The Breeding of High-Quality Dandelions by NaCl Induced Callus Variation Combined with a Drosophila Tumor Cell Migration Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Dandelion Materials
2.2. Production of Dandelion Tissue Culture Plantlet
2.2.1. Induction of Callus with Saline Treatment
2.2.2. Identification of Salt-Treated Callus
2.2.3. Regeneration of Dandelion Tissue Culture Plantlet
2.3. Determination of Candidate Dandelion Lines
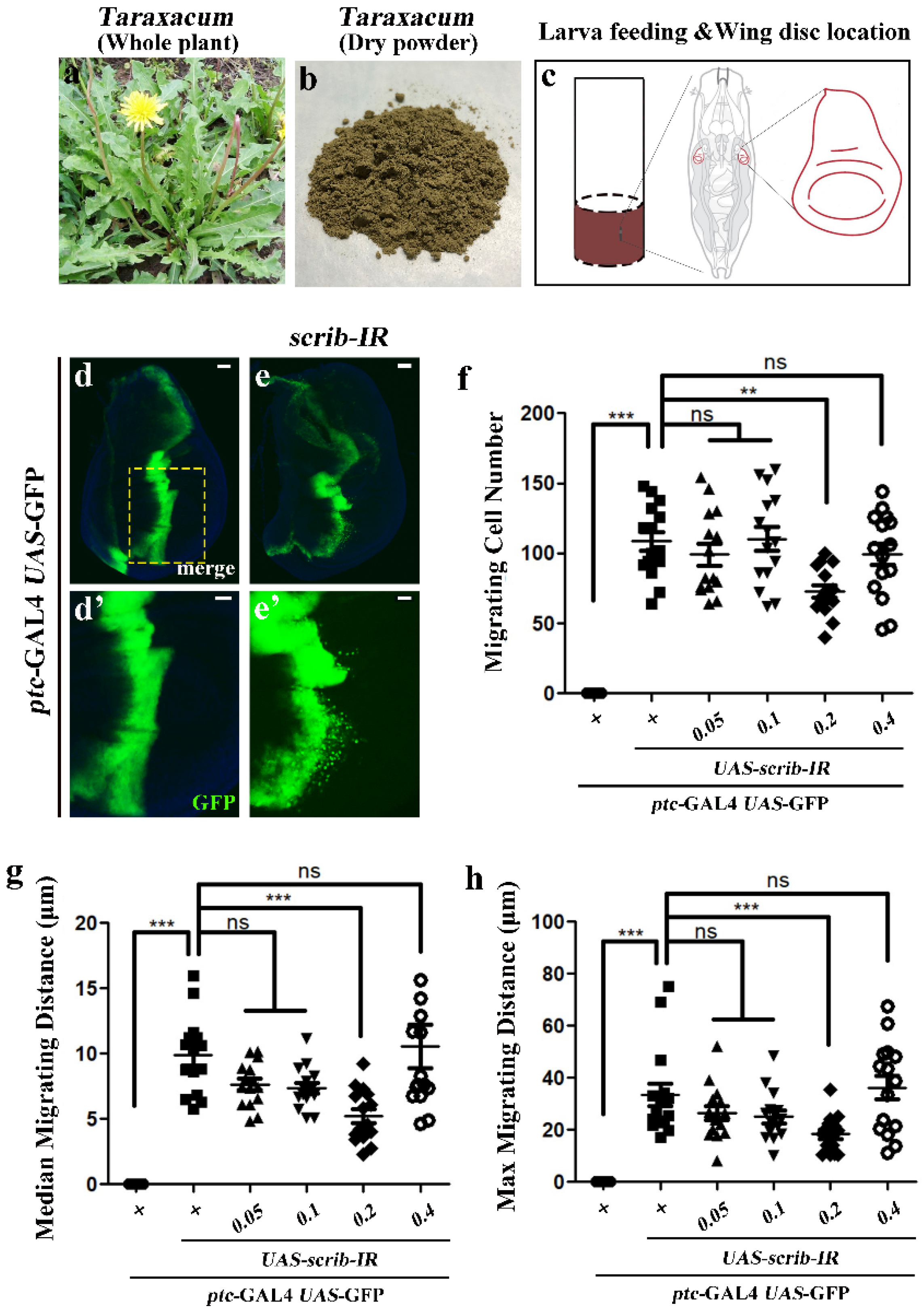
2.4. Anti-Tumor Cell Migration Evaluation
2.4.1. Preparation of Dandelion Extracts
2.4.2. Fly Strains
2.4.3. Immunostaining
2.5. Data Analysis
3. Results and Discussion
3.1. Identification of Salt-Induced Calluses
3.2. Morphological Evaluation
3.3. Yield and Bioactive Compounds Contents
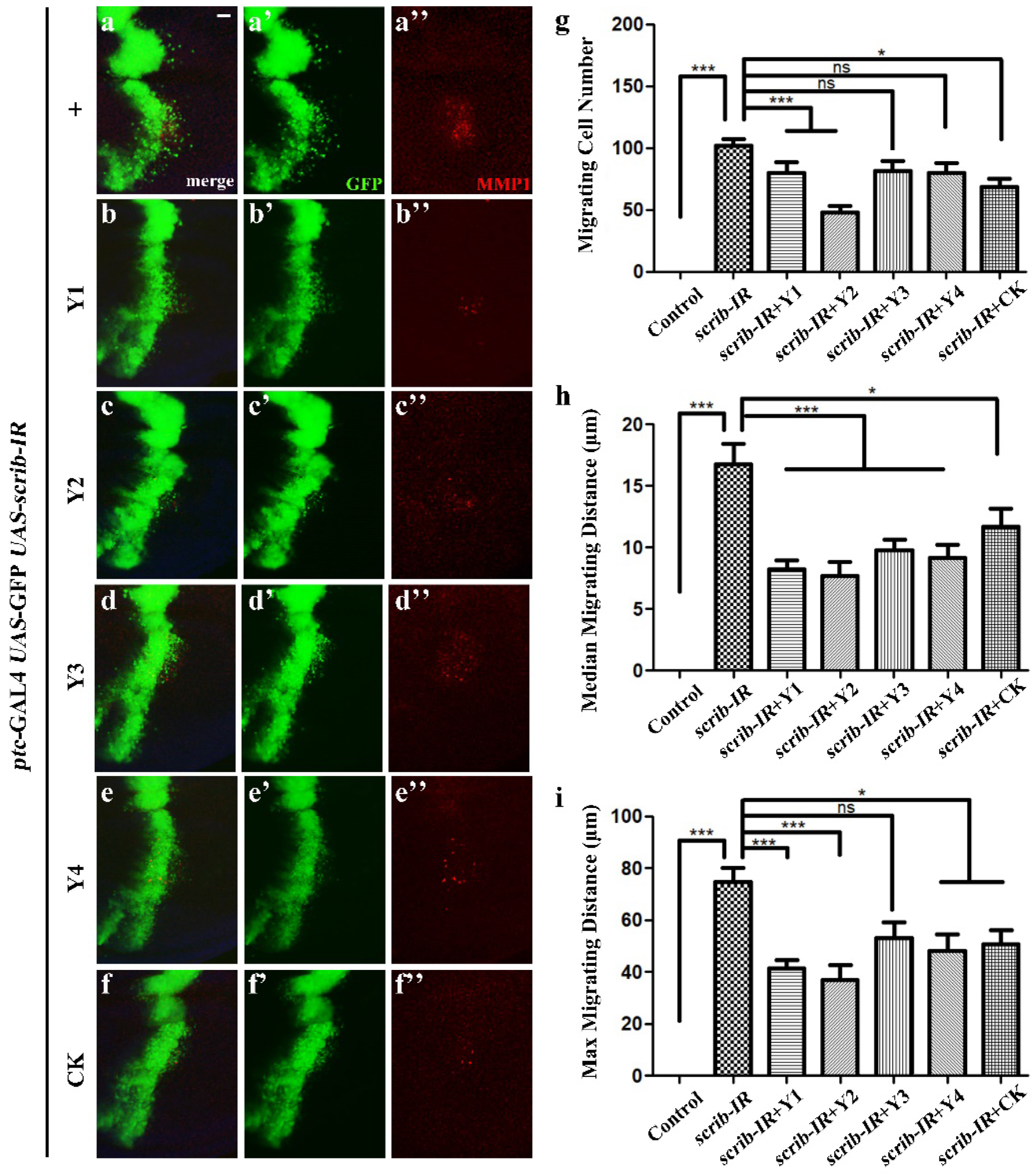
3.4. Tumor Cell Migration Evaluation
3.4.1. Inhibitory Dose of Dandelion Extract on Tumor Cell Migration
3.4.2. Evaluation of Different Dandelions on Tumor Cell Migration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Xue, Z.; Lu, X.; Liu, Y.; Liu, G.; Wu, Z. Salt leaching of heavy coastal saline silty soil by controlling the soil matric potential. Soil Water Res. 2019, 3, 132–137. [Google Scholar] [CrossRef]
- Wu, Z.; Xue, Z.; Li, H.; Zhang, X.; Wang, X.; Lu, X. Cultivation of dandelion (Taraxacum erythropodium) on coastal saline land based on the control of salinity and fertilizer. Folia Hortic. 2019, 31, 277–284. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, J.; Dai, X.; Zhao, Z.; Wang, X.; Zhang, G. Response of soil bacterial community structure and function under two salt-tolerant plants in a coastal saline soil area of eastern Hebei province of China. Int. J. Phytoremediat. 2021, 24, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xue, Z.; Lu, X.; Jia, Y.; Wang, X.; Zhang, X. Salt-tolerance identification and quality evaluation of Abelmoschus manihot (L.) Medik. Can. J. Plant Sci. 2020, 100, 568–574. [Google Scholar] [CrossRef]
- Xie, P.; Huang, L.; Zhang, C.; Ding, S.; Deng, Y.; Wang, X. Skin-care effects of dandelion leaf extract and stem extract: Antioxidant properties, tyrosinase inhibitory and molecular docking simulations. Ind. Crop. Prod. 2018, 111, 238–246. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Chen, H.; Shi, W. Selection and identification of salt-tolerant variants of Taraxacum officinale. Chin. J. Biotechnol. 2008, 24, 262–271. (In Chinese) [Google Scholar]
