Agrivoltaic System and Modelling Simulation: A Case Study of Soybean (Glycine max L.) in Italy
Abstract
1. Introduction
2. Materials and Methods
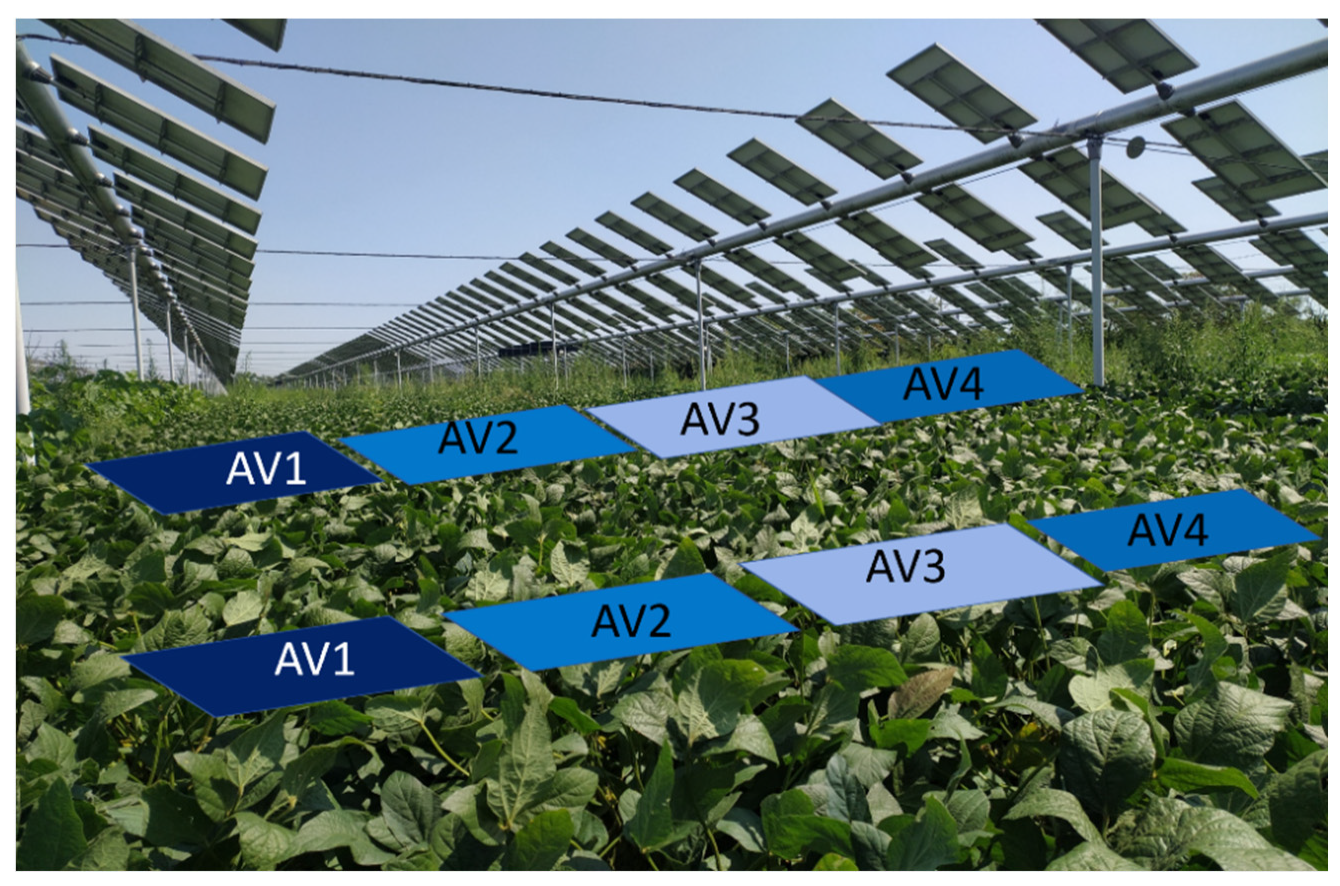
2.1. Study Area and Experimental Design
2.2. Agronomic Management
2.3. Field Data Collection
2.3.1. Crop Height
2.3.2. SPAD Chlorophyll Content
2.3.3. Leaf Area Index (LAI) and Specific Leaf Area (SLA)
2.3.4. Crop Yield Parameter: Fresh and Dry Weight of Pods
