Effects of LED Red and Blue Light Component on Growth and Photosynthetic Characteristics of Coriander in Plant Factory
Abstract
1. Introduction
2. Materials and Methods
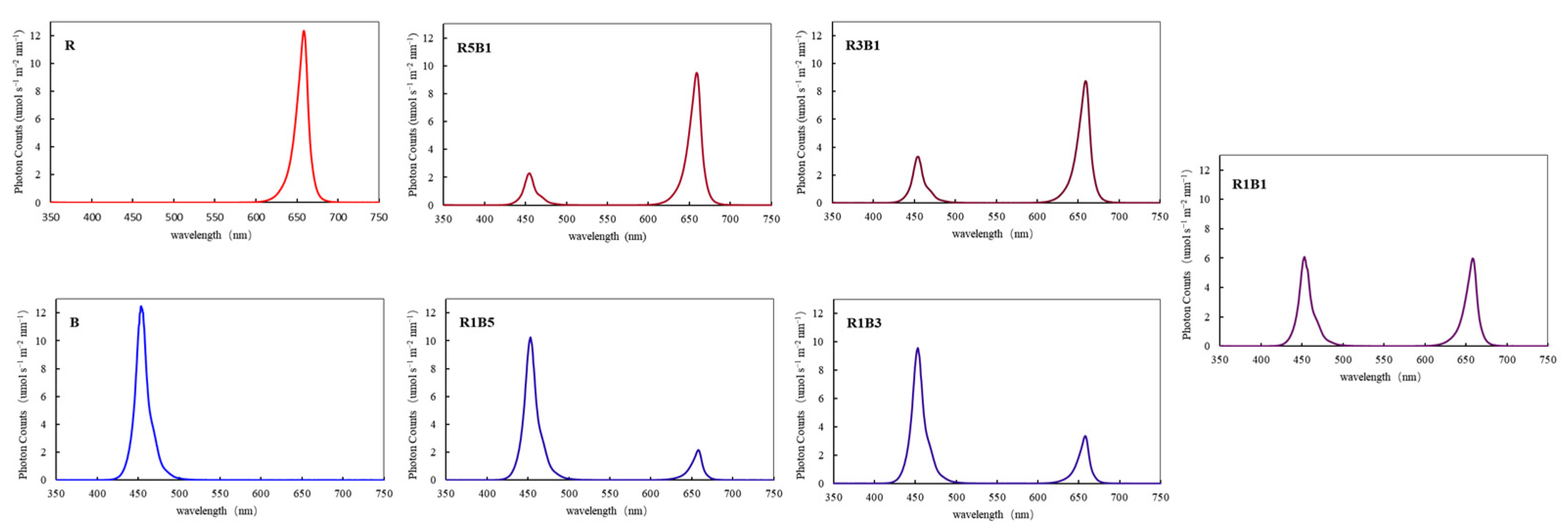
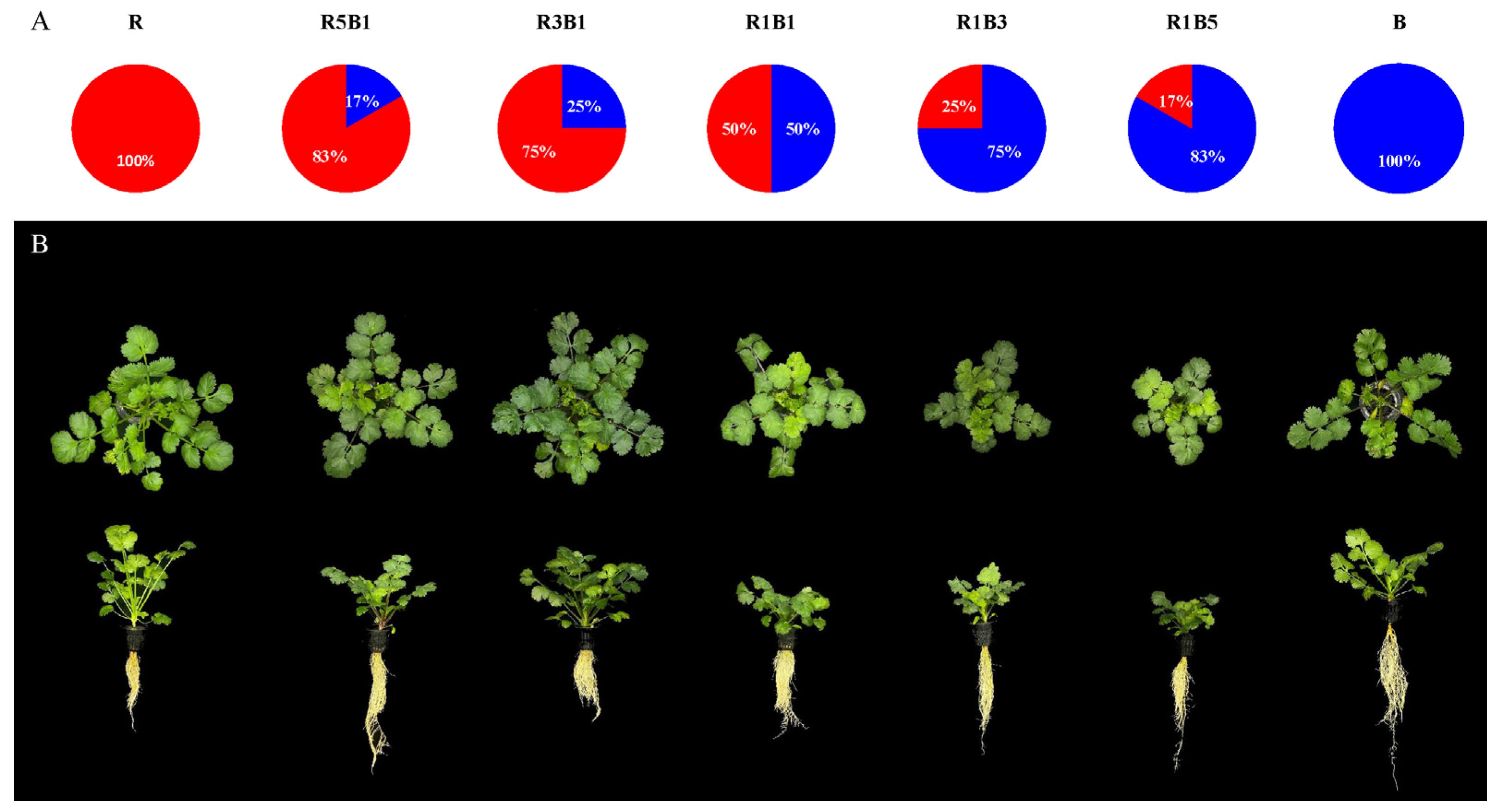
2.1. Cultivation Environment and Treatments
2.2. Plant Material and Growth Conditions
2.3. Sample Collection and Processing
2.4. Morphological Characteristics and Yield
2.5. Pigment Content
2.6. Quality Characteristics
2.7. Measurement of Physiological Characteristics
2.7.1. Stomata
2.7.2. Photosynthetic Characteristics
2.7.3. Chlorophyll a Fluorescence
2.8. Statistical Analysis
3. Results
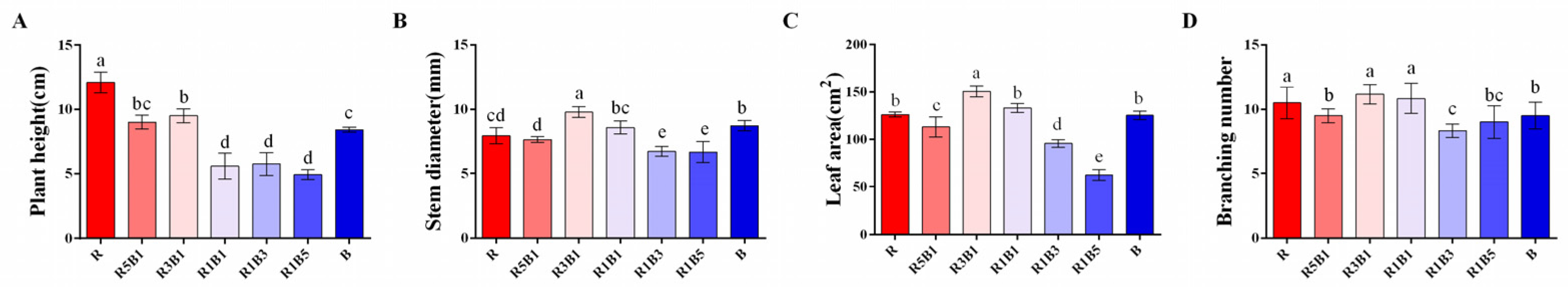
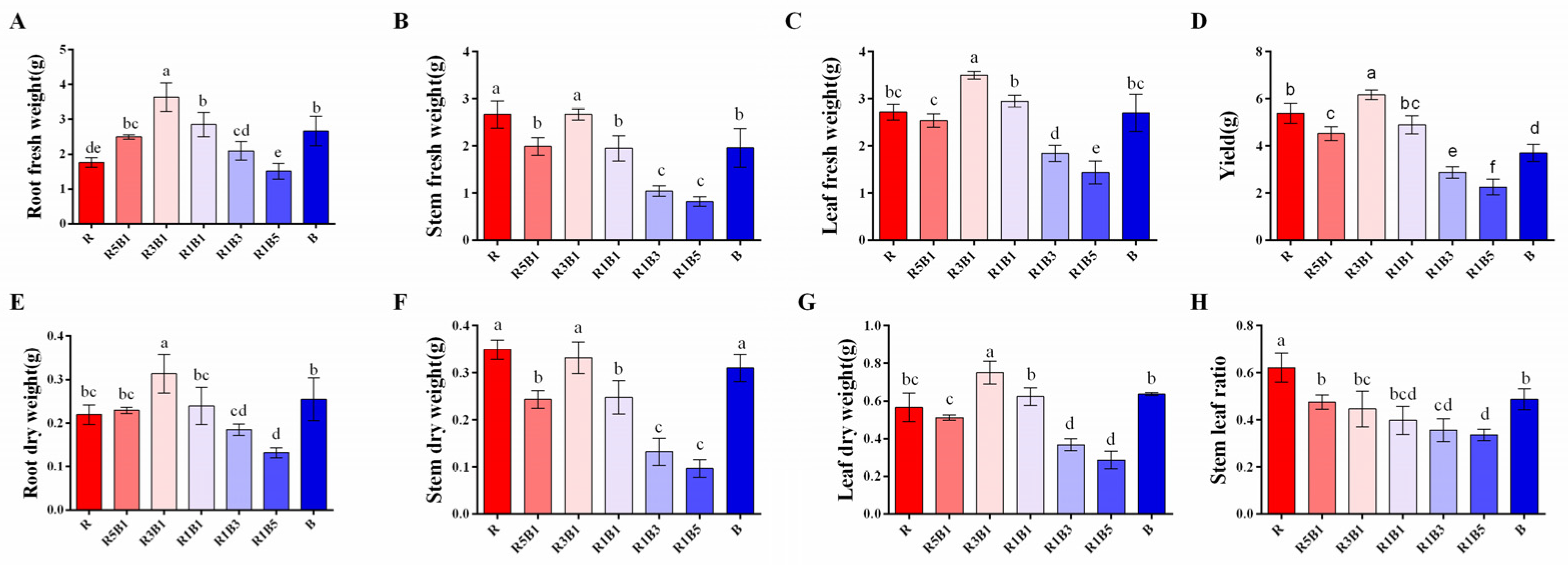
3.1. Effect of the Ratios of R to B Light on Plant Morphology and Biomass
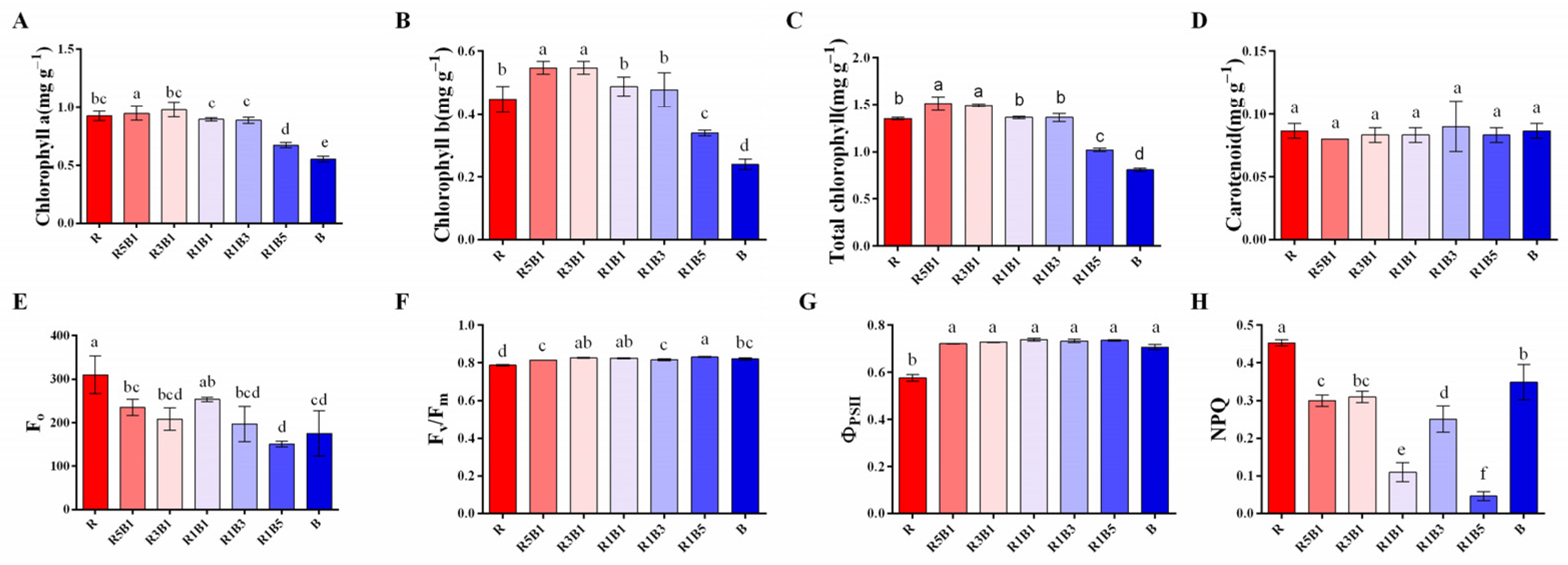
3.2. Effect of Various Ratios of R and B Light on Photosynthetic Pigments and Chlorophyll a Fluorescence
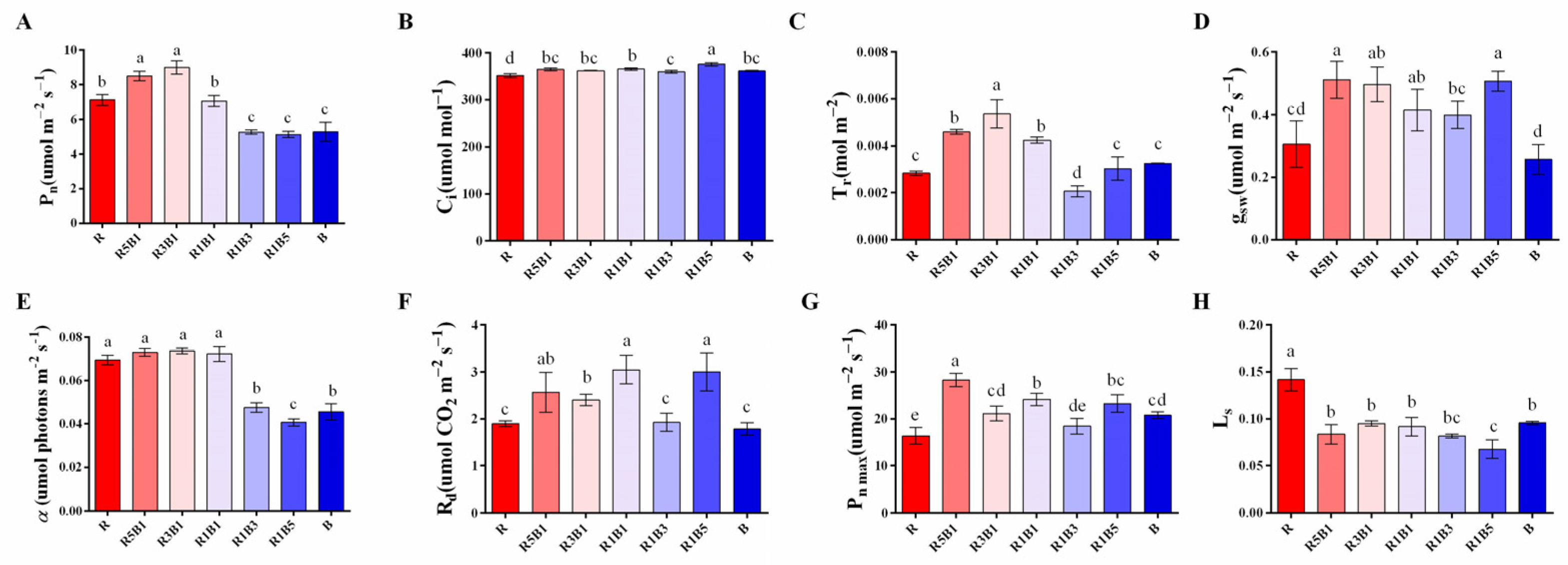
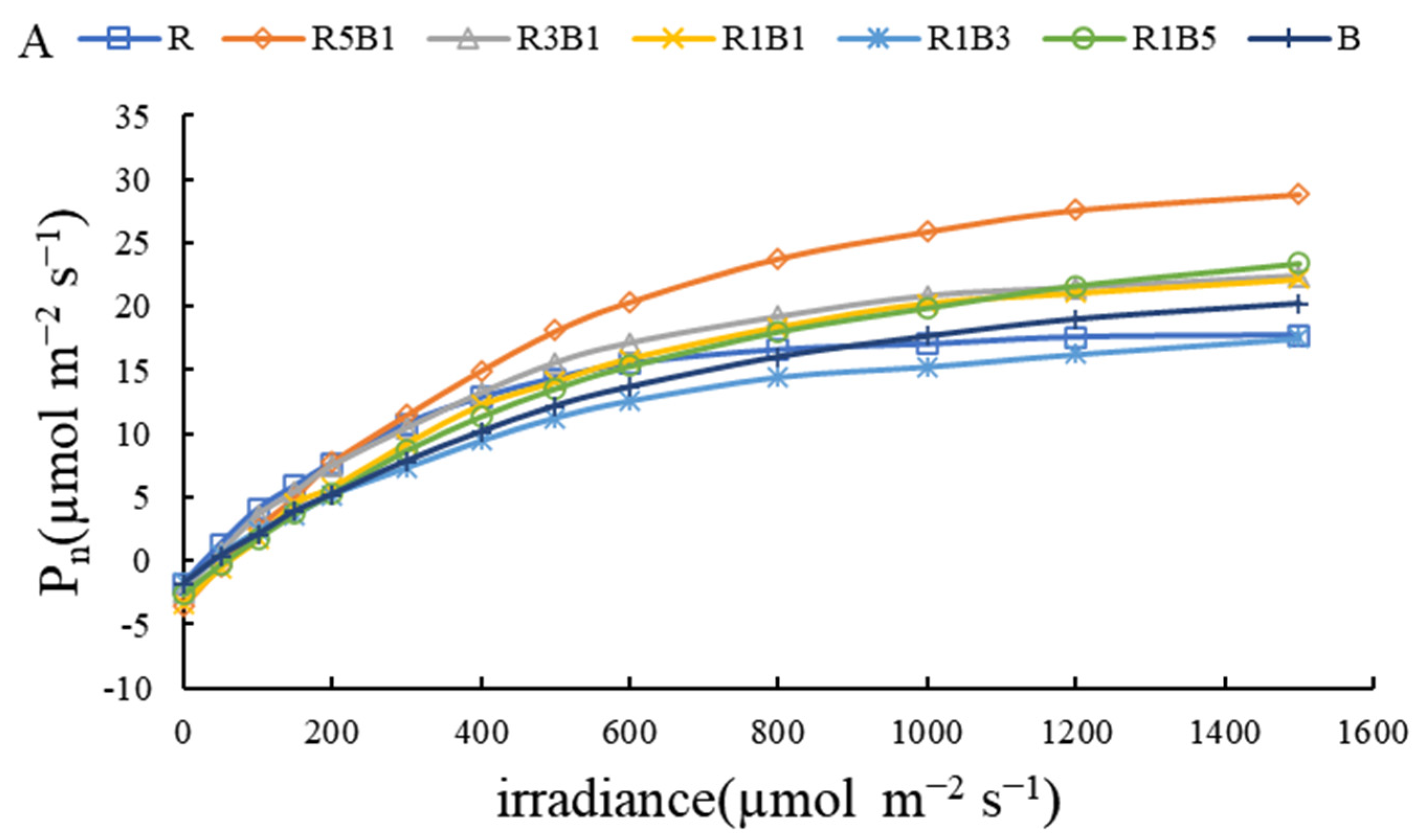
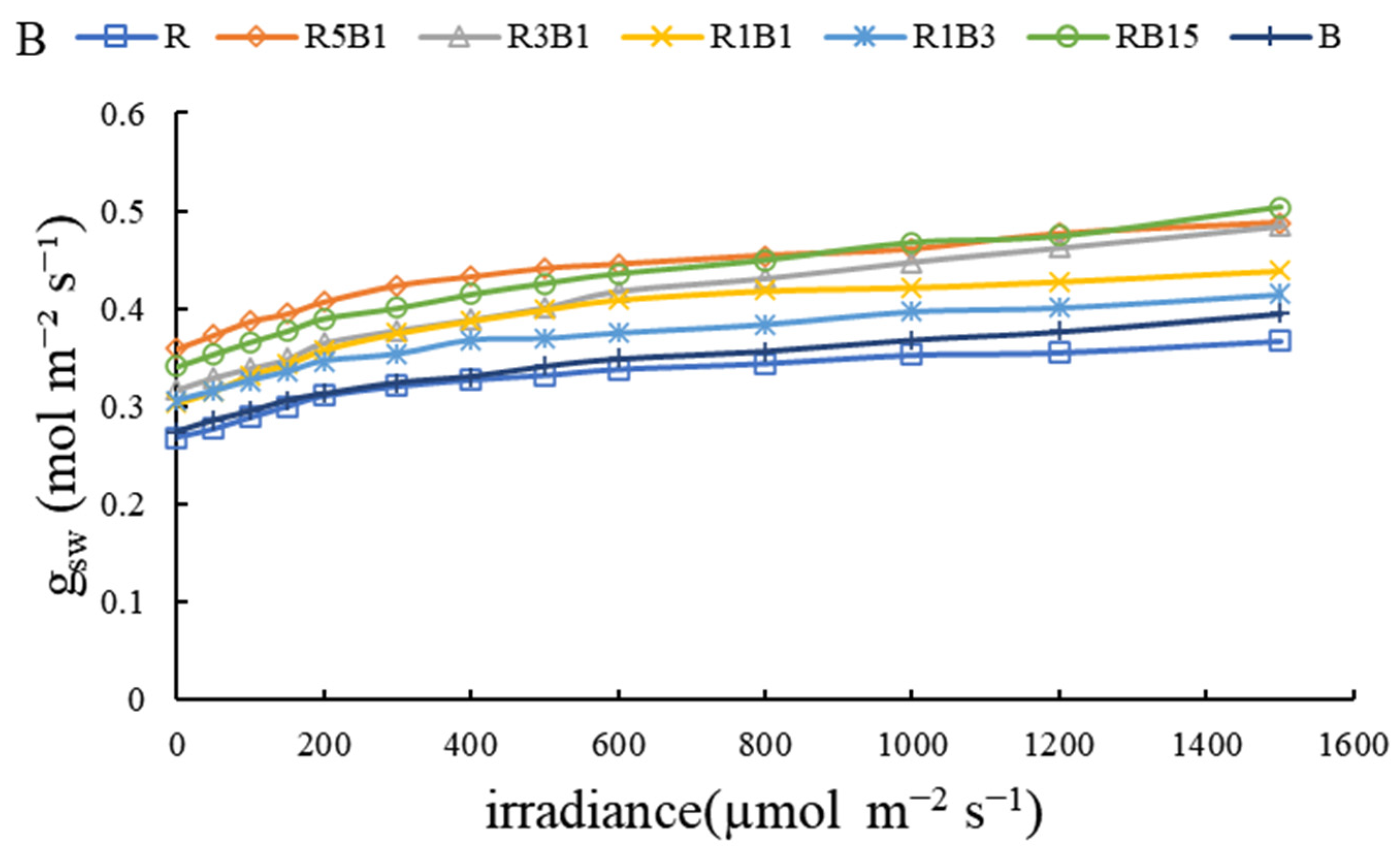
3.3. Effect of Various R and B Light Ratios on Photosynthetic Properties
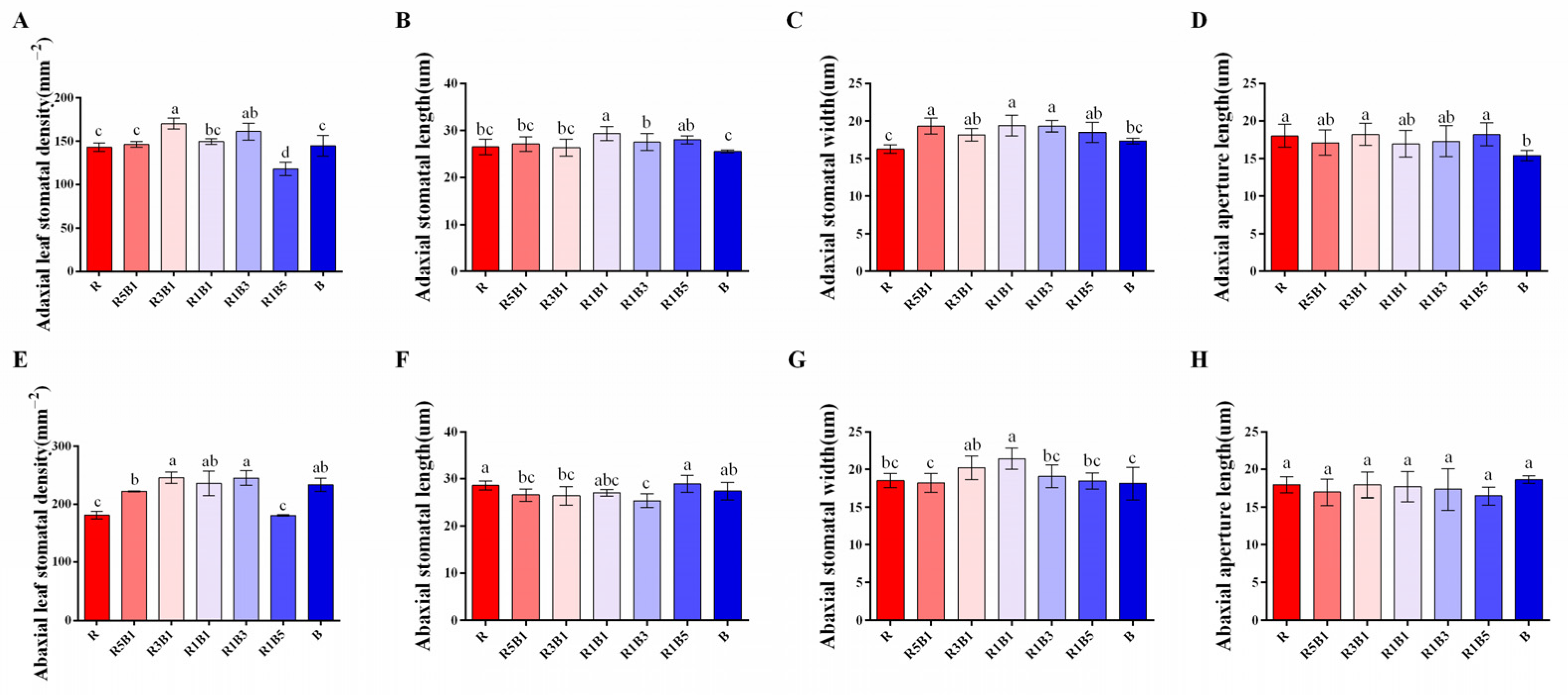
3.4. Effect of Various Ratios of R and B Light on Stomatal Development
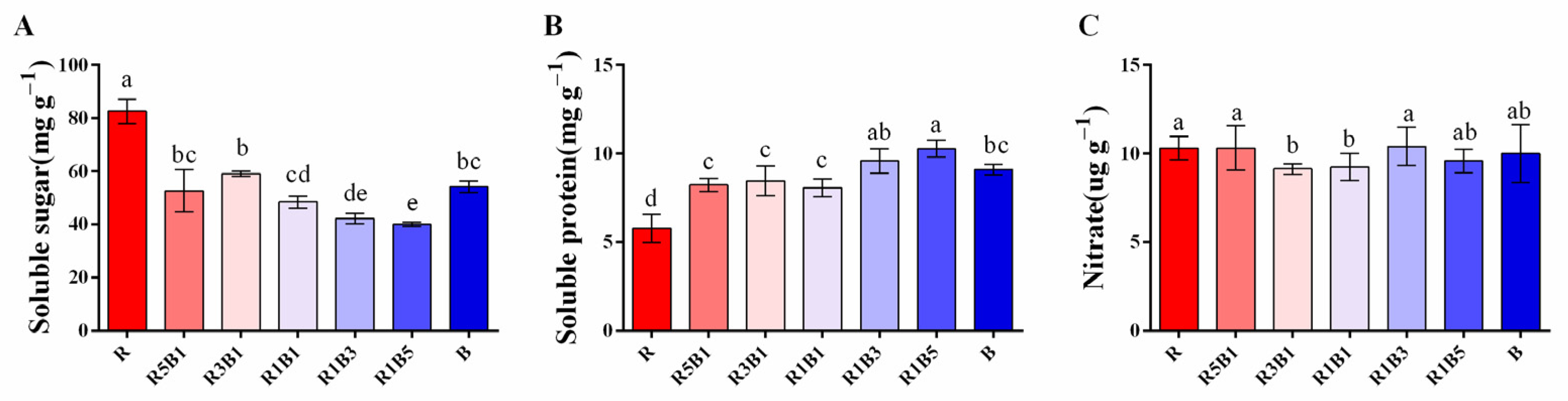
3.5. Effect of Various R and B Light Ratios on Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Treatment | Power (w s−1) | Electricity Consumption (kw h−1) |
|---|---|---|
| R | 140.5 | 2.248 |
| R5B1 | 139.2 | 2.2272 |
| R3B1 | 134.1 | 2.1456 |
| R1B1 | 129.8 | 2.0768 |
| R1B3 | 127.8 | 2.0448 |
| R1B5 | 125.4 | 2.0064 |
| B | 119.2 | 1.9072 |
References
- Bhat, S.; Kaushal, P.; Kaur, M.; Sharma, H.K. Coriander (Coriandrum sativum L.): Processing, nutritional and functional aspects. Afr. J. Plant Sci. 2014, 8, 25–33. [Google Scholar]
- Punetha, D.; Tewari, G.; Pande, C. Compositional variability in inflorescence essential oil of Coriandrum sativum from North India. J. Essent. Oil Res. 2018, 30, 113–119. [Google Scholar] [CrossRef]
- Mikhail, A.F.W.; Pereboom, M.; Utsi, L.; Hawker, J.; Lighthill, J.; Aird, H.; Swindlehurst, M.; Greig, D.R.; Jenkins, C.; Godbole, G.; et al. Utility of whole-genome sequencing during an investigation of multiple foodborne outbreaks of Shigella sonnei. Epidemiol. Infect. 2021, 149, e71. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.A.; Burns, C. Photobiology in protected horticulture. Food Energy Secur. 2016, 5, 223–238. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Fallik, E. Light quality manipulation improves vegetable quality at harvest andpostharvest: A review. Environ. Exp. Bot. 2017, 139, 79–90. [Google Scholar]
