Preliminary Study on the Impact of Non-Thermal Plasma Activated Water on the Quality of Triticum aestivum L. cv. Glosa Sprouts
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Vegetal Material
2.3. Generation of Non-Thermal Plasma-Activated Water
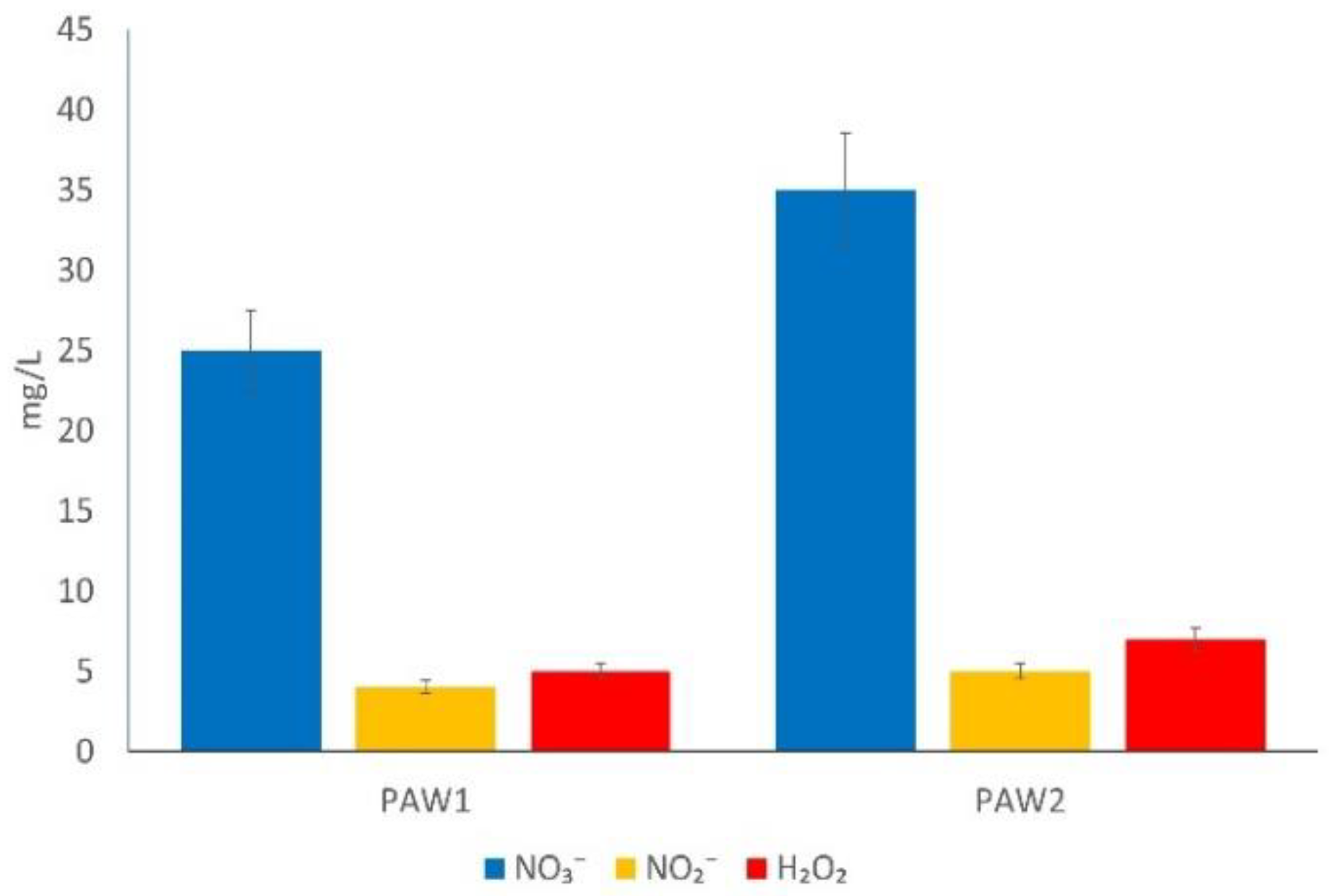
2.4. Non-Thermal Plasma-Activated Water: Composition and Analysis
2.5. Treatment and Germination of Wheat Caryopses
2.6. Biometric Measurements
2.7. Total Protein Content
2.8. Photosynthetic Pigments Content
2.9. Extraction of Free and Bound Phenolic Fractions
2.10. Total Phenolic Content
2.11. Antioxidant Activity
2.12. Superoxide Dismutase Activity
2.13. Catalase Activity
2.14. Peroxidase Activity
2.15. Statistical Analysis
3. Results
3.1. Generation and Analysis of Non-Thermal Plasma-Activated Water
3.2. Germination Rate
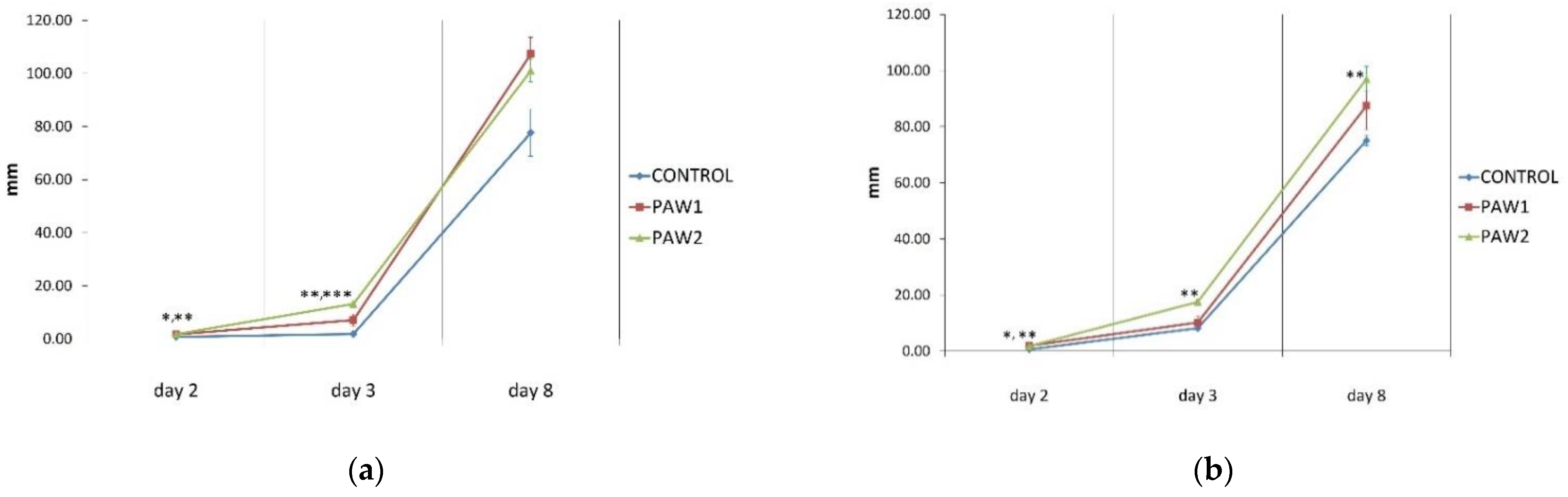
3.3. Growth Parameters
3.4. Total Protein Content
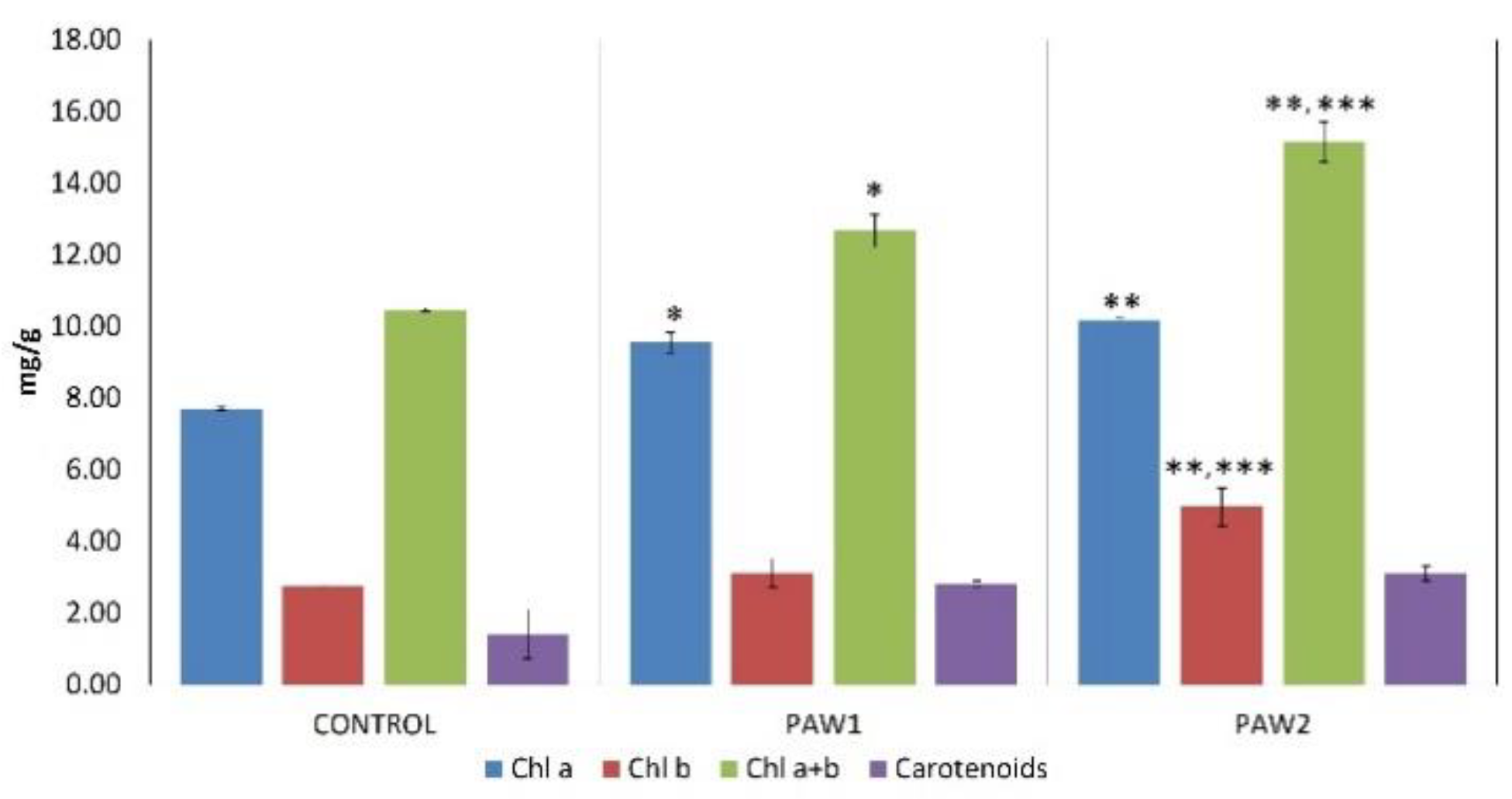
3.5. Photosynthetic Pigments Content
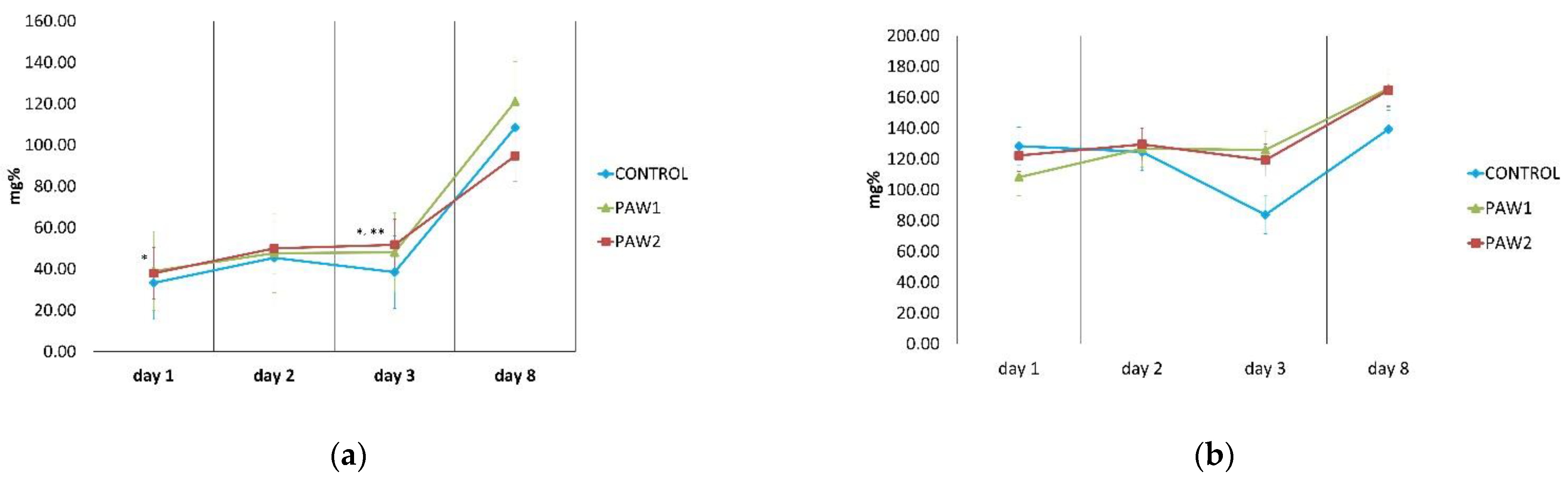
3.6. Total Phenolic Content
3.7. Antioxidant Activity
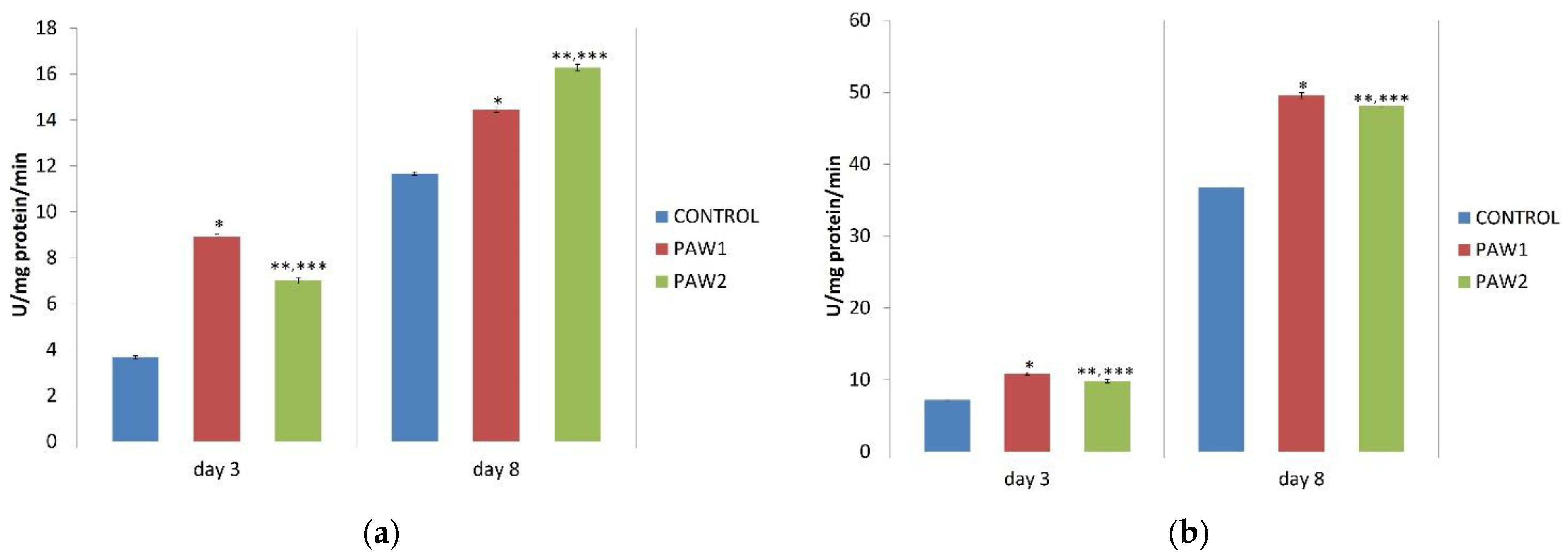
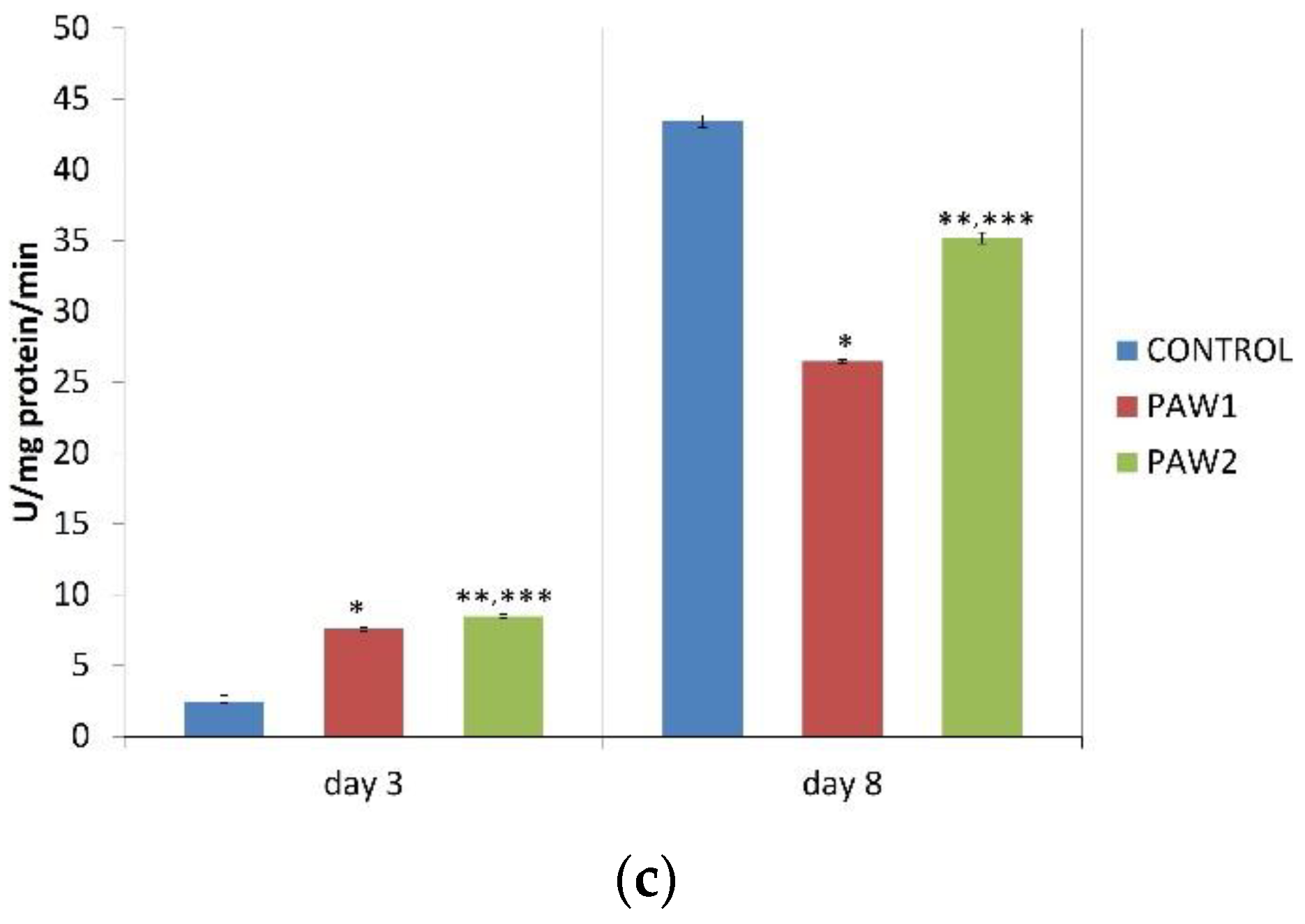
3.8. Antioxidant Enzymes Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neuhouser, M.L. The importance of healthy dietary patterns in chronic disease prevention. Nutr. Res. 2019, 70, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Whole grains and human health. Nutr. Res. Rev. 2004, 17, 99–110. [Google Scholar] [CrossRef]
- Stevenson, L.; Phillips, F.; O’Sullivan, K.; Walton, J. Wheat Bran: Its Composition and Benefits to Health, a European Perspective. Int. J. Food Sci. Nutr. 2012, 63, 1001–1013. [Google Scholar] [CrossRef]