2.4. Simulations
2.5. Statistical Analysis
3. Results
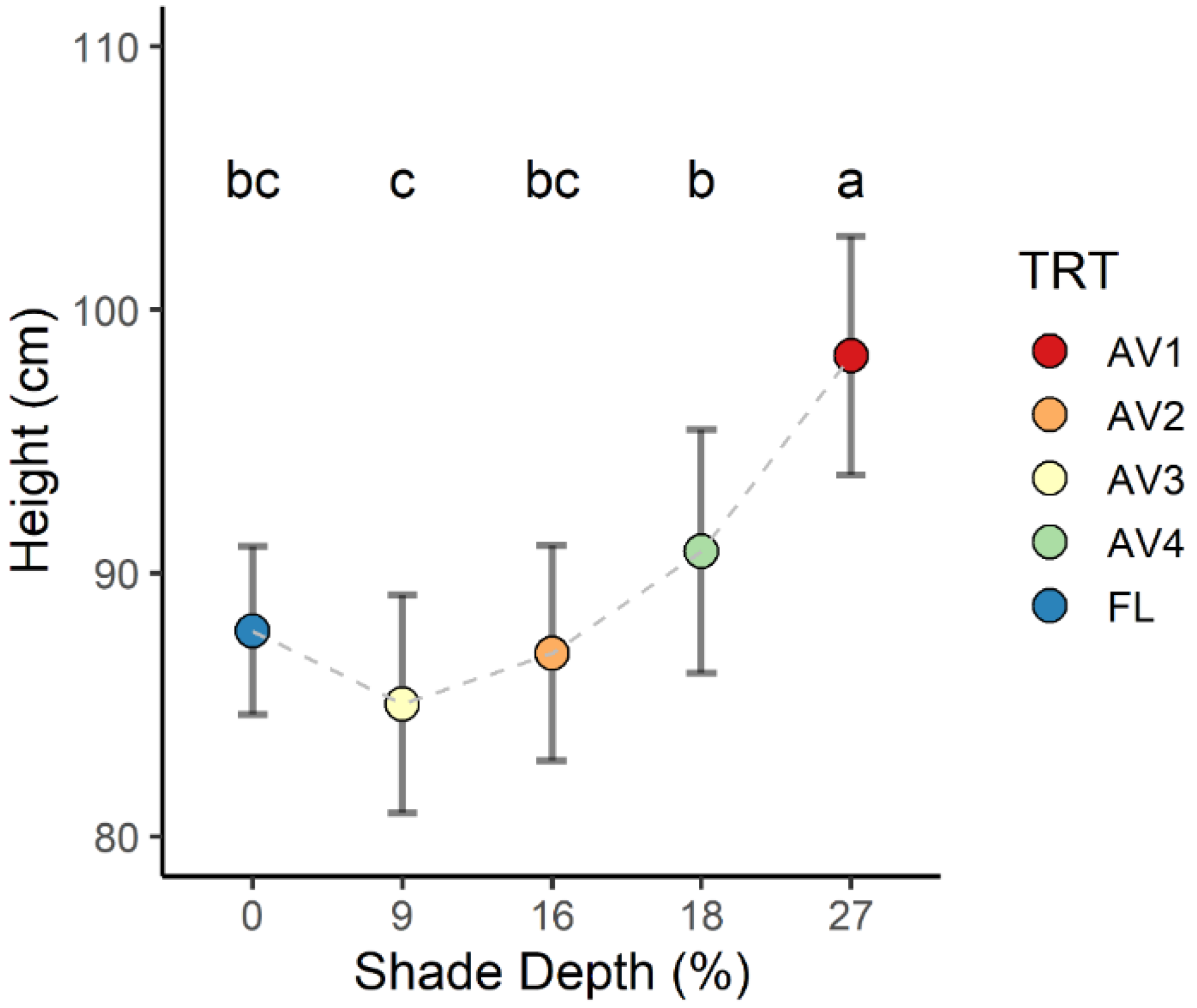
3.1. Crop Height
3.2. SPAD Chlorophyll Content
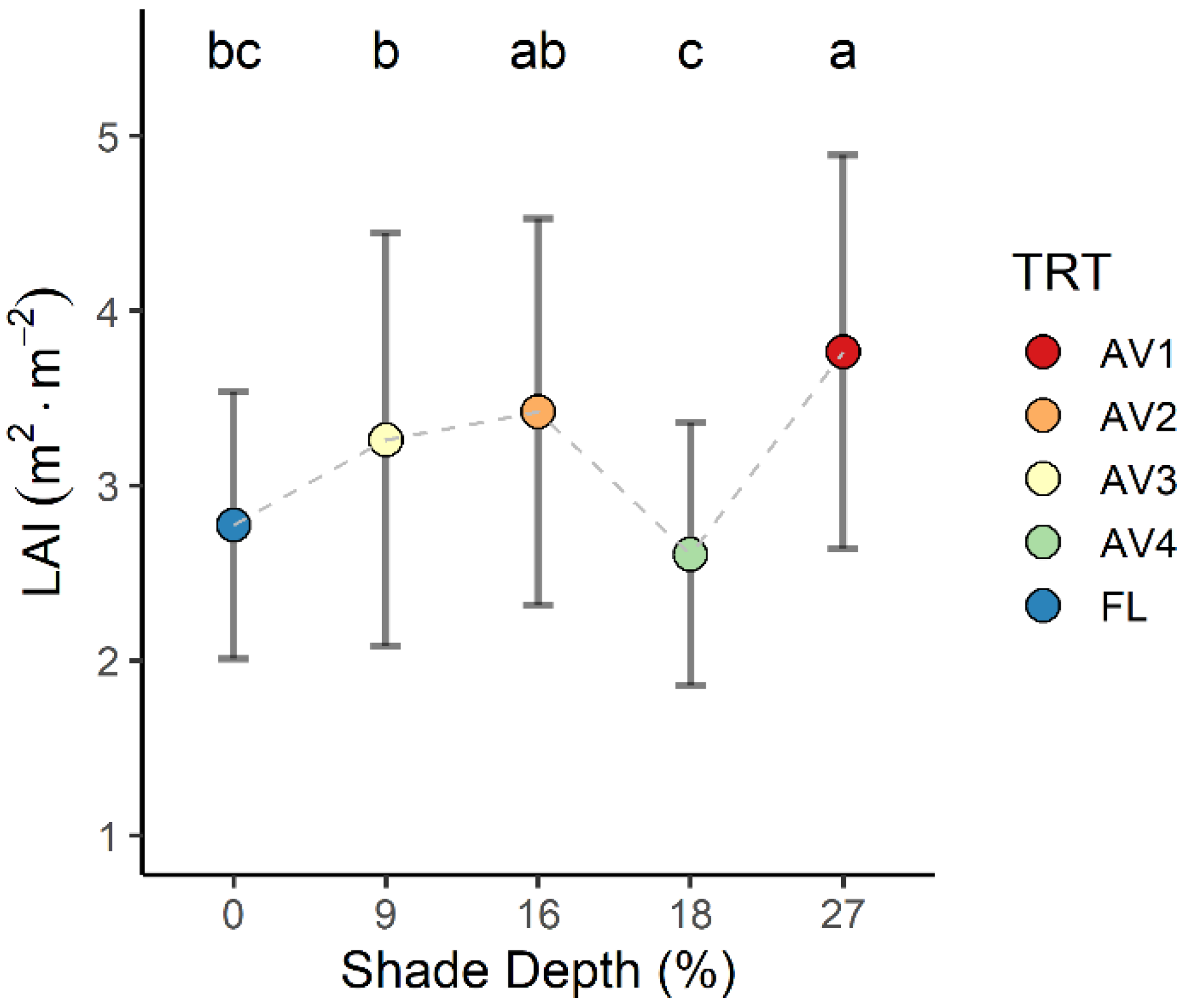
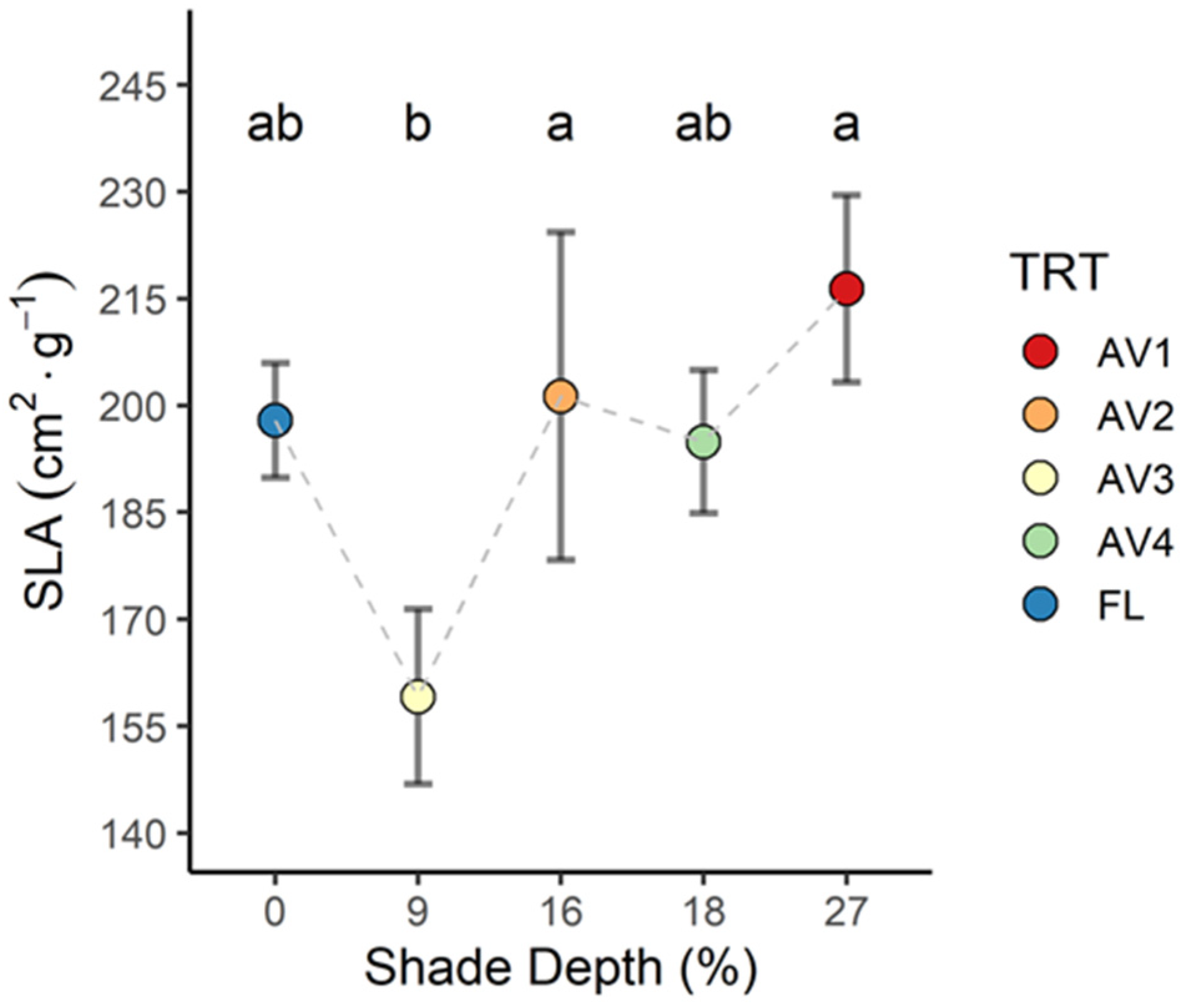
3.3. Leaf Area Index and Specific Leaf Area
3.4. Crop Yield Parameters
3.5. Modelling Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- General Assembly of the United Nations. Available online: https://www.un.org/en/ga/ (accessed on 22 September 2022).
- United Nations. Peace, Dignity and Equality on a Healthy Planet. Available online: https://www.un.org/en (accessed on 22 September 2022).
- International Renewable Energy Agency. Renewable Energy Statistics. Available online: https://www.irena.org/publications/2022/Jul/Renewable-Energy-Statistics-2022 (accessed on 15 September 2022).
