HyPRP1, A Tomato Multipotent Regulator, Negatively Regulates Tomato Resistance to Sulfur Dioxide Toxicity and Can Also Reduce Abiotic Stress Tolerance of Escherichia coli and Tobacco
Abstract
1. Introduction
2. Results
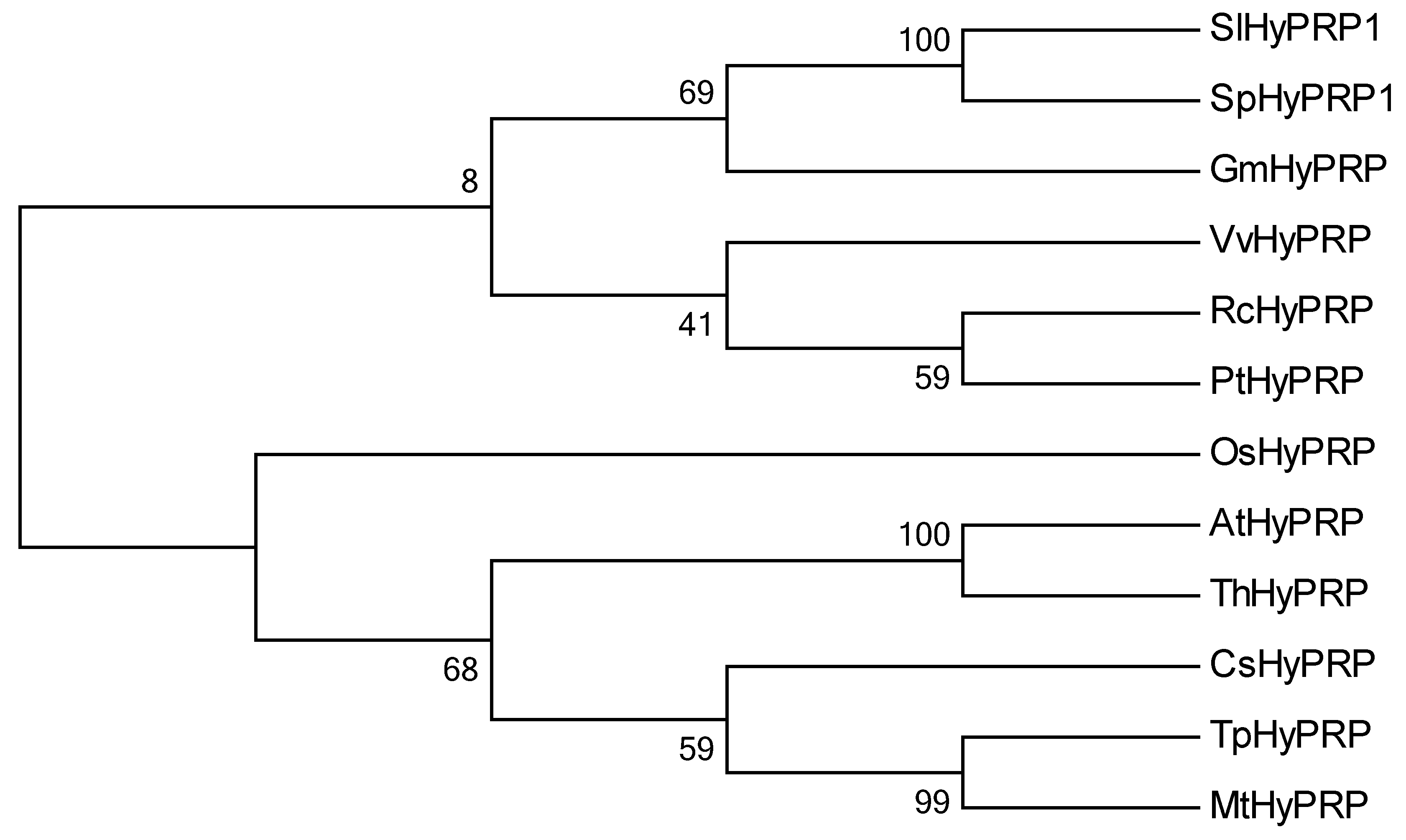
2.1. Gene Features of SlHyPRP and SpHyPRP
