbarium of College of Agriculture and Natural Resources (Tehran University, Iran). T. vulgaris plants were obtained by the Hamedan Botanical Garden (Tehran, Iran). Plants were propagated by division. For each species, propagules were obtained from the same (mother) plant and were thus considered identical clones.
The field experiment was performed in the research garden of the University of Malayer (34°15′ N, 48°51′ E, 1814 m). Plantlets were transplanted (0.2 × 0.4 m) to the field on the beginning of March 2016. In order to achieve the same starting material, all plants were pruned at 5 cm above ground level at the end of April. At the end of May (i.e., 30 d following pruning), UVA treatments were realized and performed till the final harvest. Protectant treatments were initiated 5 d after UVA treatment and were repeated (5 d intervals) till the final harvest. The final harvest was carried out at the end of August 2016. During cultivation, plants were watered once a week. When necessary, weeds were manually removed. Pesticide application was not required. Before applying the treatments, humic acid (2.5 mL L−1) fertilization was performed twice.
In control UVA plots, plants received solar light. In enriched UVA plots, plants received solar light, which was supplemented by fluorescent UVA-340 lamps (Q-Lab, Cleveland, OH, USA). The emission peak of these lamps was at 340 nm (Figure 2) [38]. Wavelengths below 320 nm (i.e., UVB) were blocked by wrapping the lamps with a specific polyester filter. Supplemental lighting was daily provided for 3 h at noon (11:00–14:00 h). In order to reduce shading (by lamps and supporting frames), plots were located in an east-west orientation. The lamps were mounted on steel frames. These were placed at the northern margin of each plot. In this way, the main axis of the lamps was perpendicular to the plant row. The distance between lamps was 0.25 m, while the distance from the center of each plot was 0.5 m. The latter distance was maintained constant throughout the experimental period. To prevent UVA contamination among plots, transparent polycarbonate sheets (allowing no transmission below 400 nm) were placed along the four sides of each plot.
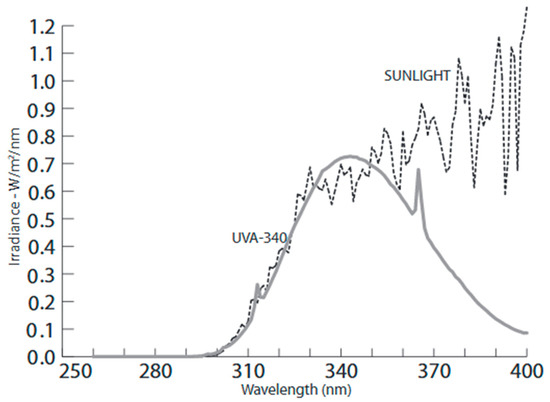
Figure 2.
The spectral irradiance emitted by the employed UVA lamps, as compared to that of sunlight assessed on a clear day at solar noon. During cultivation, supplementary light wavelengths below 320 nm (i.e., UVB) were blocked by wrapping the lamps with a specific polyester filter.
In excluded UVA plots, plants received solar light, by which UVA was excluded by using a specific filter. Filters were mounted on steel frames. The roof of the frame structure (parallel to the soil) was covered by the filter, whereas the four sides (perpendicularly to the soil) were not covered to ensure air circulation. Transparent polycarbonate sheets were employed as filters, showing no transmission below 400 nm. Over each plot, the frame structure was erected in the north–south direction (having a 26° slope towards the south). At the south-face front, a 0.5 m distance from the soil was considered, while at the north-face front the respective distance was 1.2 m. These frame orientation and dimensions ensured that solar radiation could reach the plant only after passing through the filter.
In each main plot, randomly selected sub-plots received repeated (5 d intervals) foliar spray applications of one protectant treatment (control, melatonin, glutathione, iron-zinc nanofertilizer). Each plant was sprayed to wetness by using melatonin (100 mg L−1), glutathione (550 mg L−1), or iron-zinc nanofertilizer (5 g L−1) solution. Control plants were sprayed with distilled water. All solutions, including the controls, contained 0.01% Tween 20. Tween 20, glutathione and melatonin were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA), while iron-zinc nanofertilizer (equal mix) was obtained by Khazra (Sodour Ahrar Shargh knowledge-based Co., Teheran, Iran).
2.2. Pheno-Morphological Evaluation
The UVA and protectant application effects on plant growth of the three Thymus species under study were assessed. Evaluations included plant height (from the root-shoot junction to the apical meristem), internode length, weight loss (difference between fresh and dry weights), leaf together with flowers or inflorescences (fresh and dry) weight, stem (fresh and dry) weight, and leaf to stem dry weight ratio. For measuring dry weight, samples were placed in a forced-air drying oven for 48 h at 80 °C [39]. Plants were sampled at the full flowering stage. Sampling was carried out in the morning after dew (18–21 °C during harvest) [6]. Five repetitions were assessed per treatment.
The treatment effects on plant phenology were also determined. Plants were scored according to the following scale: 0–1, early vegetative stage (active growth or 4–6 leaf stage); 1–2, late vegetative stage (stem development); 2–3, budding stage (20% budding); 3–4, full budding stage; 4–5, early flowering stage; and 5–6, full flowering stage. Evaluations were initiated 23 d following the onset of the UVA treatment (i.e., 53 d following pruning), and were repeated at 10 d intervals in five note takings. The final evaluation took place 93 d following pruning. These data are depicted in the growth column, where y-axis refers to the above-mentioned scale (Figure 3, Figure 4 and Figure 5). Four replicates were assessed per treatment. Specific treatments stimulated compaction (i.e., formation of rosette plants; for T. fedtschenkoi (see Figure 1). These phenotypes were also recorded, and data are depicted in a separate column alongside to the growth column (Figure 3, Figure 4 and Figure 5). The y-axis scale for this separate column represents the number (out of 4) of compact plants.