- Van de Ven, D.J.; Capellan-Peréz, I.; Arto, I.; Cazcarro, I.; de Castro, C.; Patel, P.; Gonzalez-Eguino, M. The potential land requirements and related land use change emissions of solar energy. Sci. Rep. 2021, 11, 2907. [Google Scholar] [CrossRef] [PubMed]
- Dupraz, C.; Marrou, H.; Talbot, G.; Dufour, L.; Nogier, A.; Ferard, Y. Combining solar photovoltaic panels and food crops for optimising land use: Towards new agrivoltaic schemes. Renew. Energy 2011, 36, 2725–2732. [Google Scholar] [CrossRef]
- Amaducci, S.; Yin, X.; Colauzzi, M. Agrivoltaic systems to optimise land use for electric energy production. Appl. Energy 2018, 220, 545–561. [Google Scholar] [CrossRef]
- Campana, P.E.; Stridh, B.; Amaducci, S.; Colauzzi, M. Optimisation of vertically mounted agrivoltaic systems. J. Clean. Prod. 2021, 325, 129091. [Google Scholar] [CrossRef]
- Trommsdorff, M.; Kang, J.; Reise, C.; Schindele, S.; Bopp, G.; Ehmann, A.; Weselek, A.; Högy, P.; Obergfell, T. Combining food and energy production: Design of an agrivoltaic system applied in arable and vegetable farming in Germany. Renew. Sustain. Energy Rev. 2021, 140, 110694. [Google Scholar] [CrossRef]
- Giri, N.C.; Mohanty, R.C. Agrivoltaic system: Experimental analysis for enhancing land productivity and revenue of farmers. Energy Sustain. Dev. 2022, 70, 54–61. [Google Scholar] [CrossRef]
- Barron-Gafford, G.A.; Pavao-Zuckerman, M.A.; Minor, R.L.; Sutter, L.F.; Barnett-Moreno, I.; Blackett, D.T.; Thompson, M.; Dimond, K.; Gerlak, A.K.; Nabhan, G.P.; et al. Agrivoltaics provide mutual benefits across the food–energy–water nexus in drylands. Nat. Sustain. 2019, 2, 848–855. [Google Scholar] [CrossRef]
- Bedbabis, S.; Ben Rouina, B.; Boukhris, M.; Ferrara, G. Effects of irrigation with treated wastewater on root and fruit mineral elements of Chemlali olive cultivar. Sci. World J. 2014, 2014, 973638. [Google Scholar] [CrossRef]
- Boselli, M.; Bahouaoui, M.A.; Lachhab, N.; Sanzani, S.M.; Ferrara, G.; Ippolito, A. Protein hydrolysates effects on grapevine (Vitis vinifera L.; cv. Corvina) performance and water stress tolerance. Sci. Hortic. 2019, 258, 108784. [Google Scholar] [CrossRef]
- Goetzberger, A.; Zastrow, A. On the Coexistence of Solar-Energy Conversion and Plant Cultivation. Int. J. Sol. Energy 1982, 1, 55–69. [Google Scholar] [CrossRef]
- Agostini, A.; Colauzzi, M.; Amaducci, S. Innovative agrivoltaic systems to produce sustainable energy: An economic and environmental assessment. Appl. Energy 2021, 281, 116102. [Google Scholar] [CrossRef]
- Marrou, H.; Wery, J.; Dufour, L.; Dupraz, C. Productivity and radiation use efficiency of lettuces grown in the partial shade of photovoltaic panels. Eur. J. Agron. 2013, 44, 54–66. [Google Scholar] [CrossRef]
- Sekiyama, T.; Nagashima, A. Solar Sharing for Both Food and Clean Energy Production: Performance of Agrivoltaic Systems for Corn, A Typical Shade-Intolerant Crop. Environments 2019, 6, 65. [Google Scholar] [CrossRef]
- Schindele, S.; Trommsdorff, M.; Schlaak, A.; Obergfell, T.; Bopp, G.; Reise, C.; Braun, C.; Weselek, A.; Bauerle, A.; Högy, P.; et al. Implementation of agrophotovoltaics: Techno-economic analysis of the price-performance ratio and its policy implications. Appl. Energy 2020, 265, 114737. [Google Scholar] [CrossRef]
- Weselek, A.; Bauerle, A.; Zikeli, S.; Lewandowski, I.; Högy, P. Effects on Crop Development, Yields and Chemical Composition of Celeriac (Apium graveolens L. var. rapaceum) Cultivated Underneath an Agrivoltaic System. Agronomy 2021, 11, 733. [Google Scholar] [CrossRef]
- Jiang, S.; Tang, D.; Zhao, L.; Liang, C.; Cui, N.; Gong, D.; Wang, Y.; Feng, Y.; Hu, X.; Peng, Y. Effects of different photovoltaic shading levels on kiwifruit growth, yield and water productivity under “agrivoltaic” system in Southwest China. Agric. Water Manag. 2022, 269, 107675. [Google Scholar] [CrossRef]
- Juillion, P.; Lopez, G.; Fumey, D.; Lesniak, V.; Génard, M.; Vercambre, G. Shading apple trees with an agrivoltaic system: Impact on water relations, leaf morphophysiological characteristics and yield determinants. Sci. Hortic. 2022, 306, 111434. [Google Scholar] [CrossRef]
- Marrou, H.; Dufour, L.; Wery, J. How does a shelter of solar panels influence water flows in a soil–crop system? Eur. J. Agron. 2013, 50, 38–51. [Google Scholar] [CrossRef]
- Elamri, Y.; Cheviron, B.; Lopez, J.M.; Dejean, C.; Belaud, G. Water budget and crop modelling for agrivoltaic systems: Application to irrigated lettuces. Agric. Water Manag. 2018, 208, 440–453. [Google Scholar] [CrossRef]
- Hassanpour Adeh, E.; Selker, J.S.; Higgins, C.W. Remarkable agrivoltaic influence on soil moisture, micrometeorology and water-use efficiency. PLoS ONE 2018, 13, e0203256. [Google Scholar] [CrossRef]
- Gonocruz, R.A.; Nakamura, R.; Yoshino, K.; Homma, M.; Doi, T.; Yoshida, Y.; Tani, A. Analysis of the Rice Yield under an Agrivoltaic System: A Case Study in Japan. Environments 2021, 8, 65. [Google Scholar] [CrossRef]
