Effect of Low-Light Stress on Sugar and Acid Accumulation during Fruit Development and Ripening of Sweet Cherry
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sampling
2.2. Determination of Relevant Indicators
2.2.1. Determination of Photosynthetic Parameters
2.2.2. Determination of Fruit Weight and Shape Index
2.2.3. Determination of Hardness, Soluble Solid Content, Vitamin C, Color, Total Anthocyanin Concentration, Trehalose, and Cellulose Content
2.2.4. Determination of Glycolic Acid Fractions and Content
2.2.5. Determination of Enzymes Related to Glycolic Acid Metabolism
2.3. Transcriptome Sequencing
2.3.1. RNA Extraction and Transcriptome Sequencing
2.3.2. Data Processing and Analysis
2.3.3. Quantitative Real-Time PCR Analysis
2.4. Data Processing and Analysis
3. Results
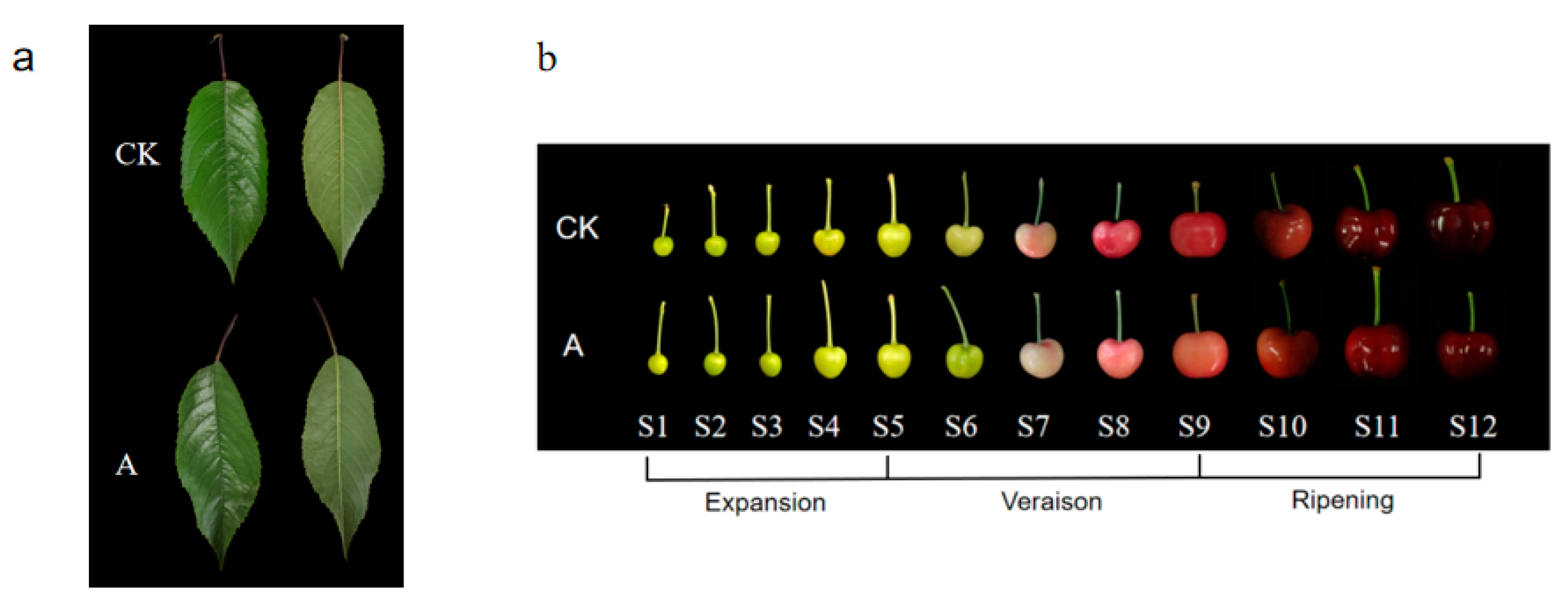
3.1. Fruit Phenotypic Changes under Low-Light Stress
3.2. Leaf Photosynthetic Properties
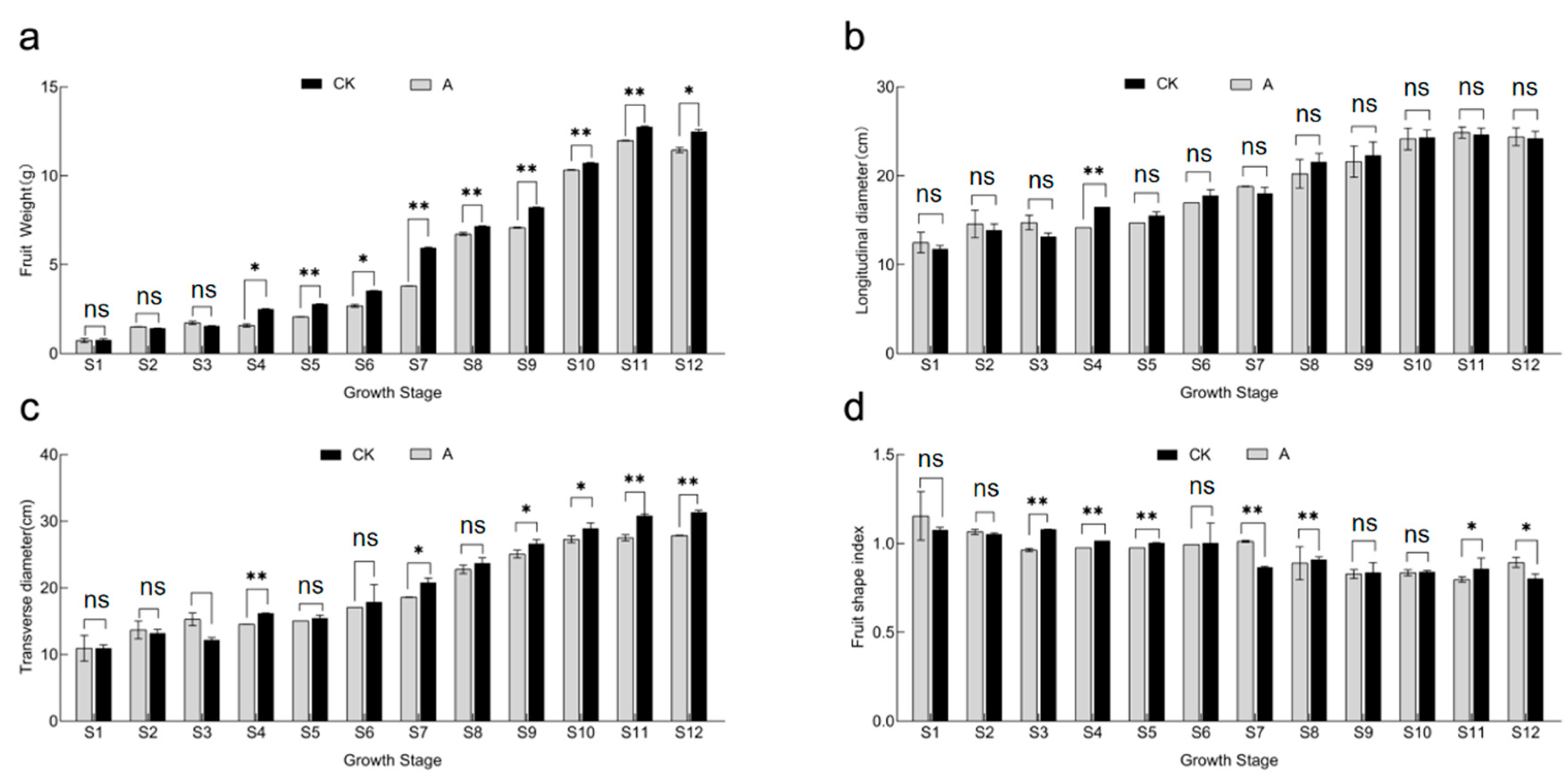
3.3. Changes in General Fruit Characteristics and Quality
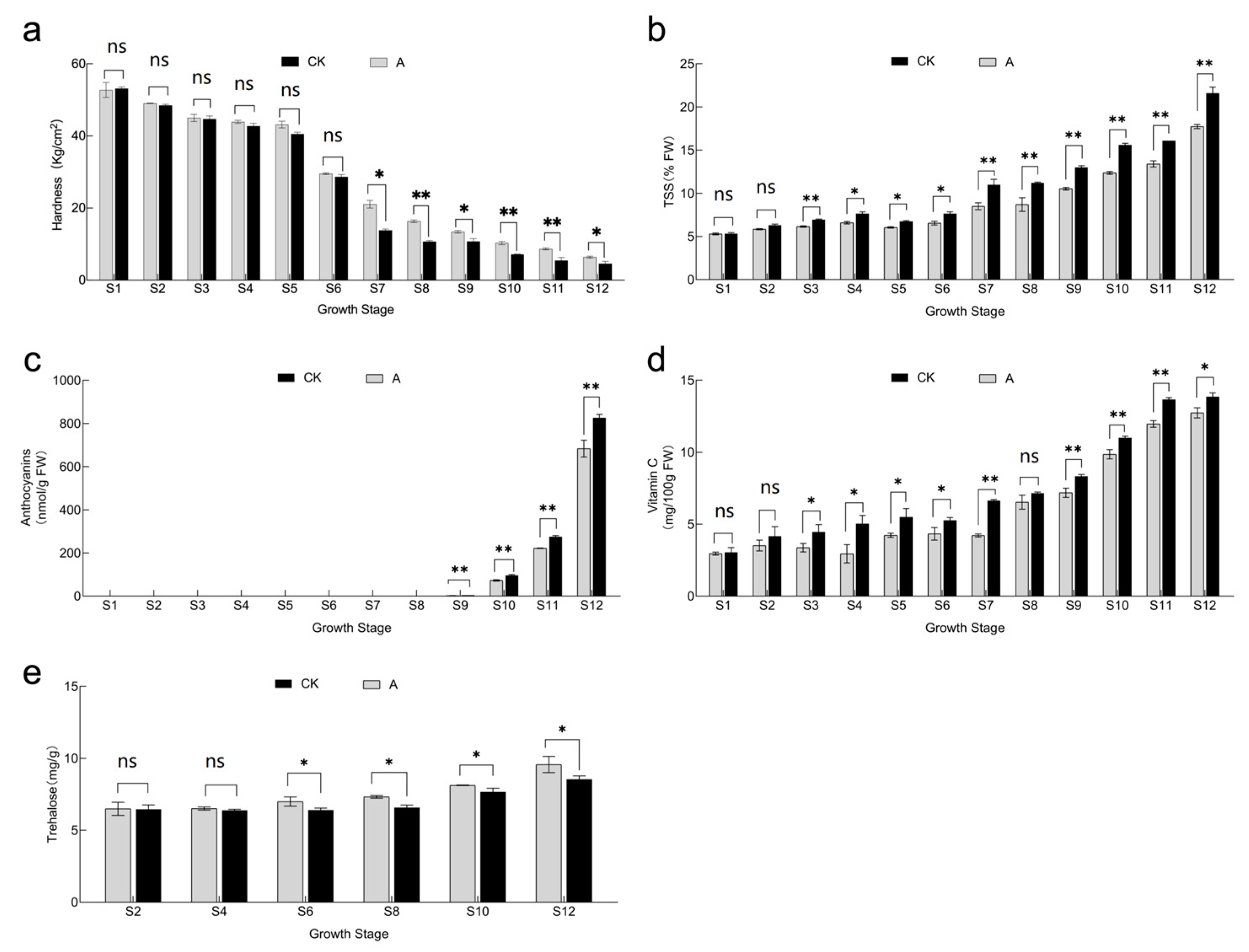
3.4. Effect of Shading Treatment on Fruit Hardness, TSS, Anthocyanin, Vitamin C and Trehalose
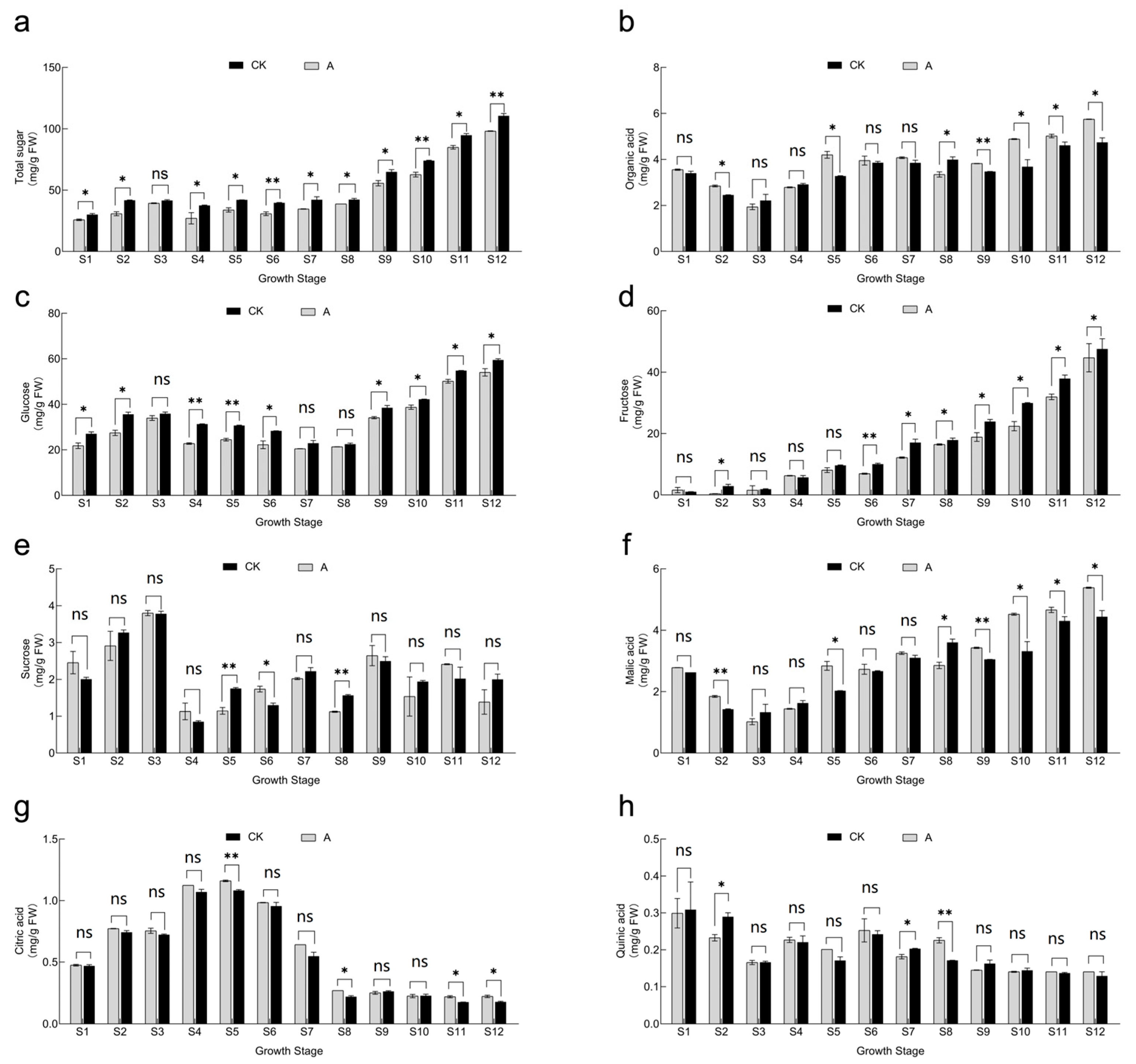
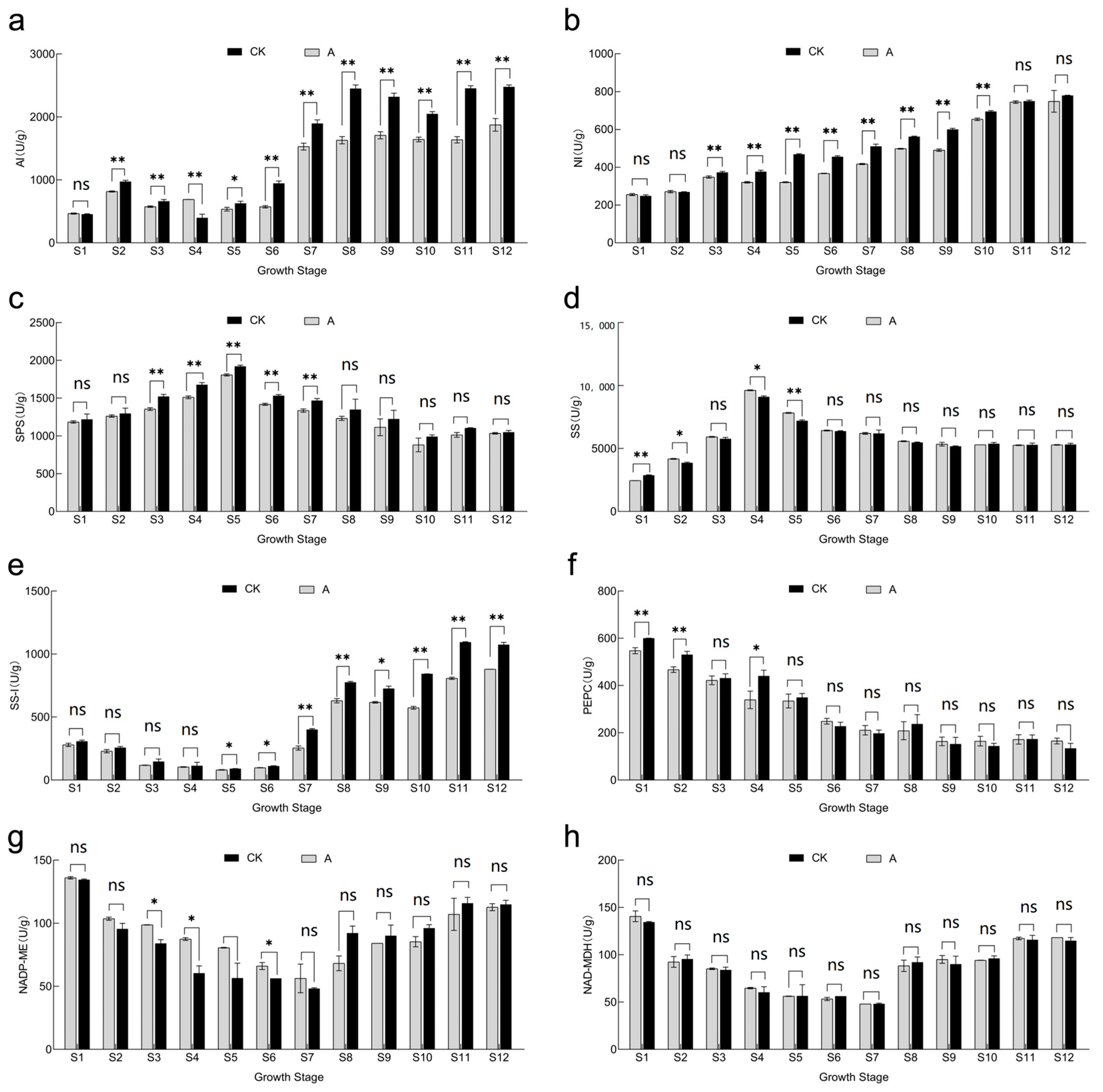
3.5. Effect of Shading Treatment on Fruit Soluble Sugar and Titratable Acid Fractions and Concentrations
3.6. Effect of Shading Treatment on the Activity of Enzymes Related to Sugar and Acid Metabolism in Fruits
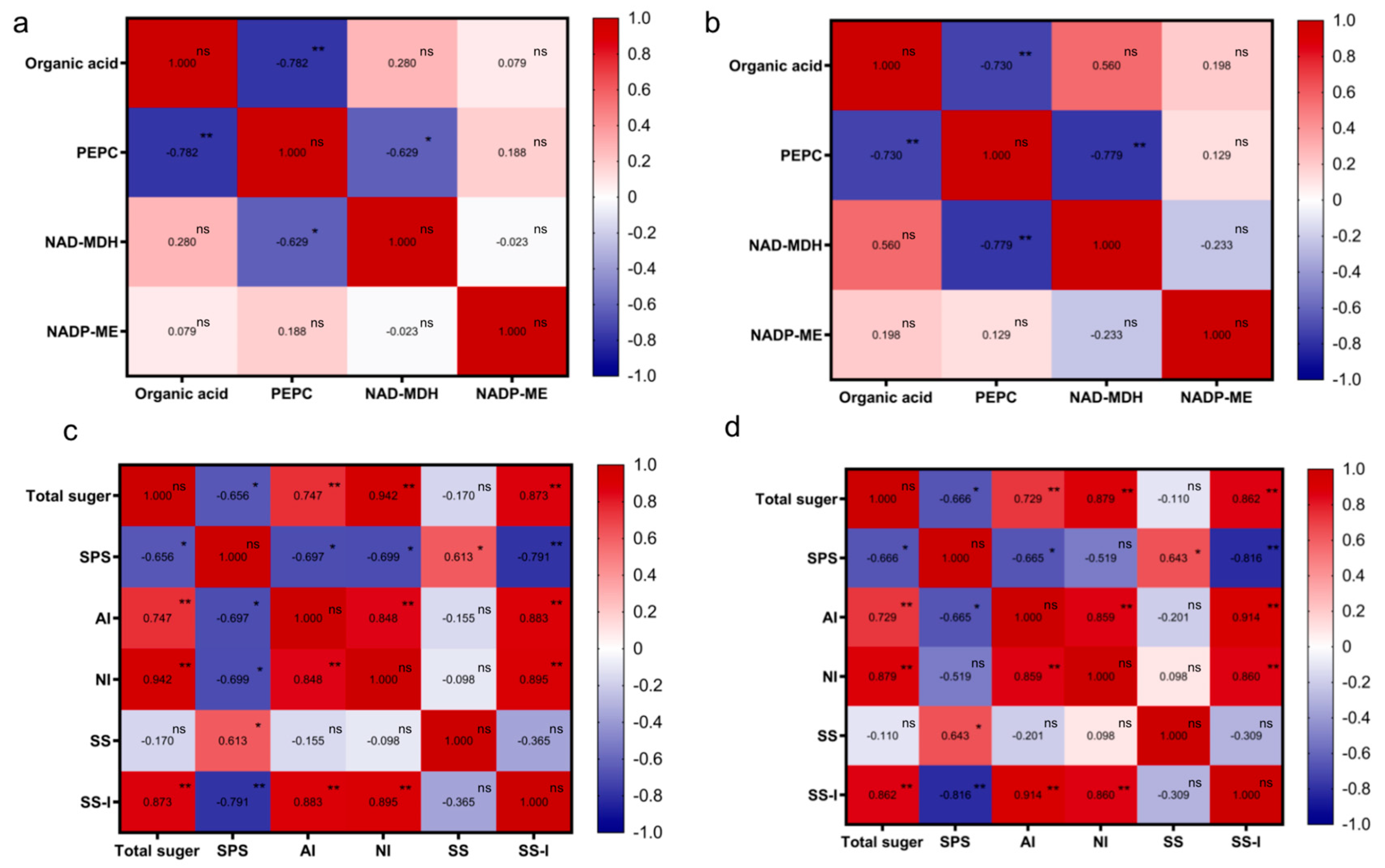
3.7. Correlations between Sugar–Acid Content and Enzyme Activities
3.8. Transcriptome Analysis
3.8.1. Sequencing Quality Analysis
3.8.2. Identification of Differentially Expressed Genes