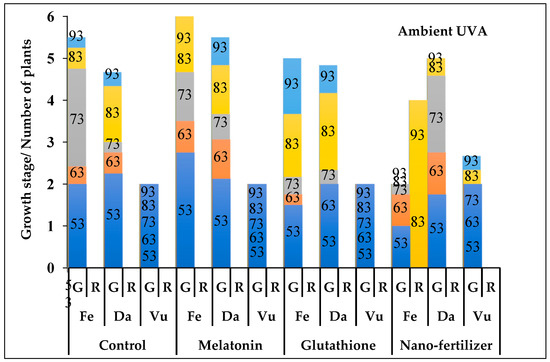
Figure 3.
The phenological stages of Thymus fedtschenkoi (Fe), T. daenensis (Da) and T. vulgaris (Vu) under ambient UVA and following application of protectant compounds [water (control), melatonin, glutathione, iron-zinc nanofertilizer]. Both the rate of growth (G) and of rosette (R) are provided. Numbers on the columns represent days after pruning. For G column, the y-axis scale represents the following stages: 0–1, early vegetative stage (active growth or 4–6 leaf stage); 1–2, late vegetative stage (stem development); 2–3, budding stage (20% budding); 3–4, full budding stage; 4–5, early flowering stage; and 5–6, full flowering stage. For R column, the y-axis scale represents the number (out of 4) of rosette plants.
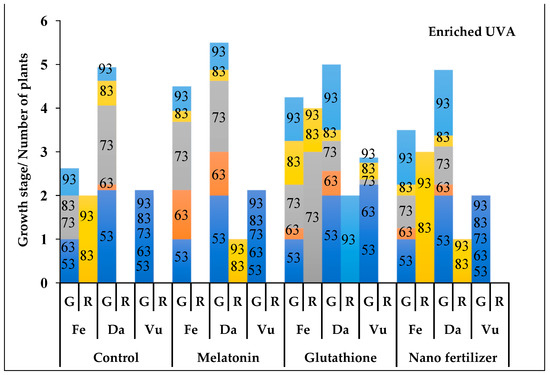
Figure 4.
The phenological stages of Thymus fedtschenkoi (Fe), T. daenensis (Da) and T. vulgaris (Vu) under enriched UVA and following application of protectant compounds [water (control), melatonin, glutathione, iron-zinc nanofertilizer]. Both the rate of growth (G) and of rosette (R) are provided. Numbers on the columns represent days after pruning. For G column, the y-axis scale represents the following stages: 0–1, early vegetative stage (active growth or 4–6 leaf stage); 1–2, late vegetative stage (stem development); 2–3, budding stage (20% budding); 3–4, full budding stage; 4–5, early flowering stage; and 5–6, full flowering stage. For R column, the y-axis scale represents the number (out of 4) of rosette plants.
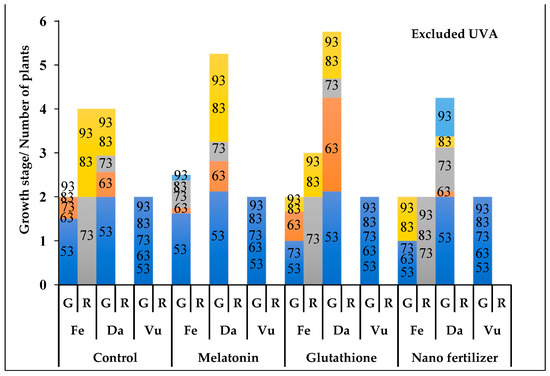
Figure 5.
The phenological stages of Thymus fedtschenkoi (Fe), T. daenensis (Da) and T. vulgaris (Vu) under excluded UVA and following application of protectant compounds [water (control), melatonin, glutathione, iron-zinc nanofertilizer]. Both the rate of growth (G) and of rosette (R) are provided. Numbers on the columns represent days after pruning. For G column, the y-axis scale represents the following stages: 0–1, early vegetative stage (active growth or 4–6 leaf stage); 1–2, late vegetative stage (stem development); 2–3, budding stage (20% budding); 3–4, full budding stage; 4–5, early flowering stage; and 5–6, full flowering stage. For R column, the y-axis scale represents the number (out of 4) of rosette plants.
2.3. Essential Oil Isolation
Samples were subjected to hydro-distillation using a Clevenger apparatus [6,7]. These (200 g air-dried material) were added to a 1 L flask containing 400 mL of distilled water. The flask was then heated for 3 h [40]. The isolated essential oils were first dried over anhydrous sodium sulfate and then kept in glass vials at 4 °C before further analysis. Three replicates were assessed per sample.
2.4. Essential Oil Composition
Gas chromatography-mass spectrometry (GC-MS) analysis was conducted using a gas chromatograph (Shimadzu GC-17A, Kyoto, Japan) coupled with a mass spectrometer (QP-5050, Shimadzu, Kyoto, Japan). A fused silica capillary column (30 m length × 0.22 mm i.d.; 0.25 μm BP-5 film thickness) was used to separate the oil compounds. The oven temperature was increased from 40 to 280 °C at a rate of 4 °C min−1, and finally held isothermal at 280 °C for 10 min. Ion source and the transfer-line temperature was 250 °C. Ultra-pure helium was used as the carrier gas. Injector and interface temperatures were 280 and 260 °C, respectively. The mass spectrum was acquired over the mass range of 35–450 amu in full-scan acquisition mode. The split ratio was 1:50. The GC-FID analysis of the essential oils was conducted using a Thermoquest Finnigan apparatus equipped with a flame ionization detector (FID) and a fused silica capillary column (30 m length × 0.22 mm i.d.; 0.25 μm BP-5 film thickness). The oven temperature was programmed as stated above. Injector and detector temperatures were 250 and 300 °C, respectively. Ultra-pure helium was used as the carrier gas with a flow rate of 1 mL min−1. The split ratio was 1:10.