- Ferrara, G.; Boselli, M.; Palasciano, M.; Mazzeo, A. Effect of shading determined by photovoltaic panels installed above the vines on the performance of cv. Corvina (Vitis vinifera L.). Sci. Hortic. 2023, 308, 111595. [Google Scholar] [CrossRef]
- Weselek, A.; Bauerle, A.; Hartung, J.; Zikeli, S.; Lewandowski, I.; Högy, P. Agrivoltaic system impacts on microclimate and yield of different crops within an organic crop rotation in a temperate climate. Agron. Sustain. Dev. 2021, 41, 59. [Google Scholar] [CrossRef]
- Gommers, C.M.; Visser, E.J.; Onge, K.R.S.; Voesenek, L.A.; Pierik, R. Shade tolerance: When growing tall is not an option. Trends Plant. Sci. 2013, 18, 65–71. [Google Scholar] [CrossRef]
- Ruberti, I.; Sessa, G.; Ciolfi, A.; Possenti, M.; Carabelli, M.; Morelli, G. Plant adaptation to dynamically changing environment: The shade avoidance response. Biotechn. Adv. 2012, 30, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.; Whitelam, G.C. The shade avoidance syndrome: Multiple responses mediated by multiple phytochromes. Plant. Cell Environ. 1997, 20, 840–844. [Google Scholar] [CrossRef]
- Niinemets, Ü. Role of foliar nitrogen in light harvesting and shade tolerance of four temperate deciduous woody species. Funct. Ecol. 1997, 11, 518–531. [Google Scholar] [CrossRef]
- Niinemets; Valladares, F. Photosynthetic Acclimation to Simultaneous and Interacting Environmental Stresses Along Natural Light Gradients: Optimality and Constraints. Plant. Biol. 2004, 6, 254–268. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, L. Shade Tolerance, a Key Plant Feature of Complex Nature and Consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Kardiman, R.; Ræbild, A. Relationship between stomatal density, size and speed of opening in Sumatran rainforest species. Tree Physiol. 2018, 18, 696–705. [Google Scholar] [CrossRef]
- Gay, A.; Hurd, R. The influence of light on stomatal density in the tomato. New Phytol. 1975, 75, 37–46. [Google Scholar] [CrossRef]
- Gregoriou, K.; Pontikis, K.; Vemmos, S. Effects of reduced irradiance on leaf morphology, photosynthetic capacity, and fruit yield in olive (Olea europaea L.). Photosynthetica 2007, 45, 172–181. [Google Scholar] [CrossRef]
- Ajmi, A.; Vázquez, S.; Morales, F.; Chaari, A.; El-Jendoubi, H.; Abadía, A.; Larbi, A. Prolonged artificial shade affects morphological, anatomical, biochemical and ecophysiological behavior of young olive trees (cv. Arbosana). Sci. Hortic. 2018, 241, 275–284. [Google Scholar] [CrossRef]
- Yang, F.; Huang, S.; Gao, R.; Liu, W.; Yong, T.; Wang, X.; Wu, X.; Yang, W. Growth of soybean seedlings in relay strip intercropping systems in relation to light quantity and red:Far-red ratio. Field Crop. Res. 2014, 155, 245–253. [Google Scholar] [CrossRef]
- Shafiq, I.; Hussain, S.; Raza, M.A.; Iqbal, N.; Asghar, M.A.; Raza, A.; Fan, Y.F.; Mumtaz, M.; Shoaib, M.; Ansar, M.; et al. Crop photosynthetic response to light quality and light intensity. J. Integr. Agric. 2021, 20, 4–23. [Google Scholar] [CrossRef]
- Ferrara, G.; Flore, J.A. Comparison between different methods for measuring transpiration in potted apple trees. Biol. Plant. 2003, 46, 41–47. [Google Scholar] [CrossRef]
- Gratani, L. Plant Phenotypic Plasticity in Response to Environmental Factors. Adv. Bot. 2014, 2014, 208747. [Google Scholar] [CrossRef]
- Evans, J.R.; Poorter, H. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant. Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Oguntunde, P.G.; van de Giesen, N. Crop growth and development effects on surface albedo for maize and cowpea fields in Ghana, West Africa. Int. J. Biometeorol. 2004, 49, 106–112. [Google Scholar] [CrossRef]
- Duursma, R.A.; Makela, A. Summary models for light interception and light-use efficiency of non-homogeneous canopies. Tree Physiol. 2007, 27, 859–870. [Google Scholar] [CrossRef]
- Bsaibes, A.; Courault, D.; Baret, F.; Weiss, M.; Olioso, A.; Jacob, F.; Hagolle, O.; Marloie, O.; Bertrand, N.; Desfond, V.; et al. Albedo and LAI estimates from FORMOSAT-2 data for crop monitoring. Remote Sens. Environ. 2009, 113, 716–729. [Google Scholar] [CrossRef]
- Ko, J.; Cho, J.; Choi, J.; Yoon, C.Y.; An, K.N.; Ban, J.O.; Kim, D.K. Simulation of Crop Yields Grown under Agro-Photovoltaic Panels: A Case Study in Chonnam Province, South Korea. Energies 2021, 14, 8463. [Google Scholar] [CrossRef]
- Cuppari, R.I.; Higgins, C.W.; Characklis, G.W. Agrivoltaics and weather risk: A diversification strategy for landowners. Appl. Energy 2021, 291, 116809. [Google Scholar] [CrossRef]
- Amaducci, S.; Potenza, E.; Colauzzi, M. Developments in agrivoltaics: Achieving synergies by combining plants with solar photovoltaic power systems. In Energy-Smart Farming: Efficiency, Renewable Energy and Sustainability; Ralph, E., Sims, H., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2022; pp. 231–262. [Google Scholar] [CrossRef]
- Remtec Energy Agrovoltaico. Available online: http://www.remtec.energy/en/agrovoltaico/ (accessed on 22 September 2022).