- Štěrbová, L.; Bradová, J.; Sedláček, T.; Holasová, M.; Fiedlerová, V.; Dvořáček, V.; Smrčková, P. Influence of technological processing of wheat grain on digestibility and resistant starch content. Starch 2016, 68, 593–602. [Google Scholar] [CrossRef]
- Russell, W.R.; Hoyles, L.; Flint, H.J.; Dumas, M.-E. Colonic bacterial metabolites and human health. Curr. Opin. Microbiol. 2013, 16, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.L.; Poutanen, K.; Gebruers, K.; Piironen, V.; Lampi, A.-M.; Nyström, L.; Andersson, A.A.M.; Åman, P.; Boros, D.; Rakszegi, M.; et al. The HEALTHGRAIN cereal diversity screen: Concept, results and prospects. J. Agric. Food Chem. 2008, 56, 9699–9709. [Google Scholar] [CrossRef]
- Gonzalez-Thuillier, I.; Salt, L.; Chope, G.; Penson, S.; Skeggs, P.; Tosi, P.; Powers, S.J.; Ward, J.L.; Wilde, P.; Shewry, P.R.; et al. Distribution of Lipids in the Grain of Wheat (cv. Hereward) Determined by Lipidomic Analysis of Milling and Pearling Fractions. J. Agric. Food Chem. 2015, 63, 10705–10716. [Google Scholar] [CrossRef]
- Prinsen, P.; Gutiérrez, A.; Faulds, C.B.; Del Río, J.C. Comprehensive Study of Valuable Lipophilic Phytochemicals in Wheat Bran. J. Agric. Food Chem. 2014, 62, 1664–1673. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- Lampi, A.-M.; Nurmi, T.; Ollilainen, V.; Piironen, V. Tocopherols and Tocotrienols in Wheat Genotypes in the HEALTHGRAIN Diversity Screen. Agric. Food Chem. 2008, 56, 9716–9721. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The role of vitamin E in human health and some diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar]
- Vasanthi, H.R.; Parameswari, R.P.; Das, D.K. Multifaceted role of tocotrienols in cardioprotection supports their structure: Function relation. Genes Nutr. 2012, 7, 19–28. [Google Scholar] [CrossRef]
