Abstract
Climate change and extreme weather affect tea growing. A competitive tea market needs quick, short-term solutions. This study evaluates the effects of various shade nets under mild and extreme cold stress on tea leaf physiology, photosynthetic alterations, antioxidant activities, and physiochemical characteristics. Tea plants were treated with SD0 (0% non-shading), SD1 (30% shading), SD2 (60% shading), and SD3 (75% shading). The 30%, 60%, and 75% shade nets shielded tea leaves from cold damage and reduced leaf injury during mild and extreme cold conditions compared with SD0% non-shading. Shading regulates photochemical capacity and efficiency and optimizes chlorophyll a and b, chlorophyll, and carotenoid contents. Moreover, carbon and nitrogen increased during mild cold and decreased in extreme cold conditions. Shading promoted antioxidant activity and physiochemical attributes. In fact, under 60% of shade, superoxide dismutase, peroxidase, catalase, and ω-3 alpha-linolenic acid were improved compared with SD0% non-shading during both mild and extreme cold conditions. From these findings, we hypothesized that the effect of different shades played an important role in the protection of tea leaves and alleviated the defense mechanism for “Zhong Cha 102” during exposure to a cold environment.
1. Introduction
Tea Camellia sinensis (L.) is a member of the Theaceae family, an important evergreen shrub or small tree covered in leaves and is the most consumable drink discovered nearly 2737 BC in China [1]. The tea tree grows in warm temperate and humid areas with temperatures between 10 and 30 °C (zone 8 climate or warmer). Due to global warming and changes in the weather, tea plants face many environmental and abiotic stresses, such as cold, chilling, high and low temperatures, which potentially harm plant growth, quality, and productivity. [2]. Cold temperatures are a frequent environmental factor that harms tea plants [3], cause undeveloped buds, wilting/yellowing leaves, and tissue death [4]. During cold stress, tea leaves are extensively damaged, producing cellular disruption, decrease in chlorophyll synthesis, photosynthetic capability, carbon, nitrogen reduction, oxidative stress, and biochemical composition leading to irregular growth, and limiting the healthy development of the tea industry [5]. Therefore, the development of new strategies controlling cold damage in tea plants is explicitly needed to overcome the loss of tea yield and production. Several studies have revealed that cold or low temperatures have a negative impact on photosynthesis, chlorophyll concentration, photosynthetic machinery, cellular adaptations, antioxidant activities, and metabolic processes in plants [6]. Cold, low-temperature exposure can result in the production of reactive oxygen species (ROS), which damage the cell membrane fluidity and disturb mitochondria, leading to cell death [7]. The cellular antioxidant system, composed of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), is essential for limiting ROS generation and protecting plants against cold stressors and abiotic conditions [8]. However, it is known that linoleic and linolenic acids modulate the plant’s defense mechanism against several abiotic stressors [9,10,11]. Linoleic and alpha-linolenic acid are the most important unsaturated fatty acids [10] that have been effective against a variety of disorders, including cancer [12] and cardiovascular disease [13].
Light intensity has a significant effect on plant growth, development, yield, and bioactive compounds [14]. However, on the other hand, high light intensity is harmful for plants [15]. In the past, several strategies concerning the protection of tea plants from various abiotic stresses have been adopted by tea growers. Shade is one of the most important and conventional techniques among those used by growers around the world [16]. In addition, shading is one of the most effective methods to protect plants from different environmental factors (e.g., cold, chilling, frost, hot, precipitation) and other harmful threats such as pest attacks, diseases causing pathogens, etc. [17].
Plants that grew under the influence of shade exhibited changes in their morphology, physiology, biochemistry, and molecular features. The use of shading nets is a common phenomenon under different climatic conditions to protect the crops both at physiological and metabolic levels [18]. Many growers used shading as protection for different horticulture crops such as tomato [19], pepper [20], lettuce [21], fig [22], and tea [23]. It has recently been reported that different shading treatments enhanced physiological attributes, including chlorophyll accumulation and, thereby, protected tea plants from cold damage [24,25]. Studies have also shown that tea plants under shading involved the chlorophyll biosynthesis pathway for improved nitrogen and carbon contents [26]. These findings recommend that shading is an important aspect of tea’s physiological attributes, including expansion of green pigments in leaves, chlorophyll synthesis, photosynthetic capacity, carbon, nitrogen contents, antioxidant activities, metabolic regulations, and secondary metabolites [27].
Over the last decade, most abiotic stress studies on tea plant completed in the lab do not represent field circumstances; thus, there may be a gap between the knowledge obtained from artificial stresses of tea plants in the laboratory and under natural abiotic stresses. A focus on physiological, metabolic, and molecular aspects of stress under natural abiotic conditions is needed to bridge this gap and to facilitate the development of tea plants with enhanced tolerance mechanisms under abiotic conditions requiring quick, short-term solutions in the field. As a result, it is critical to employ diverse tools and approaches for identifying distinct processes linked with certain cultivars under natural abiotic circumstances. Therefore, in this study we investigated the impact of different shading nets on soil plant analysis development, photosynthetic efficiency, chlorophyll and carotenoid contents, carbon and nitrogen contents, antioxidative activity, linoleic and alpha-linolenic composition in tea leaves under mild and extreme cold conditions which could promote cold-induced damage. This study will provide in-depth understanding of the impact of shading nets on fortification of winter tea leaves.
2. Materials and Methods
2.1. Plant Material and Experimental Setup
The experiment was conducted in the field located at the experimental station of Rizhao Tea Research Institute (Rizhao, Shandong, China, 35°514′ N, 119°662′ E) during winter. Five-year-old tea cultivar C. sinensis cv. “Zhong Cha 102” was used in this study under different shading conditions. In total, three shading and one control with no shade treatments were implemented in this experiment. The single-layer black polytene shades were installed above the tea leaves with 4 rows and each row was 50 m long with 4 metal fence posts (Figure 1).
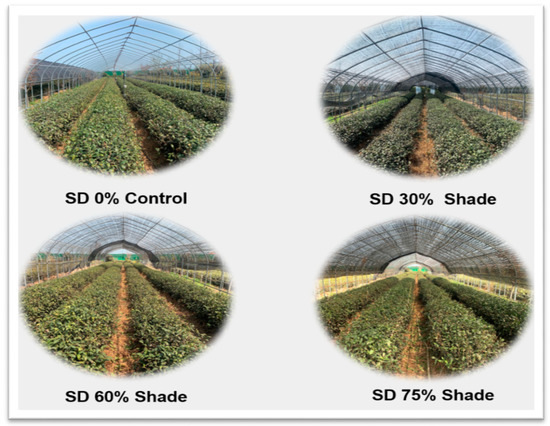
Figure 1.
Effects of shading nets on tea cultivar C. sinensis cv. “Zhong Cha 102” under different climatic conditions.
2.2. Experimental Design and Optimal Shading Percentage and Color
Site randomization and blocking were formed for all treatments including the control. Plants within rows were watered similar as required by drip irrigation. Pine bark mulch was used among rows for weed control. The only difference occurred between black shades cloths and intensity was determined by testing percentage of certain shade percentage. SD1 (0% shading), SD2 (30% shading), SD3 (60% shading), and SD4 (75% shading) were used to test the specific hypotheses (Table 1).

Table 1.
Shade specification, measurement, and PAR before sampling at mild and extreme cold conditions.
2.3. Duration of Shading
The experiment was conducted according to 24 Chinese solar terms. The shading treatments were performed under two crucial solar terms, the time interval ranged between mild and extreme weather.
2.4. Collection and Preparation of Material
Samples were collected using a hand-harvesting method. In total, 30 plants (subsamples) leaves were collected in each replication. Three to six biological replicates were created under each shade and no shade conditions. All samples were collected from each row under each shading condition including the control as the non-shading condition. Washing using distilled deionized water was followed and samples were carefully packed with high moisture under light barrier laminate packaging and shifted to the laboratory for further analysis.
2.5. Environmental Conditions
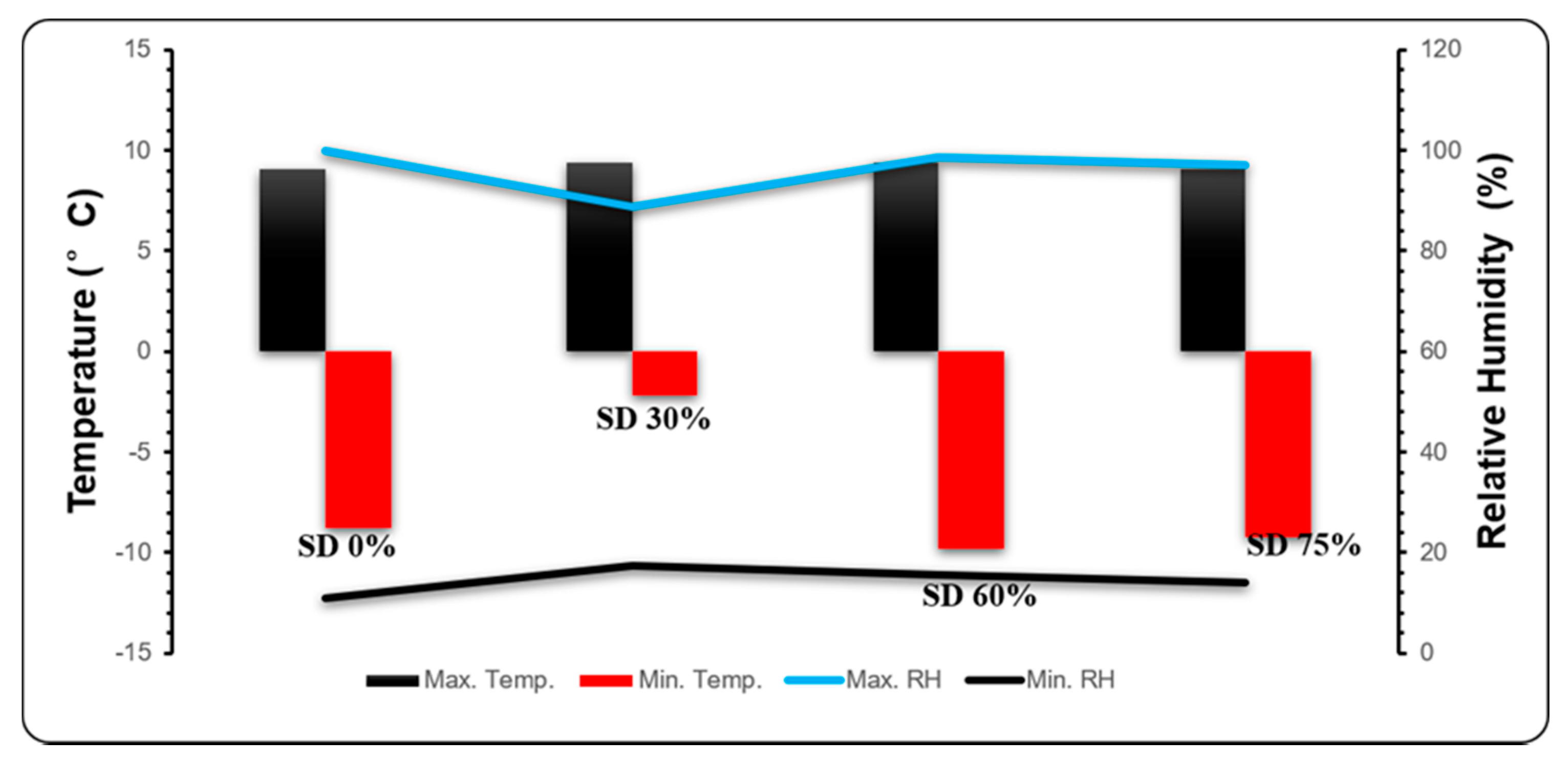
Local air temperature and relative humidity data were recorded Supplementary Table S1. The measurements were recorded during both mild and extreme cold weather under all shading conditions (Figure 2).