- Oliver, F.W.; Tansley, A.G. Methods of surveying vegetation on a large scale. New Phytologist. 1904, 3, 228–237. [Google Scholar] [CrossRef]
- Sementi RV Venturoli. Available online: https://www.rv-venturoli.com/p-IT.asp?c=4&id=78/Soia/Namaste (accessed on 22 September 2022).
- Gaspardo. Available online: https://www.maschio.com/en/web/international/pinta (accessed on 5 October 2022).
- Giannerini, G.; Genovesi, R. The water saving with Irriframe platform for thousands of Italian farms. J. Agric. Inform. 2015, 6, 49–55. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, Y.; Zhang, M.; Hong, A.; Yang, H.; Liu, Y. Shade effects on growth, photosynthesis and chlorophyll fluorescence parameters of three Paeonia species. PeerJ 2020, 8, e9316. [Google Scholar] [CrossRef]
- Muhidin; Syam’un, E.; Kaimuddin; Musa, Y.; Sadimantara, G.R.; Usman; Leomo, S.; Rakian, T.C. The effect of shade on chlorophyll and anthocyanin content of upland red rice. IOP Conf. Ser. Earth Environ. Sci. 2018, 122, 012030. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, J.; Wang, Z.; Tan, T.; Li, S.; Li, J.; Wang, B.; Zhang, J.; Cheng, Y.; Wu, X.; et al. Soybean (Glycine max L. Merr.) seedlings response to shading: Leaf structure, photosynthesis and proteomic analysis. BMC Plant. Biol. 2019, 19, 34–46. [Google Scholar] [CrossRef]
- Reich, P.; Wright, I.; Cavender-Bares, J.; Craine, J.; Oleksyn, J.; Westoby, M.; Walters, M. The Evolution of Plant Functional Variation: Traits, Spectra, and Strategies. Int. J. Plant. Sci. 2003, 164, S143–S164. [Google Scholar] [CrossRef]
- Rozendaal, D.M.A.; Hurtado, V.H.; Poorter, L. Plasticity in leaf traits of 38 tropical tree species in response to light; relationships with light demand and adult stature. Funct. Ecol. 2006, 20, 207–216. [Google Scholar] [CrossRef]
- Feng, Y.; van Kleunen, M. Responses to shading of naturalized and non-naturalized exotic woody species. Ann. Bot. 2014, 114, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dawson, W.; Prati, D.; Haeuser, E.; Feng, Y.; van Kleunen, M. Does greater specific leaf area plasticity help plants to maintain a high performance when shaded? Ann. Bot. 2016, 118, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Kenig, A.; Mishoe, J.W.; Boote, K.J.; Cook, P.W.; Reicosky, D.C.; Pettigrew, W.T.; Hodges, H.F. Development of Soybean Fresh and Dry Weight Relationships for Real Time Model Calibration. Agron. J. 1993, 85, 140–146. [Google Scholar] [CrossRef]
- Xinyou, Y.; Laar, H.H.; van Laar, H.H. Crop Systems Dynamics: An. Ecophysiological Simulation Model of Genotype-By-Environment Interactions; Wageningen Academic Publishers: Wageningen, The Netherlands, 2005. [Google Scholar]
- Wu, Y.; Chen, P.; Gong, W.; Gul, H.; Zhu, J.; Yang, F.; Wang, X.; Yong, T.; Liu, J.; Pu, T.; et al. Morphological and physiological variation of soybean seedlings in response to shade. Front. Plant. Sci. 2022, 13, 1015414. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.S.; Yang, F.; Gong, W.Z.; Ahmed, S.; Fan, Y.F.; Wu, X.L.; Yong, T.W.; Liu, W.G.; Shu, K.; Liu, J.; et al. Shade adaptive response and yield analysis of different soybean genotypes in relay intercropping systems. J. Integr. Agric. 2017, 16, 1331–1340. [Google Scholar] [CrossRef]
- Heuvelink, E. Evaluation of a Dynamic Simulation Model for Tomato Crop Growth and Development. Ann. Bot. 1999, 83, 413–422. [Google Scholar] [CrossRef]
- Breuer, L.; Eckhardt, K.; Frede, H.G. Plant parameter values for models in temperate climates. Ecol. Model. 2003, 169, 237–293. [Google Scholar] [CrossRef]
- Xu, R.; Dai, J.; Luo, W.; Yin, X.; Li, Y.; Tai, X.; Han, L.; Chen, Y.; Lin, L.; Li, G. A photothermal model of leaf area index for greenhouse crops. Agric. For. Meteorol. 2010, 150, 541–552. [Google Scholar] [CrossRef]
- Gong, W.Z.; Jiang, C.D.; Wu, Y.S.; Chen, H.H.; Liu, W.Y.; Yang, W.Y. Tolerance vs. avoidance: Two strategies of soybean (Glycine max) seedlings in response to shade in intercropping. Photosynthetica 2015, 53, 259–268. [Google Scholar] [CrossRef]
- Niinemets, L. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol. Res. 2010, 25, 693–714. [Google Scholar] [CrossRef]
- Laub, M.; Pataczek, L.; Feuerbacher, A.; Zikeli, S.; Högy, P. Contrasting yield responses at varying levels of shade suggest different suitability of crops for dual land-use systems: A meta-analysis. Agron. Sustain. Dev. 2022, 42, 20210479141. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.C.; Liao, D.P.; Lu, F.Z.; Gao, R.C.; Liu, W.G.; Yong, T.W.; Wu, X.L.; Du, J.B.; Liu, J.; et al. Yield response to different planting geometries in maize-soybean relay strip intercropping systems. Agron. J. 2015, 107, 296–304. [Google Scholar] [CrossRef]
- Schou, J.B.; Jeffers, D.L.; Streeter, J.G. Effects of Reflectors, Black Boards, or Shades Applied at Different Stages of Plant Development on Yield of Soybeans. Crop. Sci. 1978, 18, 29–34. [Google Scholar] [CrossRef]
- Egli, D. Cultivar maturity and potential yield of soybean. Field Crop. Res. 1993, 32, 147–158. [Google Scholar] [CrossRef]
- Jiang, H.; Egli, D.B. Shade induced changes in flower and pod number and flower and fruit abscission in soybean. Agron. J. 1993, 85, 221–225. [Google Scholar] [CrossRef]
- Liu, B.; Liu, X.; Wang, C.; Li, Y.; Jin, J.; Herbert, S. Soybean yield and yield component distribution across the main axis in response to light enrichment and shading under different densities. Plant. Soil Environ. 2010, 56, 384–392. [Google Scholar] [CrossRef]
- Egli, D.B.; Bruening, W.P. Shade and Temporal Distribution of Pod Production and Pod Set in Soybean. Crop. Sci. 2005, 45, 1764–1769. [Google Scholar] [CrossRef]
- DIN SPEC 91434, Agri-Photovoltaic Systems—Requirements for Primary Agricultural Use. 2021. Available online: https://www.beuth.de/en/technical-rule/din-spec-91434/337886742 (accessed on 20 December 2021).