Retention indices (RI) of each compound were calculated using a homologous series of n-alkanes (C5–C24) injected into an HP-5MS column in the same condition. Identification of oil constituents was performed by comparison of (1) their retention times with those of authentic standards, (2) their spectral mass with those of the internal reference mass spectra library (NIST08 and Wiley 9.0), and (3) their RI with those reported in the literature [41]. Quantification was conducted by the external standard method through calibration curves generated by running GC analysis of representative authentic compounds. The percentage of each compound was calculated by the area normalization method, considering an equal response factor for the different chemical classes present in the essential oil [42].
2.5. Experimental Design and Statistical Analysis
A split-split plot design with a randomized complete block (with three replications) was used. The UVA treatments (ambient, enriched, excluded) were applied as main plots, while the protectant treatments (control, melatonin, glutathione, iron-zinc nanofertilizer) as sub-plots. The three species under study were considered as sub-sub plots. Each sub-sub-plot was sized 1 × 2 m and contained four rows. Each row consisted of five plants for a total of 20 plants per sub-sub-plot (experimental unit). Analysis of variance (ANOVA) was performed by using the MSTAT-C software. The mean comparison was performed with the least significant difference (LSD) at the 0.05% level of significance. The bivariate correlation on the Pearson coefficients was estimated for all pairs of entries by using the SPSS software. Principal component analysis discrimination using variance-covariance matrix was performed by the Past software (version 3.04).
3. Results
3.1. Phenological Responses to UVA and Protectant Treatments
In nearly all UVA and protectant treatments, T. vulgaris was limited to the late vegetative stage (stem stage; Figure 3, Figure 4 and Figure 5). In T. daenensis, regardless of the protectant treatments, enriched UVA promoted early flowering as compared to ambient UVA (control), whereas excluded UVA delayed it (Figure 3, Figure 4 and Figure 5). In T. fedtschenkovi, independently of protectant treatments, both enriched and excluded UVA delayed early flowering as compared to ambient UVA (control; Figure 3, Figure 4 and Figure 5). In this species, the formation of rosette plants under both enriched and excluded UVA [2 (out of 4) and all four plants, respectively] was observed 73 and 83 d after pruning, respectively.
In all UVA treatments, melatonin promoted early flowering in both T. deanensis and T. fedtschenkoi (Figure 3, Figure 4 and Figure 5). This effect was more prominent in T. fedtschenkoi under enriched UVA. In melatonin-treated T. fedtschenkoi, no rosette plant form was observed (Figure 3, Figure 4 and Figure 5). Similar to melatonin, glutathione accelerated flowering in both T. deanensis and T. fedtschenkoi under both enriched and excluded UVA (Figure 3, Figure 4 and Figure 5). In all UVA treatments, iron-zinc nanofertilizer treatment was associated with reduced growth in T. fedtschenkoi (Figure 3, Figure 4 and Figure 5). In this species, rosette plant form was noted (83 d after pruning) in all four plants under ambient UVA, while under both enriched and excluded UVA this form was noted in two (out of four) plants. In T. fedtschenkovi, no effect of iron-zinc nanofertilizer treatment was apparent (Figure 3, Figure 4 and Figure 5).
Under ambient UVA, T. fedtschenkoi flowered earlier than T. daenensis following melatonin and glutathione treatments (Figure 3, Figure 4 and Figure 5). Under enriched UVA, the opposite trend was noted under all protectant treatments (Figure 3, Figure 4 and Figure 5). Under all protectant treatments, excluded UVA exerted the most negative effect on studied phenological traits in both T. deanensis and T. fedtschenkoi, with this effect being more prominent in the latter.
3.2. Morphological Responses to UVA and Protectant Treatments
The effect of protectant treatment was significant on plant height in T. fedtschenkoi and on leaf and stem dry weights in T. vulgaris. In T. vulgaris, melatonin and nanofertilizer treatments increased leaf dry weight, but nanofertilizer decreased stem dry weight. Ιn T. fedtschenkoi, glutathione also stimulated plant height, whereas iron-zinc nanofertilizer decreased it (Table 1).

Table 1.
Main effects of protectants compounds [water (control), melatonin, glutathione, iron-zinc nanofertilizer] on plant height of Thymus fedtschenkoi, and on leaf and stem dry weight of T. vulgaris. Within each column, different letters indicate significant differences.
The interaction effect of UVA radiation and protectants was significant on dry weight loss, leaf dry weight and stem dry weight of T. fedtschenkoi. However, the interaction effect of UVA radiation and protectants on stem dry weight, leaf/stem weight ratio and plant height was significant in T. daenensis. Under ambient UVA, glutathione generally promoted plant height, whereas iron-zinc nanofertilizer decreased it. Excluded UVA was generally associated with reduced plant height (Table 2).

Table 2.
Significant interaction effects of UVA levels (ambient, enriched, excluded) with protectant compounds [water (control), melatonin, glutathione, iron-zinc nanofertilizer] on dry weight loss, leaf dry weight, and stem dry weight of Thymus fedtschenkoi, as well as on stem dry weight, leaf to stem dry weight ratio, and plant height of T. daenensis. Within each column, different letters indicate significant differences.