- Liu, Q.; Liu, Y.; Xu, Y.; Yao, L.; Liu, Z.; Cheng, H.; Ma, M.; Wu, J.; Wang, W.; Ning, W. Overexpression of and RNA interference with hydroxycinnamoyl-CoA quinate hydroxycinnamoyl transferase affect the chlorogenic acid metabolic pathway and enhance salt tolerance in Taraxacum antungense Kitag. Phytochem. Lett. 2018, 28, 116–123. [Google Scholar] [CrossRef]
- Sigstedt, S.C.; Hooten, C.J.; Callewaert, M.C.; Jenkins, A.R.; Romero, A.E.; Pullin, M.J.; Kornienko, A.; Lowrey, T.K.; Slambrouck, S.V.; Steelant, W.F. Evaluation of aqueous extracts of Taraxacum officinale on growth and invasion of breast and prostate cancer cells. Int. J. Oncol. 2008, 32, 1085–1090. [Google Scholar] [CrossRef]
- Nassan, M.A.; Soliman, M.M.; Ismail, S.A.; El-Shazly, S. Effect of Taraxacum officinale extract on PI3K/Akt pathway in DMBA-induced breast cancer in albino rats. Biosci. Rep. 2018, 38, BSR20180334. [Google Scholar] [CrossRef]
- Menke, K.; Schwermer, M.; Felenda, J.; Beckmann, C.; Stintzing, F.; Schramm, A.; Zuzak, T. Taraxacum officinale extract shows antitumor effects on pediatric cancer cells and enhance mistletoe therapy. Complement. Ther. Med. 2018, 40, 158–164. [Google Scholar] [CrossRef]
- Gonzalez, C. Drosophila melanogaster: A model and a tool to investigate malignancy and identify new therapeutics. Nat. Rev. Cancer 2013, 13, 172–183. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, X.; Wu, H.; Sun, Y.; Ma, X.; Li, J.; Xu, Q.; Wu, C.; Li, Q.; Jiang, C.; et al. Wingless modulates activator protein-1-mediated tumor invasion. Oncogene 2019, 38, 3871–3885. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X. A new dandelion variety “Binpu 1” with high yield, good quality and salt tolerance. Xian Dai Nong Cun Ke Ji 2019, 2, 52. (In Chinese) [Google Scholar]
- Chen, G.; Zhang, X. Mechanism for salt tolerance of salt-tolerant mutant ‘BINPU 1’ of Taraxacum mongolicum. Guihaia 2021, 41, 1417–1424. (In Chinese) [Google Scholar]
- Zhang, X.; Meng, R.; Feng, W.; Li, Z.; Lu, X.; Pan, X.; Wang, X. A New Taraxacum mongolicum Cultivar ‘Binpu 2’. Acta Hortic. Sin. 2021, 48, 3025–3026. (In Chinese) [Google Scholar]
