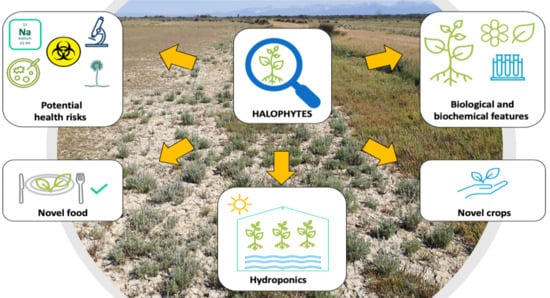
Biological and Agronomic Traits of the Main Halophytes Widespread in the Mediterranean Region as Potential New Vegetable Crops
Abstract
1. Introduction
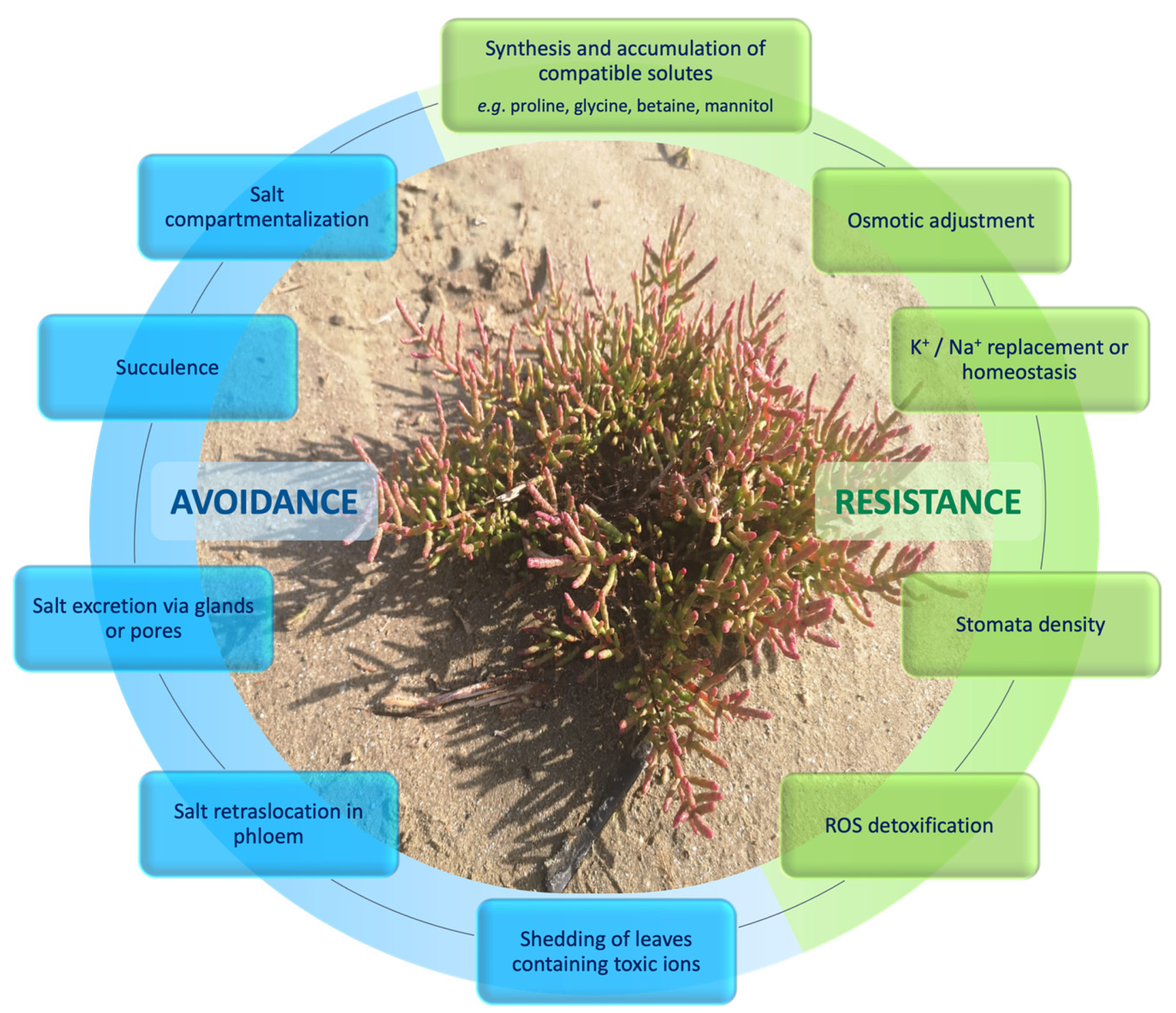
2. Halophytes: Definition and Complexity
3. Classification of Halophytes
3.1. Response to Internal Salt Content
3.2. Eco-Physiological Aspects
- Obligate halophytes, mostly the Amaranthaceae members, are species with optimal growth at moderate or high salinity (NaCl 0.1–5%); they cannot grow at lower salinity, as they require salt as part of their nutrition to activate or de-activate several salt-sensitive enzymes. These species frequently exhibit an activation of these enzymes, both at very low Na+ concentrations (below the physiological optimum) and at seawater Na+ concentrations (considered excessive).
- Facultative halophytes are plants with the ability to grow on salty soils, but their optimal growth is observed in low-salt or non-saline conditions. Many dicotyledons such as Aster tripolium, Chenopodium quinoa, Glaux maritima, and Plantago maritima, but also some Poaceae, Cyperaceae and Juncaceae, belong to this group.
- Habitat-indifferent halophytes normally grow on salt-free substrates, but in saline conditions they thrive better than sensitive species. This group includes the plants that can live in disturbed or stable habitats. In some of these, such as Festuca rubra, Agrostis stolonifera and Juncus bufonius, there are significant genetic and morphological differences between the populations living on salty soils and those on salt-free soils.
3.3. Salt Tolerance
- Eu-halophytes (extreme halophytes) are plants that can grow in seawater or tolerate more than 200 mM NaCl (up 5%), and occur almost exclusively in environments of high salinity [26]. Following the eHALOPH database, this group contains 333 species, members of 70 families of flowering plants. The 75% of eu-halophytes belong to just 19 families. Eu-halophytes are rather rare amongst flowering plants, representing just 0.4% of the 350,699 accepted names in ‘The Plant List’ within 20% of its 642 families. Some species of Atriplex, Salicornia, Suaeda, and Salsola can be included in this group.
- Mio-halophytes are plants that grow in habitats with low levels of salinity (less than 0.5% NaCl).
- Salt-excluding (root-excluding type) are halophytes (also known as pseudo-halophytes) that protect the shoot from salinity through apoplastic barriers in the roots and interveinal recycling of ions. Mangrove vegetation shows such a type of tolerance.
- Salt-excreting (endo- and eso-recretohalophytes) are plants that avoid cellular damage by releasing excess salts to the outside via specialized structures called salt glands—such as species of Limonium, Tamarix, Spartina, Avicennia, and Frankenia—or from epidermal bladders on the leaves, such as species of Atriplex and Chenopodium.
- Salt-accumulating are plants able to accumulate salts that are compartmentalized into vacuoles and used for osmotic adjustment, e.g., Salvadora persica, Sesuvium portulacastrum, Suaeda nudiflora.
3.4. Habitat and Geographical Distribution
- Hydro-halophytes are halophytic plants that need aquatic conditions or wet soil. Species growing in aquatic environments belong to this group, such as the mangrove forests, tidal marshes or coastal lagoons, and the brackish marshes of the temperate zone. Zannichellia palustris and Althenia filiformis are typical hydro-halophytes in the Mediterranean area [47,48].
- Xero-halophytes grow in environments with dry soil due to high evapotranspiration. Most plants living in desert areas and succulents belong to this group. Atriplex canescens or A. halimus are xero-halophytes that tolerate both salt and drought stress.