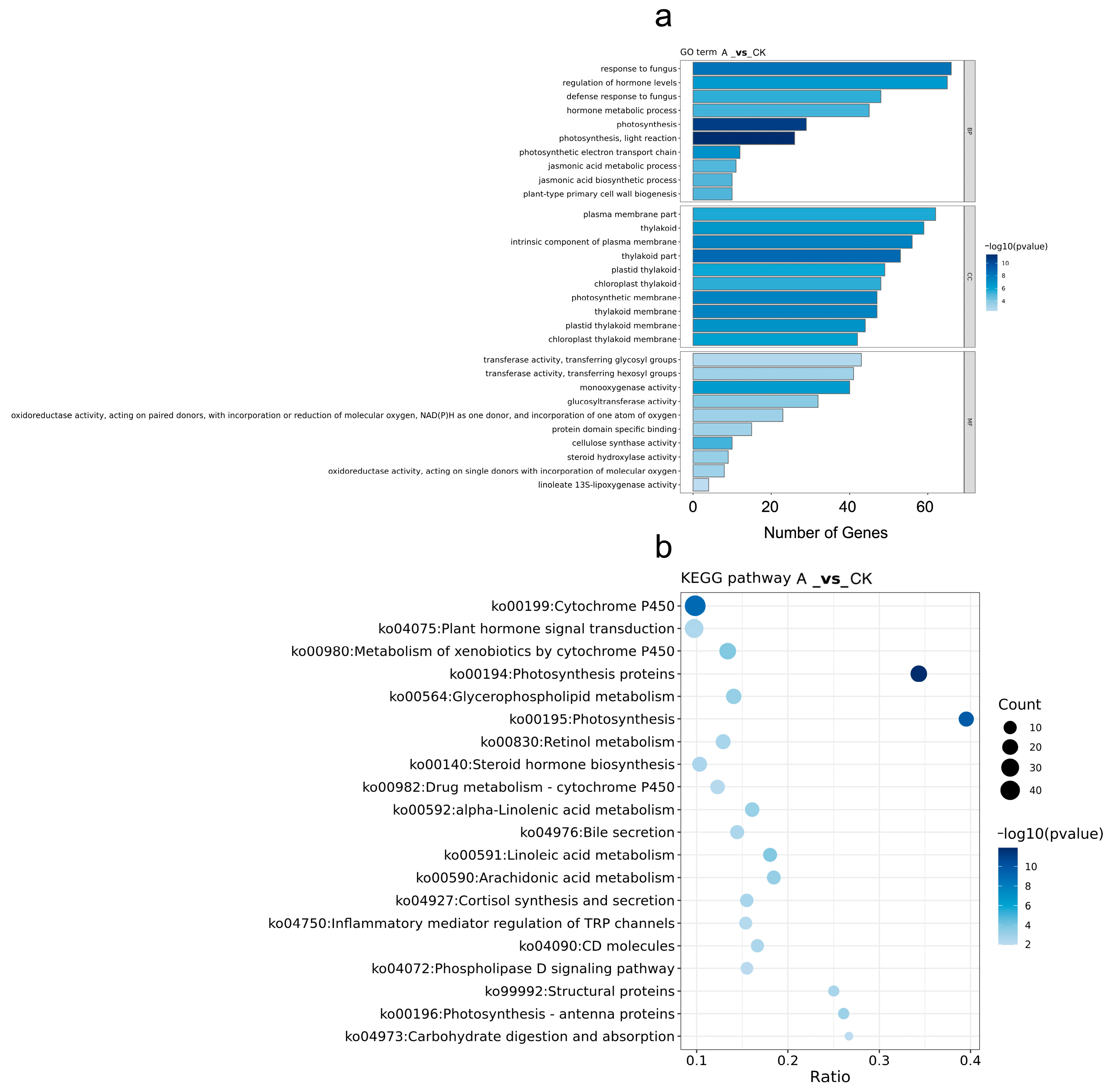
3.8.3. GO and KEGG Enrichment Analyses
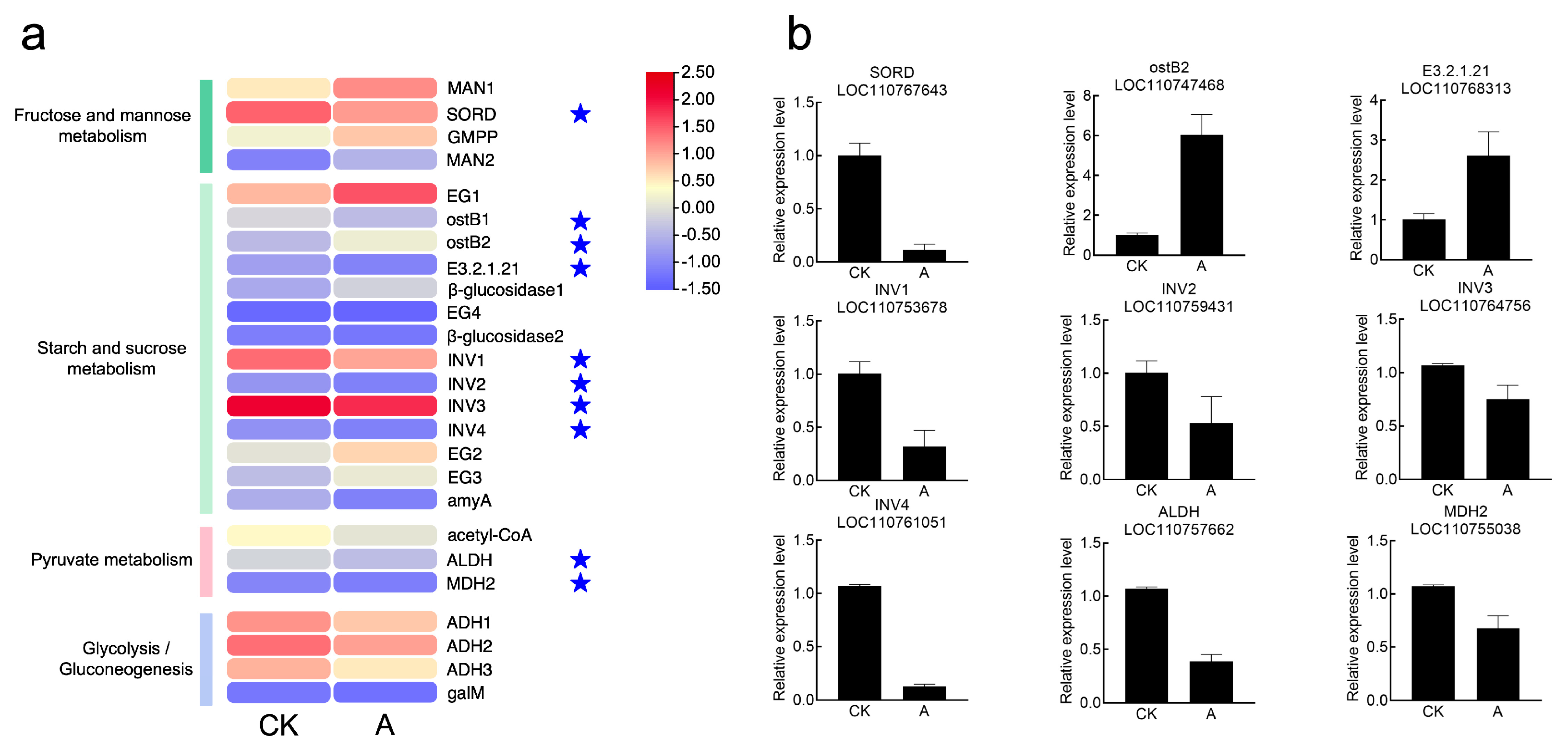
3.8.4. Differential Gene Screening and Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Acero, N.; Gradillas, A.; Beltran, M.; García, A.; Mingarro, D.M. Comparison of phenolic compounds profile and antioxidant properties of different sweet cherry (Prunus avium L.) varieties. Food Chem. 2019, 279, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Valero, D.; Serrano, M. Growth and ripening stage at harvest modulates postharvest quality and bioactive compounds with antioxidant activity. Stewart Postharvest Rev. 2013, 3, 5. [Google Scholar]
- Di, M.; Russo, R.; Graziani, G.; Ritieni, A.; Di, V.C. Characterization of autochthonous sweet cherry cultivars (Prunus avium L.) of southern Italy for fruit quality, bioactive compounds and antioxidant activity. J. Sci. Food Agric. 2017, 97, 2782–2794. [Google Scholar]
- Muccillo, L.; Colantuoni, V.; Sciarrillo, R.; Baiamonte, G.; Salerno, G.; Marziano, M.; Sabatino, L.; Guarino, C. Molecular and environmental analysis of campania (Italy) sweet cherry (Prunus avium L.) Cultivars for biocultural refugia identification and conservation. Sci. Rep. 2019, 9, 6796. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, Y.; Yıldız, K.; KüçÜker, E.; Çekic, C.; Özgen, M.; Akça, Y. Early performance of cv. Jonagold apple on m.9 in five tree training systems. Hortic. Sci. 2012, 39, 158–163. [Google Scholar] [CrossRef]
- Yan, W.; Long, L.E. Physiological and biochemical changes relating to postharvest splitting of sweet cherries affected by calcium application in hydrocooling water. Food Chem. 2015, 181, 241–247. [Google Scholar]