- Chen, X.-L.; Li, Y.-L.; Wang, L.-C.; Guo, W.-Z. Red and blue wavelengths affect the morphology, energy use efficiency and nutritional content of lettuce (Lactuca sativa L.). Sci. Rep. 2021, 11, 8374. [Google Scholar] [CrossRef]
- Cammarisano, L.; Donnison, I.S.; Robson, P.R.H. Producing Enhanced Yield and Nutritional Pigmentation in Lollo Rosso Through Manipulating the Irradiance, Duration, and Periodicity of LEDs in the Visible Region of Light. Front. Plant Sci. 2020, 11, 598082. [Google Scholar] [CrossRef]
- Sutulienė, R.; Laužikė, K.; Pukas, T.; Samuolienė, G. Effect of light intensity on the growth and antioxidant activity of sweet basil and lettuce. Plants 2022, 11, 1709. [Google Scholar] [CrossRef]
- Lillo, C.; Appenroth, K.J. Light regulation of nitrate reductase in higher plants: Which photoreceptors are involved? Plant Biol. 2001, 3, 455–465. [Google Scholar]
- Giliberto, L.; Perrotta, G.; Pallara, P.; Weller, J.L.; Fraser, P.D.; Bramley, P.M.; Fiore, A.; Tavazza, M.; Giuliano, G. Manipulation of the Blue Light Photoreceptor Cryptochrome 2 in Tomato Affects Vegetative Development, Flowering Time, and Fruit Antioxidant Content. Plant Physiol. 2005, 137, 199–208. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Zhao, B.; Yuan, X. Improved growth of Artemisia annua L hairy roots and artemisinin production under red light conditions. Biotechnol. Lett. 2001, 23, 1971–1973. [Google Scholar] [CrossRef]
- Lin, K.-H.; Huang, M.-Y.; Huang, W.-D.; Hsu, M.-H.; Yang, Z.-W.; Yang, C.-M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Urbonavičiūtė, A.; Pinho, P.; Samuolienė, G.; Duchovskis, P.; Vitta, P.; Stonkus, A.; Tamulaitis, G.; Žukauskas, A.; Halonen, L. Effect of short-wavelength light on lettuce growth and nutritional quality. Sodinink. Daržinink. 2007, 26, 157–165. [Google Scholar]
- McCree, K. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1972, 9, 191–216. [Google Scholar] [CrossRef]
- Evans, J. The Dependence of Quantum Yield on Wavelength and Growth Irradiance. Funct. Plant Biol. 1987, 14, 69–79. [Google Scholar] [CrossRef]
- Hoenecke, M.; Bula, R.; Tibbitts, T. Importance of ‘Blue’ Photon Levels for Lettuce Seedlings Grown under Red-light-emitting Diodes. HortScience 1992, 27, 427–430. [Google Scholar] [CrossRef]
- Tarakanov, I.G.; Tovstyko, D.A.; Lomakin, M.P.; Shmakov, A.S.; Sleptsov, N.N.; Shmarev, A.N.; Litvinskiy, V.A.; Ivlev, A.A. Effects of Light Spectral Quality on Photosynthetic Activity, Biomass Production, and Carbon Isotope Fractionation in Lettuce, Lactuca sativa L., Plants. Plants 2022, 11, 441. [Google Scholar] [CrossRef]