4. Mediterranean Halophytes
5. Halophytes as Potential Novel Crops
6. Cultivation of Halophytes in Hydroponic Greenhouse
| Species | Location (Country) | Growing Technique | Growing Season and Environment | Growing Cycle (Days/Month) | Plant Density (Plants/m2) | Salinity Level | Fresh Biomass (kg/m2) | Note | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Halimione portulacoides | Portugal | Hydroponics (floating system) | Growth chamber | 10 weeks | 110–220 | 20 g/L NaCl | 3.1–5.4 | [9] | |
| Halimione portulacoides | Portugal | Hydroponics (floating system) | Growth chamber | 10 weeks | 220 | 20 g/L NaCl | 3.41–5.40 | [77] | |
| Portulaca oleracea | Jordan | Soil-less substrate | Unheated greenhouse, March–July | 5 months | 5.5–6.5 dS/m | 14.6–26.9 | Total yield depended on genotypes | [80] | |
| Portulaca oleracea | Spain | Hydroponics (floating system) | Unheated greenhouse, July | 15 days | 2050 | 2.7 dS/m | 1.64–2.66 | Total yield depended on genotypes | [78] |
| Portulaca oleracea | Spain | Microgreens | Growth chamber | 4 weeks | 0–80 mM NaCl | 1.51–1.97 | Yield was greater at higher salinity | [81] | |
| Portulaca oleracea | Canada | Hydroponics (floating system) | Controlled-climate greenhouse | 26 days | 266 | 0–10 mM NaCl | 4.90–5.73 | [79] | |
| Portulaca oleracea | Alabama, US | Mixture of Jiffy mix, sand, and soil | Controlled-climate greenhouse | 60 days | 20 | Hoagland nutrient solution | 0.54 | [82] | |
| Salicornia bigelovii | Canada | Hydroponics (floating system) | Controlled-climate greenhouse | 28 days | 266 | 6–200 mM NaCl | 0.33–1.69 | The greatest yield was found at 200 mM NaCl | [73] |
| Salicornia dolichostachya | Germany | Sand culture or hydroponics (floating system) | Controlled-climate greenhouse | 42 days | 37 | 257–513 mM NaCl | 0.86–1.06 | Yield was greater in floating system than in sand | [75] |
| Salicornia europaea | Italy | Hydroponics (floating system) | Greenhouse, summer | 58 days | 60 | 0–30 g/L (artificial sea salt) | 4.80–9.84 | The lowest yield and the highest yield were found at salinity of 30 and 10 g/L | [70] |
| Salicornia ramosissima | Germany | Sand culture | Hydroponics (floating system) | 5 weeks | - | 257–513 mM NaCl | 1.06 | [69] | |
| Salicornia persica | Israel | Coconut-fiber-filled sleeves | Unheated greenhouse | 5 months | - | 4 dS/m | 18.6 | [83] | |
| Suaeda glauca | Canada | Hydroponics (floating system) | Controlled-climate greenhouse | 27 days | 266 | 6–200 mM NaCl | 4.02–5.65 | The lowest yield was found at 200 mM NaCl | [88] |
7. Health Risks Associated with the Consumption of Cultivated Halophytes
7.1. Nitrates
7.2. Sodium
7.3. Oxalate
7.4. Mycotoxigenic Fungi and Mycotoxins in Edible Halophytes: A Potential Health Risk?
7.5. Pathogenic Bacteria Potentially Associated with Edible Halophytes
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Genus Salicornia L.
Appendix A.1.1. Salicornia perennans Willd. subsp. perennans (Syn.: Salicornia europaea auct.; Salicornia patula Duval-Jouve; Salicornia herbacea (L.).) Eu-halophyte
Appendix A.1.2. Salicornia perennis Mill. subsp. perennis (Syn.: Sarcocornia perennis (Mill.) A. J. Scott subsp. perennis). Eu-halophyte
Appendix A.2. Genus Suaeda Forssk ex J.F. Gmelin
Appendix A.2.1. Suaeda maritima (L.) Dumort. (Syn.: Chenopodium maritimum L.; Schoberia maritima (L.) C.A. Mey). Eu-halophyte/halo-nitrophilous
Appendix A.2.2. Suaeda vera J. F. Gmel (Syn.: Suaeda fruticosa (L.) Forssk.; Suaeda fruticosa (L.) Forssk. subsp. vera (J. F. Gmel.) Maire & Weiller; Chenopodium fruticosum (L.). Eu-halophyte (Halo-nitrophilous)
Appendix A.3. Genus Salsola L.
Salsola soda L. (Syn.: Soda inermis Fourr.). Mio-halophyte (halo-nitrophilous)
Appendix A.4. Genus Atriplex L.
Appendix A.4.1. Atriplex littoralis L. (Syn.: Atriplex patula L. var. littoralis (L.) A. Gray). Halo-nitrophilous
Appendix A.4.2. Atriplex prostrata Boucher ex DC (Syn.: Atriplex hastata L. var. prostrata (Boucher ex DC.) Lange; Atriplex latifolia Wahlenb.). Halo-nitrophilous
Appendix A.5. Genus Halimione Aellen
Halimione portulacoides (L.) Aellen (Syn.: Atriplex portulacoides L.). Eu-halophyte
Appendix A.6. Genus Beta L.
Beta vulgaris L. subsp. Maritima (L.) Arcang. (Syn.: Beta maritima L.) Mio-halophyte
Appendix B
Appendix B.1. Genus Cakile Mill.
Cakile maritima Scop. subsp. maritima. Halo-nitrophyle (Psammophile)
Appendix C
Portulaca oleracea L. subsp. oleracea. Xerophyte
References
- Atzori, G.; Mancuso, S.; Masi, E. Seawater Potential Use in Soilless Culture: A Review. Sci. Hortic. 2019, 249, 199–207. [Google Scholar] [CrossRef]
- Ventura, Y.; Eshel, A.; Pasternak, D.; Sagi, M. The Development of Halophyte-Based Agriculture: Past and Present. Ann. Bot. 2015, 115, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Guo, J.; Shabala, S.; Wang, B. Reproductive Physiology of Halophytes: Current Standing. Front. Plant Sci. 2019, 9, 1954. [Google Scholar] [CrossRef] [PubMed]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Kumar, V.; Khare, T.; Guddimalli, R.; Parveda, M.; Solymosi, K.; Suprasanna, P.; Kavi Kishor, P.B. Engineering Salinity Tolerance in Plants: Progress and Prospects. Planta 2020, 251, 76. [Google Scholar] [CrossRef] [PubMed]
- Ventura, Y.; Wuddineh, W.A.; Myrzabayeva, M.; Alikulov, Z.; Khozin-Goldberg, I.; Shpigel, M.; Samocha, T.M.; Sagi, M. Effect of Seawater Concentration on the Productivity and Nutritional Value of Annual Salicornia and Perennial Sarcocornia Halophytes as Leafy Vegetable Crops. Sci. Hortic. 2011, 128, 189–196. [Google Scholar] [CrossRef]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet Food with Nutritional Health Benefits? J. Food Comp. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Urbano, M.; Tomaselli, V.; Bisignano, V.; Veronico, G.; Hammer, K.; Laghetti, G. Salicornia patula Duval-Jouve: From Gathering of Wild Plants to Some Attempts of Cultivation in Apulia Region (Southern Italy). Gen. Res. Crop Evol. 2017, 64, 1465–1472. [Google Scholar] [CrossRef]
- Custódio, M.; Villasante, S.; Calado, R.; Lillebø, A.I. Testing the Hydroponic Performance of the Edible Halophyte Halimione portulacoides, a Potential Extractive Species for Coastal Integrated Multi-Trophic Aquaculture. Sci. Total Environ. 2021, 766, 144378. [Google Scholar] [CrossRef]
- Centofanti, T.; Bañuelos, G. Practical Uses of Halophytic Plants as Sources of Food and Fodder. In Halophytes and Climate Change: Adaptive Mechanisms and Potential Uses; CABI: Wallingford, UK, 2019; pp. 324–342. [Google Scholar]
- Castañeda-Loaiza, V.; Oliveira, M.; Santos, T.; Schüler, L.; Lima, A.R.; Gama, F.; Salazar, M.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; et al. Wild vs Cultivated Halophytes: Nutritional and Functional Differences. Food Chem. 2020, 333, 127536. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible Halophytes of the Mediterranean Basin: Potential Candidates for Novel Food Products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- Demir Aynur and Tprdamaz, R. Halophytes as Medicinal Herbs. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.-N., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–23. ISBN 978-3-030-17854-3. [Google Scholar] [CrossRef]
- Labiad, M.H.; Giménez, A.; Varol, H.; Tüzel, Y.; Egea-Gilabert, C.; Fernández, J.A.; del Carmen Martínez-Ballesta, M. Effect of Exogenously Applied Methyl Jasmonate on Yield and Quality of Salt-Stressed Hydroponically Grown Sea Fennel (Crithmum Maritimum L.). Agronomy 2021, 11, 1083. [Google Scholar] [CrossRef]
- Custódio, M.; Lillebø, A.I.; Calado, R.; Villasante, S. Halophytes as Novel Marine Products—A Consumers’ Perspective in Portugal and Policy Implications. Mar. Policy 2021, 133, 104731. [Google Scholar] [CrossRef]
- Grigore, M.-N.; Toma, C. Anatomical Adaptations of Halophytes; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-66479-8. [Google Scholar]
- Rahman, M.M.; Mostofa, M.G.; Keya, S.S.; Siddiqui, M.N.; Ansary, M.M.U.; Das, A.K.; Rahman, M.A.; Tran, L.S.-P. Adaptive Mechanisms of Halophytes and Their Potential in Improving Salinity Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 10733. [Google Scholar] [CrossRef] [PubMed]
- Yensen, N.P. Halophyte Uses for the Twenty-First Century. In Ecophysiology of High Salinity Tolerant Plants; Springer: Dordrecht, The Netherlands, 2006; pp. 367–396. [Google Scholar]
- Uphof, J.C.T. Halophytes. Bot. Rev. 1941, 7, 1–58. [Google Scholar] [CrossRef]
- Strogonov, B.M. Structure and Function of Plant Cells in Saline Habitats-New Trends in the Study of Salt Tolerance (Translated from Russian by A. Mercado); John Wiley and Sons: New York, NY, USA, 1973. [Google Scholar]
- Stocker, O. Das Halophyten Problem. In Ergebnisse der Biologie; Springer: Berlin/Heidelberg, Germany, 1928; pp. 265–353. [Google Scholar]
- Lielh, H.; Al, A.; le Houerou, R.N. Salt-Tolerant Plants for the Arid Regions of the Mediterranean Isoclimatic Zone; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar]
- Glenn, E.P.; Brown, J.J.; Blumwald, E. Salt Tolerance and Crop Potential of Halophytes. Crit. Rev. Plant Sci. 1999, 18, 227–255. [Google Scholar] [CrossRef]
- The University of Sussex and Other Contributors EHALOPH—Halophytes Database—Version 3.22. Available online: https://www.sussex.ac.uk/affiliates/halophytes/index.php (accessed on 3 November 2021).