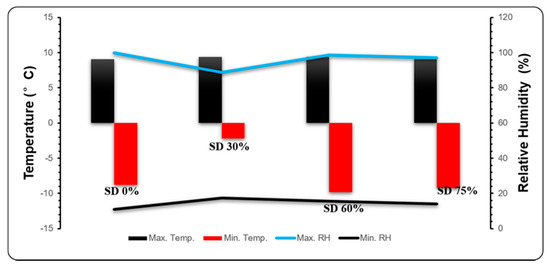
Figure 2.
Novel climatic pattern of temperature (°C), maximum temperature (Max. Temp.), minimum temperature (Min. Temp.), relative humidity (%), maximum relative humidity (Max. RH, and minimum relative humidity (Min. RH) of different shading treatments for control SD (0%), shading net SD (30%), shading net SD (60%), shading net SD (75%) during mild and extreme cold conditions.
2.6. Analysis of the Cold Damage
Plant cold injury was determined for mild and extreme weather conditions in all shade and non-shade conditions. Browning symptoms (on leaf tissue) were determined to be a cold-damage symptom with some modification [28], and the percentage of leaf suffering from cold damage on each plant was recorded according to [29,30].
2.7. Chlorophyll Fluorescence Measurement
The portable photosynthesis system (Li-6400XT, LI-COR, Inc., Lincoln, NE, USA) was used for measurement of chlorophyll fluorescence. The fourth fully expanded leaves from the shoot tip were adapted in the dark for 30 min prior to measurement. The maximum photochemical efficiency of photosystem II (Fv/Fm) was measured according to the instructions given by [31]. The relative chlorophyll content was measured with a portable chlorophyll meter (SPAD 502 Meter, Minolta Corporation, Tokyo, Japan) and values of Soil Plant Analysis Development SPAD were recorded according to [32].
2.8. Determination of Chlorophyll and Carotenoid Content
For the determination of chlorophyll and carotenoid contents, about 50 g of fresh tea leaves were cut into small pieces and incubated in a mixture of acetone and anhydrous ethanol solution (1:1) under the dark condition at 25 °C for 48 h. The absorbance for chlorophyll and carotenoid contents was measured according to the instructions given by [31].
2.9. Determination of Carbon and Nitrogen Content
For determination of carbon and nitrogen, the dried samples at 70 °C were grounded into powder and then cooled at 25 °C. A total of 1 mg of tea leaves was analyzed using an elemental analyzer (EA3000, Euro Vector, Italy) to determine the total C and N contents according to [33].
2.10. Activities of Antioxidant Enzymes
Tea fresh leaves, 0.5 g, were quickly ground with a pestle in an ice-cold mortar with 4 mL of 50 mM phosphate buffer solution (pH 7.8). The homogenate was centrifuged at 10,500 rpm for 20 min. The supernatant was used to determine the activities of antioxidant enzymes. Superoxide dismutase (SOD) activity was measured, with some modifications, based on 50% inhibition of the photochemical reduction of nitro blue tetrazolium at 560 nm. Peroxidase (POD) activity was measured as the increase in absorbance at 470 nm using the method and catalase (CAT) activity was measured as the decline in absorbance at 240 nm as described by [34].
2.11. Determination of Fatty Acid Composition through Gas Chromatography and Mass Spectrometry
About 10 mg of lyophilized power of dried leaves, 1.0 mL (1 N H2SO4), and 20 μg of internal standard (19:0) in a 10 mL screw-capped tube were mixed together. N2 was blown for 10 s in order to drive out air, the tube was sealed with the cap immediately, and heated at 80 °C for 90 min. The sample was cooled in the freezer for 10 min. After cooling, 1.5 mL of 0.9% NaCl and 1 mL of hexane were added and vortexed for 20 s, then spun at 5000 g for 5 min. Consequently, 1 μL of the upper phase (hexane phase) was taken for GC–MS analysis as described by [28].
2.12. Compound Identification
Identification of individual linoleic (18:2, ω-6 fatty acid), alpha-linolenic acid (18:3, ω-3 fatty acid) fatty acid compounds was completed using TURBOMASS 4.1.1 software (PerkinElmer Inc., Waltham, MI, USA) coupled with commercially available compound libraries: NIST 2005 (PerkinElmer Inc., Waltham, MI, USA), Wiley 7.0 (John Wiley & Sons Ltd., Hoboken, NJ, USA), as previously described [28].
2.13. Statistical Analysis
For GC–MS analysis, linoleic and alpha-linolenic acid fatty acids were identified based on retention time and comparison defined with reference spectra in mass spectral libraries. The data were statistically analyzed using analysis of variance (ANOVA), and individual treatments were compared using the least significant deference test (LSD; p = 0.05 probability level), as implemented in Statistix 8.1 software. The Graphpad Prism 5.01 software (GraphPad Software, La Jolla, CA, USA, www.graphpad.com, accessed on 1 May 2022) was used for creating figures.
3. Results
3.1. Effects of Shading Nets on Leaf Cold Damage
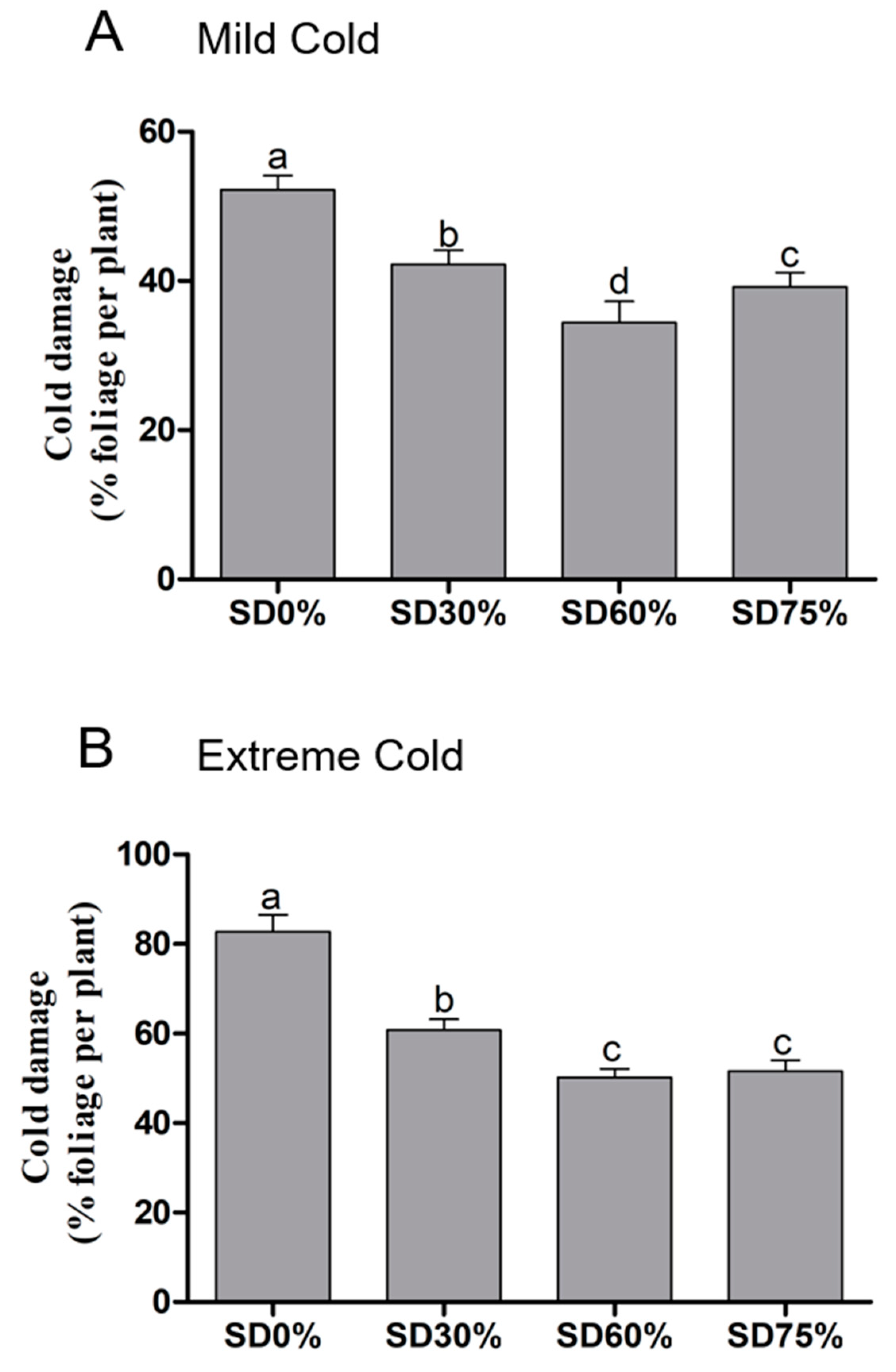
Here, tea plants were observed for cold damage during mild and extreme cold climates under shading and non-shading conditions. Compared to SD0% non-shading, leaf injury significantly decreased by 19.16%, 34.10%, and 24.90% during mild cold conditions (Figure 3A). While, 26.57%, 39.37%, and 37.68% of leaf cold injuries were recorded under SD30%, SD60%, and SD75% compared with the SD0% non-shading during extreme cold conditions (Figure 3B). The lowest and significant changes were observed in leaf injury under SD60% during both mild and extreme cold conditions compared with SD0% non-shading. However, the highest damage was observed during mild and extreme cold conditions under the SD0% non-shading condition. These results confirmed that cold weather significantly initiated leaf cold damage under the non-shading SD0% condition during both mild and extreme cold conditions, However, different shading treatments of SD30%, SD60%, and SD75% protected tea leaves from cold damage.