- Aikio, S.; Taulavuori, K.; Hurskainen, S.; Taulavuori, E.; Tuomi, J. Contributions of day length, temperature and individual variability on the rate and timing of leaf senescence in the common lilac syringa vulgaris. Tree Physiol. 2019, 39, 961–970. [Google Scholar] [CrossRef]
- Overbeck, V.; Schmitz, M.; Tartachnyk, I.; Blanke, M. Identification of light availability in different sweet cherry orchards under cover by using non-destructive measurements with a dualex™. Eur. J. Agron. 2017, 93, 50–56. [Google Scholar] [CrossRef]
- Wang, L.F. Physiological and molecular responses to variation of light intensity in rubber tree (Hevea brasiliensis Muell. Arg.). PLoS ONE 2014, 9, e89514. [Google Scholar] [CrossRef]
- Echer, F.R.; Rosolem, C.A. Cotton yield and fiber quality affected by row spacing and shading at different growth stages. Eur. J. Agron. 2015, 65, 18–26. [Google Scholar] [CrossRef]
- Overbeck, V.; Schmitz, M.; Blanke, M. Targeted forcing improves quality, nutritional and health value of sweet cherry fruit. J. Sci. Food Agric. 2017, 97, 3649–3655. [Google Scholar] [CrossRef] [PubMed]
- Szalai, G.; Majláth, I.; Pál, M.; Gondor, O.K.; Rudnóy, S.; Oláh, C.; Vanková, R.; Kalapos, B.; Janda, T. Janus-faced nature of light in the cold acclimation processes of maize. Front. Plant Sci. 2018, 9, 850. [Google Scholar] [CrossRef] [PubMed]
- Sekhar, S.; Panda, D.; Kumar, J.; Mohanty, N.; Biswal, M.; Baig, M.J.; Kumar, A.; Umakanta, N.; Samantaray, S.; Pradhan, S.K.; et al. Comparative transcriptome profiling of low light tolerant and sensitive rice varieties induced by low light stress at active tillering stage. Sci. Rep. 2019, 9, 5753. [Google Scholar] [CrossRef]
