Effect of Saline-Nutrient Solution on Yield, Quality, and Shelf-Life of Sea Fennel (Crithmum maritimum L.) Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
2.2. Analysis at Harvesting Time
2.3. Postharvest Product Handling and Analysis
2.4. Sensory Quality Panel
2.5. Statistical Analysis
3. Results
3.1. Growth, Yield, and Quality Characteristic of C. maritimum at Harvesting
3.2. Postharvest Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Renna, M.; Gonnella, M.; Caretto, S.; Mita, G.; Serio, F. Sea fennel (Crithmum maritimum L.): From underutilized crop to new dried product for food use. Genet. Resour. Crop Evol. 2017, 64, 205–216. [Google Scholar] [CrossRef]
- United States Department of Agriculture-Agriculture Research Service (USDA). U.S. National Plant Germplasm System [WWW Document]. Available online: https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomydetail?id=402237 (accessed on 15 November 2021).
- Sánchez-Hernández, E.; Buzón-Durán, L.; Andrés-Juan, C.; Lorenzo-Vidal, B.; Martín-Gil, J.; Martín-Ramos, P. Physicochemical Characterization of Crithmum maritimum L. and Daucus carota subsp. gummifer (Syme) Hook. fil. and Their Antimicrobial Activity against Apple Tree and Grapevine Phytopathogens. Agronomy 2021, 11, 886. [Google Scholar] [CrossRef]
- Atia, A.; Barhoumi, Z.; Mokded, R.; Abdelly, C.; Smaoui, A. Environmental eco-physiology and economical potential of the halophyte Crithmum maritimum L. (Apiaceae). J. Med. Plants Res. 2011, 5, 3564–3571. [Google Scholar]
- Perez-Vizcaino, F.; Duarte, J.; Jiménez, R.; Santos-Buelga, C.; Osuna, A. Antihypertensive effects of the flavonoid quercetin. Pharmacol. Rep. 2009, 61, 67–75. [Google Scholar] [CrossRef]
- Atzori, G.; de Vos, A.C.; van Rijsselberghe, M.; Vignolini, P.; Rozema, J.; Mancuso, S.; van Bodegom, P.M. Effects of increased seawater salinity irrigation on growth and quality of the edible halophyte Mesembryanthemum crystallinum L. under field conditions. Agric. Water Manag. 2017, 187, 37–46. [Google Scholar] [CrossRef]
- Renna, M.; Gonnella, M. The use of the sea fennel as a new spice-colorant in culinary preparations. Int. J. Gastron. Food Sci. 2012, 1, 111–115. [Google Scholar] [CrossRef]
- Siracusa, L.; Kulisic-Bilusic, T.; Politeo, O.; Krause, I.; Dejanovic, B.; Ruberto, G. Phenolic composition and antioxidant activity of aqueous infusions from Capparis spinosa L. and Crithmum maritimum L. before and after submission to a two-step in vitro digestion model. J. Agric. Food Chem. 2011, 59, 12453–12459. [Google Scholar] [CrossRef] [PubMed]