2.2. Expression of Tomato HyPRP1 Gene in E. coli Reduces Abiotic Stress Tolerance
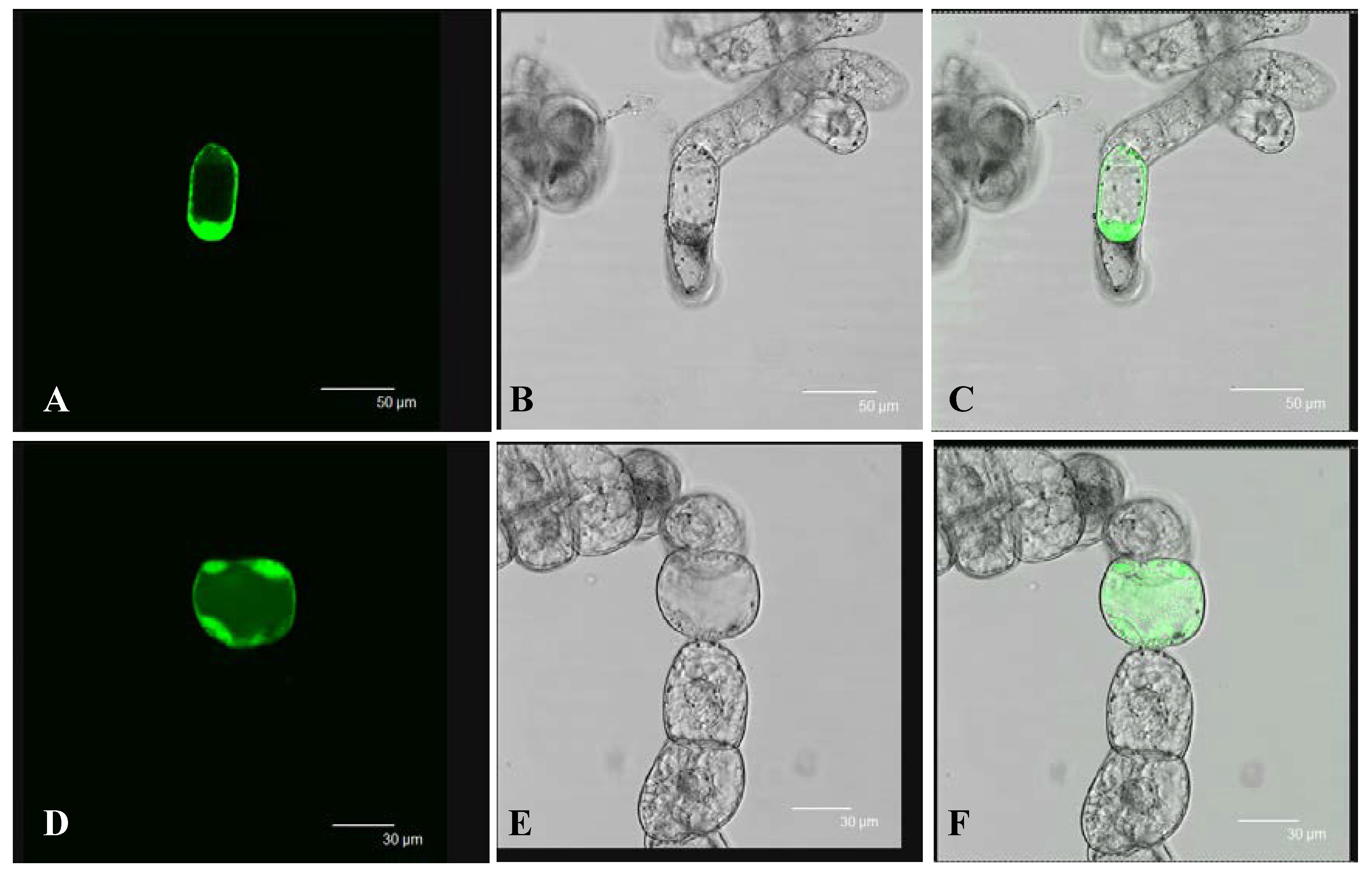
2.3. Subcellular Localization of SpHyPRP1
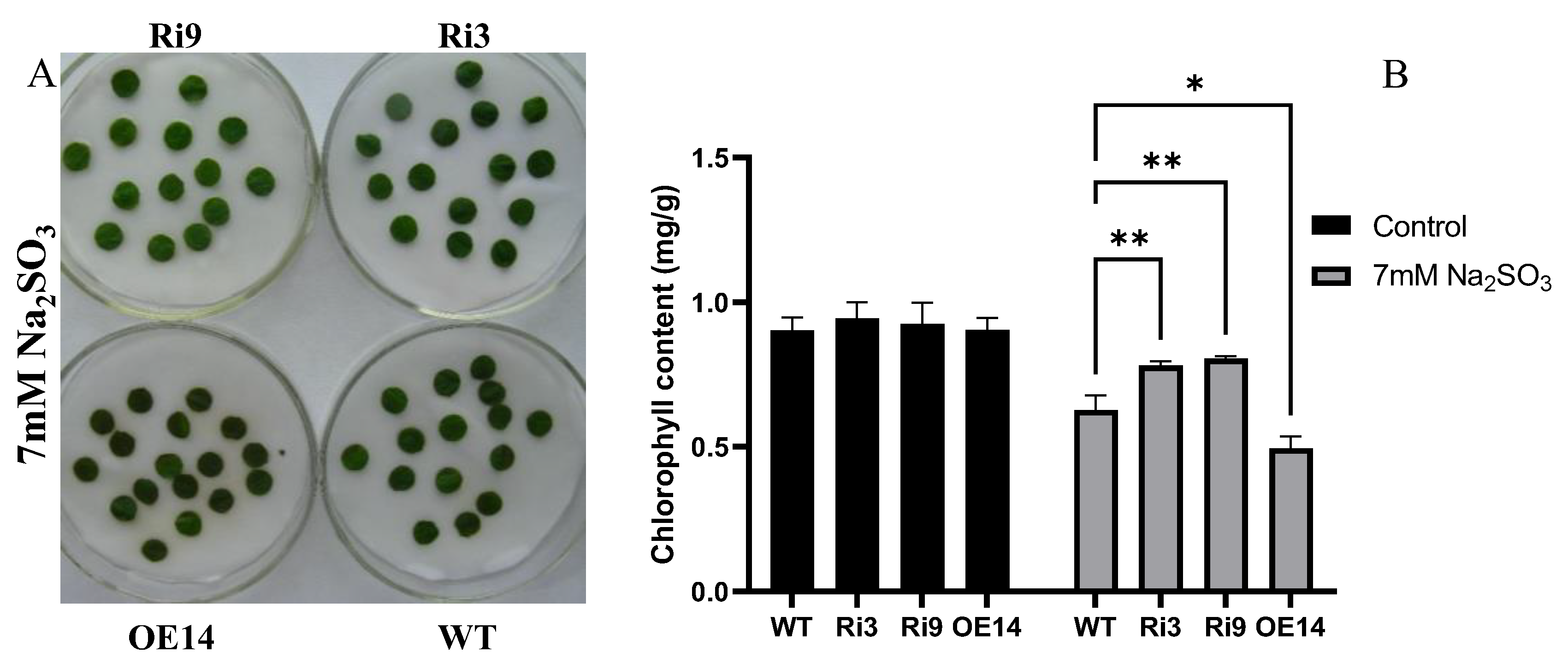
2.4. Enhanced Tolerance of HyPRP1-RNAi Lines to SO2