- Liu, Q.H.; Wu, X.; Chen, B.; Ma, J.; Gao, J. Effects of low light on agronomic and physiological characteristics of rice including grain yield and quality. Rice Sci. 2014, 21, 243–251. [Google Scholar] [CrossRef]
- Tian, T.; Qiao, G.; Deng, B.; Wen, Z.; Hong, Y.; Wen, X. The effects of rain shelter coverings on the vegetative growth and fruit characteristics of chinese cherry (Prunus pseudocerasus L.). Sci. Hortic. 2019, 254, 228–235. [Google Scholar] [CrossRef]
- Harrison, W.G.; Platt, T. Photosynthesis-irradiance relationships in polar and temperate phytoplankton populations. Polar Biol. 1986, 5, 153–164. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III; Barker, D.H.; Logan, B.A.; Bowling, D.R.; Verhoeven, A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal energy dissipation of excess excitation. Physiol. Plant. 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Yang, W.; Li, J.; Tang, W.; Gong, R. Transcriptomic and Metabolomic Analysis of Quality Changes during Sweet Cherry Fruit Development and Mining of Related Genes. Int. J. Mol. Sci. 2022, 23, 7402. [Google Scholar] [CrossRef]
- Li, M.; Li, J.; Zhang, R.; Lin, Y.; Xiong, A.; Tan, G.; Luo, Y.; Zhang, Y.; Chen, Q.; Wang, Y.; et al. Combined analysis of the metabolome and transcriptome to explore heat stress responses and adaptation mechanisms in celery (Apium graveolens L.). Int. J. Mol. Sci. 2022, 23, 3367. [Google Scholar] [CrossRef]
- Matsubara, S.; Krause, G.H.; Seltmann, M.; Virgo, A.; Kursar, T.A.; Jahns, P.; Winter, K. Lutein epoxide cycle, light harvesting and photoprotection in species of the tropical tree genus Inga. Plant Cell Environ. 2008, 31, 548–561. [Google Scholar] [CrossRef]