- Huché-Thélier, L.; Crespel, L.; Le Gourrierec, J.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue and UV radiations—Perspectives for applications in horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Wu, Q.; Su, N.; Shen, W.; Cui, J. Analyzing photosynthetic activity and growth of Solanum lycopersicum seedlings exposed to different light qualities. Acta Physiol. Plant. 2014, 36, 1411–1420. [Google Scholar] [CrossRef]
- Goins, G.D.; Yorio, N.C.; Sanwo, M.M.; Brown, C.S. Photomorphogenesis, photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LEDs) with and without supplemental blue lighting. J. Exp. Bot. 1997, 48, 1407–1413. [Google Scholar] [CrossRef]
- Muneer, S.; Kim, E.J.; Park, J.S.; Lee, J.H. Influence of Green, Red and Blue Light Emitting Diodes on Multiprotein Complex Proteins and Photosynthetic Activity under Different Light Intensities in Lettuce Leaves (Lactuca sativa L.). Int. J. Mol. Sci. 2014, 15, 4657–4670. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.-X.; Wang, X.-Z.; Gao, L.-H.; Chen, Q.-Y.; Qu, M. Blue light is more essential than red light for maintaining the activities of photosystem II and I and photosynthetic electron transport capacity in cucumber leaves. J. Integr. Agric. 2016, 15, 87–100. [Google Scholar] [CrossRef]
- Su, N.; Wu, Q.; Shen, Z.; Xia, K.; Cui, J. Effects of light quality on the chloroplastic ultrastructure and photosynthetic characteristics of cucumber seedlings. Plant Growth Regul. 2013, 73, 227–235. [Google Scholar] [CrossRef]
- Kochetova, G.; Belyaeva, O.B.; Gorshkova, D.S.; Vlasova, T.A.; Bassarskaya, E.M.; Zhigalova, T.V.; Avercheva, O.V. Long-term acclimation of barley photosynthetic apparatus to narrow-band red and blue light. Photosynthetica 2017, 56, 851–860. [Google Scholar] [CrossRef]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Biochim. Biophys. Acta (BBA) Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef]
- Zheng, Y.-J.; Zhang, Y.-T.; Liu, H.-C.; Li, Y.-M.; Liu, Y.-L.; Hao, Y.-W.; Lei, B.-F. Supplemental blue light increases growth and quality of greenhouse pak choi depending on cultivar and supplemental light intensity. J. Integr. Agric. 2018, 17, 2245–2256. [Google Scholar] [CrossRef]
- Yanagi, T.; Okamoto, K.; Takita, S. Effects of Blue, Red, and Blue/Red Lights of Two Different Ppf Levels on Growth and Morphogenesis of Lettuce Plants. Acta Hortic. 1996, 440, 117–122. [Google Scholar] [CrossRef]
- Hogewoning, S.; Trouwborst, G.; Maljaars, H.; Poorter, H.; Van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Kitayama, M.; Lu, N.; Takagaki, M. Improving secondary metabolite accumulation, mineral content, and growth of coriander (Coriandrum sativum L.) by regulating light quality in a plant factory. J. Hortic. Sci. Biotechnol. 2020, 95, 356–363. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.; Liu, C.; Shi, Q.; Yang, F.; Wei, M. Effects of red and blue light on leaf anatomy, CO2 assimilation and the photosynthetic electron transport capacity of sweet pepper (Capsicum annuum L.) seedlings. BMC Plant Biol. 2020, 20, 318. [Google Scholar] [CrossRef]