- Aronson, J.A. HALOPH: A Data Base of Salt Tolerant Plants of the World, 1st ed.; Office of Arid Lands Studies, University of Arizona: Tucson, AZ, USA, 1989. [Google Scholar]
- Santos, J.; Al-Azzawi, M.; Aronson, J.; Flowers, T.J. EHALOPH a Database of Salt-Tolerant Plants: Helping Put Halophytes to Work. Plant Cell Physiol. 2016, 57, e10. [Google Scholar] [CrossRef]
- Cheeseman, J.M. The Evolution of Halophytes, Glycophytes and Crops, and Its Implications for Food Security under Saline Conditions. New Phytol. 2015, 206, 557–570. [Google Scholar] [CrossRef]
- Janssen, J.A.M.; Rodwell, J.S.; García Criado, M.; Gubbay, S.; Haynes, T.; Nieto, A.; Sanders, N.; Calix, M. European Red List of Habitats, 1st ed.; Commission, E., Ed.; Publications Office of the European Union: Luxembourg, 2016; ISBN 9789279615887. [Google Scholar]
- FAO. Salt-Affected Soils. Available online: http://www.fao.org/soils-portal/soil-management/management-of-some-problem-soils/salt-affected-soils/more-information-on-salt-affected-soils/en/2018 (accessed on 15 September 2021).
- Colla, G.; Roupahel, Y.; Cardarelli, M.; Rea, E. Effect of Salinity on Yield, Fruit Quality, Leaf Gas Exchange, and Mineral Composition of Grafted Watermelon Plants. HortScience 2006, 41, 622–627. [Google Scholar] [CrossRef]
- Karakas, S.; Bolat, I.; Dikilitas, M. The Use of Halophytic Companion Plant (Portulaca oleracea L.) on Some Growth, Fruit, and Biochemical Parameters of Strawberry Plants under Salt Stress. Horticulturae 2021, 7, 63. [Google Scholar] [CrossRef]
- Waisel, Y. Biology of Halophytes, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012; p. 410. [Google Scholar]
- Flowers, T.J.; Colmer, T.D. Plant Salt Tolerance: Adaptations in Halophytes. Ann. Bot. 2015, 115, 327–331. [Google Scholar] [CrossRef]
- Guo, J.; Suo, S.; Wang, B.-S. Sodium Chloride Improves Seed Vigour of the Euhalophyte Suaeda salsa. Seed Sci. Res. 2015, 25, 335–344. [Google Scholar] [CrossRef]
- Debez, A.; Hamed, K.B.; Grignon, C.; Abdelly, C. Salinity Effects on Germination, Growth, and Seed Production of the Halophyte Cakile maritima. Plant Soil 2004, 262, 179–189. [Google Scholar] [CrossRef]
- Zhang, H.; Irving, L.J.; Tian, Y.; Zhou, D. Influence of Salinity and Temperature on Seed Germination Rate and the Hydrotime Model Parameters for the Halophyte, Chloris virgata, and the Glycophyte, Digitaria sanguinalis. S. Afr. J. Bot. 2012, 78, 203–210. [Google Scholar] [CrossRef]
- Guo, J.; Du, M.; Lu, C.; Wang, B. NaCl Improves Reproduction by Enhancing Starch Accumulation in the Ovules of the Euhalophyte Suaeda salsa. BMC Plant Biol. 2020, 20, 262. [Google Scholar] [CrossRef]
- Zhang, S.; Song, J.; Wang, H.; Feng, G. Effect of Salinity on Seed Germination, Ion Content and Photosynthesis of Cotyledons in Halophytes or Xerophyte Growing in Central Asia. J. Plant Ecol. 2010, 3, 259–267. [Google Scholar] [CrossRef]
- Balnokin, Y.V.; Kurkova, E.B.; Myasoedov, N.A.; Lun’kov, R.V.; Shamsutdinov, N.Z.; Egorova, E.A.; Bukhov, N.G. Structural and Functional State of Thylakoids in a Halophyte Suaeda altissima before and after Disturbance of Salt-Water Balance by Extremely High Concentrations of NaCl. Russ. J. Plant Physiol. 2004, 51, 815–821. [Google Scholar] [CrossRef]
- Koyro, H.-W.; Geißler, N.; Hussin, S.; Huchzermeyer, B. Survival at Extreme Locations: Life Strategies of Halophytes—The Long Way from System Ecology, Whole Plant Physiology, Cell Biochemistry and Molecular Aspects Back to Sustainable Utilization at Field Sites. In Biosaline Agriculture and High Salinity Tolerance; Birkhäuser: Basel, Switzerland, 2008; pp. 1–20. [Google Scholar]
- Flowers, T.J.; Muscolo, A. Introduction to the Special Issue: Halophytes in a Changing World. AoB Plants 2015, 7, plv020. [Google Scholar] [CrossRef]
- Steiner, M. To the Ecology of the Salt March of the Nordost lichen United Countries of Nordamerika. Jahrb. Know Offer. 1934, 81, 94. [Google Scholar]
- Chapman, V.J. The New Perspective in the Halophytes. Q. Rev. Biol. 1942, 17, 291–311. [Google Scholar] [CrossRef]
- Grigore, M.; Toma, C. A Proposal for a New Halophytes Classification, Based on Integrative Anatomy Observations. Muz Olten. Craiova Stud. Şi Comun. Ştiinţele Nat. 2010, 26, 45–50. [Google Scholar]
- Walter, H. Salinity Problems in the Acid Zones. The Adaptations of Plants to Saline Soils. Arid Zone Res. 1961, 14, 65–68. [Google Scholar]
- Aslam, R.; Bostan, N.; Nabgha-e-Amen; Maria, M.; Safdar, W. A Critical Review on Halophytes: Salt Tolerant Plants. J. Med. Plant Res. 2011, 5, 7108–7118. [Google Scholar]
- Lombardi, T.; Bedini, S.; Bertacchi, A. Germination Ecology of the Aromatic Halophyte Artemisia caerulescens L.: Influence of Abiotic Factors and Seed after-Ripening Time. Folia Geobot. 2019, 54, 115–124. [Google Scholar] [CrossRef]
- Lombardi, T.; Bedini, S. Seed Germination Strategies of Mediterranean Halophytes under Saline Condition. In Handbook of Halophytes; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–19. [Google Scholar] [CrossRef]
- Bertacchi, A.; Lombardi, T.; Saggese, A.; Lazzeri, V. The Vegetation of a Relict Salt Marsh Area in the Pisan Coast in the Context of Brackish Wetlands of Tuscany. Plant Sociol. 2021, 58, 41–53. [Google Scholar] [CrossRef]
- Frondoni, R.; Iberite, M. The Halophile Vegetation of the Sedimentary Coast of Lazio (Central Tyrrhenian District, Italy). Plant Biosyst. 2002, 136, 49–67. [Google Scholar] [CrossRef]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An Updated Checklist of the Vascular Flora Native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Byng, J.W.; Chase, M.W.; Christenhusz, M.J.; Fay, M.F.; Judd, W.S.; Mabberley, D.J.; Sennikov, A.N.; Soltis, D.E.; Soltis, P.S.; Stevens, P.F.; et al. An Update of the Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants: APG IV. The Angiosperm Phylogeny Group. Bot. J. Linn. Soc. 2016, 35, 1–20. [Google Scholar]
- Guarrera, P.M.; Salerno, G.; Caneva, G. Food, Flavouring and Feed Plant Traditions in the Tyrrhenian Sector of Basilicata, Italy. J. Ethnobiol. Ethnomed. 2006, 2, 37. [Google Scholar] [CrossRef]
- Simopoulos, A.P.; Artemis, D.; Simopoulos, P.B.R. Omega-3 Fatty Acids and Antioxidants in Edible Wild Plants. Biol. Res. 2004, 37, 263–277. [Google Scholar] [CrossRef]
- Tardío, J.; Pardo-de-santayana, M.; Morales, R. Ethnobotanical Review of Wild Edible Plants in Spain. Bot. J. Linn. Soc. 2006, 152, 27–71. [Google Scholar] [CrossRef]
- Tug, A.E.; Yaprak, G.N. Halophytes as a Potential Food Source. ANADOLU J. AARI 2017, 27, 78–81. [Google Scholar]
- Agudelo, A.; Carvajal, M.; del Carmen Martinez-Ballesta, M. Halophytes of the Mediterranean Basin-Underutilized Species with the Potential to be Nutritious Crops in the Scenario of the Climate Change. Foods 2021, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.; Divyanshu, K.; Kumar, S.; Swapnil, P.; Zehra, A.; Shukla, V.; Yadav, M.; Upadhyay, R.S. Regulation of L-Proline Biosynthesis, Signal Transduction, Transport, Accumulation and Its Vital Role in Plants during Variable Environmental Conditions. Heliyon 2019, 5, e02952. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Centofanti, T.; Bañuelos, G. Evaluation of the Halophyte Salsola soda as an Alternative Crop for Saline Soils High in Selenium and Boron. J. Environ. Manag. 2015, 157, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Oueslati, S.; Karray-Bouraoui, N.; Attia, H.; Rabhi, M.; Ksouri, R.; Lachaal, M. Physiological and Antioxidant Responses of Mentha pulegium (Pennyroyal) to Salt Stress. Acta Physiol. Plant 2010, 32, 289–296. [Google Scholar] [CrossRef]