- Giungato, P.; Renna, M.; Rana, R.; Licen, S.; Barbieri, P. Characterization of dried and freeze-dried sea fennel (Crithmum maritimum L.) samples with headspace gas-chromatography/mass spectrometry and evaluation of an electronic nose discrimination potential. Food Res. Int. 2019, 115, 65–72. [Google Scholar] [CrossRef]
- Souid, A.; Della Croce, C.M.; Frassinetti, S.; Gabriele, M.; Pozzo, L.; Ciardi, M.; Abdelly, C.; Hamed, K.B.; Magné, C.; Longo, V. Nutraceutical potential of leaf hydro-ethanolic extract of the edible halophyte Crithmum maritimum L. Molecules 2021, 26, 5380. [Google Scholar] [CrossRef]
- Pistrick, K. Notes on neglected and underutilized crops Current taxonomical overview of cultivated plants in the families Umbelliferae and Labiatae. Genet. Resour. Crop Evol. 2002, 49, 211–221. [Google Scholar] [CrossRef]
- Ozcan, B.; Tzeremes, P.G.; Tzeremes, N.G. Energy consumption, economic growth and environmental degradation in OECD countries. Econ. Model. 2020, 84, 203–213. [Google Scholar] [CrossRef]
- Tran, D.Q.; Konishi, A.; Cushman, J.C.; Morokuma, M.; Toyota, M.; Agarie, S. Ion accumulation and expression of ion homeostasis-related genes associated with halophilism, NaCl-promoted growth in a halophyte Mesembryanthemum crystallinum L. Plant Prod. Sci. 2020, 23, 91–102. [Google Scholar] [CrossRef]
- Jiménez-Becker, S.; Ramírez, M.; Plaza, B.M. The influence of salinity on the vegetative growth, osmolytes and chloride concentration of four halophytic species. J. Plant Nutr. 2019, 42, 1838–1849. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- Renna, M.; Cocozza, C.; Gonnella, M.; Abdelrahman, H.; Santamaria, P. Elemental characterization of wild edible plants from countryside and urban areas. Food Chem. 2015, 177, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Renna, M. Reviewing the prospects of sea fennel (Crithmum maritimum L.) as emerging vegetable crop. Plants 2018, 7, 92. [Google Scholar] [CrossRef]
- Giménez, A.; Martínez-Ballesta, M.D.; Egea-Gilabert, C.; Gómez, P.A.; Artés-Hernández, F.; Pennisi, G.; Orsini, F.; Crepaldi, A.; Fernández, J.A. Combined Effect of Salinity and LED Lights on the Yield and Quality of Purslane (Portulaca oleracea L.) Microgreens. Horticulturae 2021, 7, 180. [Google Scholar] [CrossRef]
- Nicola, S.; Fontana, E. Fresh-cut produce quality: Implications for a systems approach. In Postharvest Handling: A System Approach; Florkowski, W.J., Shewfelt, R., Breuckner, B., Prussia, S.E., Eds.; Academic Press: San Diego, CA, USA; Elsevier: Amsterdam, The Netherlands, 2014; pp. 217–273. [Google Scholar]