- Hussain, A.; Larsson, H.; Johansson, E. Carotenoid Extraction from Locally and Organically Produced Cereals Using Saponification Method. Processes 2021, 9, 783. [Google Scholar] [CrossRef]
- Trono, D. Carotenoids in Cereal Food Crops: Composition and Retention throught Grain Storage and Food Processing. Plants 2019, 8, 551. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Lui, W.-Y.; Wu, K.; Chan, C.-L.; Dai, S.-H.; Sui, Z.-Q.; Corke, H. Bioactive compounds and bioactivities of germinated edible seeds and sprouts: An updated review. Trends Food Sci. Technol. 2017, 59, 1–14. [Google Scholar] [CrossRef]
- Likes, R.; Madl, R.L.; Zeisel, S.H.; Craig, S.A.S. The betaine and choline content of a whole wheat flour compared to other mill streams. J. Cereal Sci. 2007, 46, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Tinelli, C.; Di Pino, A.; Ficulle, E.; Marcelli, S.; Feligioni, M. Hyperhomocysteinemia as a Risk Factor and Potential Nutraceutical Target for Certain Pathologies. Front. Nutr. 2019, 6, 49. [Google Scholar] [CrossRef]
- Calinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review in Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef]
- Mpofu, A.; Sapirstein, H.D.; Beta, T. Genotype and Environmental Variation in Phenolic Content, Phenolic Acid Composition, and Antioxidant Activity of Hard Spring Wheat. J. Agric. Food Chem. 2006, 54, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shewry, P.R.; Ward, J.L. Phenolic Acids in Wheat Varieties in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2008, 56, 9732–9739. [Google Scholar] [CrossRef]
- Okarter, N.; Liu, C.-S.; Sorrells, M.E.; Liu, R.H. Phytochemical Content and Antioxidant Activity of Six Diverse Varieties of Whole Wheat. Food Chem. 2010, 119, 249–257. [Google Scholar] [CrossRef]