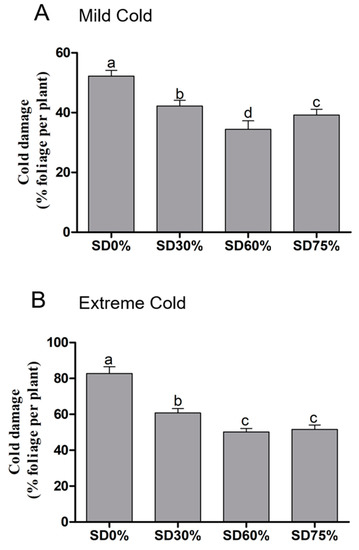
Figure 3.
Effects of shading nets on cold-damage symptoms of tea leaves during (A) mild cold and (B) extreme cold conditions. Different letters on the top of the columns within each figure represent significant differences between the treatments indicated by LSD test at p < 0.05.
3.2. Effects of Shading Nets on Soil Plant Analysis Development (SPAD) Measurements and Photochemical Efficiency (Fv/Fm)
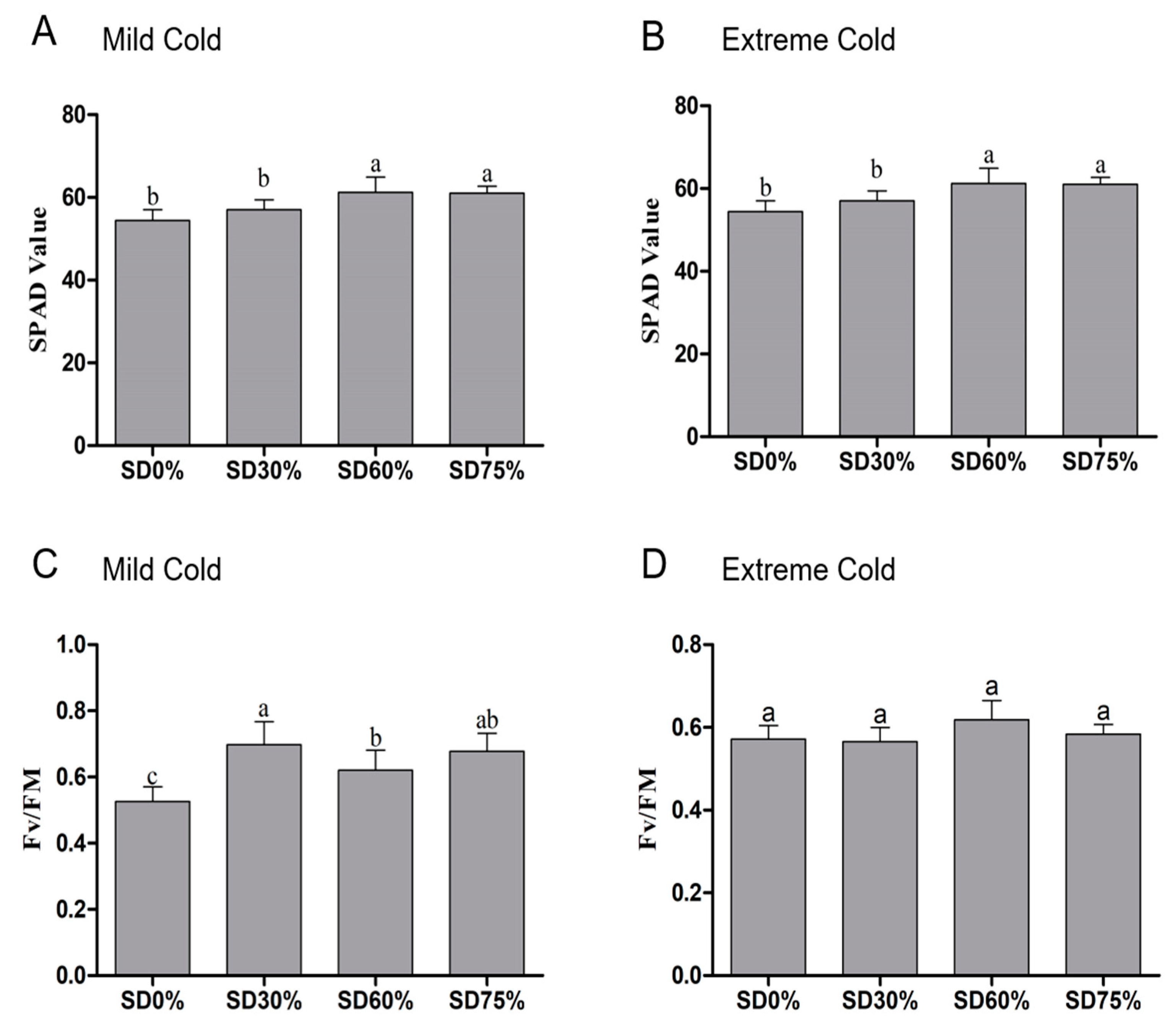
Photosynthetic capacity of chlorophyll or SPAD values were slightly increased by 4.97% non-significantly under SD30% of shading as compared to SD0 % non-shading. However, compared to the SD0% non-shading condition, 12.69% and 12.10% were significantly improved under SD60 % and SD75 %, but no significance changes were found between SD60% and SD75% during mild cold conditions (Figure 4A). On the other hand, compared to SD0 % non-shading, 15.78% of SPAD values were seen to be non-significant under SD30 %. However, 22.52% of SPAD values under SD60% and 21.81% under SD75% were significantly increased compared to SD0% non-shading. However, no such significant variations in SPAD were found between SD60% and SD75% of shading during extreme cold conditions (Figure 4B).
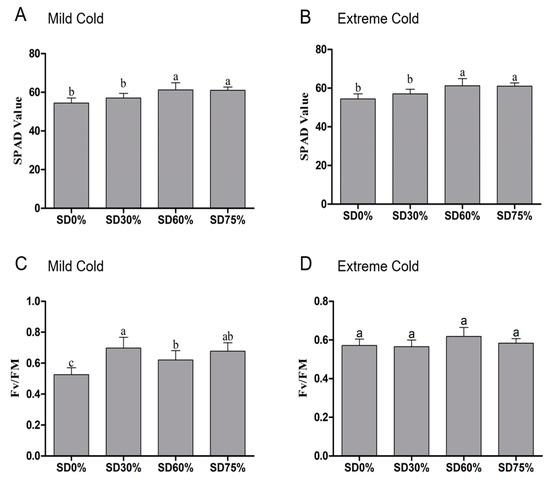
Figure 4.
Effects of shading nets on soil plant development analysis values recorded during (A) mild cold and (B) extreme cold conditions. Photochemical efficiency was recorded during (C) mild cold and (D) extreme cold conditions. Different letters on the top of the columns within each figure represent significant differences between the treatments indicated by LSD test at p < 0.05.
We also evaluated the chlorophyll photochemical efficiency under different shading groups and the results showed that photochemical efficiency was significantly distinct among all shading and non-shading conditions during mild cold. Compared with SD0% non-shading, the photochemical efficiency was significantly increased by 32.70%, 17.87%, and 28.90% during the mild cold condition (Figure 4C). While, during the extreme cold condition, no such significant changes were observed between all shading and no-shading conditions. However, an 8.04% increase in photochemical efficiency was found under SD60% compared with SD0% non-shading during the extreme cold condition (Figure 4D).
3.3. Effects of Shading Nets on Chlorophyll and Carotenoid Content
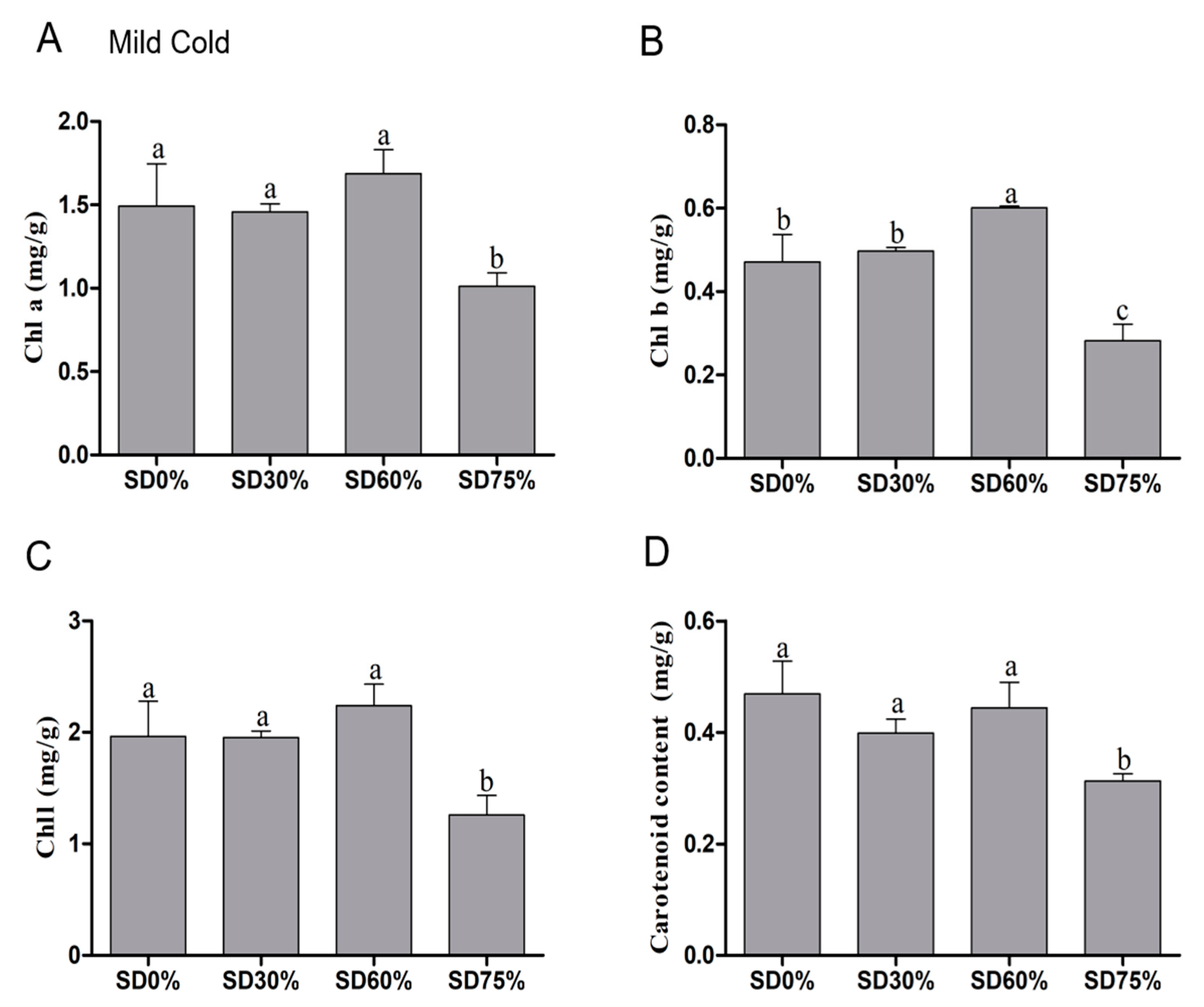
The impact of different shades diversely affects the tea leaves under mild and extreme cold conditions. Therefore, several physiological, photosynthetic, and biochemical attributes were measured. The composition of chlorophyll and carotenoid was tremendously affected under abiotic stress and degraded under cold stress conditions, which eventually diminished photosynthetic capacity. Our results showed that the content of chlorophyll a was non-significant under SD0%, SD30%, and SD60%. However, SD75% significantly decreased 32.22% and SD30% slightly declined 2.41% of the chlorophyll a content and SD60% increased 13.06% of chlorophyll a content as compared with SD0% non-shading during the mild cold condition (Figure 5A). The content of chlorophyll b was increased non-significantly by 5.52% under SD30% and significantly increased by 27.60% under SD60%. However, a dramatic reduction of 40.13% was found under SD75% during the mild cold condition (Figure 5B).