- Ali, A.Y.A.; Ibrahim, M.E.H.; Zhou, G.; Nimir, N.E.A.; Elsiddig, A.M.I.; Jiao, X.; Zhu, G.; Salih, E.G.I.; Suliman, M.S.E.S.; Elradi, S.B.M. Gibberellic acid and nitrogen efficiently protect early seedlings growth stage from salt stress damage in Sorghum. Sci. Rep. 2021, 11, 6672. [Google Scholar] [CrossRef]
- Machado, R.; Serralheiro, R. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Egea-Gilabert, C.; Fernández, J.A.; Migliaro, D.; Martínez-Sánchez, J.J.; Vicente, M.J. Genetic variability in wild vs. cultivated Eruca vesicaria populations as assessed by morphological, agronomical and molecular analyses. Sci. Hortic. 2009, 121, 260–266. [Google Scholar] [CrossRef]
- Lara, L.J.; Egea-Gilabert, C.; Niñirola, D.; Conesa, E.; Fernández, J.A. Effect of aeration of the nutrient solution on the growth and quality of purslane (Portulaca oleracea). J. Hortic. Sci. Biotechnol. 2011, 86, 603–610. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the folin-Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- López-Marín, J.; Gálvez, A.; del Amor, F.M.; Albacete, A.; Fernández, J.A.; Egea-Gilabert, C.; Pérez-Alfocea, F. Selecting vegetative/generative/dwarfing rootstocks for improvingfruit yield and quality in water stressed sweet peppers. Sci. Hortic. 2017, 214, 9–17. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Tomás-Callejas, A.; López-Velasco, G.; Camacho, A.B.; Artés, F.; Artés-Hernández, F.; Suslow, T.V. Survival and distribution of Escherichia coli on diverse fresh-cut baby leafy greens under preharvest through postharvest conditions. Int. J. Food Microbiol. 2011, 151, 216–222. [Google Scholar] [CrossRef]
- Hamed, K.B.; Debez, A.; Chibani, F.; Abdelly, C. Salt response of Crithmum maritimum, an oleagineous halophyte. Trop. Ecol. 2004, 45, 151–159. [Google Scholar]
- Hamed, K.B.; Castagna, A.; Salem, E.; Ranieri, A.; Abdelly, C. Sea fennel (Crithmum maritimum L.) under salinity conditions: A comparison of leaf and root antioxidant responses. Plant Growth Regul. 2007, 53, 185–194. [Google Scholar] [CrossRef]
- Amanullah; Khan, I.; Jan, A.; Jan, M.T.; Khalil, S.K.; Shah, Z.; Afzal, M. Compost and nitrogen management influence productivity of spring maize (Zea mays L.) under deep and conventional tillage systems in Semi-arid regions. Commun. Soil Sci. Plant Anal. 2015, 46, 1566–1578. [Google Scholar] [CrossRef]
- Yepes, L.; Chelbi, N.; Vivo, J.-M.; Franco, M.; Agudelo, A.; Carvajal, M.; del Carmen Martinez-Ballesta, M. Analysis of physiological traits in the response of Chenopodiaceae, Amaranthaceae, and Brassicaceae plants to salinity stress. Plant Physiol. Biochem. 2018, 132, 145–155. [Google Scholar] [CrossRef]