- Wu, Z.; Li, Z.; Xue, Z.; Lu, X.; Wang, X. Optimization of extraction technology for determination of caffeic and chlorogenic acid in dandelion. Banats J. Biotechnol. 2020, 11, 26–37. [Google Scholar] [CrossRef]
- Liu, N.; Song, M.; Wang, N.; Wang, Y.; Wang, R.; An, X.; Qi, J. The effects of solid-state fermentation on the content, composition and in vitro antioxidant activity of flavonoids from dandelion. PLoS ONE 2020, 15, e0239076. [Google Scholar] [CrossRef]
- Ma, X.; Yang, L.; Yang, Y.; Li, M.; Li, W.; Xue, L. dUev1a modulates TNF-JNK mediated tumor progression and cell death in Drosophila. Dev. Biol. 2013, 380, 211–221. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Chen, Y.; Huang, X.; Hu, Y.; Zhang, R.; Ho, M.S.; Xue, L. FoxO mediates APP-induced AICD-dependent cell death. Cell Death Dis. 2014, 5, e1233. [Google Scholar] [CrossRef]
- González-Castejón, M.; Visioli, F.; Rodriguez-Casado, A. Diverse biological activities of dandelion. Nutr. Rev. 2012, 70, 534–547. [Google Scholar] [CrossRef]
- Ma, X.; Shao, Y.; Zheng, H.; Li, M.; Li, W.; Xue, L. Src42A modulates tumor invasion and cell death via Ben/dUev1a-mediated JNK activation in Drosophila. Cell Death Dis. 2013, 4, e864. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, W.; Yu, H.; Yang, Y.; Li, M.; Xue, L.; Xu, T. Bendless modulates JNK-mediated cell death and migration in Drosophila. Cell Death Differ. 2014, 21, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Szafranski, P.; Hall, C.A.; Goode, S. Basolateral junctions utilize warts signaling to control epithelial-mesenchymal transition and proliferation crucial for migration and invasion of Drosophila ovarian epithelial cells. Genetics 2008, 178, 1947–1971. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef]

| Index | Y1 * | Y2 | Y3 | Y4 | Y5 | Y6 | CK |
|---|---|---|---|---|---|---|---|
| Plant shape | <45° | 75–90° | 45–75° | <30° | <45° | <30° | <45° |
| Leaf pubescence | no | yes | no | no | no | only front side | no |
| Leaf margin color | green | light purple | green | green | green | green | green |
| Phyllopodium color | purple | purple | purple | purple | light purple | purple | purple |
| Leaf margin toothed | yes | light cleft | yes | yes | yes | yes | yes |