- Tian, W.; Li, Y. Phenolic Acid Composition and Antioxidant Activity of Hard Red Winter Wheat Varieties. J. Food Biochem. 2018, 42, e12682. [Google Scholar] [CrossRef]
- Van Hung, P.; Hatcher, D.W.; Barker, W. Phenolic acid composition of sprouted wheats by ultra-performance liquid chromatography (UPLC) and their antioxidant activities. Food Chem. 2011, 126, 1896–1901. [Google Scholar] [CrossRef]
- Ding, J.; Feng, H. Controlled germination for enhancing the nutritional value of sprouted grains. In Sprouted Grains: Nutritional Value, Production, and Applications; Feng, H., Nemzer, B., DeVries, J.W., Eds.; Woodhead Publishing: Duxford, UK, 2019; pp. 91–112. [Google Scholar] [CrossRef]
- Van Hung, P.; Maeda, T.; Morita, N. Improvement of Nutritional Composition and Antioxidant Capacity of High-Amylose Wheat during Germination. J. Food Sci. Technol. 2015, 52, 6756–6762. [Google Scholar] [CrossRef] [PubMed]
- Ceccaroni, D.; Alfeo, V.; Bravi, E.; Sileoni, V.; Perreti, G.; Marconi, O. Effect of the time and temperature of germination on the phenolic compounds of Triticum aestivum L. and Panicum miliaceum L. LWT Food Sci. Technol. 2020, 127, 109396. [Google Scholar] [CrossRef]
- Perez, S.M.; Biondi, E.; Laurita, R.; Proto, M.; Sarti, F.; Gherardi, M.; Bertaccini, A.; Colombo, V. Plasma activated water as resistance inducer against bacterial leaf spot of tomato. PLoS ONE 2019, 14, e0217788. [Google Scholar] [CrossRef]
- Yodpitaka, S.; Mahatheeranonta, S.; Boonyawand, D.; Sookwonga, P.; Roytrakule, S.; Norkaew, O. Cold plasma treatment to improve germination and enhance the bioactive phytochemical content of germinated brown rice. Food Chem. 2019, 289, 328–339. [Google Scholar] [CrossRef]