- Mzoughi, Z.; Abdelhamid, A.; Rihouey, C.; le Cerf, D.; Bouraoui, A.; Majdoub, H. Optimized Extraction of Pectin-like Polysaccharide from Suaeda fruticosa Leaves: Characterization, Antioxidant, Anti-Inflammatory and Analgesic Activities. Carbohydr. Polym. 2018, 185, 127–137. [Google Scholar] [CrossRef]
- Guerrero, J.L.G.; Madrid, P.C.; Isasa, M.E.T. Mineral Elements Determination in Wild Edible Plants. Ecol. Food Nutr. 1999, 38, 209–222. [Google Scholar] [CrossRef]
- Zhou, Y.-X.; Xin, H.-L.; Rahman, K.; Wang, S.-J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A Review of Phytochemistry and Pharmacological Effects. Biomed. Res. Int. 2015, 2015, 925631. [Google Scholar] [CrossRef]
- Fernández, J.A.; Orsini, F.; Baeza, E.; Oztekin, G.B.; Muñoz, P.; Contreras, J.; Montero, J.I. Current Trends in Protected Cultivation in Mediterranean Climates. Eur. J. Hortic. Sci. 2018, 83, 294–305. [Google Scholar] [CrossRef]
- Savvas, D.; Gruda, N. Application of Soilless Culture Technologies in the Modern Greenhouse Industry—A Review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Kotzen, B.; Emerenciano, M.G.C.; Moheimani, N.; Burnell, G.M. Aquaponics: Alternative Types and Approaches. In Aquaponics Food Production Systems; Springer International Publishing: Cham, Switzerland, 2019; pp. 301–330. [Google Scholar]
- Custódio, M.; Villasante, S.; Cremades, J.; Calado, R.; Lillebø, A. Unravelling the Potential of Halophytes for Marine Integrated Multi-Trophic Aquaculture (IMTA)—A Perspective on Performance, Opportunities and Challenges. Aquac. Environ. Interact. 2017, 9, 445–460. [Google Scholar] [CrossRef]
- Singh, D.; Buhmann, A.K.; Flowers, T.J.; Seal, C.E.; Papenbrock, J. Salicornia as a Crop Plant in Temperate Regions: Selection of Genetically Characterized Ecotypes and Optimization of Their Cultivation Conditions. AoB Plants 2014, 6, plu071. [Google Scholar] [CrossRef]
- Boni, A. Uno Studio Sulla Coltura Idroponica e la Conservazione Post-Raccolta della Salicornia Europaea. Master’s Thesis, Department of Food Agriculture Environment, University of Pisa, Pisa, Italy, 2020. [Google Scholar]
- Nie, L.; Feng, J.; Lü, S.; Jiang, P.; Fan, P.; Tai, F.; Li, Y. The Response of Euhalophyte Salicornia europaea L. to Different Nitrogen Forms. Acta Ecol. Sin. 2012, 32, 5703–5712. [Google Scholar] [CrossRef][Green Version]
- Lima, A.R.; Castañeda-Loaiza, V.; Salazar, M.; Nunes, C.; Quintas, C.; Gama, F.; Pestana, M.; Correia, P.J.; Santos, T.; Varela, J.; et al. Influence of Cultivation Salinity in the Nutritional Composition, Antioxidant Capacity and Microbial Quality of Salicornia ramosissima Commercially Produced in Soilless Systems. Food Chem. 2020, 333, 127525. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Potential of Producing Salicornia bigelovii Hydroponically as a Vegetable at Moderate NaCl Salinity. Hortic. Sci. 2014, 49, 1154–1157. [Google Scholar] [CrossRef]
- Beyer, C.P.; Gómez, S.; Lara, G.; Monsalve, J.P.; Orellana, J.; Hurtado, C.F. Sarcocornia neei: A Novel Halophyte Species for Bioremediation of Marine Aquaculture Wastewater and Production Diversification in Integrated Systems. Aquaculture 2021, 543, 736971. [Google Scholar] [CrossRef]
- Doncato, K.B.; Costa, C.S.B. Micronutrient Supplementation Needs for Halophytes in Saline Aquaponics with BFT System Water. Aquaculture 2021, 531, 735815. [Google Scholar] [CrossRef]
- Custódio, M.; Cartaxana, P.; Villasante, S.; Calado, R.; Lillebø, A.I. LED Lighting and High-Density Planting Enhance the Cost-Efficiency of Halimione portulacoides Extraction Units for Integrated Aquaculture. Appl. Sci. 2021, 11, 4995. [Google Scholar] [CrossRef]
- Custódio, M.; Maciel, E.; Domingues, M.R.; Lillebø, A.I.; Calado, R. Nutrient Availability Affects the Polar Lipidome of Halimione portulacoides Leaves Cultured in Hydroponics. Sci. Rep. 2020, 10, 6583. [Google Scholar] [CrossRef] [PubMed]
- Egea-Gilabert, C.; Ruiz-Hernández, M.V.; Parra, M.Á.; Fernández, J.A. Characterization of Purslane (Portulaca Oleracea L.) Accessions: Suitability as Ready-to-Eat Product. Sci. Hortic. 2014, 172, 73–81. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Hydroponic Production of Purslane as a Sodium-Removing Vegetable in NaCl-Rich Nutrient Solution. HortScience 2014, 49, 201–206. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Alhamad, M.N.; Al-Tawaha, A.; Al-Tawaha, A.R.; Gammoh, S.; Ereifej, K.I.; Al-Karaki, G.; Hamasha, H.R.; Tranchant, C.C.; et al. Herbal Yield, Nutritive Composition, Phenolic Contents and Antioxidant Activity of Purslane (Portulaca oleracea L.) Grown in Different Soilless Media in a Closed System. Ind. Crops Prod. 2019, 141, 111746. [Google Scholar] [CrossRef]
- Giménez, A.; Martínez-Ballesta, M.D.C.; Egea-Gilabert, C.; Gómez, P.A.; Artés-Hernández, F.; Pennisi, G.; Orsini, F.; Crepaldi, A.; Fernández, J.A. Combined Effect of Salinity and Led Lights on the Yield and Quality of Purslane (Portulaca oleracea L.) Microgreens. Horticulturae 2021, 7, 180. [Google Scholar] [CrossRef]
- Mortley, D.G.; Oh, J.H.; Johnson, D.S.; Bonsi, C.K.; Hill, W.A. Influence of Harvest Intervals on Growth Responses and Fatty Acid Content of Purslane (Portulaca oleracea). HortScience 2012, 47, 437–439. [Google Scholar] [CrossRef]
- Ventura, Y.; Sagi, M. Halophyte Crop Cultivation: The Case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C. Enhancing Quality of Fresh Vegetables through Salinity Eustress and Biofortification Applications Facilitated by Soilless Cultivation. Front. Plant. Sci. 2018, 9, 1254. [Google Scholar] [CrossRef]
- Oztekin, G.B.; Uludag, T.; Tuzel, Y. Impact of Nutrient Solution Concentration and Growth Period on Baby Leaf Purslane Production in Floating System. Acta Hortic. 2020, 1273, 65–74. [Google Scholar] [CrossRef]
- Nicola, S.; Egea-Gilabert, C.; Niñirola, D.; Conesa, E.; Pignata, G.; Fontana, E.; Fernández, J.A. Nitrogen and Aeration Levels of the Nutrient Solution in Soilless Cultivation Systems as Important Growing Conditions Affecting Inherent Quality of Baby Leaf Vegetables: A Review. Acta Hortic. 2015, 167–177. [Google Scholar] [CrossRef]
- Ceccanti, C.; Landi, M.; Incrocci, L.; Pardossi, A.; Venturi, F.; Taglieri, I.; Ferroni, G.; Guidi, L. Comparison of Three Domestications and Wild-Harvested Plants for Nutraceutical Properties and Sensory Profiles in Five Wild Edible Herbs: Is Domestication Possible? Foods 2020, 9, 1065. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zheng, Y. Suaeda glauca Can Be Produced Hydroponically at Moderate NACL Salinity. HortScience 2015, 50, 847–850. [Google Scholar] [CrossRef]
- Santamaria, P. Nitrate in Vegetables: Toxicity, Content, Intake and EC Regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Natesh, H.N.; Abbey, L.; Asiedu, S.K. An Overview of Nutritional and Anti Nutritional Factors in Green Leafy Vegetables. Hortic. Int. J. 2017, 1, 58–65. [Google Scholar] [CrossRef]