- Lü, J.H.; Wang, X.; Feng, Y.M.; Li, Y.F.; Zhao, H.X.; Wang, Y. Effects of shading on the photosynthetic characteristics and anatomical structure of Trolliu schinensis Bunge. Acta Ecol. Sin. 2012, 32, 6033–6043. [Google Scholar]
- Hu, Y.; Chu, H.J.; Li, J.Q. Response of leaf anatomy characteristics and its plasticity to different soil-water conditions of Medicago ruthenica in four populations. Plant Sci. J. 2011, 29, 218–225. [Google Scholar]
- Chen, X.; Zhang, Q.; Zeng, S.M.; Chen, Y.; Guo, Y.Y.; Huang, X.Z. Rain-shelter cultivation affects fruit quality of pear, and the chemical properties and microbial diversity of rhizosphere soil. Can. J. Plant Sci. 2020, 100. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. BBA-Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Jiao, L.L.; Lu, B.S.; Zhou, R.J.; Bai, Z.Y.; Liang, H.Y.; Zhen, H.W. Effects of shading on net photosynthetic rate and chlorophyII fluorescence parameters of leaf in david maple (Acer davidii Franch). Acta Hortic. Sin. 2007, 34, 173–178. [Google Scholar]
- Busch, F.; Hüner Norman, P.A.; Ensminger, I. Biochemical constrains limit the potential of the photochemical reflectance index as a predictor of effective quantum efficiency of photosynthesis during the winter spring transition in Jack pine seedlings. Funct. Plant Biol. 2009, 36, 115–119. [Google Scholar] [CrossRef]
- Osório, M.L.; Osório, J.; Vieira, A.C.; Gonçalves, S.; Romano, A. Influence of enhanced temperature on photosynthesis, photooxidative damage, and antioxidant strategies in Ceratonia siliqua L. seedlings subjected to water deficit and rewatering. Photosynthetica 2011, 49, 3–12. [Google Scholar] [CrossRef]