- Klein, S.; Fiebig, A.; Noga, G.; Hunsche, M. Influence of light quality on leaf physiology of sweet pepper plants grown under drought. Theor. Exp. Plant Physiol. 2018, 30, 287–296. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, O.K. Blue Light added with Red LEDs Enhance Growth Characteristics, Pigments Content, and Antioxidant Capacity in Lettuce, Spinach, Kale, Basil, and Sweet Pepper in a Controlled Environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Caferri, R.; Bassi, R. Plants and water in a changing world: A physiological and ecological perspective. Rend. Lincei Sci. Fis. Nat. 2022, 33, 479–487. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, J.; Zou, H.; Zhang, L.; Li, S.; Wang, Y. The combination of blue and red LED light improves growth and phenolic acid contents in Salvia miltiorrhiza Bunge. Ind. Crop. Prod. 2020, 158, 112959. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the Role of Red:Blue LED Lights on Resource Use Efficiency and Nutritional Properties of Indoor Grown Sweet Basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Spalholz, H.; Perkins-Veazie, P.; Hernández, R. Impact of sun-simulated white light and varied blue: Red spectrums on the growth, morphology, development, and phytochemical content of green-and red-leaf lettuce at different growth stages. Sci. Hortic. 2020, 264, 109195. [Google Scholar] [CrossRef]
- Liang, Y.; Kang, C.; Kaiser, E.; Kuang, Y.; Yang, Q.; Li, T. Red/blue light ratios induce morphology and physiology alterations differently in cucumber and tomato. Sci. Hortic. 2021, 281, 109995. [Google Scholar] [CrossRef]
- Naznin, M.; Lefsrud, M.; Gravel, V.; Hao, X. Different ratios of red and blue LED light effects on coriander productivity and antioxidant properties. Acta Hortic. 2016, 1134, 223–230. [Google Scholar] [CrossRef]
- McAusland, L.; Lim, M.-T.; Morris, D.E.; Smith-Herman, H.L.; Mohammed, U.; Hayes-Gill, B.R.; Crowe, J.A.; Fisk, I.D.; Murchie, E.H. Growth Spectrum Complexity Dictates Aromatic Intensity in Coriander (Coriandrum sativum L.). Front. Plant Sci. 2020, 11, 462. [Google Scholar] [CrossRef]
- Asao, T.; Asaduzzaman, M.; Mondal, F.; Tokura, M.; Adachi, F.; Ueno, M.; Kawaguchi, M.; Yano, S.; Ban, T. Impact of reduced potassium nitrate concentrations in nutrient solution on the growth, yield and fruit quality of melon in hydroponics. Sci. Hortic. 2013, 164, 221–231. [Google Scholar] [CrossRef]