- Manzocco, L.; Foschia, M.; Tomasi, N.; Maifreni, M.; Dalla Costa, L.; Marino, M.; Cortella, G.; Cesco, S. Influence of Hydroponic and Soil Cultivation on Quality and Shelf Life of Ready-to-Eat Lamb’s Lettuce (Valerianella locusta L. Laterr). J. Sci. Food Agric. 2011, 91, 1373–1380. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; Pannico, A.; El-Nakhel, C.; Fascella, G.; Duri, L.G.; Cristofano, F.; Gentile, B.R.; Giordano, M.; Rouphael, Y.; et al. Nutrient Solution Deprivation as a Tool to Improve Hydroponics Sustainability: Yield, Physiological, and Qualitative Response of Lettuce. Agronomy 2021, 11, 1469. [Google Scholar] [CrossRef]
- Urrestarazu, M.; Postigo, A.; Salas, M.; Sánchez, A.; Carrasco, G. Nitrate Accumulation Reduction Using Chloride in the Nutrient Solution on Lettuce Growing by NFT in Semiarid Climate Conditions. J. Plant Nutr. 1998, 21, 1705–1714. [Google Scholar] [CrossRef]
- Mori, S.; Kobayashi, N.; Arao, T.; Higuchi, K.; Maeda, Y.; Yoshiba, M.; Tadano, T. Enhancement of Nitrate Reduction by Chlorine Application in Suaeda salsa (L.) Pall. Soil Sci. Plant Nutr. 2008, 54, 903–909. [Google Scholar] [CrossRef][Green Version]
- He, J.; You, X.; Qin, L. High Salinity Reduces Plant Growth and Photosynthetic Performance but Enhances Certain Nutritional Quality of Halophyte Portulaca oleracea L. Grown Hydroponically Under LED Lighting. Front. Plant Sci. 2021, 12, 457. [Google Scholar] [CrossRef]
- Abrol, I.P.; Massoud, F.I.; Yadav, J.S.P. Salt-Affected Soils and Their Management. In Food and Agriculture Organization of the United Nations (FAO) Soils Bulletin; FAO: Rome, Italy, 1988; No. 39. [Google Scholar]
- Yuan, F.; Xu, Y.; Leng, B.; Wang, B. Beneficial Effects of Salt on Halophyte Growth: Morphology, Cells, and Genes. Open Life Sci. 2019, 14, 191–200. [Google Scholar] [CrossRef]
- WHO. World Health Organization Guideline: Sodium Intake for Adults and Children; WHO: Geneva, Switzerland, 2012; pp. 1–56. [Google Scholar]
- Lopes, M.; Cavaleiro, C.; Ramos, F. Sodium Reduction in Bread: A Role for Glasswort (Salicornia ramosissima J. Woods). Compr. Rev. Food Sci. Food Saf. 2017, 16, 1056–1071. [Google Scholar] [CrossRef] [PubMed]
- Seong, P.N.; Seo, H.W.; Cho, S.H.; Kim, Y.S.; Kang, S.M.; Kim, J.H.; Kang, G.H.; Park, B.Y.; Moon, S.S.; Hoa, V.B. Potential Use of Glasswort Powder as a Salt Replacer for the Production of Healthier Dry-Cured Ham Products. Czech J. Food Sci. 2017, 35, 149–159. [Google Scholar] [CrossRef]
- Savage, G.; Klunklin, W. Oxalates Are Found in Many Different European and Asian Foods—Effects of Cooking and Processing. J. Food Res. 2018, 7, 76. [Google Scholar] [CrossRef]
- Siener, R.; Hönow, R.; Seidler, A.; Voss, S.; Hesse, A. Oxalate Contents of Species of the Polygonaceae, Amaranthaceae and Chenopodiaceae Families. Food Chem. 2006, 98, 220–224. [Google Scholar] [CrossRef]
- Marcason, W. Where Can I Find Information on the Oxalate Content of Foods? J. Am. Diet. Assoc. 2006, 106, 627–628. [Google Scholar] [CrossRef]
- Uddin, M.M.; Chen, Z.; Huang, L. Cadmium Accumulation, Subcellular Distribution and Chemical Fractionation in Hydroponically Grown Sesuvium portulacastrum [Aizoaceae]. PLoS ONE 2021, 15, e0244085. [Google Scholar] [CrossRef]
- Fontana, E.; Hoeberechts, J.; Nicola, S.; Cros, V.; Palmegiano, G.B.; Peiretti, P.G. Nitrogen Concentration and Nitrate/Ammonium Ratio Affect Yield and Change the Oxalic Acid Concentration and Fatty Acid Profile of Purslane (Portulaca oleracea L.) Grown in a Soilless Culture System. J. Sci. Food Agric. 2006, 86, 2417–2424. [Google Scholar] [CrossRef]
- Kaşkar, Ç.; Fernándeza, J.A.; Ochoa, J.; Niñirola, D.; Conesa, E.; Tüzel, Y. Agronomic Behaviour and Oxalate and Nitrate Content of Different Purslane Cultivars (Portulaca oleracea) Grown in a Hydroponic Floating System. Acta Hortic. 2009, 807, 521–526. [Google Scholar] [CrossRef]
- Guil, J.L.; Rodríguez-Garcí, I.; Torija, E. Nutritional and Toxic Factors in Selected Wild Edible Plants. Plant Foods Hum. Nutr. 1997, 51, 99–107. [Google Scholar] [CrossRef]
- Carvalho, I.S.; Teixeira, M.; Brodelius, M. Effect of Salt Stress on Purslane and Potential Health Benefits: Oxalic Acid and Fatty Acids Profile. In Proceedings of the International Plant Nutrition Colloquium XVI, U.C Davis, Sacramento, CA, USA, 26–30 August 2009. [Google Scholar]
- Szalai, G.; Dai, N.; Danin, A.; Dudai, N.; Barazani, O. Effect of Nitrogen Source in the Fertilizing Solution on Nutritional Quality of Three Members of the Portulaca oleracea Aggregate. J. Sci. Food Agric. 2010, 90, 2039–2045. [Google Scholar] [CrossRef]
- Palaniswamy, U.R.; Bible, B.B.; McAvoy, R.J. Oxalic Acid Concentrations in Purslane (Portulaca oleraceae L.) Is Altered by the Stage of Harvest and the Nitrate to Ammonium Ratios in Hydroponics. Sci. Hortic. 2004, 102, 267–275. [Google Scholar] [CrossRef]
- Ponzilacqua, B.; Rottinghaus, G.E.; Landers, B.R.; Oliveira, C.A.F. Effects of Medicinal Herb and Brazilian Traditional Plant Extracts on in Vitro Mycotoxin Decontamination. Food Control 2019, 100, 24–27. [Google Scholar] [CrossRef]
- Medina, Á.; Rodríguez, A.; Magan, N. Climate Change and Mycotoxigenic Fungi: Impacts on Mycotoxin Production. Curr. Opin. Food Sci. 2015, 5, 99–104. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Drusch, S.; Ragab, W. Mycotoxins in Fruits, Fruit Juices, and Dried Fruits. J. Food Prot. 2003, 66, 1514–1527. [Google Scholar] [CrossRef] [PubMed]
- Enikova, R.K.; Stoynovska, M.R.; Karcheva, M.D. Mycotoxins in Fruits and Vegetables. J. IMAB 2020, 26, 3139–3143. [Google Scholar] [CrossRef]
- Yeni, F.; Yavaş, S.; Alpas, H.; Soyer, Y. Most Common Foodborne Pathogens and Mycotoxins on Fresh Produce: A Review of Recent Outbreaks. Crit. Rev. Food Sci. Nutr. 2016, 56, 1532–1544. [Google Scholar] [CrossRef]
- Paster, N.; Barkai-Golan, R. Mouldy Fruits and Vegetables as a Source of Mycotoxins: Part 2. World Mycotoxin J. 2008, 1, 385–396. [Google Scholar] [CrossRef]
- Restani, P. Diffusion of Mycotoxins in Fruits and Vegetables. In Mycotoxins Fruits Veg; Barkai-Golan, R., Paster, N., Eds.; Elsevier: San Diego, CA, USA, 2008; pp. 105–114. ISBN 9780123741264. [Google Scholar]
- Strobel, G.A. Endophytes as Sources of Bioactive Products. Microbes Infect. 2003, 5, 535–544. [Google Scholar] [CrossRef]
- Zhang, H.W.; Song, Y.C.; Tan, R.X. Biology and Chemistry of Endophytes. Nat. Prod. Rep. 2006, 23, 753–771. [Google Scholar] [CrossRef]
- Mousa, W.K.; Raizada, M.N. The Diversity of Anti-Microbial Secondary Metabolites Produced by Fungal Endophytes: An Interdisciplinary Perspective. Front. Microbiol. 2013, 4, 65. [Google Scholar] [CrossRef] [PubMed]
- Manganiello, G.; Marra, R.; Staropoli, A.; Lombardi, N.; Vinale, F.; Nicoletti, R. The Shifting Mycotoxin Profiles of Endophytic Fusarium Strains: A Case Study. Agriculture 2019, 9, 143. [Google Scholar] [CrossRef]
- Thirumalai, E.; Venkatachalam, A.; Suryanarayanan, T.S. Fungal Endophytes of Betel Leaves: The Need to Study Mycotoxin-Producing Endophytes in Leafy Vegetables. Sydowia 2020, 66, 2. [Google Scholar] [CrossRef]
- Cantrell, S.A.; Casillas-Martínez, L.; Molina, M. Characterization of Fungi from Hypersaline Environments of Solar Salterns Using Morphological and Molecular Techniques. Mycol. Res. 2006, 110, 962–970. [Google Scholar] [CrossRef]
- Biango-Daniels, M.N.; Hodge, K.T. Sea Salts as a Potential Source of Food Spoilage Fungi. Food Microbiol. 2018, 69, 89–95. [Google Scholar] [CrossRef]
- Maciá-Vicente, J.G.; Jansson, H.B.; Abdullah, S.K.; Descals, E.; Salinas, J.; Lopez-Llorca, L. v Fungal Root Endophytes from Natural Vegetation in Mediterranean Environments with Special Reference to Fusarium spp. FEMS Microbiol. Ecol. 2008, 64, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Calabon, M.S.; Jones, E.B.G.; Promputtha, I.; Hyde, K.D. Fungal Biodiversity in Salt Marsh Ecosystems. J. Fungi 2021, 7, 648. [Google Scholar] [CrossRef]