| Leaf vein | green | purple at lower | green | green | purple at lower | green | green |
| Distance of top leaf lobe to 2nd lobe | larger | smaller | middle | larger | larger | larger | larger |
| Leaf crack depth | deep | deep | deep | deep | deep | deep | deep |
| Leaf flatness | intimate smooth | intimate smooth | intimate smooth | intimate smooth | intimate smooth | intimate smooth | intimate smooth |
| Leaf length (cm) | 23.9 ± 1.4 | 22.9 ± 1.4 | 28.1 ± 1.7 | 23.3 ± 1.4 | 22.2 ± 1.3 | 23.6 ± 1.4 | 23.2 ± 1.4 |
| Leaf width (cm) | 4.7 ± 0.3 | 5.3 ± 0.3 | 6.1 ± 0.4 | 4.8 ± 0.3 | 4.5 ± 0.3 | 4.8 ± 0.3 | 4.7 ± 0.3 |
| Name 1 | Total Flavonoids (mg/g) | Caffeic Acid (mg/g) | Chlorogenic Acid (mg/g) | Cichoric Acid (mg/g) | Fresh Leaf Yield (t/ha) |
|---|---|---|---|---|---|
| CK | 4.51 ± 0.25 c | 0.07 ± 0.005 c | 0.28 ± 0.02 c | 4.8 ± 0.27 b | 13.2 ± 1.14 ab |
| Y2 | 3.01 ± 0.17 d | 0.32 ± 0.02 a | 0.81 ± 0.06 a | 6.7 ± 0.38 a | 14.2 ± 1.23 a |
| Y3 | 6.61 ± 0.37 b | 0.06 ± 0.004 c | 0.49 ± 0.04 b | 3.8 ± 0.22 c | 14.9 ± 1.29 a |
| Anguo | 8.72 ± 0.48 a | 0.1 ± 0.007 b | 0.26 ± 0.02 c | 4.9 ± 0.28 b | 11.6 ± 1.01 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Li, Z.; Feng, W.; Meng, R.; Wang, X.; Wu, C. The Breeding of High-Quality Dandelions by NaCl Induced Callus Variation Combined with a Drosophila Tumor Cell Migration Test. Horticulturae 2022, 8, 1167. https://doi.org/10.3390/horticulturae8121167
Wu Z, Li Z, Feng W, Meng R, Wang X, Wu C. The Breeding of High-Quality Dandelions by NaCl Induced Callus Variation Combined with a Drosophila Tumor Cell Migration Test. Horticulturae. 2022; 8(12):1167. https://doi.org/10.3390/horticulturae8121167
Chicago/Turabian StyleWu, Zhe, Zhaojia Li, Wei Feng, Ran Meng, Xiuping Wang, and Chenxi Wu. 2022. "The Breeding of High-Quality Dandelions by NaCl Induced Callus Variation Combined with a Drosophila Tumor Cell Migration Test" Horticulturae 8, no. 12: 1167. https://doi.org/10.3390/horticulturae8121167
APA StyleWu, Z., Li, Z., Feng, W., Meng, R., Wang, X., & Wu, C. (2022). The Breeding of High-Quality Dandelions by NaCl Induced Callus Variation Combined with a Drosophila Tumor Cell Migration Test. Horticulturae, 8(12), 1167. https://doi.org/10.3390/horticulturae8121167