- Zhang, S.R. A discussion on chlorophyll fluorescence kinetics parameters and their significance. Chin. Bull. Bot. 1999, 16, 444–448. [Google Scholar]
- Zhou, Y.H.; Hang, L.F.; Yu, J.Q. Effects of sustained chilling and low light on gas exchange, chlorophyll fluorescence quenching and absorbed light allocation in cucumber leaves. J. Plant Physiol. Mol. Biol. 2004, 30, 153–160. [Google Scholar]
- Chen, Z.; Deng, H.; Xiong, B.; Li, S.; Yang, L.; Yang, Y.; Huang, S.; Tan, L.; Sun, G.; Wang, Z. Rootstock Effects on Anthocyanin Accumulation and Associated Biosynthetic Gene Expression and Enzyme Activity during Fruit Development and Ripening of Blood Oranges. Agriculture 2022, 12, 342. [Google Scholar] [CrossRef]
- Morales, J.; Tárrega, A.; Salvador, A.; Navarro, P.; Besada, C. Impact of ethylene degreening treatment on sensory properties and consumer response to citrus fruits. Food Res. Int. 2020, 127, 108641. [Google Scholar] [CrossRef]
- Pangborn, R.M. Relative taste intensities of selected sugars and organic acids. J. Food Sci. 2006, 28, 726–733. [Google Scholar] [CrossRef]
- Lamikanra, O.; Inyang, I.D.; Leong, S. Distribution and effect of grape maturity on organic acid content of red muscadine grapes. J. Agric. Food Chem. 1995, 43, 3026–3028. [Google Scholar] [CrossRef]
- Qian, Z.; Xi, X.J.; Yani, H.E.; Jiang, A.L. Bagging affecting sugar and anthocyanin metabolism in the ripening period of grape berries. Not. Bot. Horti Agrobot. 2019, 47, 1194–1205. [Google Scholar]
- Suran, P.; Vavra, R.; Jonas, M.; Zeleny, L.; Skrivanova, A. Effect of rain protective covering of sweet cherry orchard on fruit quality and cracking. Acta Hortic. 2019, 1235, 189–195. [Google Scholar] [CrossRef]