- Bell, A.A.; Wheeler, M.H. Biosynthesis and Functions of Fungal Melanins. Annu. Rev. Phytopathol. 1986, 24, 411–451. [Google Scholar] [CrossRef]
- Papizadeh, M.; Wijayawardene, N.N.; Amoozegar, M.A.; Saba, F.; Fazeli, S.A.S.; Hyde, K.D. Neocamarosporium jorjanensis, N. persepolisi, and N. Solicola spp. nov. (Neocamarosporiaceae, Pleosporales) Isolated from Saline Lakes of Iran Indicate the Possible Halotolerant Nature for the Genus. Mycol. Prog. 2018, 17, 661–679. [Google Scholar] [CrossRef]
- Gonçalves, M.F.M.; Aleixo, A.; Vicente, T.F.L.; Esteves, A.C.; Alves, A. Three New Species of Neocamarosporium Isolated from Saline Environments: N. aestuarinum sp. nov., N. endophyticum sp. nov. and N. halimiones sp. nov. Mycosphere 2019, 10, 608–621. [Google Scholar] [CrossRef]
- Chalbi, A.; Sghaier-Hammamil, B.; Meca, G.; Quiles, J.M.; Abdelly, C.; Marangi, C.; Logrieco, A.N.F.; Moretti, A.; Masiello, M. Characterization of Mycotoxigenic Alternaria Species Isolated from the Tunisian Halophyte Cakile maritima. Phytopathol. Mediterr. 2020, 59, 107–118. [Google Scholar] [CrossRef]
- Lopes, M.; da Conceição Castilho, M.; Sanches-Silva, A.; Freitas, A.; Barbosa, J.; Gonçalves, M.J.; Cavaleiro, C.; Ramos, F. Evaluation of the Mycotoxins Content of Salicornia spp.: A Gourmet Plant Alternative to Salt. Food Addit. Contam. Part B Surveill. 2020, 13, 162–170. [Google Scholar] [CrossRef]
- Murphy, P.A.; Hendrich, S.; Landgren, C.; Bryant, C.M. Food Mycotoxins: An Update. J. Food Sci. 2006, 71, R51–R65. [Google Scholar] [CrossRef]
- Bhat, R.; Rai, R.V.; Karim, A.A. Mycotoxins in Food and Feed: Present Status and Future Concerns. Compr. Rev. Food Sci. Food Saf. 2010, 9, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate Outbreaks of Foodborne Illness in the United States Associated with Fresh Produce From 2010 to 2017. Front. Microbiol. 2019, 10, 2667. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Abu Ghoush, M.; Al-Holy, M.; Abu Hilal, H.; Al-Nabulsi, A.A.; Osaili, T.M.; Ayyash, M.; Holley, R.A. Survival and Growth of Listeria monocytogenes and Staphylococcus aureus in Ready-to-Eat Mediterranean Vegetable Salads: Impact of Storage Temperature and Food Matrix. Int. J. Food Microbiol. 2021, 346, 109149. [Google Scholar] [CrossRef] [PubMed]
- European Commission (EC) Regulation No 1441/2007 of 5 December 2007 Amending Regulation (EC) No 2073/2005 on Microbiological Criteria for Foodstuffs. Off. J. Eur. Union 2007, L322, 12–29.
- Castro-Ibáñez, I.; Gil, M.I.; Allende, A. Ready-to-Eat Vegetables: Current Problems and Potential Solutions to Reduce Microbial Risk in the Production Chain. LWT-Food Sci. Technol. 2017, 85, 284–292. [Google Scholar] [CrossRef]
- Aiyedun, S.O.; Onarinde, B.A.; Swainson, M.; Dixon, R.A. Foodborne Outbreaks of Microbial Infection from Fresh Produce in Europe and North America: A Systematic Review of Data from This Millennium. Int. J. Food Sci. Technol. 2021, 56, 2215–2223. [Google Scholar] [CrossRef]
- Kadereit, G.; Piirainen, M.; Lambinon, J.; Vanderpoorten, A. Cryptic Taxa Should Have Names: Reflections in the Glasswort Genus Salicornia (Amaranthaceae). TAXON 2012, 61, 1227–1239. [Google Scholar] [CrossRef]
- Steffen, S.; Ball, P.; Mucina, L.; Kadereit, G. Phylogeny, Biogeography and Ecological Diversification of Sarcocornia (Salicornioideae, Amaranthaceae). Ann. Bot. 2015, 115, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Piirainen, M.; Salicornia. In Euro+Med Plantbase—The Information Resource for Euro-Mediterranean Plant Diversity. Available online: http://ww2.bgbm.org/EuroPlusMed/PTaxonDetail.asp?NameCache=Salicornia+europaea#2 (accessed on 20 February 2022).
- Lv, S.; Jiang, P.; Chen, X.; Fan, P.; Wang, X.; Li, Y. Multiple Compartmentalization of Sodium Conferred Salt Tolerance in Salicornia europaea. Plant Physiol. Biochem. 2012, 51, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Boo, H.O.; Jeon, M.W.; KO, J.Y. Chemical Components of Native Plant, Salicornia herbacea L. Korean J. Plant Res. 2002, 15, 216–220. [Google Scholar]
- Wang, X.; Bai, J.; Wang, W.; Zhang, G.; Yin, S.; Wang, D. A Comparative Metabolomics Analysis of the Halophyte Suaeda salsa and Salicornia europaea. Environ. Geochem. Health 2021, 43, 1109–1122. [Google Scholar] [CrossRef]
- Araus, J.L.; Rezzouk, F.Z.; Thushar, S.; Shahid, M.; Elouafi, I.A.; Bort, J.; Serret, M.D. Effect of Irrigation Salinity and Ecotype on the Growth, Physiological Indicators and Seed Yield and Quality of Salicornia europaea. Plant Sci. 2021, 304, 110819. [Google Scholar] [CrossRef] [PubMed]
- Aghaleh, M.; Niknam, V.; Ebrahimzadeh, H.; Razavi, K. Salt Stress Effects on Growth, Pigments, Proteins and Lipid Peroxidation in Salicornia persica and S. europaea. Biol. Plant 2009, 53, 243–248. [Google Scholar] [CrossRef]
- Aghaleh, M.; Niknam, V.; Ebrahimzadeh, H.; Razavi, K. Antioxidative Enzymes in Two in Vitro Cultured Salicornia Species in Response to Increasing Salinity. Biol. Plant 2014, 58, 391–394. [Google Scholar] [CrossRef]
- Patel, S. Salicornia: Evaluating the Halophytic Extremophile as a Food and a Pharmaceutical Candidate. 3 Biotech 2016, 6, 104. [Google Scholar] [CrossRef]
- Essaidi, I.; Brahmi, Z.; Snoussi, A.; ben Haj Koubaier, H.; Casabianca, H.; Abe, N.; el Omri, A.; Chaabouni, M.M.; Bouzouita, N. Phytochemical Investigation of Tunisian Salicornia herbacea L., Antioxidant, Antimicrobial and Cytochrome P450 (CYPs) Inhibitory Activities of Its Methanol Extract. Food Control 2013, 32, 125–133. [Google Scholar] [CrossRef]
- Furtado, B.U.; Szymańska, S.; Hrynkiewicz, K. A Window into Fungal Endophytism in Salicornia europaea: Deciphering Fungal Characteristics as Plant Growth Promoting Agents. Plant Soil 2019, 445, 577–594. [Google Scholar] [CrossRef]
- Furtado, B.U.; Gołebiewski, M.; Skorupa, M.; Hulisz, P.; Hrynkiewicz, K. Bacterial and Fungal Endophytic Microbiomes of Salicornia europaea. Appl. Environ. Microbiol. 2019, 85, e00305-19. [Google Scholar] [CrossRef] [PubMed]
- Okane, I.; Nakagiri, A. Assemblages of Endophytic Fungi on Salicornia europaea Disjunctively Distributed in Japan: Towards Clarification of the Ubiquity of Fungal Endophytes on Halophytes and Their Ecological Roles. Curr. Sci. 2015, 109, 62–71. [Google Scholar] [CrossRef]
- Yucel, C.; Farhan, M.; Khairo, A.; Ozer, G.; Cetin, M.; Ortas, I.; Islam, K. Evaluating Salicornia as a Potential Forage Crop to Remediate High Groundwater-Table Saline Soil under Continental Climates. Int. J. Plant Soil Sci. 2017, 16, 1–10. [Google Scholar] [CrossRef][Green Version]
- Petrini, O.; Fisher, P.J. Fungal Endophytes in Salicornia perennis. Trans. Br. Mycol. Soc. 1986, 87, 647–651. [Google Scholar] [CrossRef]
- Boulos, L. Notes on Suaeda Forssk. Ex Scop. Studies in the Chenopodiaceae of Arabia: 2. Kew Bull. 1991, 46, 291. [Google Scholar] [CrossRef]
- Wang, S.-M.; Zhang, J.-L.; Flowers, T.J. Low-Affinity Na+ Uptake in the Halophyte Suaeda maritima. Plant Physiol. 2007, 145, 559–571. [Google Scholar] [CrossRef]
- 158. The European Parliament, the European Council. Regulation (EU) No 1169/2011 of 25 October 2011 on the provision of food information to consumers, 2011. Off. J. Eur. Union, 2011; L 304, 18–63.