- Kučerová, K.; Henselová, M.; Slováková, L.; Hensel, K. Effects of plasma activated water on wheat: Germination, growth parameters, photosynthetic pigments, soluble protein content, and antioxidant enzymes activity. Plasma Process. Polym. 2019, 16, e1800131. [Google Scholar] [CrossRef]
- Yang, F.; Basu, T.K.; Ooraikul, B. Studies on germination conditions and antioxidant contents of wheat grain. Int. J. Food Sci. Nutr. 2001, 52, 319–330. [Google Scholar] [CrossRef]
- Hsieh, K.C.; Wandell, R.J.; Bresch, S.; Locke, B.R. Analysis of hydroxyl radical formation in a gas-liquid electrical discharge plasma reactor utilizing liquid and gaseous radical scavengers. Plasma Process. Polym. 2017, 14, e1600171. [Google Scholar] [CrossRef]
- Wandell, R.J.; Wang, H.; Tachibana, K.; Makled, B.; Locke, B.R. Nanosecond pulsed plasma discharge over a flowing water film: Characterization of hydrodynamics, electrical, and plasma properties and their effect on hydrogen peroxide generation. Plasma Process. Polym. 2018, 15, e1800008. [Google Scholar] [CrossRef]
- Burlica, R.; Shih, K.Y.; Locke, B.R. Formation of H2 and H2O2 in a water-spray gliding arc nonthermal plasma reactor. Ind. Engin. Chem. Res. 2010, 49, 6342–6349. [Google Scholar] [CrossRef]
- Cretu, D.E.; Astanei, D.; Burlica, R.; Beniuga, O.; Tesoi, D. The Influence of NTP Reactor Geometry on H2O2 Generation in Water. In Proceedings of the 2020 International Conference and Exposition on Electrical and Power Engineering (EPE), Iasi, Romania, 22–23 October 2020; pp. 632–635. [Google Scholar] [CrossRef]
- Colorimetric Test Kit VISOCOLOR ECO Nitrate. Available online: https://www.mn-net.com/media/pdf/a5/a5/8f/Instruction-931041-931241-Colorimetric-test-kit-VISOCOLOR-ECO-Nitrate.pdf (accessed on 15 October 2022).