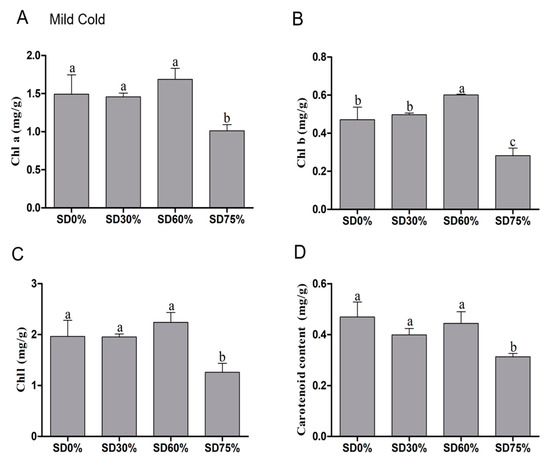
Figure 5.
Effects of shading nets on (A) chlorophyll a, (B) chlorophyll b, (C) chlorophyll, and (D) carotenoid content during the mild cold condition. Different letters on the top of the columns within each figure represent significant differences between the treatments indicated by LSD test at p < 0.05.
The content of chlorophyll was non-significant among SD0%, SD30%, and SD60%. Compared with SD0% non-shading, the amount of chlorophyll slightly decreased by 0.51% under SD30% and significantly reduced by 35.85% under SD75%. However, a 13.95% increase was found under SD60% compared with SD0% non-shade during the mild cold condition (Figure 5C). In addition to this, a non-significant variation in carotenoid content was also observed under SD0%, SD30%, and SD60% during mild and extreme cold conditions. A non-significant variation in carotenoid content was also observed under SD0%, SD30%, and SD60% during mild and extreme cold conditions. The reduced amount of carotenoid content was 14.93% and 5.33% found under SD30% and SD60% compared with SD0%. However, a 33.26% significant reduction was observed under SD75% compared with SD0% non-shading during the mild cold condition (Figure 5D). The results showed that effect of shading improved chlorophyll contents to reduce cold damage and shielded the tea leaves during the mild cold condition.
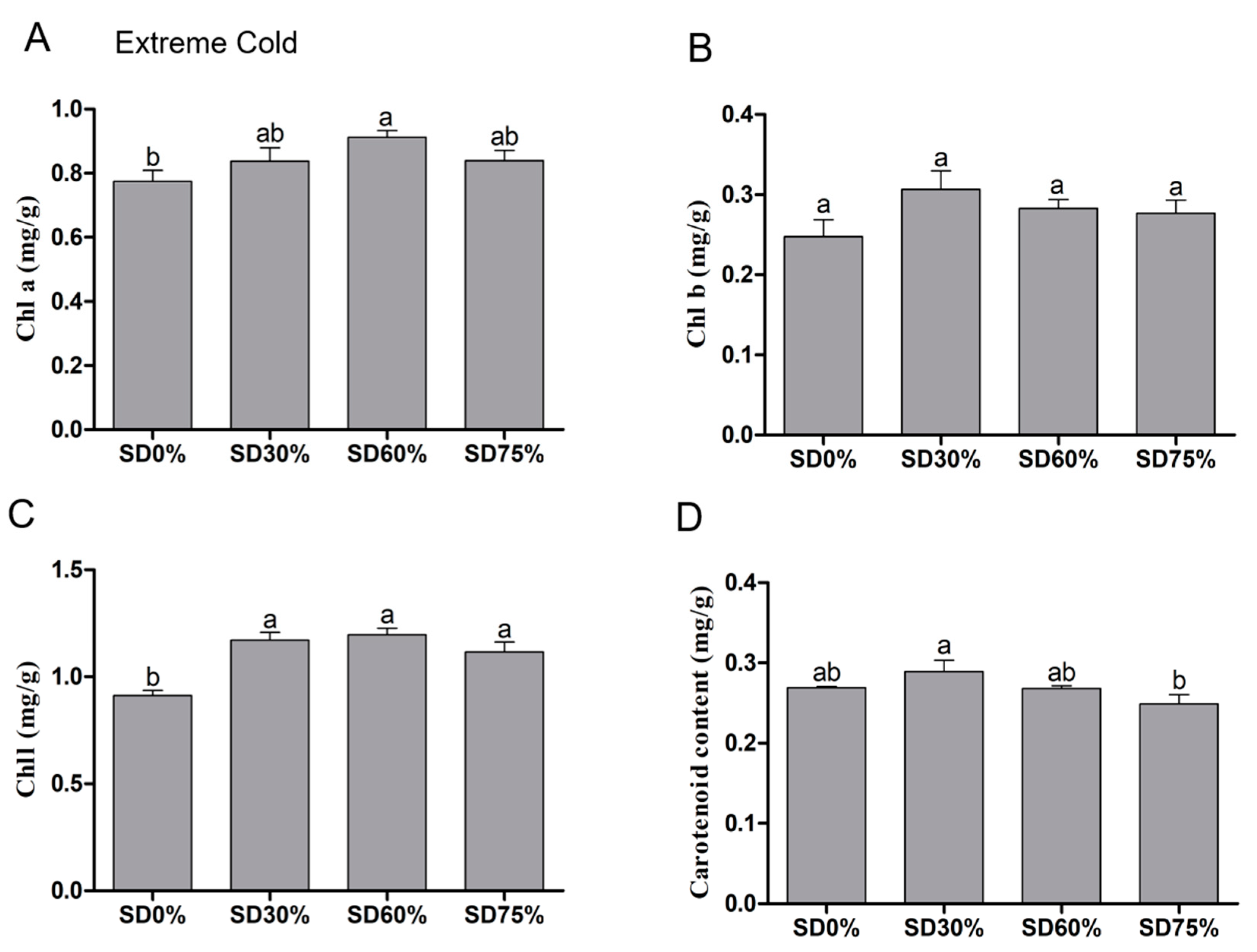
On the other hand, significance variations were observed in chlorophyll a content under shading and non-shading conditions during extreme cold. Compared with SD0% non-shading, the contents of chlorophyll a were increased slightly by 8.09% under SD30%. While, significantly increases of 17. 75% under SD60% and 8.35% were observed under SD75% during extreme cold (Figure 6A). The content of chlorophyll b was non-significant under SD0%, SD30%, SD60%, and SD75% during the extreme cold condition. However, 23.93%, 14.13%, and 11.61% increases were noticed under SD30%, SD60%, and SD75% compared with SD0% non-shading during the extreme cold condition (Figure 6B).
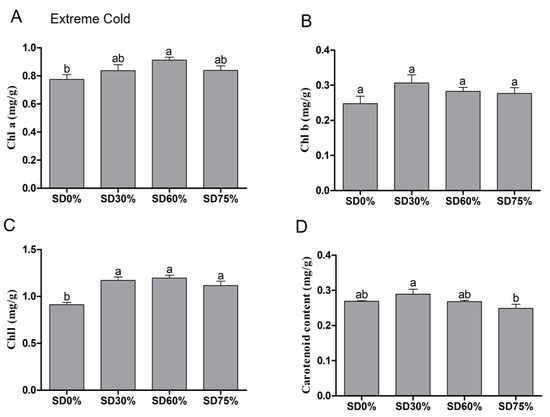
Figure 6.
Effects of shading nets on (A) chlorophyll a, (B) chlorophyll b, (C) chlorophyll, and (D) carotenoid content during the extreme cold condition. Different letters on the top of the columns within each figure represent significant differences between the treatments indicated by LSD test at p < 0.05.
The contents of chlorophyll were also non-significant under SD30%, SD60%, and SD75% compared with SD0% non-shading during extreme cold. However, 28.46%, 31.12%, and 22.44% increases were observed under SD30%, SD60%, and SD75% compared with SD0% non-shading during extreme cold conditions (Figure 6C). Significant variations occurred in the content of carotenoid under all shading and non-shading conditions during extreme cold. The decreased amount of carotenoid was observed under SD60% and SD75%, which counted as 0.43% and 7.46%. However, compared with SD0% non-shading, SD30% increased 7.56% of carotenoid content during the extreme cold condition (Figure 6D).