- Damerum, A.; Chapman, M.A.; Taylor, G. Innovative breeding technologies in lettuce for improved post-harvest quality. Postharvest Biol. Technol. 2020, 168, 111266. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, F.; Derridj, A.; Rogers, H.J. Diverse salinity responses in Crithmum maritimum tissues at different salinities over time. J. Soil Sci. Plant Nutr. 2017, 17, 716–734. [Google Scholar] [CrossRef][Green Version]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef] [PubMed]
- Rea, P.A.; Poole, R.J. Vacuolar H+-translocating pyrophosphatase. Ann. Rev. Plant Physiol. Mol. Biol. 1993, 44, 157–180. [Google Scholar] [CrossRef]
- Ben Hamed, K.; Ben Youssef, N.; Ranieri, A.; Zarrouk, M.; Abdelly, C. Changes in content and fatty acid profiles of total lipids and sulfolipids in the halophyte Crithmum maritimum under salt stress. J. Plant Physiol. 2005, 162, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Loaiza, V.; Oliveira, M.; Santos, T.; Schüler, L.; Lima, A.R.; Gama, F.; Salazar, M.; Neng, N.R.; Nogueira, J.M.F.; Varela, J. Wild vs cultivated halophytes: Nutritional and functional differences. Food Chem. 2020, 333, 127536. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Faure, A.; Calvo, M.M.; Pérez-Jiménez, J.; Martín-Diana, A.B.; Rico, D.; Montero, M.P.; del Carmen Gómez-Guillén, M.; López-Caballero, M.E.; Martínez-Alvarez, O. Exploring the potential of common iceplant, seaside arrowgrass and sea fennel as edible halophytic plants. Food Res. Int. 2020, 137, 109613. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Effects of calcium on antioxidant activities and water relations associated with heat tolerance in two cool-season grasses. J. Exp. Bot. 2001, 52, 341–349. [Google Scholar] [CrossRef]
- Hernández, J.A.; Aguilar, A.B.; Portillo, B.; López-Gómez, E.; Beneyto, J.M.; García-Legaz, M.F. The effect of calcium on the antioxidant enzymes from salt-treated loquat and anger plants. Funct. Plant Biol. 2003, 30, 1127–1137. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Jean, Y.-K.; Kabir, I.; Tolay, I.; Torun, A.A.; Kronzucker, H.J. K+ efflux and retention in response to NaCl stress do not predict salt tolerance in contrasting genotypes of rice (Oryza sativa L.). PLoS ONE 2013, 8, e57767. [Google Scholar] [CrossRef] [PubMed]
- Yuriko, O.; Osakabe, K.; Shinozaki, K.; Tran, L. SP Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar]
- Chrysargyris, A.; Tzionis, A.; Xylia, P.; Tzortzakis, N. Effects of salinity on tagetes growth, physiology, and shelf life of edible flowers stored in passive modified atmosphere packaging or treated with ethanol. Front. Plant Sci. 2018, 9, 1765. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Huffaker, R.C.; Rains, D.W. Early effects of salinity on nitrate assimilation in barley seedlings. Plant Physiol. 1984, 76, 321–325. [Google Scholar] [CrossRef]