In T. fedtschenkoi under all three UVA levels, the highest dry weight loss was noted following glutathione treatment. Instead, the lowest dry weight loss was observed in iron-zinc nanofertilizer under ambient and excluded UVA treatments (Table 2).
3.3. Essential Oil Composition Responses to UVA and Protectant Treatments
In T. daenensis, the highest amount of thymol (the main essential oil ingredient) was noted under enriched UVA, and especially following glutathione application (88.20%; Table 3). In this species under enriched UVA, the overall amount of p-cymene, borneol, carvacrol methyl ether, and trans-caryophyllene was lower as compared to ambient UVA (Table 3). The highest total amount of these compounds, and of β-myrcene, γ-terpinene was obtained under ambient UVA. Under ambient UVA, the highest total amount of main compounds (33 compounds above 1%), monoterpene hydrocarbons (11.41%), sesquiterpenes [both hydrocarbon (2.62%) and oxygenated (1.47%)] and the lowest total amount of oxygenated monoterpenes (82.68%) were observed.

Table 3.
The essential oil profile of Thymus daenensis under different UVA levels (ambient, enriched, excluded) in combination with spraying protectants [water (control), melatonin, glutathione, iron-zinc nanofertilizer].
Under ambient UVA, iron-zinc nanofertilizer application was the representative of the highest and lowest levels of the above-mentioned compounds, besides sesquiterpene hydrocarbons, where the highest amount was noted following melatonin application (Table 3 and Table 4).

Table 4.
Key total essential oil characteristics at each UVA level (ambient, enriched, excluded), regardless of the application with protectant compounds [water (control), melatonin, glutathione, iron-zinc nanofertilizer] in the three Thymus species under study.
Scatter plots based on the first two principal components of chemical-composition data also demonstrated that the treatments under ambient UVA were placed between vectors of compounds above 1% and monoterpene hydrocarbons and between vectors of sesquiterpene hydrocarbons and oxygenated sesquiterpenes (Figure 6). Instead, under enriched UVA, the maximum total amount of oxygenated monoterpenes (92.36%), the total number of compounds below 1% and the minimum total amount of monoterpene hydrocarbons (4.78%), sesquiterpene hydrocarbons (1.16%), and oxygenated sesquiterpenes (0.30%) were obtained. Under enriched UVA, the treatments that had the highest and lowest levels of the above-mentioned compounds were as follows: no protectant application (94.08%), glutathione application (2.68%), melatonin (0.51%), and no protectant application UVA (0.11%), respectively.
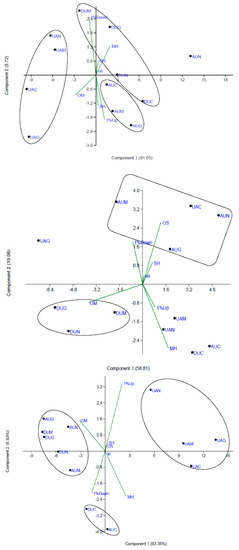
Figure 6.
Scatter plot based on the first two principal components of chemical profile data, demonstrating the relationships among some important characteristics in the essential oil of Thymus daenensis (top panel), T. fedtschenkoi (middle panel) and T. vulgaris (bottom panel) and employed treatments. During growth, three UVA levels (ambient, enriched, excluded) were realized in combination with spraying protectants [water (control), melatonin, glutathione, iron−zinc nanofertilizer]. AUM, melatonin under ambient UVA; AUG, glutathione under ambient UVA; AUN, iron−zinc nanofertilizer under ambient UVA; AUC, control under ambient UVA; UAM, melatonin under enriched UVA; UAG, glutathione under enriched UVA; UAN, iron−zinc nanofertilizer under enriched UVA; UAC: control under enriched UVA; DUM, melatonin under excluded UVA; DUG, glutathione under excluded UVA; DUN, iron−zinc nanofertilizer under excluded UVA; DUC, control under excluded UVA.
As expected, the treatments under enriched UVA, and contrary to ambient UVA, were situated between vectors of oxygenated monoterpenes and compounds below 1% (Figure 6). The highest and lowest essential oil contents were obtained in iron-zinc nanofertilizer application under excluded UVA (2.08%) and glutathione application under enriched UVA (0.54%), respectively. The essential oil content under enriched UVA was generally low (Table 3 and Table 4). The pattern changes in ambient UVA were generally the opposite of those in enriched UVA, while excluded UVA was in between these two conditions with a tendency towards ambient UVA (Table 4; Figure 6).
In T. fedtschenkoi, the maximum amount of linalool (the main essential oil component) was obtained following glutathione application under excluded UVA (75.76%). The minimum amount of linalool was found following melatonin application under excluded UVA (28.14%) and no protectant application under ambient UVA (31.84%). In these two treatments, the main component was Z-dihydrocarvone (46.18 and 35.46%, respectively). In addition, following linalool, Z-sbinene hydrate had the highest percentage in the iron-zinc nanofertilizer application under ambient UVA (14.47%) and no protectant application under enriched UVA (10.42%) (Table 5). With few notable exceptions, the amount of linalool was generally the lowest under enriched UVA.

Table 5.
The essential oil profile of Thymus fedtschenkoi under different UVA levels (ambient, enriched, excluded) in combination with spraying protectants [water (control), melatonin, glutathione, iron-zinc nanofertilizer].