- D’Ambrosio, C.; Stigliani, A.L.; Giorio, G. Food from Genetically Engineered Plants: Tomato with Increased β-Carotene, Lutein, and Xanthophylls Contents. In Genetically Modified Organisms in Food: Production, Safety, Regulation and Public Health; Watson, R.R., Preedy, V.R., Eds.; Elsevier Inc.: London, UK, 2015; pp. 361–380. ISBN 978-012802259-7/978-012802530-7. [Google Scholar]
- US Department of Agriculture (USDA); Agricultural Research Service; Nutrient Data Laboratory. Composition of Foods: Raw, Processed, Prepared. USDA National Nutrient Database for Standard Reference, Legacy (2018). Documentation and User Guide; USDA: Beltsville, MD, USA, 2018.
- Khalmuratova, I.; Choi, D.H.; Woo, J.R.; Jeong, M.J.; Oh, Y.; Kim, Y.G.; Lee, I.J.; Choo, Y.S.; Kim, J.G. Diversity and Plant Growth-Promoting Effects of Fungal Endophytes Isolated from Salt-Tolerant Plants. J. Microbiol. Biotechnol. 2020, 30, 1680–1687. [Google Scholar] [CrossRef]
- Khalmuratova, I.; Kim, H.; Nam, Y.J.; Oh, Y.; Jeong, M.J.; Choi, H.R.; You, Y.H.; Choo, Y.S.; Lee, I.J.; Shin, J.H.; et al. Diversity and Plant Growth Promoting Capacity of Endophytic Fungi Associated with Halophytic Plants from the West Coast of Korea. Mycobiology 2015, 43, 373–383. [Google Scholar] [CrossRef]
- Suryanarayanan, T.S.; Kumaresan, V. Endophytic Fungi of Some Halophytes from an Estuarine Mangrove Forest. Mycol. Res. 2000, 104, 1465–1467. [Google Scholar] [CrossRef]
- Hameed, A.; Hussain, T.; Gulzar, S.; Aziz, I.; Gul, B.; Khan, M.A. Salt Tolerance of a Cash Crop Halophyte Suaeda Fruticosa: Biochemical Responses to Salt and Exogenous Chemical Treatments. Acta Physiol. Plant 2012, 34, 2331–2340. [Google Scholar] [CrossRef]
- Qasim, M.; Abideen, Z.; Adnan, M.Y.; Gulzar, S.; Gul, B.; Rasheed, M.; Khan, M.A. Antioxidant Properties, Phenolic Composition, Bioactive Compounds and Nutritive Value of Medicinal Halophytes Commonly Used as Herbal Teas. S. Afr. J. Bot. 2017, 110, 240–250. [Google Scholar] [CrossRef]
- Shahi, M.; Saaghari, M.; Esfahan, E.Z.; Jaimand, K. Investigation on Potential of Suaeda fruticosa as a Source of Edible Oil. J. Biodivers. Environ. Sci. 2013, 3, 101–107. [Google Scholar]
- Ozcan, T. Fatty Acid Composition of Seed Oils in Some Sand Dune Vegetation Species from Turkey. Chem. Nat. Compd. 2014, 50, 804–809. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity Effects on Polyphenol Content and Antioxidant Activities in Leaves of the Halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Fisher, P.J.; Petrini, O. Location of Fungal Endophytes in Tissues of Suaeda fruticosa: A Preliminary Study. Trans. Br. Mycol. Soc. 1987, 89, 246–249. [Google Scholar] [CrossRef]
- Karakas, S.; Çullu, M.A.; Dikilitas, M. Comparison of Two Halophyte Species (Salsola soda and Portulaca oleracea) for Salt Removal Potential under Different Soil Salinity Conditions. Turk. J. Agric. For. 2017, 41, 183–190. [Google Scholar] [CrossRef]
- Hamed, A.I.; Masullo, M.; Sheded, M.G.; Mahalel, U.A.; Tawfik, M.M.; Perrone, A.; Piacente, S. Triterpene Saponins from Salsola imbricata. Phytochem. Lett. 2011, 4, 353–356. [Google Scholar] [CrossRef]
- Tundis, R.; Menichini, F.; Conforti, F.; Loizzo, M.R.; Bonesi, M.; Statti, G.; Menichini, F. A Potential Role of Alkaloid Extracts from Salsola Species (Chenopodiaceae) in the Treatment of Alzheimer’s Disease. J. Enzym. Inhib. Med. Chem. 2009, 24, 818–824. [Google Scholar] [CrossRef]
- Rasheed, D.M.; el Zalabani, S.M.; Koheil, M.A.; El-Hefnawy, H.M.; Farag, M.A. Metabolite Profiling Driven Analysis of Salsola Species and Their Anti-Acetylcholinesterase Potential. Nat. Prod. Res. 2013, 27, 2320–2327. [Google Scholar] [CrossRef]
- Iannuzzi, A.M.; Moschini, R.; de Leo, M.; Pineschi, C.; Balestri, F.; Cappiello, M.; Braca, A.; Del-Corso, A. Chemical Profile and Nutraceutical Features of Salsola soda (Agretti): Anti-Inflammatory and Antidiabetic Potential of Its Flavonoids. Food Biosci. 2020, 37, 100713. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Statti, G.A.; Menichini, F. Inhibitory Effects on the Digestive Enzyme Alpha-Amylase of Three Salsola Species (Chenopodiaceae) in Vitro. Pharmazie 2007, 62, 473–475. [Google Scholar] [PubMed]
- Ungar, I.A. Effect of Salinity on Seed Germination, Growth, and Ion Accumulation of Atriplex patula (Chenopodiaceae). Am. J. Bot. 1996, 83, 604. [Google Scholar] [CrossRef]
- Mann, E.; Rutter, A.; Zeeb, B. Evaluating the Efficacy of Atriplex spp. in the Phytoextraction of Road Salt (NaCl) from Contaminated Soil. Environ. Pollut. 2020, 265, 114963. [Google Scholar] [CrossRef] [PubMed]
- Bylka, W. A New Acylated Flavonol Diglycoside from Atriplex littoralis. Acta Physiol. Plant 2004, 26, 393–398. [Google Scholar] [CrossRef]
- Bueno, M.; Lendínez, M.L.; Aparicio, C.; Cordovilla, M.P. Effect of Salinity on Polyamines and Ethylene in Atriplex prostrata and Plantago coronopus. Biol. Plant 2015, 59, 596–600. [Google Scholar] [CrossRef]
- Egan, T.P.; Ungar, I.A. Competition between Salicornia europaea and Atriplex prostrata (Chenopodiaceae) along an Experimental Salinity Gradient. Wetl. Ecol. Manag. 2004, 9, 457–461. [Google Scholar] [CrossRef]
- Wang, L.; Showalter, A.; Ungar, I. Effect of Salinity on Growth, Ion Content, and Cell Wall Chemistry in Atriplex prostrata (Chenopodiaceae). Am. J. Bot. 1997, 84, 1247. [Google Scholar] [CrossRef]
- Bueno, M.; Lendínez, M.L.; Calero, J.; del Pilar Cordovilla, M. Salinity Responses of Three Halophytes from Inland Saltmarshes of Jaén (Southern Spain). Flora 2020, 266, 151589. [Google Scholar] [CrossRef]
- Bueno, M.; Lendínez, M.L.; Aparicio, C.; Cordovilla, M.P. Germination and Growth of Atriplex prostrata and Plantago coronopus: Two Strategies to Survive in Saline Habitats. Flora 2017, 227, 56–63. [Google Scholar] [CrossRef]
- Rodrigues, M.; Gangadhar, K.; Vizetto-Duarte, C.; Wubshet, S.; Nyberg, N.; Barreira, L.; Varela, J.; Custódio, L. Maritime Halophyte Species from Southern Portugal as Sources of Bioactive Molecules. Mar. Drugs 2014, 12, 2228–2244. [Google Scholar] [CrossRef]
- Benzarti, M.; ben Rejeb, K.; Debez, A.; Messedi, D.; Abdelly, C. Photosynthetic Activity and Leaf Antioxidative Responses of Atriplex portulacoides Subjected to Extreme Salinity. Acta Physiol. Plant 2012, 34, 1679–1688. [Google Scholar] [CrossRef]
- Maciel, E.; Lillebø, A.; Domingues, P.; da Costa, E.; Calado, R.; Domingues, M.R.M. Polar Lipidome Profiling of Salicornia ramosissima and Halimione portulacoides and the Relevance of Lipidomics for the Valorization of Halophytes. Phytochemistry 2018, 153, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.I.; Caçador, I.; Lillebø, A.I.; Pardal, M.A. Heavy Metal Accumulation in Halimione portulacoides: Intra- and Extra-Cellular Metal Binding Sites. Chemosphere 2008, 70, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, C.H. The Mycoflora Associated with Halimione portulacoides: III. fungi on green and moribund leaves. Trans. Br. Mycol. Soc. 1965, 48, 381–390. [Google Scholar] [CrossRef]