- Roell, M.-S.; von Borzyskowski, L.S.; Westhoff, P.; Plett, A.; Paczia, N.; Claus, P.; Schlueter, U.; Erb, T.J.; Weber, A.P. A synthetic C4 shuttle via the β-hydroxyaspartate cycle in C3 plants. Proc. Natl. Acad. Sci. USA 2021, 118, e2022307118. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, J.; Jandova, V.; Macek, M.; Liancourt, P. Contrasting biomass allocation responses across ontogeny and stress gradients reveal plant adaptations to drought and cold. Funct. Ecol. 2020, 35, 32–42. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Song, S.W.; Liao, G.X.; Liu, H.C.; Sun, G.W.; Chen, R.Y. Effect of Ammonium and Nitrate Ratio on Nutritional Quality of Chinese Kale. Adv. Mater. Res. 2012, 461, 13–16. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Savvides, A.; Fanourakis, D.; van Ieperen, W. Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves. J. Exp. Bot. 2011, 63, 1135–1143. [Google Scholar] [CrossRef]
- Xu, J.; Peng, S.; Wei, Z.; Jiao, X. Intercellular CO2 concentration and stomatal or non-stomatal limitation of rice under water saving irrigation. Trans. Chin. Soc. Agric. Eng. 2010, 26, 76–80. [Google Scholar]
- Ye, Z.P.; Suggett, D.J.; Robakowski, P.; Kang, H.J. A mechanistic model for the photosynthesis–light response based on the photosynthetic electron transport of photosystem II in C3 and C4 species. New Phytol. 2013, 199, 110–120. [Google Scholar] [CrossRef]
- Ko, S.-S.; Jhong, C.-M.; Shih, M.-C. Blue Light Acclimation Reduces the Photoinhibition of Phalaenopsis aphrodite (Moth Orchid). Int. J. Mol. Sci. 2020, 21, 6167. [Google Scholar] [CrossRef]
- Warner, R.M. Applied Statistics: From Bivariate through Multivariate Techniques; Sage Publications: Washington, DC, USA, 2012. [Google Scholar]
- Arena, C.; Tsonev, T.; Doneva, D.; De Micco, V.; Michelozzi, M.; Brunetti, C.; Centritto, M.; Fineschi, S.; Velikova, V.; Loreto, F. The effect of light quality on growth, photosynthesis, leaf anatomy and volatile isoprenoids of a monoterpene-emitting herbaceous species (Solanum lycopersicum L.) and an isoprene-emitting tree (Platanus orientalis L.). Environ. Exp. Bot. 2016, 130, 122–132. [Google Scholar] [CrossRef]
- LaNoue, J.; Leonardos, E.D.; Grodzinski, B. Effects of Light Quality and Intensity on Diurnal Patterns and Rates of Photo-Assimilate Translocation and Transpiration in Tomato Leaves. Front. Plant Sci. 2018, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, R.; Ohashi-Kaneko, K.; Fujiwara, K.; Goto, E.; Kurata, K. Photosynthetic Characteristics of Rice Leaves Grown under Red Light with or without Supplemental Blue Light. Plant Cell Physiol. 2004, 45, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Ouzounis, T.; Fretté, X.; Rosenqvist, E.; Ottosen, C.-O. Spectral effects of supplementary lighting on the secondary metabolites in roses, chrysanthemums, and campanulas. J. Plant Physiol. 2014, 171, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Anuchai, J.; Hsieh, C.-H. Effect of Change in Light Quality on Physiological Transformation of in vitro Phalaenopsis ‘Fortune Saltzman’ Seedlings during the Growth Period. Hortic. J. 2017, 86, 395–402. [Google Scholar] [CrossRef]