- Børve, J.; Skaar, E.; Sekse, L.; Meland, M.; Vangdal, E. Rain protective covering of sweet cherry trees-effects of different covering methods on fruit quality and microclimate. Horttechnology 2003, 13, 143–148. [Google Scholar] [CrossRef]
- Menon, S.V.; Rao TV, R. Health—Promoting components and related enzyme activities of muskmelon fruit during its development and ripening. J. Food Biochem. 2014, 38, 415–423. [Google Scholar] [CrossRef]
- Xie, Z.S.; Li, B.; Forney, C.F.; Xu, W.; Wang, S. Changes in sugar content and relative enzyme activity in grape berry in response to root restriction. Sci. Hortic. 2009, 123, 39–45. [Google Scholar] [CrossRef]
- Klages, K. Diurnal changes in non-structural carbohydrates in leaves, phloem exudates and fruit in ‘Braeburn’ apple. Funct. Plant Biol. 2001, 28, 131–139. [Google Scholar] [CrossRef]
- Ali, K.; Maltese, F.; Choi, Y.H.; Verpoorte, R. Metabolic constituents of grapevine and grape-derived products. Phytochem. Rev. 2010, 9, 357–378. [Google Scholar] [CrossRef]
- Okamoto, G.; Fujii, Y.; Shimamura, K. Effect of levels of fertilizer application on tree growth and fruit quality of peach trees on Prunus tomentasa. Environ. Control. Biol. 2010, 27, 83–87. [Google Scholar] [CrossRef]


| Treatment | (Pn)/(µmol·m−2·s−1) | (Tr)/(mol·m−2·s−1) | (Gs)/(mol·m−2·s−1) | (Ci)/(µmol·mol−1) |
|---|---|---|---|---|
| CK | 11.1 ± 0.062 a | 0.001 ± 0.001 a | 0.140 ± 0.002 a | 244 ± 2.31 a |
| A | 10.2 ± 0.171 b | 0.001 ± 0.001 b | 0.126 ± 0.002 b | 247 ± 3.88 a |
| Treatment | Fv/Fm | ΦPSII | ETR | NPQ | qP |
|---|---|---|---|---|---|