- Dickinson, C.H.; Pugh, G.J.F. The Mycoflora Associated with Halimione Portulacoides: II. Root surface fungi of mature and excised plants. Trans. Br. Mycol. Soc. 1965, 48, 595–602. [Google Scholar] [CrossRef]
- Aleixo, A.M.F. Biodiversidade de Fungos Endofíticos Em Halimione portulacoides. Master’s Thesis, Departamento de Biologia da Universidade de Aveiro, Aveiro, Portugal, 2013. [Google Scholar]
- Morales, P.; Ferreira, I.C.F.R.; Carvalho, A.M.; Sánchez-Mata, M.C.; Cámara, M.; Fernández-Ruiz, V.; Pardo-de-Santayana, M.; Tardío, J. Mediterranean Non-Cultivated Vegetables as Dietary Sources of Compounds with Antioxidant and Biological Activity. LWT-Food Sci. Technol. 2014, 55, 389–396. [Google Scholar] [CrossRef]
- Niazi, B.H.; Athar, M.; Salim, M.; Rozema, J. Growth and Ionic Relations of Fodderbeet and Seabeet under Saline Environments. Int. J. Environ. Sci. Technol. 2005, 2, 113–120. [Google Scholar] [CrossRef]
- Zardi-Bergaoui, A.; ben Nejma, A.; Harzallah-Skhiri, F.; Flamini, G.; Ascrizzi, R.; ben Jannet, H. Chemical Composition and Biological Studies of the Essential Oil from Aerial Parts of Beta vulgaris subsp. maritima (L.) Arcang. Growing in Tunisia. Chem. Biodivers. 2017, 14, e1700234. [Google Scholar] [CrossRef]
- Arbelet-Bonnin, D.; Blasselle, C.; Rose Palm, E.; Redwan, M.; Ponnaiah, M.; Laurenti, P.; Meimoun, P.; Gilard, F.; Gakière, B.; Mancuso, S.; et al. Metabolism Regulation during Salt Exposure in the Halophyte Cakile maritima. Environ. Exp. Bot. 2020, 177, 104075. [Google Scholar] [CrossRef]
- Ellouzi, H.; ben Hamed, K.; Cela, J.; Munné-Bosch, S.; Abdelly, C. Early Effects of Salt Stress on the Physiological and Oxidative Status of Cakile maritima (Halophyte) and Arabidopsis thaliana (Glycophyte). Physiol. Plant 2011, 142, 128–143. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Giménez-Martínez, J.J.; Torija-Isasa, M.E. Nutritional Composition of Wild Edible Crucifer Species. J. Food Biochem. 1999, 23, 283–294. [Google Scholar] [CrossRef]
- Welcome, A.K.; van Wyk, B.-E. An Inventory and Analysis of the Food Plants of Southern Africa. S. Afr. J. Bot. 2019, 122, 136–179. [Google Scholar] [CrossRef]
- Kumar, A.; Sreedharan, S.; Singh, P.; Achigan-Dako, E.G.; Ramchiary, N. Improvement of a Traditional Orphan Food Crop, Portulaca oleracea L. (Purslane) Using Genomics for Sustainable Food Security and Climate-Resilient Agriculture. Front. Sustain. Food Syst. 2021, 5, 711820. [Google Scholar] [CrossRef]
- Calvo, M.M.; Tzamourani, A.; Martínez-Alvarez, O. Halophytes as a Potential Source of Melanosis-Inhibiting Compounds. Mechanism of Inhibition of a Characterized Polyphenol Extract of Purslane (Portulaca oleracea). Food Chem. 2021, 355, 129649. [Google Scholar] [CrossRef]
- Mandlaa; Zhang, Y.; Wan, Y.; Tie, Y.; Zhang, B.; Wang, R.; Wang, G. Isolation and Characterization of Endophytic Fungi from Purslane and the Effects of Isolates on the Growth of the Host. Adv. Microbiol. 2019, 9, 438–453. [Google Scholar] [CrossRef]
- Abdel-Sater, M.A.; Abdel-Latif, A.M.A.; Abdel-Wahab, D.A.; Al-Bedak, O.A. Endophytic Mycobiota of Wild Medicinal Plants from New Valley Governorate, Egypt and Quantitative Assessment of Their Cell Wall Degrading Enzymes. Stud. Fungi 2021, 6, 78–91. [Google Scholar] [CrossRef]

| Botanical Name | Family | Common Name in English, French, German and Italian | Type of Halophytism | Edible Organs |
|---|---|---|---|---|
| Atriplex littoralis L. (Syn.: Atriplex patula L. var. littoralis (L.) A. Gray) | Amaranthaceae | Grassleaf orache, Arroche du littoral, Strand-meide, Atriplice litorale. | Psammophyte | Leaves |
| Atriplex prostrata Boucher ex DC (Syn.: Atriplex latifolia Wahlenb. = Atriplex hastata L. var. prostrata (Boucher ex DC.) Lange) | Amaranthaceae | Hastate orache, Arroche couché, Spiess-meide, Atriplice prostata | Eu-halophyte Meso-hydrohalophile | Leaves |
| Beta vulgaris L. subsp. maritima (L.) Arcang. (Tuscany, Sardinia, Sicily) | Amaranthaceae | Sea beet, Bette maritime, Wilde übe, Bietola marittima | Mio-halophyte | Leaves |
| Cakile maritima Scop. subsp. maritima | Brassicaceae | Searocket, Roquette de mer, Strandrauke, Ravastrello di mare | Psammophile Halo-nitrophilous | Leaves |
| Halimione portulacoides (L.) Aellen (Syn.: Atriplex portulacoides L.) | Amaranthaceae | Sea pursiane, Arroche faux-pourpier, Strand-salzmeide, Porcellana di mare | Eu-halophyte Hydro-halophyte | Leaves |
| Portulaca oleracea L. subsp. oleracea | Portulacaceae | Common pursiane, Purcelane, Portulach, Porcellana | Xero-halophyte | Leaves Stem |
| Salicornia perennans Willd. subsp. perennans (Syn.: Salicornia europaea auct.; Salicornia patula Duval-Jouve). | Amaranthaceae | Grasswort, Salicorne etaleé, Pannonien glasschmaiz, Salicornia patula | Eu-halophyte Xero-halophyte | Stem |
| Salicornia perennis Mill. subsp. perennis (Syn.: Sarcocornia perennis (Mill.) A.J.Scott subsp. perennis). | Amaranthaceae | Perennial grasswort, Salicorne vivace, Ausdauernde gliedermeide, Salicornia radicante | Eu-halophyte Hydro-halophyte | Stem |
| Salsola soda L. (Syn: Soda inermis Fourr.) | Amaranthaceae | Monk’s beard, Soude commune, Soda-salzicraut, Agretto | Eu-halophyte | Leaves Young stem |
| Suaeda maritima (L.) Dumort. (Syn.: Chenopodium maritimum L.) | Amaranthaceae | Sea-blite, Soude maritime, Strand-sode, Sueda marittima | Eu-halophyte Mesohydro-halophile | Leaves Young stem |
| Suaeda vera J.F. Gmel (Syn.: Suaeda fruticosa (L.) Forssk. subsp. vera (J.F. Gmel.) Maire & Weiller). | Amaranthaceae | Shrubby sea-blite, Soude vraie, Strauchige sode, Sueda vera | Eu-halophyte Halo-nitrophilous | Leaves Young stem |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardi, T.; Bertacchi, A.; Pistelli, L.; Pardossi, A.; Pecchia, S.; Toffanin, A.; Sanmartin, C. Biological and Agronomic Traits of the Main Halophytes Widespread in the Mediterranean Region as Potential New Vegetable Crops. Horticulturae 2022, 8, 195. https://doi.org/10.3390/horticulturae8030195
Lombardi T, Bertacchi A, Pistelli L, Pardossi A, Pecchia S, Toffanin A, Sanmartin C. Biological and Agronomic Traits of the Main Halophytes Widespread in the Mediterranean Region as Potential New Vegetable Crops. Horticulturae. 2022; 8(3):195. https://doi.org/10.3390/horticulturae8030195
Chicago/Turabian StyleLombardi, Tiziana, Andrea Bertacchi, Laura Pistelli, Alberto Pardossi, Susanna Pecchia, Annita Toffanin, and Chiara Sanmartin. 2022. "Biological and Agronomic Traits of the Main Halophytes Widespread in the Mediterranean Region as Potential New Vegetable Crops" Horticulturae 8, no. 3: 195. https://doi.org/10.3390/horticulturae8030195
APA StyleLombardi, T., Bertacchi, A., Pistelli, L., Pardossi, A., Pecchia, S., Toffanin, A., & Sanmartin, C. (2022). Biological and Agronomic Traits of the Main Halophytes Widespread in the Mediterranean Region as Potential New Vegetable Crops. Horticulturae, 8(3), 195. https://doi.org/10.3390/horticulturae8030195