3.4. Effects of Shading Nets on Carbon and Nitrogen Content
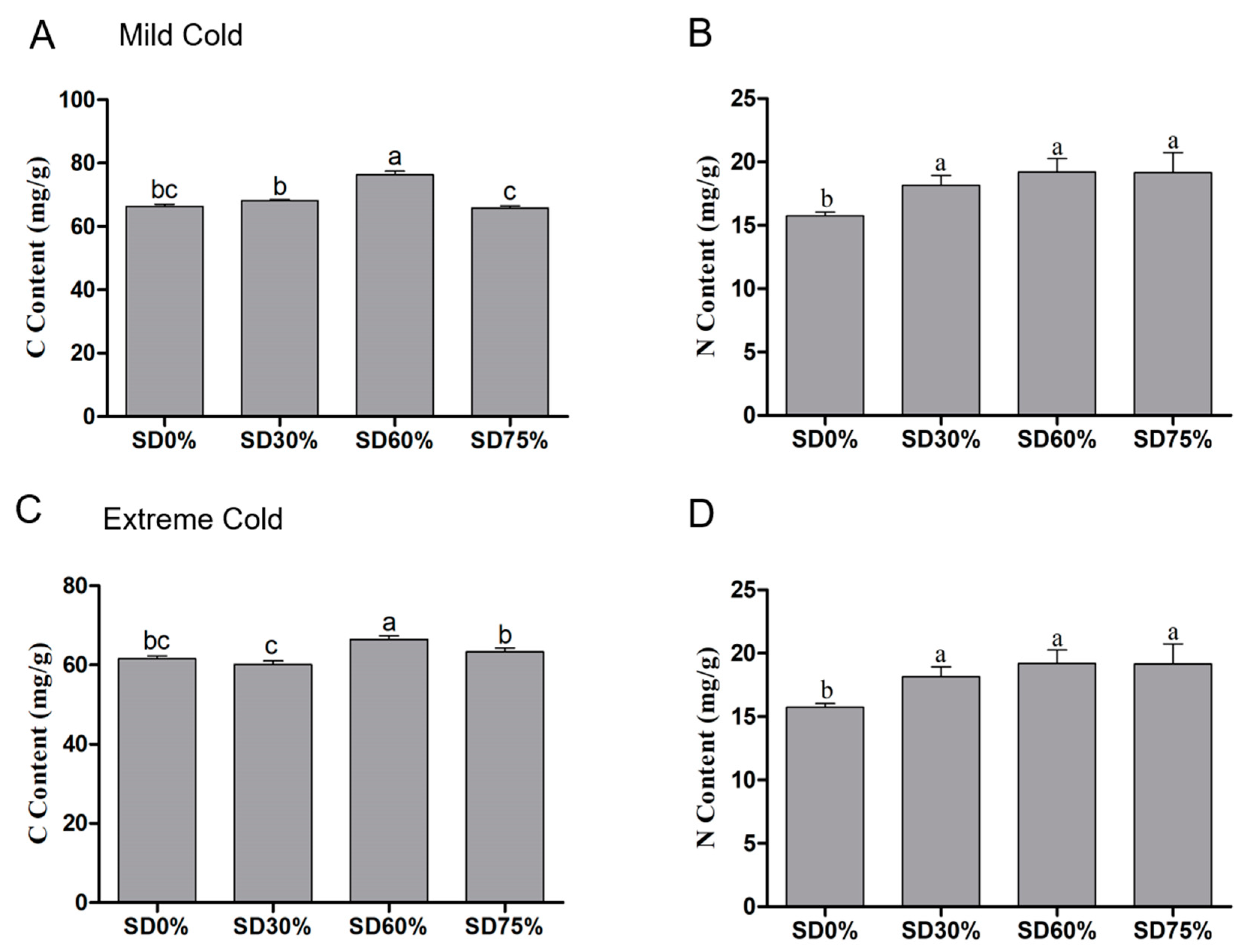
The carbon and nitrogen contents of tea leaves were determined during mild and extreme cold conditions. Significance variations were observed in carbon content under shading and non-shading conditions. Compared with SD0% non-shading, the contents of carbon increased by 2.68% and 14.94% under SD30% and SD60% of shading and a minor reduction of 0.93% was found under SD75% during mild cold conditions (Figure 7A). However, non-significant differences were observed under SD30%, SD60%, and SD75% regarding the content of nitrogen. The content of nitrogen was recorded as 4.62%, 10.90%, and 13.63% under SD30%, SD60%, and SD75% compared with SD0% non-shading during the mild cold condition (Figure 7B).
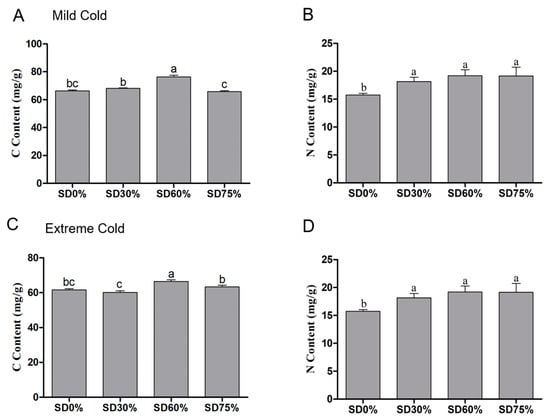
Figure 7.
Effects of shading nets on (A) carbon content and (B) nitrogen content, during mild cold condition, and (C) carbon content and (D) nitrogen content during extreme cold conditions. Different letters on the top of the columns within each figure represent significant differences between the treatments indicated by LSD test at p < 0.05.
On the other hand, during the extreme cold condition the content of carbon was enriched by 7.99% and 2.86% under SD60% and SD75%; however, a 2.31% reduction in carbon content was found under SD30% compared with SD0% non-shading during the extreme cold condition (Figure 7C). The amount of nitrogen was non-significant under SD30%, SD60%, and SD75% during the extreme cold condition. However, 15.37%, 21.98%, and 21.73% of nitrogen was observed under SD30%, SD60%, and SD75% compared with SD0% non-shading (Figure 7D). These findings shown that the SD60% shading condition amplified the carbon and nitrogen contents during mild and extreme cold conditions, indicating that shading stabilizes the biosynthesis of carbon and nitrogen metabolism together under abiotic stresses.
3.5. Effects of Shading on Leaf Antioxidant Activities
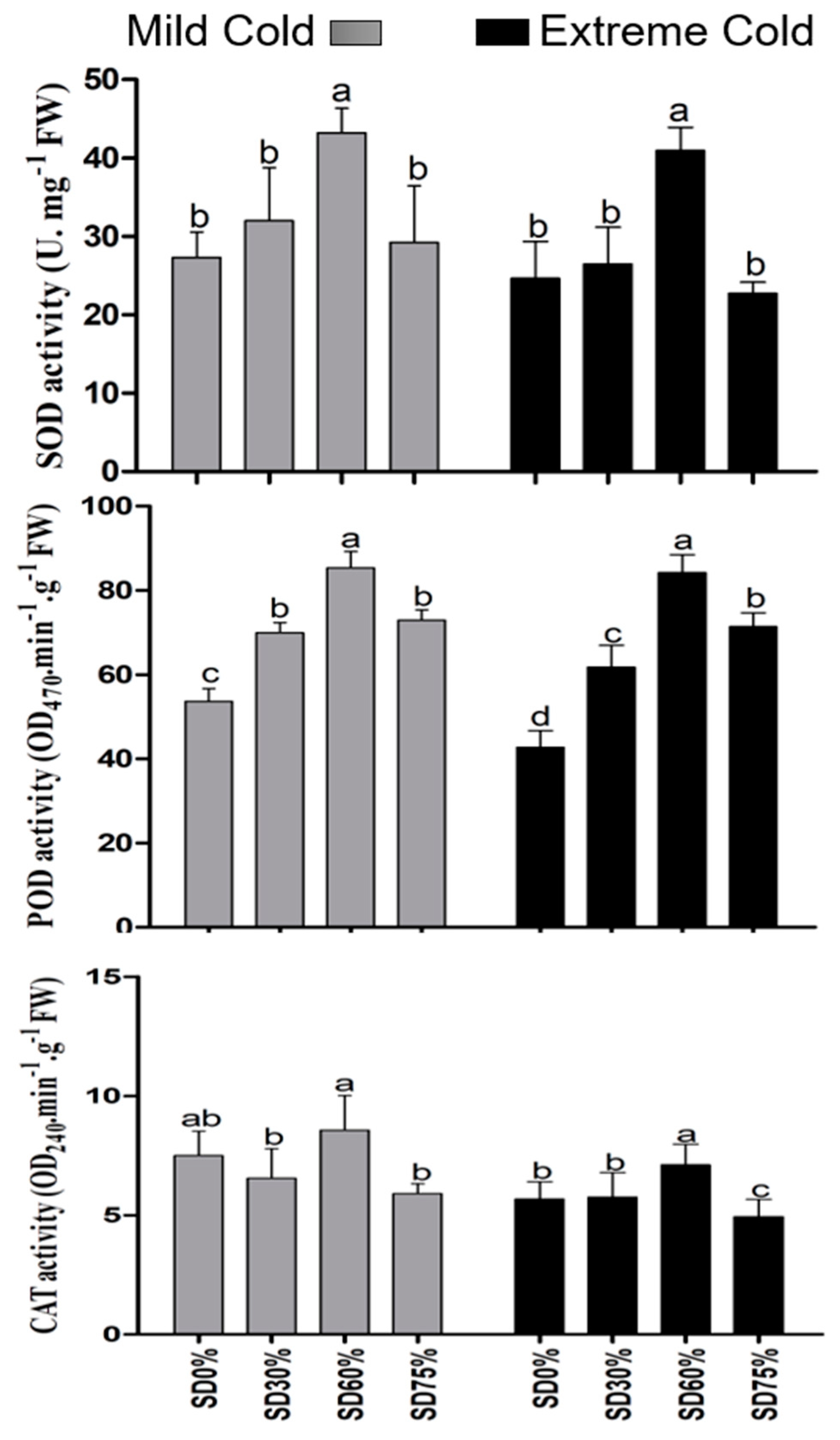
In the present study, antioxidant enzyme superoxide dismutase, peroxidase, and catalase were significantly improved under shading treatments compared with those of non-shading during both mild and extreme cold conditions. Compared with SD0% non-shading, the SD30%, SD60%, and SD75% increases SOD activity by 17.11%, 58.12%, and 6.89% during the mild cold condition. However, 7.46% and 66.43% increases were noticed under SD30% and SD60%. Additionally, a 7.76% decline in SOD activity was found under SD75% during the extreme cold condition. The activity of POD was increased by 30.31%, 22.10%, and 36.01% under SD30%, SD60%, and SD75% during mild cold and 44.67%, 97.29%, and 67.62% increases under SD30%, SD60%, and SD75% during the extreme cold condition. The activity of CAT was significantly decreased by 12.67% and 21.18% under SD30% and SD75% compared with SD0% non-shading during mild cold. However, no such variations were observed under SD60% compared with SD0% non-shading during mild cold. However, SD30% and SD60% increases of 1.36% and 25.33% and SD75% decrease of 13.12% in CAT activity during the extreme cold condition compared to the SD0% non-shading condition (Figure 8). In fact, SD60% of shading increased the antioxidant activity during the extreme cold conditions. These findings indicated that various shade treatments regulate the plant defense system and protect tea leaves from cold weather.