- Pang, K.; Van Sambeek, J.W.; Navarrete-Tindall, N.E.; Lin, C.H.; Jose, S.; Garrett, H.E. Responses of legumes and grasses to non-, moderate, and dense shade in Missouri, USA. I. Forage yield and its species-level plasticity. Agrofor. Syst. 2019, 93, 11–24. [Google Scholar] [CrossRef]
- Gholamhoseini, M.; Ebrahimian, E.; Habibzadeh, F.; Ataei, R.; Dezfulizadeh, M.S. Interactions of shading conditions and irrigation regimes on photosynthetic traits and seed yield of soybean (Glycine max L.). Legum Res. 2018, 41, 230–238. [Google Scholar] [CrossRef]
- Liu, X.; Rahman, T.; Song, C.; Su, B.; Yang, F.; Yong, T.; Wu, Y.; Zhang, C.; Yang, W. Changes in light environment, morphology, growth and yield of soybean in maize-soybean intercropping systems. Field Crop. Res. 2017, 200, 38–46. [Google Scholar] [CrossRef]



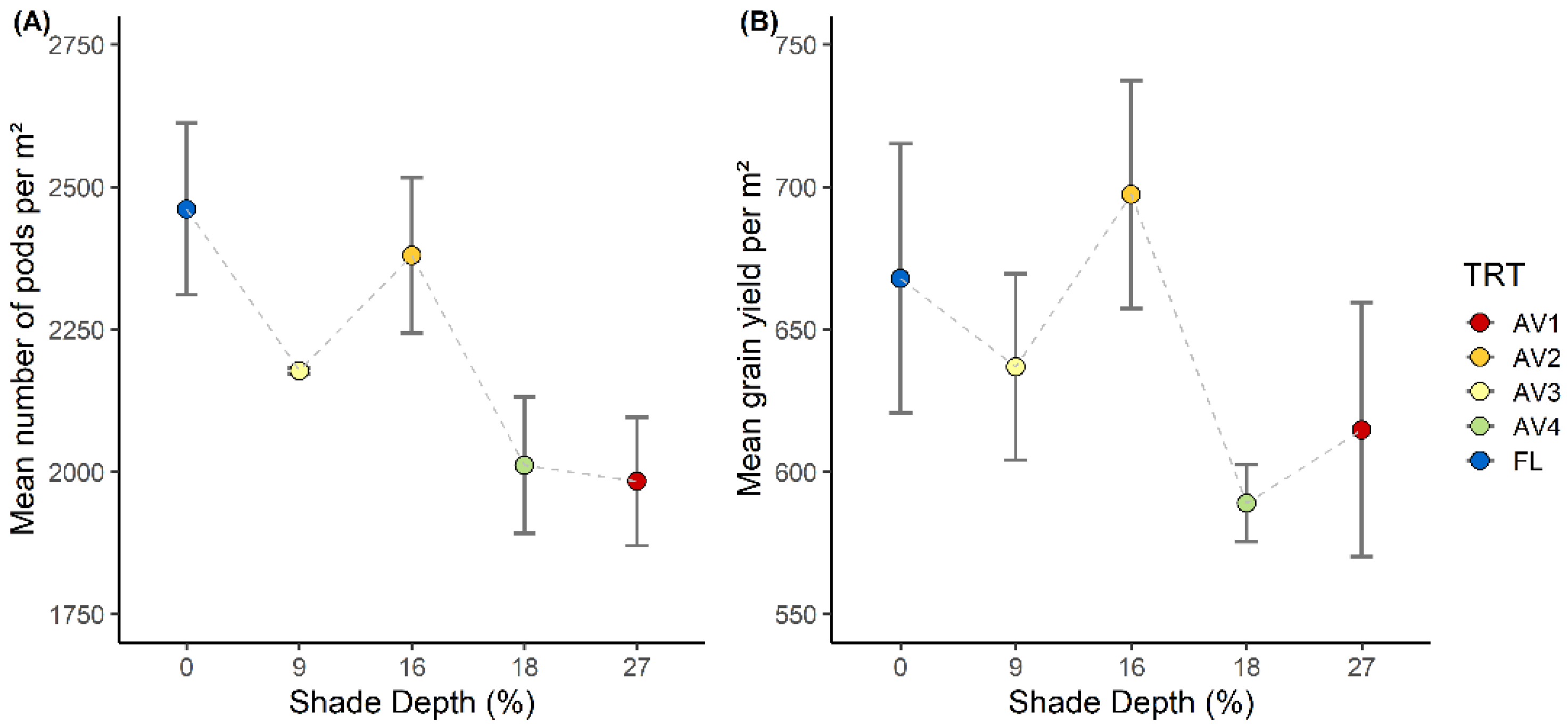
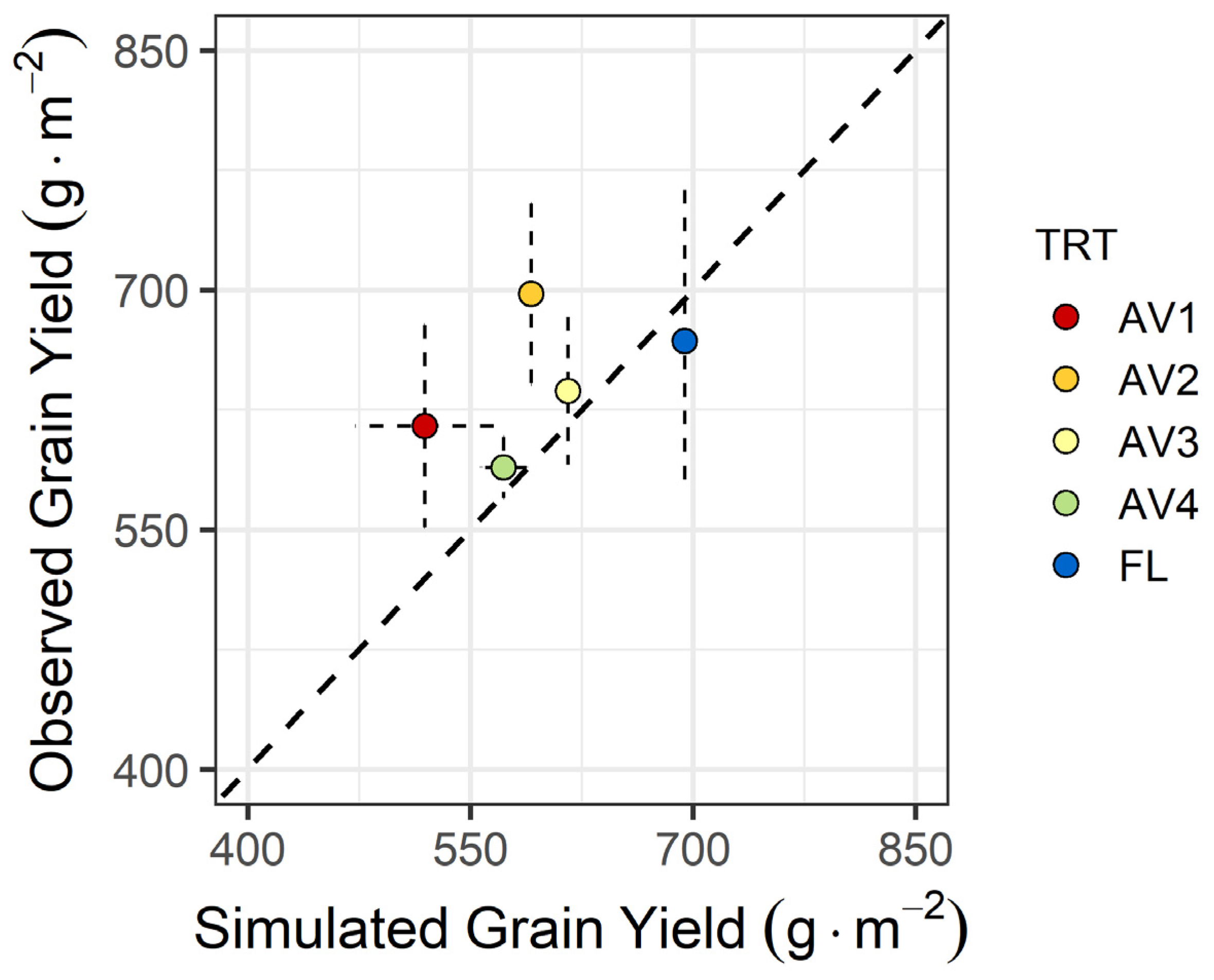
| TRT | SD (%) | RMSE | nRMSE |
|---|---|---|---|
| FL | 0% | 86.2 | 12.9% |
| AV1 | 27% | 96.3 | 15.7% |
| AV2 | 16% | 115.00 | 16.5% |
| AV3 | 9% | 42.7 | 6.71% |
| AV4 | 18% | 16.6 | 2.82% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potenza, E.; Croci, M.; Colauzzi, M.; Amaducci, S. Agrivoltaic System and Modelling Simulation: A Case Study of Soybean (Glycine max L.) in Italy. Horticulturae 2022, 8, 1160. https://doi.org/10.3390/horticulturae8121160
Potenza E, Croci M, Colauzzi M, Amaducci S. Agrivoltaic System and Modelling Simulation: A Case Study of Soybean (Glycine max L.) in Italy. Horticulturae. 2022; 8(12):1160. https://doi.org/10.3390/horticulturae8121160
Chicago/Turabian StylePotenza, Eleonora, Michele Croci, Michele Colauzzi, and Stefano Amaducci. 2022. "Agrivoltaic System and Modelling Simulation: A Case Study of Soybean (Glycine max L.) in Italy" Horticulturae 8, no. 12: 1160. https://doi.org/10.3390/horticulturae8121160
APA StylePotenza, E., Croci, M., Colauzzi, M., & Amaducci, S. (2022). Agrivoltaic System and Modelling Simulation: A Case Study of Soybean (Glycine max L.) in Italy. Horticulturae, 8(12), 1160. https://doi.org/10.3390/horticulturae8121160