The maximum number of compounds above 1% was noted in melatonin application under enriched UVA (14 compounds), and its maximum total was also observed under enriched UVA (44 treatments). The highest amount of monoterpene hydrocarbons were detected in no protectant application under ambient UVA (8.83%), iron-zinc nanofertilizer application under enriched UVA (7.17%), and no protectant application under excluded UVA (8.67%), while the highest amount of oxygenated monoterpenes was detected in glutathione application under enriched UVA (91.68%). Instead, the highest total amount of these compounds were obtained under excluded UVA conditions (5.50% and 88.51%, respectively). Regarding sesquiterpenes, the maximum amount of sesquiterpene hydrocarbons and oxygenated sesquiterpenes were observed following application of glutathione (8.15%) and iron-zinc nanofertilizer (8.52%) under ambient UVA, respectively. Their minimum values were noted following the application of iron-zinc nanofertilizer (0.93%) and glutathione (1.67%) under excluded UVA conditions, respectively. Similarly, the highest total amount of sesquiterpenes (both hydrocarbon and oxygenated) was obtained under ambient UVA, whereas the opposite was noted under excluded UVA (Table 4). The maximum essential oil content was obtained from no protectant application under enriched UVA (2.40%) and iron-zinc nanofertilizer application under ambient UVA (2.26%), while the highest total was apparent under ambient UVA (7.15%). Based on scatter biplot, the closeness of excluded UVA treatments, besides the no protectant application treatment, and the distance of glutathione application under enriched UVA were associated with an enhanced level of oxygenated monoterpenes. The pattern changes under ambient UVA were generally the opposite of those under excluded UVA (Table 4).
In T. vulgaris, the highest amount of carvacrol was found following glutathione application under ambient (62.07%) and excluded (60.47%) UVA, while the lowest amount was detected in glutathione under enriched UVA (43.35%; Table 6). After carvacrol, γ-terpinene was the constituent with the highest percentage in the essential oil. Under enriched UVA, the total amount of carvacrol and γ-terpinene was low, whereas the highest total amounts of α-thujene, 1-octen-3-ol, β-myrcene, Z-sbinene hydrate, and borneol were obtained. Under all UVA levels, the maximum amount of p-cymene was found following no protectant application. The most and least essential oil contents were obtained from iron-zinc nanofertilizer application under excluded UVA (1.80%) and glutathione application under excluded UVA (0.80%), respectively. The maximum total number of compounds above 1% was observed in treatments under enriched UVA (42 compounds), and its minimum number was observed in treatments under excluded (12 compounds) and ambient (15 compounds) UVA. Regarding the compounds below 1%, the opposite trend was noted. The highest amount of monoterpene hydrocarbons (22.95%) and the lowest amount of oxygenated monoterpenes (71.05%) were found following glutathione application under enriched UVA. In this way, the highest and lowest totals were obtained in treatments under enriched UVA (21.13 and 73.78%, respectively). Similarly, the highest contents of sesquiterpene hydrocarbons (1.53%) and oxygenated sesquiterpenes (0.89%) were obtained with no protectant application under enriched UVA. In a comparable manner, the highest total sesquiterpenes [2.09%, including both hydrocarbon (1.38%) and oxygenated (0.71%)] was observed in treatments under enriched UVA (Table 4). In this species, the scatter plot indicated that the treatments under enriched UVA were situated between vectors of compounds above 1% and monoterpene hydrocarbons (Figure 6). In contrast, the treatments of ambient and decreased UVA, besides no protectant application, were placed between vectors of compounds below 1% and oxygenated monoterpenes. Therefore, the pattern changes in enriched UVA conditions were the opposite of ambient and excluded UVA treatments.

Table 6.
The essential oil profile of Thymus vulgaris under different UVA levels (ambient, enriched, excluded) in combination with spraying protectants [water (control), melatonin, glutathione, iron-zinc nanofertilizer].
4. Discussion
For all protectant treatments (melatonin, glutathione, iron-zinc nanofertilizer), enriched UVA stimulated quicker completion of the growth cycle in T. daenensis, as denoted by early flowering [43]. While T. daenensis plants flowered later than plants of T. fedtschenkoi under ambient UVA at all protectant treatments, the opposite was noted under enriched UVA. In addition to the promotive effect of melatonin and the negative effect of iron-zinc nanofertilizer on early flowering, these findings are similar to a large extent to the earlier reported UVB effects [20]. As noted in that study, the origin and natural habitat (collection site) evidently play an essential role in this regard, as justified by the different responses of the three species under study in various aspects (i.e., phenological, morphological and phytochemical). The natural habitats of T. daenensis are often warmer microclimates on the southern slopes, whereas T. fedtschenkoi grows on the northern slopes with cool microclimates at high altitudes [20,33]. Evidently, these two different climate zones, letting aside edaphic factors, create their own ecological niche climatic variables, with their specific favorable conditions and stresses for each species. The ecological niche of T. daenensis has a greater potential to receive high levels of UV radiation compared to the T. fedtschenkoi [44]. Hence, it seems that enriched UVA radiation is more compatible and favorable for T. daenensis than T. fedtschenkoi. Owing to early flowering, the average plant height was also generally increased, especially in the absence of protectants. Contrary to the current findings, enriched UVB was associated with reduced plant height [20]. The early flowering of T. fedtschenkoi under ambient UVA conditions is probably associated with the experimental site’s low altitude and warm conditions. Regardless of UVA level, the adverse effect of iron-zinc nanofertilizer application on the phenological stage potentially suggests the use of an inappropriate dose [27]. In combination with flowering delay, this negative effect in T. fedtschenkoi, led to the formation of short and dense plants, resembling rosette form (Figure 1).