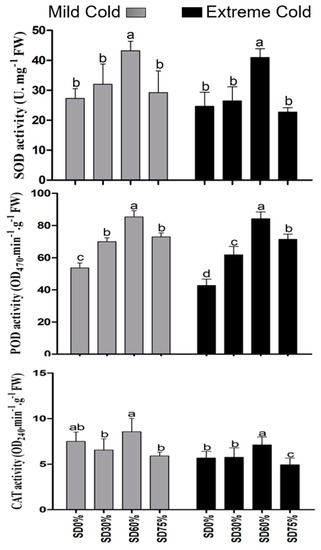
Figure 8.
Effects of shading nets on antioxidant enzyme superoxide dismutase, peroxidase, and catalase, during mild and extreme cold conditions. Different letters on the top of the columns within each figure represent significant differences between the treatments indicated by LSD test at p < 0.05.
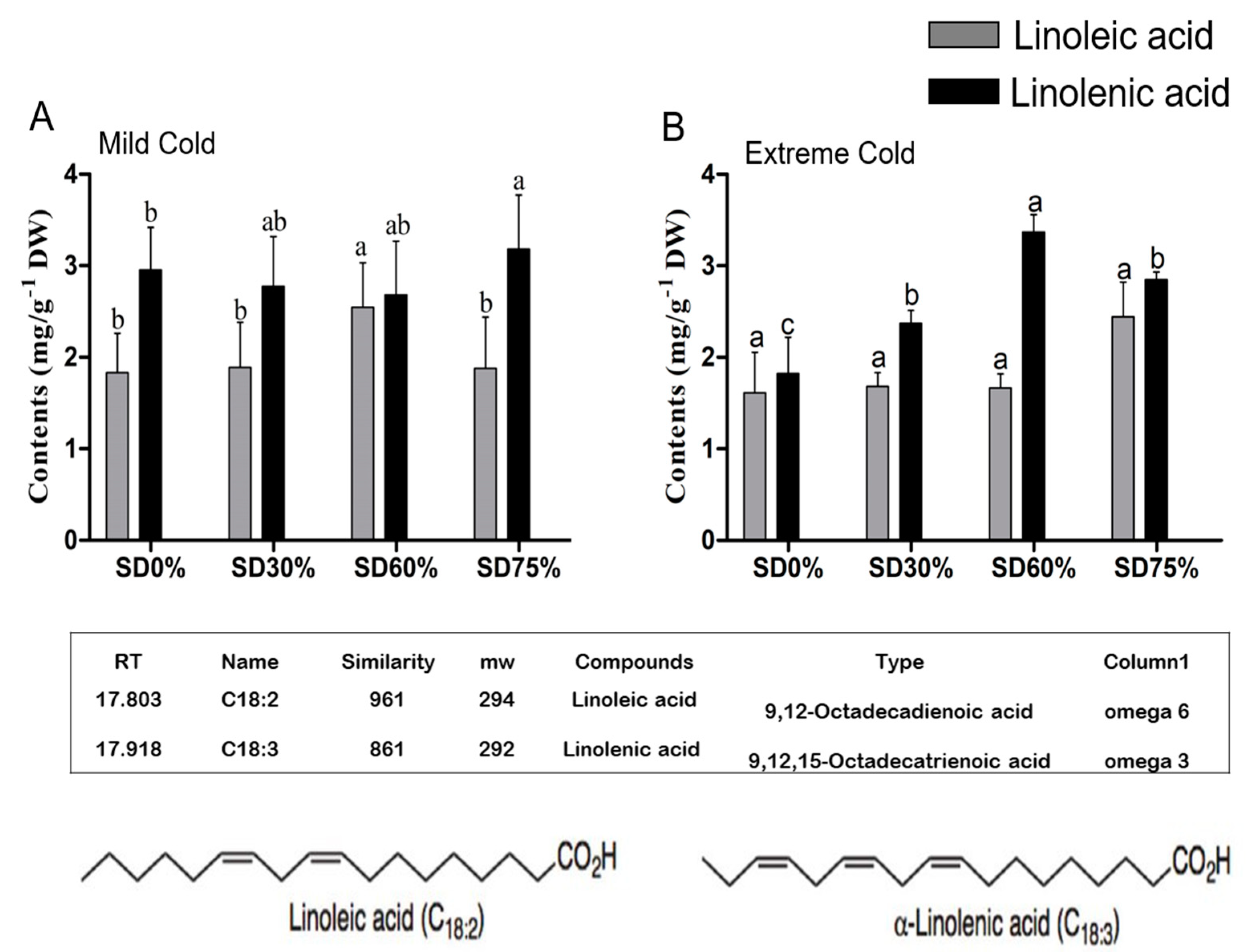
3.6. Effects of Shading Nets on Fatty Acid Composition
In present study, two types of important unsaturated fatty acids, linoleic and alpha-linolenic acid, in tea leaves were identified and their compositions were determined under non-shade and shading conditions. Effect of shading influenced both ω-3 and ω-6 fatty acid contents during mild and extreme cold conditions. Linoleic acid and alpha-linolenic acid are important fatty acids and they are known to play an important role in plant growth and development under different abiotic stress conditions. Our results showed that, compared with SD0% non-shading, the amount of linoleic acid was increased by 3.17%, 38.91%, and 2.49% under SD30%, SD60%, and SD75%, and alpha-linolenic acid contents were increased by 21.24%, 17.28%, and 39.09% under SD30%, SD60%, and SD75% during the mild cold condition (Figure 9A). On the other hand, during extreme cold, the content of linoleic acid was non-significant under shade and non-shade conditions. However, 4.36%, 3.20%, and 51.33% increases were observed under SD30%, SD60%, and SD75% and alpha-linolenic acid were increases 30.16%, 84.67% and 56.25% under SD30%. SD60%, and SD75% respectively (Figure 9B). Moreover, a higher composition of ω-3 alpha-linolenic acid was increased under SD60% during the extreme cold condition. The augmentation in the contents of both linoleic and alpha-linolenic fatty acid compositions under all shading conditions indicated that shading is beneficial for protecting tea plants during mild and extreme cold conditions. However, degradation in linoleic and alpha-linolenic fatty acid composition under SD0% non-shading conditions specified that cold weather influenced and reduced the composition of unsaturated fatty acids in tea plants.
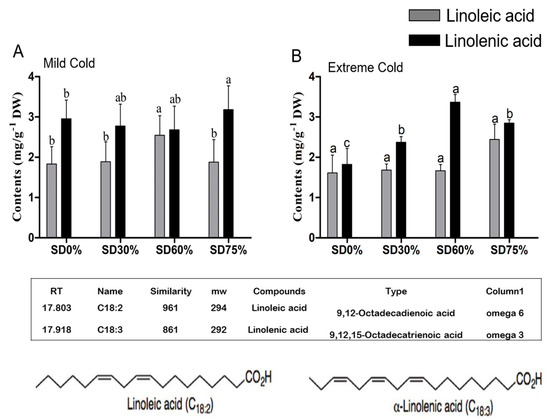
Figure 9.
Effects of shading nets on ω-6 linoleic fatty acid and ω-3 alpha-linolenic acid fatty acid composition of tea during (A) mild cold and (B) extreme cold conditions. Different letters on the top of the columns within each figure represent significant differences between the treatments indicated by LSD test at p < 0.05.
4. Discussion
Shading is one of the best ways to protect plants from different temperatures such as cold, hot, highs and low temperatures, and hence decrease cold damage or leaf injury in plants [35]. In present research, cold damage was detected under non-shading conditions during both mild and extreme conditions. However, shaded tea leaves were protected from cold damage throughout mild and extreme cold conditions, demonstrating that shade protected tea leaves against ice accumulation in extracellular tissues [36], and therefore promoted tolerance of plants. Plant tolerance to high PAR or low PAR conditions is regulated under varying shade conditions, which modify intensity and affect the photosynthetic capacity of crop growth and development in climatic zones. [37]. Previous studies stated that higher PAR was observed under the control condition compared to different shade levels [38]. Consistent with the previous studies, our findings also confirmed that the PAR (μmol m−2 s−1) was significantly higher under non-shading conditions while it decreased under increased shading conditions.
Cold stress and low temperature have adverse effects on tea leaves, which results in a substantial decline in photochemical efficiency and soil plant analysis development capacity, as well as chlorophyll accumulation [39]. In recent years, a great consideration has been given to variations in photosynthetic traits in response to cold stress. The photochemical efficiency is a consistent indicator of plant adaptation to stress [36]. Previously, the chlorophyll photochemical efficiency was implemented following the instruction of [37], in order to categorize cold tolerance in nine Arabidopsis thaliana accessions [36] and Stevia rebaudiana cultivars [38]. Our findings also suggested that the photochemical efficiency was significantly decreased under SD0% non-shade conditions during mild and extreme cold conditions, while it significantly increased under all shading levels during the mild cold condition. During extreme cold conditions, dramatic decreases were also found under SD30% and increases in photochemical efficiency were found under shading conditions. The photochemical efficiency was protected and significantly increased during mild and extreme cold conditions indicating that different shades played an important role in protection of leaves and alleviating defense mechanisms for cold-tolerance in tea plants.