- Li, Y.; Xin, G.; Wei, M.; Shi, Q.; Yang, F.; Wang, X. Carbohydrate accumulation and sucrose metabolism responses in tomato seedling leaves when subjected to different light qualities. Sci. Hortic. 2017, 225, 490–497. [Google Scholar] [CrossRef]
- Neff, M.M.; Fankhauser, C.; Chory, J. Light: An indicator of time and place. Genes Dev. 2000, 14, 257–271. [Google Scholar] [CrossRef]
- Ian, Z.H.; Bo LE, I.; Cheng, R.F.; Yu WA, N.G.; Tao, L.I.; Yang, Q.C. Selenium distribution and nitrate metabolism in hydroponic lettuce (Lactuca sativa L.): Effects of selenium forms and light spectra. J. Integr. Agric. 2020, 19, 133–144. [Google Scholar]
- Mengxi, L.; Zhigang, X.; Yang, Y.; Yijie, F. Effects of different spectral lights on Oncidium PLBs induction, proliferation, and plant regeneration. Plant Cell Tissue Organ Cult. (PCTOC) 2010, 106, 1–10. [Google Scholar] [CrossRef]
- Riikonen, J.; Kettunen, N.; Gritsevich, M.; Hakala, T.; Särkkä, L.; Tahvonen, R. Growth and development of Norway spruce and Scots pine seedlings under different light spectra. Environ. Exp. Bot. 2016, 121, 112–120. [Google Scholar] [CrossRef]
- Guo, X.; Xue, X.; Chen, L.; Li, J.; Wang, Z.; Zhang, Y. Effects of LEDs Light Spectra on the Growth, Yield, and Quality of Winter Wheat (Triticum aestivum L.) Cultured in Plant Factory. J. Plant Growth Regul. 2022, 1–15. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Zhang, L.; Kato, M.; Yamawaki, K.; Kiriiwa, Y.; Yahata, M.; Ikoma, Y.; Matsumoto, H. Effect of Blue and Red LED Light Irradiation on β-Cryptoxanthin Accumulation in the Flavedo of Citrus Fruits. J. Agric. Food Chem. 2011, 60, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.D.; Barman, M.; Mitra, A. Photosynthetic apparatus plays a central role in photosensitive physiological acclimations affecting spinach (Spinacia oleracea L.) growth in response to blue and red photon flux ratios. Environ. Exp. Bot. 2018, 156, 170–182. [Google Scholar] [CrossRef]
- Wang, X.Y.; Xu, X.M.; Cui, J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. Photosynthetica 2014, 53, 213–222. [Google Scholar] [CrossRef]
- Huang, B.; Lin, B.; Li, C.; Liu, X.; Liao, Z.; Liu, Y.; Fan, C. Effects of LED light quality on growth and photosynthetic physiological characteristics in spinach. J. Fujian Agric. For. Univ. (Nat. Sci. Ed.) 2018, 47, 403–408. [Google Scholar]