Regardless of the protectant treatment effects, enriched UVA increased the total amount of thymol and monoterpenes in T. daenensis (monoterpene hydrocarbons less, oxygenated monoterpenes more), while the total number of compounds above 1% and total sesquiterpenes (both hydrocarbon and oxygenated) were decreased. In the case of T. fedtschenkoi, except for the total number of compounds above 1% (with a slight difference), the changes in the composition of monoterpenes and sesquiterpenes were similar to T. daenensis, whereas in T. vulgaris the opposite trend was noted. Notably, similar results were found in the earlier report on the effects of UVB in the same Thymus species [20]. In T. daenensis, the pattern changes under excluded UV were between ambient and enriched UVA (close to ambient and opposite to enriched). In T. fedtschenkoi, the pattern changes under excluded UV were opposed to ambient UVA (enriched UVA was between ambient and excluded UVA). In T. vulgaris, it was quite close to ambient UVA and opposed to enriched UVA (Table 4). In T. fedtschenkoi, the total amount of linalool decreased under enriched UVA. Hence, enriched UVA, similarly to enriched UVB, reduced the alcoholic compound linalool and increased the phenolic compound thymol.
UVA activates the accumulation of specific phenolic compounds rather than total phenolics [11,45,46]. In T. vulgaris, enriched UVA reduced the total percentages of carvacrol, similarly to enriched UVB, and γ-terpinene, while the overall percentage of the other components, which were often monoterpene hydrocarbons (e.g., α-thujene, 1-octen-3-ol, β-myrcene, p-cymene, Z-sabinene hydrate, and borneol), was increased. Hence, as stated above, in contrast to T. daenensis and T. fedtschenkoi, the amount of monoterpene hydrocarbons increased in T. vulgaris. Enriched and decreased UVA conditions enhanced the essential oil quality in both T. daenensis and T. fedtschenkoi by enriching the valuable oxygenated monoterpenes.
In both T. daenensis and T. fedtschenkoi, enriched and decreased UVA conditions reduced the amount of major compounds in essential oil, and increased the amount of miscellaneous or alternative compounds, correspondingly. It seems that the enhancement of oxygenated monoterpenes is correlated with the reduction of the compounds above 1%. Similar effects were earlier noted for enriched UVB [20]. In both T. daenensis and T. fedtschenkoi, total essential oil yield changes corresponded with the changes in the total number of compounds above 1%.
T. daenensis and T. fedtschenkoi differentially responded to UVA as compared to T. vulgaris. Although genetic and origin differences may contribute to the above-mentioned pattern among species, the underlying processes require further investigations.
5. Conclusions
In this field study, an attempt was made to evaluate the phenological, morphological, and essential oil component responses of three Thymus species with distinguished plant types and divergent origins to three UVA levels (ambient, enriched, excluded). High temperature and drought are common and prevalent events in natural habitats of T. daenensis, whereas occasional cold and frost occur in T. fedtschenkoi ecological niche. UV stress often occurs in parallel with high temperature events and is evident in native habitats of warmer micro-climates, herein T. daenensis, which are equipped with mechanisms of response to elevated temperature and water deprivation. Stress-induced flowering (i.e., growth cycle completion) can be regarded as an ultimate adaptation to stress and should be considered a central component, along with tolerance. In T. fedtschenkoi, the main UVA response was associated with dense bush formation and delayed flowering. This may be due to a lower adaptation potential to high temperature which often coincides with exposure to enhanced UVA. Cultivation UVA level also affected essential oil quality (composition) and quantity based on the species. These effects were more prominent in T. deanensis than T. fedtschenkoi.
Author Contributions
H.M., conceptualization, methodology, investigation, writing—original draft, writing—review and editing; A.S., validation, software, formal analysis, writing—original draft; G.T., methodology, formal analysis, writing—review and editing; G.T., Z.E.B., D.F. and S.N., data interpretation, enhancing the quality of the manuscript and editing and final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to appreciate the invaluable support of Lorestan University and Malayer University in this study. We are grateful to the laboratory staff for their contributions, continued diligence, and dedication to their craft. The authors also wish to thank Zanghaee for assisting analytical evaluations. The valuable comments of the editor and three anonymous reviewers are greatly acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fowler, M.W. Plants, medicines and man. J. Sci. Food Agric. 2006, 86, 1797–1804. [Google Scholar] [CrossRef]
- Sharmeen, J.; Mahomoodally, F.; Zengin, G.; Maggi, F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules 2021, 26, 666. [Google Scholar] [CrossRef]
- Zhang, W.J.; Björn, L.O. The effect of ultraviolet radiation on the accumulation of medicinal compounds in plants. Fitoterapia 2009, 80, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Thamkaew, G.; Sjöholm, I.; Galindo, F.G. A review of drying methods for improving the quality of dried herbs. Crit. Rev. Food Sci. Nutr. 2021, 61, 1763–1786. [Google Scholar] [CrossRef]
- Dutta, T.; Anand, U.; Saha, S.C.; Mane, A.B.; Prasanth, D.A.; Kandimalla, R.; Proćków, J.; Dey, A. Advancing urban ethnopharmacology: A modern concept of sustainability, conservation and cross-cultural adaptations of medicinal plant lore in the urban environment. Conserv. Physiol. 2021, 9, 073. [Google Scholar] [CrossRef]