- Varma, S.; Rogers, D.M.; Pratt, L.R.; Rempe, S.B. Design principles for K+ selectivity in membrane transport. J. Gen. Physiol. 2011, 137, 479–488. [Google Scholar] [CrossRef]
- Rubinigg, M.; Posthumus, F.; Ferschke, M.; Elzenga, J.T.M.; Stulen, I. Effects of NaCl salinity on 15 N-nitrate fluxes and specific root length in the halophyte Plantago maritima L. Plant Soil 2003, 250, 201–213. [Google Scholar] [CrossRef]
- De Abreu, I.N.; Mazzafera, P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Biochem. 2005, 43, 241–248. [Google Scholar] [CrossRef]
- Labiad, M.H.; Giménez, A.; Varol, H.; Tüzel, Y.; Egea-Gilabert, C.; Fernández, J.A.; Martínez-Ballesta, M.-C. Effect of exogenously applied methyl jasmonate on yield and quality of salt-stressed hydroponically grown sea fennel (Crithmum maritimum L.). Agronomy 2021, 11, 1083. [Google Scholar] [CrossRef]
- Yuan, G.; Wang, X.; Guo, R.; Wang, Q. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010, 121, 1014–1019. [Google Scholar] [CrossRef]
- Emami Bistgani, Z.; Hashemi, M.; DaCosta, M.; Craker, L.; Filippo, M.; Morshedloo, M.R. Effect of salinity stress on the physiological characteristics, phenolic compounds and antioxidant activity of Thymus vulgaris L. and Thymus daenensis Celak. Ind. Crops Prod. 2019, 135, 311–320. [Google Scholar] [CrossRef]
- Rakhmankulova, Z.F.; Shuyskaya, E.V.; Shcherbakov, A.V.; Fedyaev, V.; Biktimerova, G.Y.; Khafisova, R.R.; Usmanov, I.Y. Content of proline and flavonoids in the shoots of halophytes inhabiting the South Urals. Russ. J. Plant Physiol. 2021, 62, 71–79. [Google Scholar] [CrossRef]
- Grace, S.G.; Logan, B.A. Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2000, 355, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Gago, C.; Sousa, A.R.; Juliao, M.; Miguel, G.; Antunes, D.C.; Panagopoulos, T. Sustainable use of energy in the storage of halophytes used for food. Int. J. Energy Environ. 2011, 4, 592–599. [Google Scholar]
- D’Imperio, M.; Renna, M.; Cardinali, A.; Buttaro, D.; Serio, F.; Santamaria, P. Calcium biofortification and bioaccessibility in soilless “baby leaf” vegetable production. Food Chem. 2016, 213, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Sanches-Silva, A.; Castilho, M.; Cavaleiro, C.; Ramos, F. Halophytes as source of bioactive phenolic compounds and their potential applications. Crit. Rev. Food Sci. Nutr. 2021, 2, 1–24. [Google Scholar] [CrossRef]
- Abadias, M.; Usall, J.; Anguera, M.; Solsona, C.; Viñas, I. Microbiological quality of fresh, minimally-processed fruit and vegetables, and sprouts from retail establishments. Int. J. Food Microbiol. 2008, 123, 121–129. [Google Scholar] [CrossRef] [PubMed]