- Colorimetric Test Kit VISOCOLOR ECO Nitrite. Available online: https://www.mn-net.com/media/pdf/ee/4f/f2/Instruction-931044-931244-Colorimetric-test-kit-VISOCOLOR-ECO-Nitrite.pdf (accessed on 15 October 2022).
- Mughal, I.; Shah, Y.; Tahir, S.; Haider, W.; Fayyaz, M.; Yasmin, T.; Ilyas, M.; Farrakh, S. Protein quantification and enzyme activity estimation of Pakistani wheat landraces. PLoS ONE 2020, 15, e0239375. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic membranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Vochita, G.; Oprica, L.; Gherghel, D.; Mihai, C.-T.; Boukherroub, R.; Lobiuc, A. Graphene oxide effects in early ontogenetic stages of Triticum aestivum L. seedlings. Ecotoxicol. Environ. Safety 2019, 181, 345–352. [Google Scholar] [CrossRef]
- Stagnari, F.; Glieni, A.; D’Egidio, S.; Falcinelli, B.; Pagnani, G.; Pace, R. Effects of sprouting and salt stress on polyphenol composition and antiradical activity of einkorn, emmer and durum wheat. Ital. J. Agron. 2017, 12, 293–301. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Vasincu, A.; Paulsen, B.S.; Diallo, D.; Vasincu, I.; Aprotosoaie, A.C.; Bild, V.; Charalambous, C.; Constantinou, A.I.; Miron, A.; Gavrilescu, C.M. Veronia kotschyana Roots: Therapeutic Potential via Antioxidant Activity. Molecules 2014, 19, 19114–19136. [Google Scholar] [CrossRef]
- Naidoo, C.M.M.; Naidoo, Y.; Dewir, Y.H.; Singh, M.; Daniels, A.N.; El-Ramady, H. In Vitro Investigation of the Antioxidant and Cytotoxic Potential of Tabernaemontana ventricosa Hochst. ex A. DC. Leaf, Stem, and Latex Extracts. Horticulturae 2022, 8, 91. [Google Scholar] [CrossRef]
- Unal, M.S.; Gundesli, M.A.; Ercisli, S.; Kupe, M.; Assouguem, A.; Ullah, R.; Almeer, R.; Najda, A. Cultivar Differences on Nutraceuticals of Grape Juices and Seeds. Horticulturae 2022, 8, 267. [Google Scholar] [CrossRef]
- Winterbourn, C.; Hawkins, R.; Brian, M.; Carrel, R. The estimation of red cell superoxide dismutase activity. J. Lab.Clin. Med. 1975, 85, 337–341. [Google Scholar]
- Artenie, V.; Ungureanu, E.; Negură, A.M. Metode de Investigare a Metabolismului Glucidic şi Lipidic; PIM: Iaşi, Romania, 2008; p. 182. [Google Scholar]
- Beers, R.; Sizer, I. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Su, L.; Lan, Q.; Pritchard, H.W.; Xue, H.; Wang, X. Reactive oxygen species induced by cold stratification promote germination of Hedysarum scoparium seeds. Plant Physiol. Biochem. 2016, 109, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Šírová, J.; Sedlářová, M.; Piterková, J.; Luhová, L.; Petřivalský, M. The role of nitric oxide in the germination of plant seeds and pollen. Plant Sci. 2011, 181, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cheng, J.-H.; Sun, D.-W. Enhancement of Wheat Germination, Seedling Growth and Nutritional Proprieties of Wheat Plantlet Juice by Plasma Activated Water. J. Plant Growth Regul. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Rousseau, A.; Dufour, T. Promoting lentil germination and stem growth by plasma activated tap water, demineralized water and liquid fertilizer. RSC Adv. 2017, 7, 31244. [Google Scholar] [CrossRef]