- Taheri-Garavand, A.; Mumivand, H.; Fanourakis, D.; Fatahi, S.; Taghipour, S. An artificial neural network approach for noninvasive estimation of essential oil content and composition through considering drying processing factors: A case study in Mentha aquatica. Ind. Crops Prod. 2021, 171, 113985. [Google Scholar] [CrossRef]
- Mumivand, H.; Ebrahimi, A.; Shayganfar, A.; Khoshro, H.H. Screening of tarragon accessions based on physiological and phytochemical responses under water deficit. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef]
- Kovács, V.; Gondor, O.; Szalai, G.; Majláth, I.; Janda, T.; Pál, M. UV-B radiation modifies the acclimation processes to drought or cadmium in wheat. Environ. Exp. Bot. 2014, 100, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Mumivand, H.; Ebrahimi, A.; Morshedloo, M.R.; Shayganfar, A. Water deficit stress changes in drug yield, antioxidant enzymes activity and essential oil quality and quantity of Tarragon (Artemisia dracunculus L.). Ind. Crop. Prod. 2021, 164, 113381. [Google Scholar] [CrossRef]
- Brune, D.; Hellborg, R.; Persson, B.R.R.; Pääkkönen, R. Radiation: At Home, Outdoors and in the Workplace. Am. J. Phys. 2003, 71, 189–190. [Google Scholar] [CrossRef]
- Verdaguer, D.; Jansen, M.A.; Llorens, L.; Morales, L.O.; Neugart, S. UV-A radiation effects on higher plants: Exploring the known unknown. Plant Sci. 2017, 255, 72–81. [Google Scholar] [CrossRef]
- Kataria, S.; Devi Ahilya University; Baroniya, S.S.; Kanungo, M.; Bhaghel, L. Effect of Exclusion of Solar UV radiation on Plants. Plant Sci. Today 2014, 1, 224–232. [Google Scholar] [CrossRef]
- Helsper, J.P.F.G.; De Vos, C.H.R.; Maas, F.M.; Jonker, H.H.; Broeck, H.C.V.D.; Jordi, W.; Pot, C.S.; Keizer, L.C.P.; Schapendonk, A.H.C.M. Response of selected antioxidants and pigments in tissues of Rosa hybrida and Fuchsia hybrida to supplemental UV-A exposure. Physiol. Plant. 2003, 117, 171–178. [Google Scholar] [CrossRef]
- Yang, L.; Fanourakis, D.; Tsaniklidis, G.; Li, K.; Yang, Q.; Li, T. Contrary to Red, Blue Monochromatic Light Improves the Bioactive Compound Content in Broccoli Sprouts. Agronomy 2021, 11, 2139. [Google Scholar] [CrossRef]
- Chen, Y.; Fanourakis, D.; Tsaniklidis, G.; Aliniaeifard, S.; Yang, Q.; Li, T. Low UVA intensity during cultivation improves the lettuce shelf-life, an effect that is not sustained at higher intensity. Postharvest Biol. Technol. 2021, 172, 111376. [Google Scholar] [CrossRef]
- Shiozaki, N.; Hattori, I.; Gojo, R.; Tezuka, T. Activation of growth and nodulation in a symbiotic system between pea plants and leguminous bacteria by near-UV radiation. J. Photochem. Photobiol. B Biol. 1999, 50, 33–37. [Google Scholar] [CrossRef]
- Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Kalogiannakis, K.; Debouba, F.J.; Ipsilantis, I.; Tsoktouridis, G.; et al. Pilot Cultivation of the Vulnerable Cretan Endemic Verbascum arcturus L. (Scrophulariaceae): Effect of Fertilization on Growth and Quality Features. Sustainability 2021, 13, 14030. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, Y.; Ren, J.; Li, C. UV-spectra dependence of seedling injury and photosynthetic pigment change in Cucumis sativus and Glycine max. Environ. Exp. Bot. 2005, 57, 160–167. [Google Scholar] [CrossRef]
- Krizek, D.T.; Chalker-Scott, L. Ultraviolet Radiation and Terrestrial Ecosystems. Photochem. Photobiol. 2005, 81, 1021–1025. [Google Scholar] [CrossRef]
- Shayganfar, A.; Azizi, M.; Rasouli, M. Various strategies elicited and modulated by elevated UV-B radiation and protectant compounds in Thymus species: Differences in response over treatments, acclimation and interaction. Ind. Crop. Prod. 2018, 113, 298–307. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Nawaz, K.; Chaudhary, R.; Sarwar, A.; Ahmad, B.; Gul, A.; Hano, C.; Abbasi, B.; Anjum, S. Melatonin as Master Regulator in Plant Growth, Development and Stress Alleviator for Sustainable Agricultural Production: Current Status and Future Perspectives. Sustainability 2020, 13, 294. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef]
- Paramo, L.A.; Feregrino-Pérez, A.A.; Guevara, R.; Mendoza, S.; Esquivel, K. Nanoparticles in Agroindustry: Applications, Toxicity, Challenges, and Trends. Nanomaterials 2020, 10, 1654. [Google Scholar] [CrossRef]
- Sangwan, N.S.; Farooqi, A.H.A.; Shabih, F.; Sangwan, R.S. Regulation of essential oil production in plants. Plant Growth Regul. 2001, 34, 3–21. [Google Scholar] [CrossRef]
- Prado, F.E.; Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M. UV-B Radiation, Its Effects and Defense Mechanisms in Terrestrial Plants. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M., Eds.; Springer: New York, NY, USA, 2011; pp. 57–83. [Google Scholar]
- El-Saadony, M.T.; Almoshadak, A.S.; Shafi, M.E.; Albaqami, N.M.; Saad, A.M.; El-Tahan, A.M.; Desoky, E.-S.M.; Elnahal, A.S.; Almakas, A.; El-Mageed, T.A.A.; et al. Vital roles of sustainable nano-fertilizers in improving plant quality and quantity-an updated review. Saudi J. Biol. Sci. 2021, 28, 7349–7359. [Google Scholar] [CrossRef]
- Verdaguer, D.; Llorens, L.; Bernal, M.; Badosa, J. Photomorphogenic effects of UVB and UVA radiation on leaves of six Mediterranean sclerophyllous woody species subjected to two different watering regimes at the seedling stage. Environ. Exp. Bot. 2012, 79, 66–75. [Google Scholar] [CrossRef]
- Kataria, S.; Dehariya, P.; Guruprasad, K.; Pandey, G. Effect of Exclusion of Ambient Solar Uv-A/B Components on Growth and Antioxidant Response of Cotton (Gossypium Hirsutum L.). Acta Biol. Cracoviensia Ser. Bot. 2012, 54, 47–53. [Google Scholar] [CrossRef]
- Morales, R.V. The history, botany and taxonomy of the genus Thymus. In Thyme: The genus Thymus; Stahl-Biskup, E., Saez, F., Eds.; Taylor & Francis: London, UK, 2002; pp. 1–43. Available online: https://scirp.org/reference/ReferencesPapers.aspx?ReferenceID=2581722 (accessed on 16 February 2020).