| CK | 0.808 ± 0.003 a | 0.466 ± 0.000 a | 134± 0.115 a | 1.19 ± 0.001 a | 0.740 ± 0.003 a |
| A | 0.795 ± 0.005 b | 0.495 ± 0.001 b | 117 ± 0.253 b | 0.909 ± 0.010 b | 0.792 ± 0.002 b |
| Treatment | Antenna Thermal Dissipation (D) | Photochemical Reaction Dissipation(P) | Non-Photochemical Reaction Dissipation (Ex) |
|---|---|---|---|
| CK | 42.9 ± 0.291 a | 42.3 ± 0.098 a | 14.9 ± 0.263 a |
| A | 34.3 ± 0.115 b | 52.1 ± 0.103 b | 13.6 ± 0.107 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Chen, C.; Zhang, Y.; Chu, Y.; Yang, W.; Cui, Y.; Kou, G.; Chen, H.; Song, H.; Gong, R. Effect of Low-Light Stress on Sugar and Acid Accumulation during Fruit Development and Ripening of Sweet Cherry. Horticulturae 2023, 9, 654. https://doi.org/10.3390/horticulturae9060654
Tang W, Chen C, Zhang Y, Chu Y, Yang W, Cui Y, Kou G, Chen H, Song H, Gong R. Effect of Low-Light Stress on Sugar and Acid Accumulation during Fruit Development and Ripening of Sweet Cherry. Horticulturae. 2023; 9(6):654. https://doi.org/10.3390/horticulturae9060654
Chicago/Turabian StyleTang, Wenjing, Chaoqun Chen, Yao Zhang, Yuanqi Chu, Wenlong Yang, Yiling Cui, Guanqiong Kou, Hongxu Chen, Haiyan Song, and Ronggao Gong. 2023. "Effect of Low-Light Stress on Sugar and Acid Accumulation during Fruit Development and Ripening of Sweet Cherry" Horticulturae 9, no. 6: 654. https://doi.org/10.3390/horticulturae9060654
APA StyleTang, W., Chen, C., Zhang, Y., Chu, Y., Yang, W., Cui, Y., Kou, G., Chen, H., Song, H., & Gong, R. (2023). Effect of Low-Light Stress on Sugar and Acid Accumulation during Fruit Development and Ripening of Sweet Cherry. Horticulturae, 9(6), 654. https://doi.org/10.3390/horticulturae9060654