- Schreiber, U. Chlorophyll fluorescence yield changes as a tool in plant physiology I. The measuring system. Photosynth. Res. 1983, 4, 361–373. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Rinderle, U. The Role of Chlorophyll Fluorescence in The Detection of Stress Conditions in Plants. CRC Crit. Rev. Anal. Chem. 1988, 19, S29–S85. [Google Scholar] [CrossRef]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Mann, M. Mass Spectrometric Sequencing of Proteins from Silver-Stained Polyacrylamide Gels. Anal. Chem. 1996, 68, 850–858. [Google Scholar] [CrossRef]
- Inada, K. Action spectra for photosynthesis in higher plants. Plant Cell Physiol. 1976, 17, 355–365. [Google Scholar] [CrossRef]
- Urban, O.; Hlaváčová, M.; Klem, K.; Novotná, K.; Rapantová, B.; Smutná, P.; Horáková, V.; Hlavinka, P.; Škarpa, P.; Trnka, M. Combined effects of drought and high temperature on photosynthetic characteristics in four winter wheat genotypes. Field Crop. Res. 2018, 223, 137–149. [Google Scholar] [CrossRef]
- Lee, G.; Yoon, J.; Lee, M. Effects of irradiation quantities of mixed red and blue LEDs on the growth, total flavonoids and phenolic compound contents of leaf lettuce grown in plant factory. Acta Hortic. 2014, 1107, 245–250. [Google Scholar] [CrossRef]
- Zhang, Y.; Kaiser, E.; Zhang, Y.; Yang, Q.; Li, T. Red/blue light ratio strongly affects steady-state photosynthesis, but hardly affects photosynthetic induction in tomato (Solanum lycopersicum). Physiol. Plant. 2019, 167, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Di, Q.; Li, J.; Du, Y.; Wei, M.; Shi, Q.; Li, Y.; Yang, F. Combination of Red and Blue Lights Improved the Growth and Development of Eggplant (Solanum melongena L.) Seedlings by Regulating Photosynthesis. J. Plant Growth Regul. 2020, 40, 1477–1492. [Google Scholar] [CrossRef]
- Wada, M.; Kagawa, T.; Sato, Y. Chloroplast movement. Annu. Rev. Plant Biol. 2003, 54, 455–468. [Google Scholar] [CrossRef]
- Loreto, F.; Tsonev, T.; Centritto, M. The impact of blue light on leaf mesophyll conductance. J. Exp. Bot. 2009, 60, 2283–2290. [Google Scholar] [CrossRef]
- Xu, W.; Liu, X.; Jiao, X.; Xu, Z. Effect of blue light quantity on growth and quality of lettuce. J. Nanjing Agric. Univ. 2015, 38, 890–895. [Google Scholar]
- Matthews, J.S.A.; Vialet-Chabrand, S.; Lawson, T. Role of blue and red light in stomatal dynamic behaviour. J. Exp. Bot. 2020, 71, 2253–2269. [Google Scholar] [CrossRef]
- Shimazaki, K.-I.; Doi, M.; Assmann, S.M.; Kinoshita, T. Light Regulation of Stomatal Movement. Annu. Rev. Plant Biol. 2007, 58, 219–247. [Google Scholar] [CrossRef]
- Lawson, T. Guard cell photosynthesis and stomatal function. New Phytol. 2008, 181, 13–34. [Google Scholar] [CrossRef]