- Foyo-Moreno, I.; Alados, I.; Olmo, F.; Alados-Arboledas, L. The influence of cloudiness on UV global irradiance (295–385 nm). Agric. For. Meteorol. 2003, 120, 101–111. [Google Scholar] [CrossRef]
- (IPCC) Climate IP on C Change. Climate Change 2007: The Physical Science Basis Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/ar4-wg1-frontmatter-1.pdf (accessed on 18 February 2020).
- Mumivand, H.; Shayganfar, A.; Hasanvand, F.; Maggi, F.; Alizadeh, A.; Darvishnia, M. Antimicrobial Activity and Chemical Composition of Essential Oil from Thymus daenensis and Thymus fedtschenkoi During Phenological Stages. J. Essent. Oil Bear. Plants 2021, 24, 469–479. [Google Scholar] [CrossRef]
- Rustaiee, A.R.; Mirahmadi, S.F.; Sefidkon, F.; Tabatabaei, M.F.; Omidbaigi, R. Essential Oil Content and Composition of Thymus fedtschenkoi Ronniger at Different Phenological Stages. J. Essent. Oil Bear. Plants 2011, 14, 625–629. [Google Scholar] [CrossRef]
- Jalas, J. Notes on Thymus L.(Labiatae) in Europe. I. Supraspecific classification and nomenclature. Bot. J. Linn. Soc. 1971, 64, 199–215. [Google Scholar]
- Rustaiee, A.R.; Yavari, A.; Nazeri, V.; Shokrpour, M.; Sefidkon, F.; Rasouli, M. Genetic Diversity and Chemical Polymorphism of SomeThymusSpecies. Chem. Biodivers. 2013, 10, 1088–1098. [Google Scholar] [CrossRef]
- Satyal, P.; Murray, B.L.; McFeeters, R.L.; Setzer, W.N. Essential Oil Characterization of Thymus vulgaris from Various Geographical Locations. Foods 2016, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- q-lab. q-lab.com. 2018. Available online: https://www.q-lab.com/products/lamps-optical-filters/lamps-and-optical-filters (accessed on 16 October 2018).
- Fanourakis, D.; Papadopoulou, E.; Valla, A.; Tzanakakis, V.A.; Nektarios, P.A. Partitioning of transpiration to cut flower organs and its mediating role on vase life response to dry handling: A case study in chrysanthemum. Postharvest Biol. Technol. 2021, 181, 111636. [Google Scholar] [CrossRef]
- Mumivand, H.; Khanizadeh, P.; Morshedloo, M.R.; Sierka, E.; Żuk-Gołaszewska, K.; Horaczek, T.; Kalaji, H.M. Improvement of Growth, Yield, Seed Production and Phytochemical Properties of Satureja khuzistanica Jamzad by Foliar Application of Boron and Zinc. Plants 2021, 10, 2469. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation Carol Stream: Carol Stream, IL, USA, 2007. [Google Scholar]
- Mumivand, H.; Aghemiri, A.; Aghemiri, A.; Morshedloo, M.R.; Nikoumanesh, K. Ferulago angulata and Tetrataenium lasiopetalum: Essential oils composition and antibacterial activity of the oils and extracts. Biocatal. Agric. Biotechnol. 2019, 22, 101407. [Google Scholar] [CrossRef]
- Takeno, K. Stress-induced flowering. In Abiotic Stress Responses in Plants; Ahmad, P., Prassad, M.N.V., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 331–345. Available online: https://link.springer.com/book/10.1007/978-1-4614-0634-1#about (accessed on 22 February 2020).
- Migicovsky, Z.; Kovalchuk, I. Transgenerational changes in plant physiology and in transposon expression in response to UV-C stress in Arabidopsis thaliana. Plant Signal. Behav. 2014, 9, e976490. [Google Scholar] [CrossRef] [Green Version]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB Light Doses and Harvesting Time Differentially Tailor Glucosinolate and Phenolic Profiles in Broccoli Sprouts. Molecules 2017, 22, 1065. [Google Scholar] [CrossRef]
- Neugart, S.; Schreiner, M. UVB and UVA as eustressors in horticultural and agricultural crops. Sci. Hortic. 2018, 234, 370–381. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).