- Fan, L.; Liu, X.; Ma, Y.; Xiang, Q. Effects of plasma-activated water treatment on seed germination and growth of mung bean sprouts. J. Taibah Univ. Sci. 2020, 14, 823–830. [Google Scholar] [CrossRef]
- Lo Porto, C.; Ziuzina, D.; Los, A.; Boehm, D.; Palumbo, F.; Favia, P.; Tiwari, B.; Bourke, P.; Cullen, P.J. Plasma activated water and airborne ultrasound treatments for enhanced germination and growth of soybean. Innov. Food Sci. Emerg. Technol. 2018, 49, 13–19. [Google Scholar] [CrossRef]
- Naumova, I.K.; Maksimov, A.I.; Khlyustova, A.V. Stimulation of the Germinability of Seeds and Germ Growth under Treatment with Plasma-Activated Water. Surf. Eng. Appl. Elecvtrochem. 2011, 47, 263–265. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Sinclair, A.J.; Cahill, D.M.; Wang, X.; Dai, X.J. Nitrate and Hydrogen Peroxide Generated in Water by Electrical Discharges Stimulate Wheat Seedling Growth. Plasma Chem. Plasma Process. 2017, 37, 1393–1404. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Khacef, A. Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: Combined effect of seed and water treatment. RSC Adv. 2017, 7, 1822. [Google Scholar] [CrossRef]
- Sajib, S.A.; Billah, M.; Mahmud, S.; Mlah, M.; Hossain, F.; Omar, F.B.; Roy, N.C.; Hoque, K.M.F.; Talukder, M.R.; Kabir, A.H.; et al. Plasma activated water: The next generation eco-friendly stimulant for enhancing plant seed germination, vigor and increased enzyme activity, a study on black gram (Vigna mungo L.). Plasma Chem. Plasma Process. 2020, 40, 119–143. [Google Scholar] [CrossRef]
- Stoleru, V.; Burlică, R.; Mihalache, G.; Dîrlău, I.D.; Pădureanu, S.; Teliban, G.-C.; Astanei, D.; Cojocaru, A.; Beniugă, O.; Patraș, A.J. Plant growth promotion effect of plasma activated water on Lactuca sativa L. cultivated in two different volumes of substrate. Nat. Sci. Rep. 2020, 10, 20920. [Google Scholar] [CrossRef]
- Nurnaeimah, N.; Mat, N.; Suryati Mohd, S.; Badaluddin, N.A.; Yusoff, N.; Sajili, M.H.; Mahmud, K.; Mohd Adnan, A.F.; Khandaker, M.M. The Effects of Hydrogen Peroxide on Plant Growth, Mineral Accumulation, as Well as Biological and Chemical Properties of Ficus deltoidea. Agronomy 2020, 10, 599. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Fang, G.; Zhao, Q.; Zeng, Q.; Li, X.; Gong, H.; Li, Y. Nitrite Promotes the Growth and Decreases the Lignin Content of indica Rice Calli: A Comprehensive Transcriptome Analysis of Nitrite-Responsive Genes during In Vitro Culture of Rice. PLoS ONE 2014, 9, e95105. [Google Scholar] [CrossRef] [PubMed]
- Punith, N.; Harsha, R.; Lakshminarayana, R.; Hemanth, M.; Anand, M.S.; Dasappa, S. Plasma Activated Water Generation and its Application in Agriculture. Adv. Matter. Lett. 2019, 10, 700–704. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Agnihotri, A.; Seth, C.S. Exogenously applied nitrate improves the photosynthetic performance and nitrogen metabolism in tomato (Solanum lycopersicum L. cv Pusa Rohini) under arsenic (V) toxicity. Physiol. Mol. Biol. Plants 2016, 22, 341–349. [Google Scholar] [CrossRef]