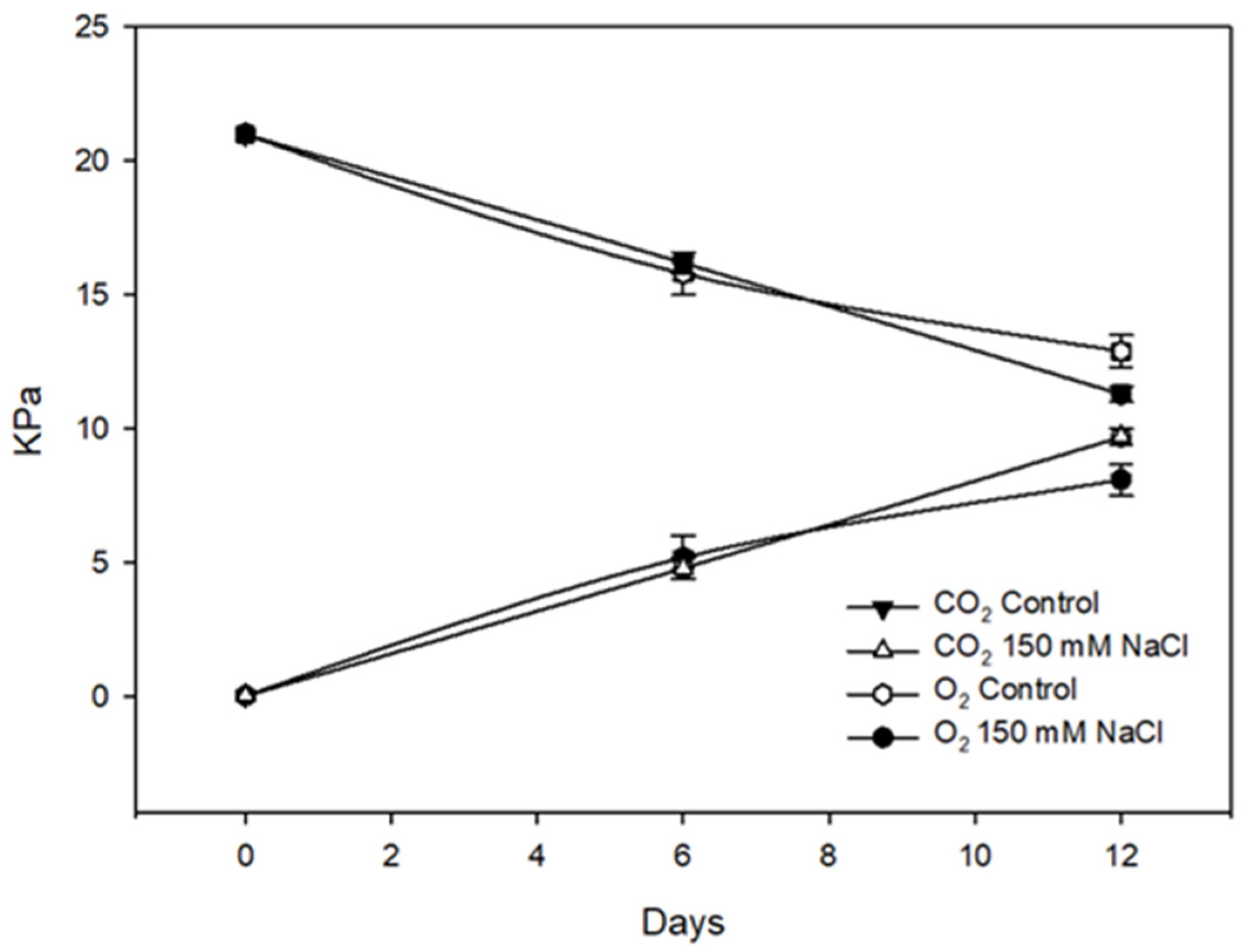
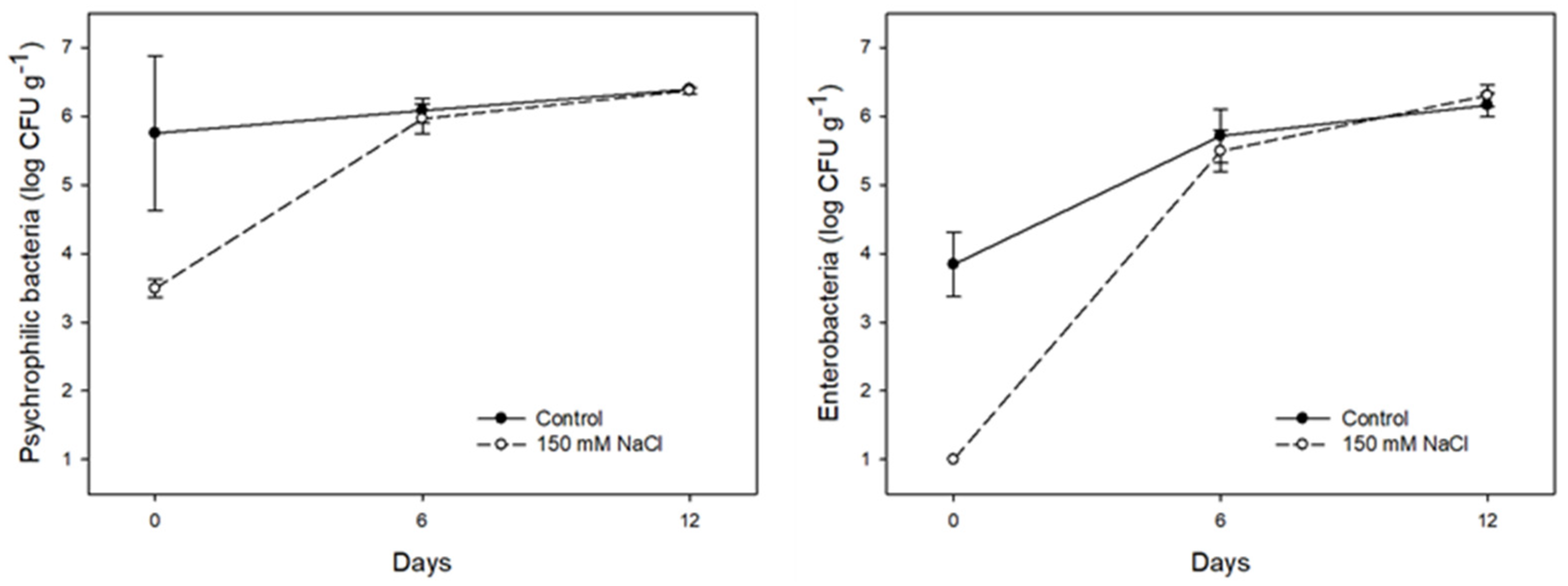
| Treatments | Shoot Fresh Weight (g plant−1) | Shoot Dry Weight (g plant−1) | Leaf Area (cm2 plant−1) | SLA (m2 kg−1) | Total Root Length (cm) | Root Diameter (mm) | Root Volume (cm3) |
|---|---|---|---|---|---|---|---|
| Control | 2.10 ± 0.47 a | 0.205 ± 0.005 a | 3.43 ± 0.21 b | 0.19 ± 0.012 b | 112.24 ± 6.31 a | 0.37 ± 0.01 a | 0.59 ± 0.07 a |
| 150 mM NaCl | 2.45 ± 0.42 a | 0.235 ± 0.005 a | 2.37 ± 0.09 a | 0.11 ± 0.005 a | 109.92 ± 5.84 a | 0.33 ± 0.02 a | 0.46 ± 0.05 a |
| Treatments | NO3− | Cl− | Br− | PO43− | SO42− | C2O42− |
|---|---|---|---|---|---|---|
| Control | 1530.31 ± 586.47 b | 1810.97 ± 120.56 a | 152.00 ± 2.82 b | 947.01 ± 94.14 a | 1699.55 ± 32.28 b | 88.19 ± 33.59 a |
| 150 mM NaCl | 1263.03 ± 19.79 a | 6718.18 ± 1029.31 b | 125.98 ± 3.46 a | 1154.39 ± 142.73 a | 671.25 ± 47.66 a | 88.70 ± 28.87 a |
| Treatments | Na+ | K+ | Ca2+ | Mg2+ |
|---|---|---|---|---|
| Control | 777.57 ± 54.46 a | 3642.49 ± 109.48 b | 1108.29 ± 20.91 b | 379.10 ± 12.46 b |
| 150 mM NaCl | 4639.45 ± 703.02 b | 1070.45 ± 345.86 a | 532.05 ± 143.54 a | 203.42 ± 10.47 a |
| Treatments | Total Phenolics (mg GA kg−1 FW) | Total Flavonoids (mg Rutin kg−1 FW) | Total Antioxidant Capacity (mg DPPHreduced kg−1 FW) |
|---|---|---|---|
| Control | 887.43 ± 11.95 b | 1966.89 ± 45.17 a | 112.24 ± 6.31 a |
| 150 mM NaCl | 833.53 ± 9.42 a | 2167.24 ± 22.09 b | 109.92 ± 5.84 a |
| Sea Fennel Firmness (g) | |
|---|---|
| Salinity Treatment (A) | |
| Control | 596.27 ± 20.43 b x |
| 150 mM NaCl | 457.94 ± 28.27 a |
| Storage (B) | |
| 0 days | 494.48 ± 35.26 a y |
| 6 days | 515.05 ± 34.01 a |
| 12 days | 571.80 ± 28.37 a |
| Significant Differences | |
| A | *** |
| B | ns |
| A × B | ns |
| L | a | b | HUE | Chroma | |
|---|---|---|---|---|---|
| Salinity Treatment (A) | |||||
| Control | 37.20 ± 0.66 a x | −11.31 ± 0.39 a | 18.32 ± 1.01 a | 121.98 ± 1.08 b | 22.26 ± 0.76 a |
| 150 mM NaCl | 39.14 ± 0.40 b | −10.94 ± 0.27 a | 20.62 ± 0.55 a | 118.01 ± 0.70 a | 23.35 ± 0.54 a |
| Storage (B) | |||||
| 0 days | 38.51 ± 0.62 a y | 11.29 ± 0.42 a | 20.25 ± 1.12 a | 119.43 ± 1.96 a | 21.09 ± 0.94 a |
| 6 days | 37.95 ± 1.07 a | 10.84 ± 0.36 a | 18.98 ± 1.28 a | 120.04 ± 1.14 a | 23.58 ± 0.56 a |
| 12 days | 38.04 ± 0.66 a | 11.26 ± 0.49 a | 19.18 ± 0.94 a | 120.51 ± 1.04 a | 22.71 ± 0.98 a |
| Significant Differences | |||||
| A | * | ns | ns | * | ns |
| B | ns | ns | ns | ns | ns |
| A × B | ns | ns | ns | ns | ns |