- Zeiger, E.; Talbott, L.D.; Frechilla, S.; Srivastava, A.; Zhu, J. The guard cell chloroplast: A perspective for the twenty-first century. N. Phytol. 2002, 153, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D.; Raschke, K. Effect of Light Quality on Stomatal Opening in Leaves of Xanthium strumarium L. Plant Physiol. 1981, 68, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wei, H.; Jeong, B.R. Lighting direction affects leaf morphology, stomatal characteristics, and physiology of head lettuce (Lactuca sativa L.). Int. J. Mol. Sci. 2021, 22, 3157. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kim, J. Light Quality Affects Water Use of Sweet Basil by Changing Its Stomatal Development. Agronomy 2021, 11, 303. [Google Scholar] [CrossRef]
- Boccalandro, H.E.; Giordano, C.; Ploschuk, E.L.; Piccoli, P.N.; Bottini, R.; Casal, J. Phototropins but Not Cryptochromes Mediate the Blue Light-Specific Promotion of Stomatal Conductance, while both Enhance Photosynthesis and Transpiration under Full Sunlight. Plant Physiol. 2011, 158, 1475–1484. [Google Scholar] [CrossRef]
- Pillitteri, L.J.; Torii, K.U. Mechanisms of Stomatal Development. Annu. Rev. Plant Biol. 2012, 63, 591–614. [Google Scholar] [CrossRef]
- Kim, S.-J.; Hahn, E.-J.; Heo, J.-W.; Paek, K.-Y. Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci. Hortic. 2003, 101, 143–151. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jung, J.-H.; Park, C.-M. Light Inhibits COP1-Mediated Degradation of ICE Transcription Factors to Induce Stomatal Development in Arabidopsis. Plant Cell 2017, 29, 2817–2830. [Google Scholar] [CrossRef]
- Liu, T.D.; Zhang, X.W.; Xu, Y. Influence of red light on the expression of genes on stomatal formation in maize seedlings. Can. J. Plant Sci. 2020, 100, 296–303. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Q.; Liao, Q.; Li, Q.; Yang, Q.; Wang, F.; Li, J. Effects of LED Red and Blue Light Component on Growth and Photosynthetic Characteristics of Coriander in Plant Factory. Horticulturae 2022, 8, 1165. https://doi.org/10.3390/horticulturae8121165
Gao Q, Liao Q, Li Q, Yang Q, Wang F, Li J. Effects of LED Red and Blue Light Component on Growth and Photosynthetic Characteristics of Coriander in Plant Factory. Horticulturae. 2022; 8(12):1165. https://doi.org/10.3390/horticulturae8121165
Chicago/Turabian StyleGao, Qi, Qiuhong Liao, Qingming Li, Qichang Yang, Fang Wang, and Jianming Li. 2022. "Effects of LED Red and Blue Light Component on Growth and Photosynthetic Characteristics of Coriander in Plant Factory" Horticulturae 8, no. 12: 1165. https://doi.org/10.3390/horticulturae8121165
APA StyleGao, Q., Liao, Q., Li, Q., Yang, Q., Wang, F., & Li, J. (2022). Effects of LED Red and Blue Light Component on Growth and Photosynthetic Characteristics of Coriander in Plant Factory. Horticulturae, 8(12), 1165. https://doi.org/10.3390/horticulturae8121165