- Zhang, R.-Q.; Zhu, H.-H.; Zhao, H.-Q.; Yao, Q. Arbuscular mycorrhizal fungal inoculation increases phenolic synthesis in clover roots via hydrogen peroxide, salicylic acid and nitric oxide signaling pathways. J. Plant Physiol. 2013, 170, 74–79. [Google Scholar] [CrossRef]
- Niu, L.; Liao, W. Hydrogen Peroxide Signaling in Plant Development and Abiotic Responses: Crosstalk with Nitric Oxide and Calcium. Front. Plant. Sci. 2016, 7, 230. [Google Scholar] [CrossRef]
- Bogdanović, J.; Radotić, K.; Mitrović, A. Changes in activities of antioxidant enzymes during Chenopodium murale seed germination. Biol. Plant. 2008, 52, 396–400. [Google Scholar] [CrossRef]
- Puač, N.; Škoro, N.; Spasić, K.; Živković, S.; Miltunović, M.; Malović, G.; Petrović, Z.L.J. Activity of catalase enzyme in Paulownia tomentosa seeds during the process of germination after treatments with low pressure plasma and plasma activated water. Plasma Process. Polym. 2018, 15, e1700082. [Google Scholar] [CrossRef]
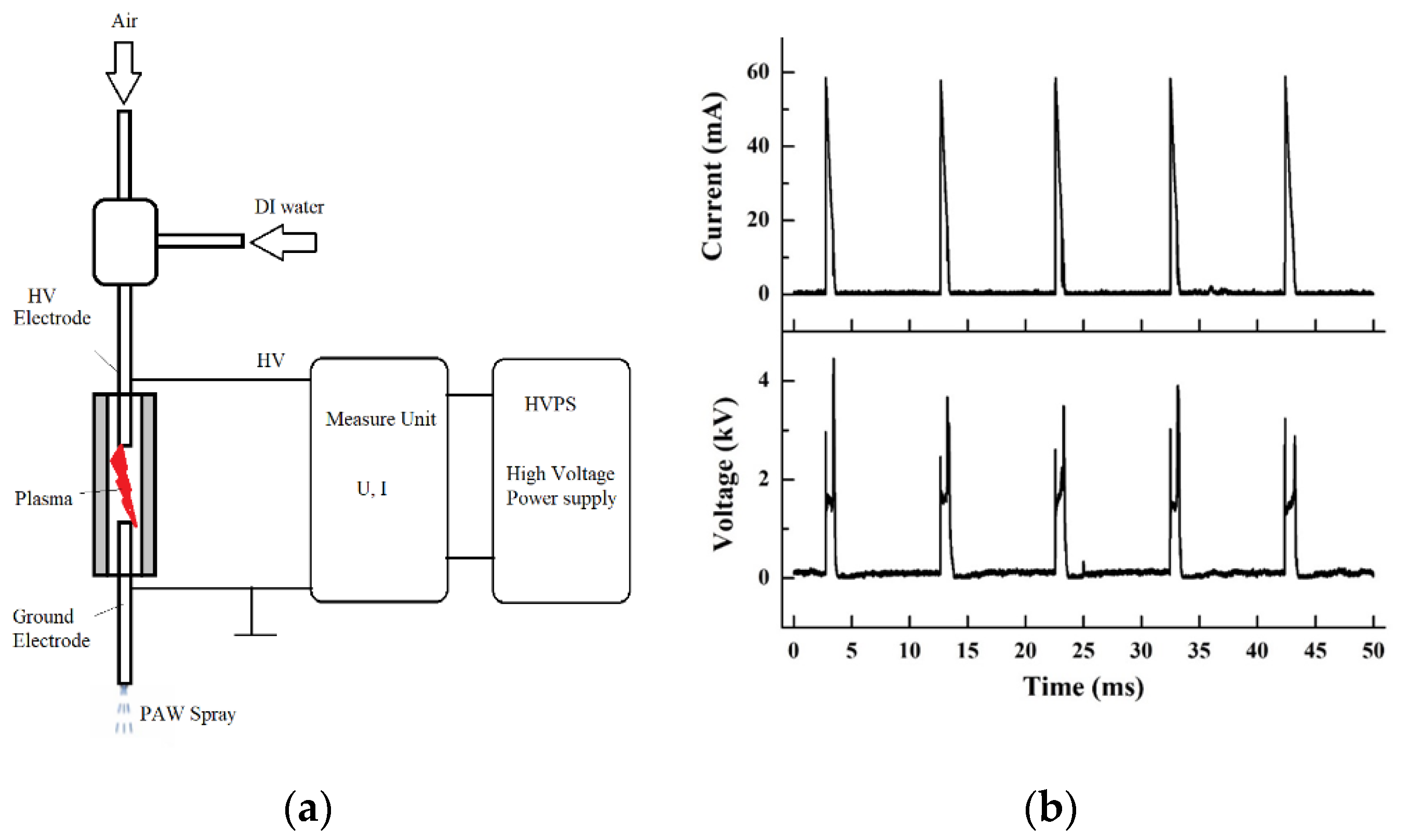


| Frequency (Hz) | Imax (mA) | Iavg (mA) | Umax (V) | Uavg (V) | Pavg (W) |
|---|---|---|---|---|---|
| 60 | 58 | 2.5 | 6049 | 153 | 3 |
| 100 | 59 | 2.8 | 7200 | 250 | 4 |
| PAW | Frequency (Hz) | EEf (g/kWh) | ||
|---|---|---|---|---|
| H2O2 | NO2− | NO3− | ||
| PAW1 | 60 | 1.7 | 1.4 | 8.6 |
| PAW2 | 100 | 1.6 | 1.2 | 8.1 |
| Treatment | Germination (%) |
|---|---|
| Control | 42.67 ± 1.89 |
| PAW1 | 47.33 ± 4.99 |
| PAW2 | 48.00 ± 3.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandici, A.; Cretu, D.E.; Burlica, R.; Astanei, D.; Beniuga, O.; Rosu, C.; Topa, D.C.; Aostacioaei, T.G.; Aprotosoaie, A.C.; Miron, A. Preliminary Study on the Impact of Non-Thermal Plasma Activated Water on the Quality of Triticum aestivum L. cv. Glosa Sprouts. Horticulturae 2022, 8, 1158. https://doi.org/10.3390/horticulturae8121158
Mandici A, Cretu DE, Burlica R, Astanei D, Beniuga O, Rosu C, Topa DC, Aostacioaei TG, Aprotosoaie AC, Miron A. Preliminary Study on the Impact of Non-Thermal Plasma Activated Water on the Quality of Triticum aestivum L. cv. Glosa Sprouts. Horticulturae. 2022; 8(12):1158. https://doi.org/10.3390/horticulturae8121158
Chicago/Turabian StyleMandici, Alexandru, Daniel Eusebiu Cretu, Radu Burlica, Dragos Astanei, Oana Beniuga, Craita Rosu, Denis Constantin Topa, Tudor George Aostacioaei, Ana Clara Aprotosoaie, and Anca Miron. 2022. "Preliminary Study on the Impact of Non-Thermal Plasma Activated Water on the Quality of Triticum aestivum L. cv. Glosa Sprouts" Horticulturae 8, no. 12: 1158. https://doi.org/10.3390/horticulturae8121158
APA StyleMandici, A., Cretu, D. E., Burlica, R., Astanei, D., Beniuga, O., Rosu, C., Topa, D. C., Aostacioaei, T. G., Aprotosoaie, A. C., & Miron, A. (2022). Preliminary Study on the Impact of Non-Thermal Plasma Activated Water on the Quality of Triticum aestivum L. cv. Glosa Sprouts. Horticulturae, 8(12), 1158. https://doi.org/10.3390/horticulturae8121158