| Psychrophilic Bacteria (log UFC g−1) | Mesophilic Bacteria (log UFC g−1) | Enterobacteria (log UFC g−1) | Yeast and Moulds (log UFC g−1) | |
|---|---|---|---|---|
| Salinity Treatment (A) | ||||
| Control | 5.81 ± 0.28 b x | 5.40 ± 0.31 a | 5.15 ± 0.38 b | 3.89 ± 0.18 b |
| 150 mM NaCl | 5.25 ± 0.45 a | 5.24 ± 0.36 a | 3.90 ± 0.98 a | 3.34 ± 0.12 a |
| Storage (B) | ||||
| 0 days | 4.23 ± 0.44 a y | 4.05 ± 0.11 a | 1.89 ± 0.83 a | 3.09 ± 0.16 a |
| 6 days | 5.99 ± 0.08 b | 5.61 ± 0.09 b | 5.61 ± 0.13 b | 3.82 ± 0.10 b |
| 12 days | 6.38 ± 0.01 b | 6.30 ± 0.01 c | 6.21 ± 0.07 c | 3.84 ± 0.21 b |
| Significant Differences | ||||
| A | * | ns | *** | ** |
| B | *** | *** | *** | ** |
| A × B | * | ns | *** | ns |
| Sensorial Quality | ||||||
|---|---|---|---|---|---|---|
| Day 0 | Day 6 | Day 12 | ||||
| Control | 150 mM NaCl | Control | 150 mM NaCl | Control | 150 mM NaCl | |
| Acceptance | ||||||
| Visual appearance | 5 | 4 | 4.5 | 4 | 4 | 3.5 |
| Colour | 4 | 4 | 4 | 4 | 4 | 4 |
| Texture (Crispness) | 5 | 5 | 4 | 3.5 | 3 | 3 |
| Flavour (Freshness) | 5 | 4 | 4 | 4 | 3 | 3 |
| Aroma | 5 | 5 | 5 | 5 | 5 | 5 |
| Global acceptance | 5 | 4.5 | 4 | 3.5 | 3.5 | 3 |
| Alterations | ||||||
| Off-odours | 5 | 5 | 5 | 5 | 5 | 5 |
| Mechanical damage | 4 | 4 | 4 | 4 | 3.5 | 3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amoruso, F.; Signore, A.; Gómez, P.A.; Martínez-Ballesta, M.d.C.; Giménez, A.; Franco, J.A.; Fernández, J.A.; Egea-Gilabert, C. Effect of Saline-Nutrient Solution on Yield, Quality, and Shelf-Life of Sea Fennel (Crithmum maritimum L.) Plants. Horticulturae 2022, 8, 127. https://doi.org/10.3390/horticulturae8020127
Amoruso F, Signore A, Gómez PA, Martínez-Ballesta MdC, Giménez A, Franco JA, Fernández JA, Egea-Gilabert C. Effect of Saline-Nutrient Solution on Yield, Quality, and Shelf-Life of Sea Fennel (Crithmum maritimum L.) Plants. Horticulturae. 2022; 8(2):127. https://doi.org/10.3390/horticulturae8020127
Chicago/Turabian StyleAmoruso, Fabio, Angelo Signore, Perla A. Gómez, María del Carmen Martínez-Ballesta, Almudena Giménez, José A. Franco, Juan A. Fernández, and Catalina Egea-Gilabert. 2022. "Effect of Saline-Nutrient Solution on Yield, Quality, and Shelf-Life of Sea Fennel (Crithmum maritimum L.) Plants" Horticulturae 8, no. 2: 127. https://doi.org/10.3390/horticulturae8020127
APA StyleAmoruso, F., Signore, A., Gómez, P. A., Martínez-Ballesta, M. d. C., Giménez, A., Franco, J. A., Fernández, J. A., & Egea-Gilabert, C. (2022). Effect of Saline-Nutrient Solution on Yield, Quality, and Shelf-Life of Sea Fennel (Crithmum maritimum L.) Plants. Horticulturae, 8(2), 127. https://doi.org/10.3390/horticulturae8020127