Similarly, chlorophyll and carotenoid pigmentations determine leaf color variation under shade. Chlorophyll is very sensitive to abiotic stress and decomposition of chlorophyll and photosynthetic capacity is associated with degradation in chloroplast advancement and enhancement of photosynthetic machinery [40,41]. An earlier study demonstrated that different shade conditions increased chlorophyll a and b contents and chlorophyll accumulation but no such differences were observed in the contents of carotenoids in C. sinensis [24]. In our findings, the contents of chlorophyll a, b, and chlorophyll were significantly improved under SD60% of shading during the mild cold condition. However, dramatic degradation was found under SD75% compared with SD0% non-shading. The reasons are unknown. However, a significant reduction in the content of chlorophyll was found under SD0% non-shading conditions during extreme cold conditions, which means that cold stress affects the tea leaves and might damage the membrane fluidity in cell compartments during cold weather. These findings proposed that SD60% of shading increased chlorophyll protection during mild and extreme cold conditions, the contents of carotenoids were decreased under SD60% but increased under other shading conditions, and non-shade conditions degraded the accumulation of both chlorophyll and carotenoids in the leaves of tea during extreme cold conditions. It is also speculated that several genes and transcription factors are involved in photosynthesis, flavonoid biosynthesis, amino acid and carotenoid biosynthesis in tea leaves under shade and non-shading conditions as revealed by several transcriptomic data [17,42]. To better understand the molecular mechanisms of tea plants under abiotic stressors, it may be interesting to investigate the regulation and expression levels of genes involved under different shading treatments in the future.
In the development of tea plants, different shade treatments had a substantial impact on chlorophyll and biochemical components, as well as carbon and nitrogen regulation [24,35,43]. Photosynthesis and photosynthetic pigments can deliver energy for specialized metabolism of plants and carbon and nitrogen are both crucial for synthesis of photosynthetic products, enzymes, and chloroplasts in plants [33]. We also observed that both carbon and nitrogen contents are stable under different shading conditions in tea plants. However, the outcome of SD60% shading coordinately improved carbon and nitrogen contents during both mild and extreme cold conditions compared with SD0% non-shading conditions. Our result is in agreement with [26], who reported that shading treatment improved the tea quality and differentially regulated the content of carbon and nitrogen for metabolic regulation. It is possible that both carbon and nitrogen regulated together under shading conditions and serve as a substrate for tea polyphenol biosynthesis via the regulation of shikimic acid and phenylpropane pathways through the plant metabolism process [44]. Thus, we assume that the regulation of carbon and nitrogen metabolism is jointly integrated and promotes tea leaf quality under shade during mild and slight cold conditions.
Plants accumulate reactive oxygen species and malondialdehyde in response to abiotic stress, which is very toxic and has a negative influence on photosynthetic, lipids, protein, and carbohydrates synthesis [7]. The reactive oxygen species scavenging mechanism is critical in protecting cells from photooxidative damage [45]. Different crops might regulate the active balance of reactive oxygen species via antioxidant enzyme superoxide dismutase, peroxidase, and the catalase system, diminishing membrane peroxidation and the degree of oxidative stress induced by abiotic stressors [46], and may cause cell death [6]. We found that the increased amount of SOD, POD, and CAT enzymes under shading treatments might lead to survival of tea plants under mild and extreme cold conditions, and produce plant defense mechanisms throughout a fluctuating climate. However, the decline in SOD, POD, and CAT activities under non-shading treatments, suggest that tea plants generate reactive oxygen species instability and membrane deterioration, promoting leaf injury and withering [47], during mild and extreme cold conditions. Under the SD60% shading condition, the increments in SOD and POD antioxidant activities during mild and extreme cold conditions signify that shade nets were effective in enhancing antioxidant enzyme activities.
Abiotic climatic factors disturb the chemical composition and produce different biological functions in plants. Consequently, in plants, fatty acid biosynthesis is generally present in plastidial compartments [48] and the content of fatty acids assorted with phenotypic plasticity and ω-3 desaturase catalyzes the synthesis of alpha-linolenic acid ω-3 from linoleic acid ω-6 [31]. In our results, non-shading and shading conditions significantly influenced the global composition of fatty acid contents, especially ω-6 linoleic acid. However, the ω-3 alpha-linolenic contents were improved under different shading conditions in both mild and extreme cold conditions. These results indicate that when tea leaves were subjected to extreme cold conditions without shade, the membrane fluidity in the cell compartment of tea leaves was reduced. However, different shading conditions protected tea leaves from cold damage and increased the amount of fatty acid composition to maintain membrane fluidity in cell compartments. In addition, both ω-3 alpha-linolenic acid and ω-6 linoleic acid are unsaturated fatty acids and the unsaturation level of plants’ ability is used to maintain or regulate membrane fluidity, which was positively related with plant-tolerance to abiotic stress [31]. These findings are in line with those of our previous studies, in which 94 genes were identified and participate in the pathway of biosynthesis of unsaturated fatty acids and FAD2 family genes regulated for unsaturation of fatty acid composition and played an important role in ω-3 and ω-6 fatty acid desaturase to increase stress tolerance [10]. Some plants have fatty acid desaturations that regulate chloroplast fluidity and increase tolerance mechanisms in specific cultivars. Moreover, the content of ω-3 alpha-linolenic acid significantly improved under SD60% during the extreme cold condition. This could be because omega-3 desaturase promotes chloroplast abundance and membrane fluidity in leaves [31,49], under protective shade conditions during mild and extreme cold conditions. Altogether, our results indicated that shading played an important role and protected tea leaves from cold damage and improved photosynthetic capacity, green pigments, antioxidant activities, and sustained membrane fluidity in cell compartments for fatty acid composition in tea plants under mild and extreme cold conditions.
5. Conclusions
This paper investigated how tea leaf shading promotes cold protection. Cold weather and non-shading reduced photosynthetic capacity, photochemical efficiency, chlorophyll levels, carbon and nitrogen contents, antioxidant enzyme activities, and physiochemical characteristics during mild and extreme cold conditions. Shade nets protected tea leaves from cold damage by modulating photosynthetic capacity, efficiency, chlorophyll levels, carbon and nitrogen contents, antioxidant activities, and physiochemical qualities. SD60 % of shade protected tea plants against cold temperature by enhancing the plant defense system and improving chlorophyll, carbon, nitrogen, and ω-3 alpha-linolenic acid during extreme cold. Thus, shades played an important role in protection of tea leaves and alleviated the defense mechanism for “Zhong Cha 102” during exposure to a cold environment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8070637/s1, Table S1: Climatic data.
Author Contributions
Conceptualization, S.Z. and Z.D.; methodology, S.W.; software, D.S.; validation, H.W., X.P. and M.W.; formal analysis, J.S.; investigation, D.S.; resources, Z.D.; data curation, S.Z.; writing—original draft preparation, S.Z.; writing—review and editing, S.Z.; visualization, Y.W.; supervision, Z.D.; project administration, S.D.; funding acquisition, Z.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded internally by the Tea Research Institute, SAAS, for postdoctoral research number “300948”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Kindly contact the first author of the manuscript.
Acknowledgments
We thank Rizhao Tea Research Institute, Shandong, China for technical assistance and Naveed Ahmed (SAAS), for revision of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chow, K.B.; Kramer, I. All the Tea in China; China Books & Periodicals Inc.: South San Francisco, CA, USA, 1990. [Google Scholar]
- Joshi, R.; Rana, A.; Gulati, A. Studies on quality of orthodox teas made from anthocyanin-rich tea clones growing in Kangra valley, India. Food Chem. 2015, 176, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Q.; Ou, L.; Ji, D.; Liu, T.; Lan, R.; Li, X.; Jin, L. Response to the Cold Stress Signaling of the Tea Plant (Camellia sinensis) Elicited by Chitosan Oligosaccharide. Agronomy 2020, 10, 915. [Google Scholar] [CrossRef]
- Li, X.; Ahammed, G.J.; Li, Z.-X.; Zhang, L.; Wei, J.-P.; Yan, P.; Zhang, L.-P.; Han, W.-Y. Freezing stress deteriorates tea quality of new flush by inducing photosynthetic inhibition and oxidative stress in mature leaves. Sci. Hortic. 2017, 230, 155–160. [Google Scholar] [CrossRef]
- Yin, Y.; Ma, Q.-P.; Zhu, Z.-X.; Cui, Q.-Y.; Chen, C.-S.; Chen, X.; Fang, W.-P.; Li, X.-H. Functional analysis of CsCBF3 transcription factor in tea plant (Camellia sinensis) under cold stress. Plant Growth Regul. 2016, 80, 335–343. [Google Scholar] [CrossRef]
- Anwar, A.; Wang, J.; Yu, X.; He, C.; Li, Y. Substrate Application of 5-Aminolevulinic Acid Enhanced Low-temperature and Weak-light Stress Tolerance in Cucumber (Cucumis sativus L.). Agronomy 2020, 10, 472. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Yan, Y.; Liu, Y.; Li, Y.; Yu, X. 5-Aminolevulinic Acid Improves Nutrient Uptake and Endogenous Hormone Accumulation, Enhancing Low-Temperature Stress Tolerance in Cucumbers. Int. J. Mol. Sci. 2018, 19, 3379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conconi, A.; Miquel, M.; Browse, J.; Ryan, C.A. Intracellular Levels of Free Linolenic and Linoleic Acids Increase in Tomato Leaves in Response to Wounding. Plant Physiol. 1996, 111, 797–803. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Zaman, S.; Hu, S.; Che, S. Single-Molecule Long-Read Sequencing of Purslane (Portulaca oleracea) and Differential Gene Expression Related with Biosynthesis of Unsaturated Fatty Acids. Plants 2021, 10, 655. [Google Scholar] [CrossRef]
- Golizadeh, F.; Kumleh, H.H. Physiological Responses and Expression Changes of Fatty Acid Metabolism–Related Genes in Wheat (Triticum aestivum) Under Cold Stress. Plant Mol. Biol. Rep. 2019, 37, 224–236. [Google Scholar] [CrossRef]
- Arshad, A.; Isherwood, J.; Dennison, A. Could omega-3 fatty acids improve quality of life in cancer patients? Future Med. 2015, 11, 3225–3228. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Tatsuno, I. Omega-3 polyunsaturated fatty acids for cardiovascular diseases: Present, past and future. Expert Rev. Clin. Pharmacol. 2017, 10, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Ayatullah Leghari, S.K.; Shaukat, K.; Khattak, M.I.; Panezai, M.A.; Marri, A.A.; Ismail, T. Influence of sun and shade on the growth, yield and quality of Vitis vinifera L. (grapes) under semi-arid environmental conditions. App. Ecol. Environ. Res. 2019, 17, 8847–8864. [Google Scholar]
- Tmušić, N.; Ilić, Z.S.; Milenković, L.; Šunić, L.; Lalević, D.; Kevrešan, Ž.; Mastilović, J.; Stanojević, L.; Cvetković, D. Shading of Medical Plants Affects the Phytochemical Quality of Herbal Extracts. Horticulturae 2021, 7, 437. [Google Scholar] [CrossRef]
- Mohotti, A. Shade in Tea. Is It Beneficial? 2004. Reproduced from the Proceedings of the First Symposium on Plantation Crop Research. Available online: http://cri.nsf.ac.lk//handle/1/2547 (accessed on 8 May 2022).
- Jiang, X.; Zhao, H.; Guo, F.; Shi, X.; Ye, C.; Yang, P.; Liu, B.; Ni, D. Transcriptomic analysis reveals mechanism of light-sensitive albinism in tea plant Camellia sinensis ‘Huangjinju’. BMC Plant Biol. 2020, 20, 216. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.-G.; Lee, Y.-R.; Lee, M.-S.; Hwang, K.H.; Park, C.Y.; Kim, E.-H.; Park, J.S.; Hong, Y.-S. Diverse Metabolite Variations in Tea (Camellia sinensis L.) Leaves Grown Under Various Shade Conditions Revisited: A Metabolomics Study. J. Agric. Food Chem. 2018, 66, 1889–1897. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Šunić, L.; Fallik, E. Effect of coloured shade-nets on plant leaf parameters and tomato fruit quality. J. Sci. Food Agric. 2014, 95, 2660–2667. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Šunić, L.; Barać, S.; Mastilović, J.; Kevrešan, Ž.; Fallik, E. Effect of shading by coloured nets on yield and fruit quality of sweet pepper. Zemdirb. Agric. 2016, 104, 53–62. [Google Scholar] [CrossRef]
- Ilić, S.; Milenković, L.; Dimitrijević, A.; Stanojević, L.; Cvetković, D.; Kevrešan, Ž.; Fallik, E.; Mastilović, J. Light modification by color nets improve quality of lettuce from summer production. Sci. Hortic. 2017, 226, 389–397. [Google Scholar] [CrossRef]
- Jokar, A.; Zare, H.; Zakerin, A.; Aboutalebi Jahromi, A. Effects of Shade Net Colors on Mineral Elements and Postharvest Shelf Life and Quality of Fresh Fig (Ficus carica L.) under Rain-Fed Condition. Horticulturae 2021, 7, 93. [Google Scholar] [CrossRef]
- Zhang, Q. Growing Tea in Mississippi. 2022. Available online: https://scholarsjunction.msstate.edu/td/5423/ (accessed on 8 May 2022).
- Li, Y.; Jeyaraj, A.; Yu, H.; Wang, Y.; Ma, Q.; Chen, X.; Sun, H.; Zhang, H.; Ding, Z.; Li, X. Metabolic regulation profiling of carbon and nitrogen in tea plants [Camellia sinensis (L.) O. Kuntze] in response to shading. J. Agric. Food Chem. 2020, 68, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Di, Q.; Yan, Y.; He, C.; Li, Y.; Yu, X. Exogenous 24-epibrassinolide alleviates the detrimental effects of suboptimal root zone temperature in cucumber seedlings. Arch. Agron. Soil Sci. 2019, 65, 1927–1940. [Google Scholar] [CrossRef]
- Shao, C.; Jiao, H.; Chen, J.; Zhang, C.; Liu, J.; Chen, J.; Li, Y.; Huang, J.; Yang, B.; Liu, Z.; et al. Carbon and Nitrogen Metabolism Are Jointly Regulated During Shading in Roots and Leaves of Camellia Sinensis. Front. Plant Sci. 2022, 13, 894840. [Google Scholar] [CrossRef]
- Xu, P.; Su, H.; Jin, R.; Mao, Y.; Xu, A.; Cheng, H.; Wang, Y.; Meng, Q. Shading Effects on Leaf Color Conversion and Biosynthesis of the Major Secondary Metabolites in the Albino Tea Cultivar “Yujinxiang”. J. Agric. Food Chem. 2020, 68, 2528–2538. [Google Scholar] [CrossRef] [PubMed]
- Alisoltani, A.; Shiran, B.; Fallahi, H.; Ebrahimie, E. Gene regulatory network in almond (Prunus dulcis Mill.) in response to frost stress. Tree Genet. Genomes 2015, 11, 100. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, T.; Wang, Q.; LeCompte, J.; Harkess, R.L.; Bi, G. Screening Tea Cultivars for Novel Climates: Plant Growth and Leaf Quality of Camellia sinensis Cultivars Grown in Mississippi, United States. Front. Plant Sci. 2020, 11, 280. [Google Scholar] [CrossRef]
- Bielsa, B.; Ávila-Alonso, J.I.; Fernández i Martí, Á.; Grimplet, J.; Rubio-Cabetas, M.J. Gene Expression Analysis in Cold Stress Conditions Reveals BBX20 and CLO as Potential Biomarkers for Cold Tolerance in Almond. Horticulturae 2021, 7, 527. [Google Scholar] [CrossRef]
- Zaman, S.; Hu, S.; Alam, A.; Du, H.; Che, S. The accumulation of fatty acids in different organs of purslane under salt stress. Sci. Hortic. 2019, 250, 236–242. [Google Scholar] [CrossRef]
- Yang, H.; Ge, C.; Ying, W.; Yang, J.; Li, J.; He, J.J. Effect of shading on leaf SPAD values and the characteristics of photosynthesis and morphology of rice canopy. Plant Nutr. Fert. Sci. 2014, 20, 580–587. [Google Scholar]
- Li, Z.; Jiang, H.; Yan, H.; Jiang, X.; Ma, Y.; Qin, Y. Carbon and nitrogen metabolism under nitrogen variation affects flavonoid accumulation in the leaves of Coreopsis tinctoria. PeerJ 2021, 9, e12152. [Google Scholar] [CrossRef]
- Anwar, A.; Bai, L.; Miao, L.; Liu, Y.; Li, S.; Yu, X.; Li, Y. 24-Epibrassinolide Ameliorates Endogenous Hormone Levels to Enhance Low-Temperature Stress Tolerance in Cucumber Seedlings. Int. J. Mol. Sci. 2018, 19, 2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Bi, G.; Li, T.; Wang, Q.; Xing, Z.; LeCompte, J.; Harkess, R.L. Color Shade Nets Affect Plant Growth and Seasonal Leaf Quality of Camellia sinensis Grown in Mississippi, the United States. Front. Nutr. 2022, 9, 786421. [Google Scholar] [CrossRef] [PubMed]
- Zuther, E.; Lee, Y.P.; Erban, A.; Kopka, J.; Hincha, D.K. Natural Variation in Freezing Tolerance and Cold Acclimation Response in Arabidopsis thaliana and Related Species. Surviv. Strateg. Extrem. Cold Desiccation 2018, 1081, 81–98. [Google Scholar] [CrossRef]
- Rezai, S.; Etemadi, N.; Nikbakht, A.; Yousefi, M.; Majidi, M.M. Effect of Light Intensity on Leaf Morphology, Photosynthetic Capacity, and Chlorophyll Content inSage (Salvia officinalis L.). Korean J. Hortic. Sci. 2018, 36, 46–57. [Google Scholar] [CrossRef]
- Thakur, M.; Bhatt, V.; Kumar, R. Effect of shade level and mulch type on growth, yield and essential oil composition of damask rose (Rosa damascena Mill.) under mid hill conditions of Western Himalayas. PLoS ONE 2019, 14, e0214672. [Google Scholar] [CrossRef]
- Li, N.; Yang, Y.; Ye, J.; Lu, J.; Zheng, X.; Liang, Y. Effects of sunlight on gene expression and chemical composition of light-sensitive albino tea plant. Plant Growth Regul. 2015, 78, 253–262. [Google Scholar] [CrossRef]
- Pandey, S.; Fartyal, D.; Agarwal, A.; Shukla, T.; James, D.; Kaul, T.; Negi, Y.K.; Arora, S.; Reddy, M.K. Abiotic Stress Tolerance in Plants: Myriad Roles of Ascorbate Peroxidase. Front. Plant Sci. 2017, 8, 581. [Google Scholar] [CrossRef] [Green Version]
- Ogweno, J.O.; Song, X.S.; Shi, K.; Hu, W.H.; Mao, W.H.; Zhou, Y.H.; Yu, J.Q.; Nogués, S. Brassinosteroids Alleviate Heat-Induced Inhibition of Photosynthesis by Increasing Carboxylation Efficiency and Enhancing Antioxidant Systems in Lycopersicon esculentum. J. Plant Growth Regul. 2007, 27, 49–57. [Google Scholar] [CrossRef]
- Ye, J.-H.; Lv, Y.-Q.; Liu, S.-R.; Jin, J.; Wang, Y.-F.; Wei, C.-L.; Zhao, S.-Q. Effects of Light Intensity and Spectral Composition on the Transcriptome Profiles of Leaves in Shade Grown Tea Plants (Camellia sinensis L.) and Regulatory Network of Flavonoid Biosynthesis. Molecules 2021, 26, 5836. [Google Scholar] [CrossRef]
- Huang, S.; Zuo, T.; Xu, W.; Zhang, Y.; Ni, W. Improving Albino Tea Quality by Foliar Application of Glycinebetaine as a Green Regulator under Lower Temperature Conditions. J. Agric. Food Chem. 2021, 69, 1242–1250. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.-P.; Zhang, L.; Yan, P.; Ahammed, G.J.; Han, W.-Y. Methyl Salicylate Enhances Flavonoid Biosynthesis in Tea Leaves by Stimulating the Phenylpropanoid Pathway. Molecules 2019, 24, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of Waterlogging Tolerance in Plants: Research Progress and Prospects. Front. Plant Sci. 2021, 11, 627331. [Google Scholar] [CrossRef]
- Farooq, M.A.; Islam, F.; Yang, C.; Nawaz, A.; Athar, H.-U.; Gill, R.A.; Ali, B.; Song, W.; Zhou, W. Methyl jasmonate alleviates arsenic-induced oxidative damage and modulates the ascorbate–glutathione cycle in oilseed rape roots. Plant Growth Regul. 2018, 84, 135–148. [Google Scholar] [CrossRef]
- Ohlrogge, J.; Browse, J. Lipid biosynthesis. Plant Cell 1995, 7, 957. [Google Scholar] [PubMed] [Green Version]
- Ivanova, T.; Maiorova, O.V.; Orlova, Y.V.; Kuznetsova, E.I.; Khalilova, L.A.; Myasoedov, N.A.; Balnokin, Y.V.; Tsydendambaev, V.D. Cell ultrastructure and fatty acid composition of lipids in vegetative organs of Chenopodium album L. under salt stress conditions. Russ. J. Plant Physiol. 2016, 63, 763–775. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).