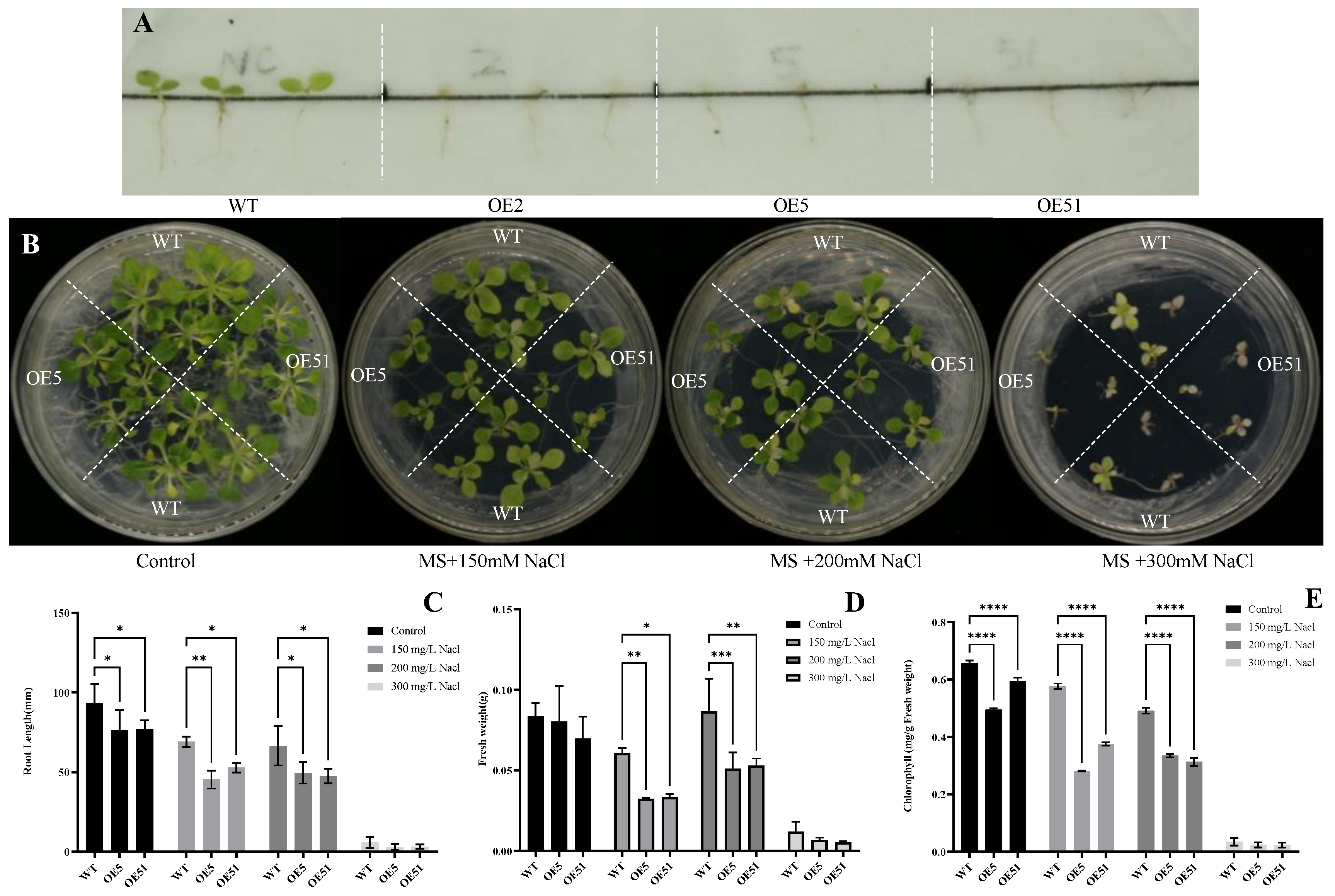
2.5. OE of the SpHyPRP1 in Tobacco Reduces Salt Stress Resistance
3. Discussion
4. Materials and Methods
4.1. Phylogenetic Analysis of HyPRP1, Prediction of cis-element, and Methylation Analysis of HyPRP1
4.2. HyPRP1 Expression in E. coli and Abiotic Stress Treatment
4.3. Subcellular Localization of SpHyPRP1
4.4. Leaf Disc and SO2 Treatment of HyPRP1 Transgenic Plants
4.5. Salt Stress Resistance Analysis of Heterologous Expression HyPRP1 in Tobacco
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical Priming of Plants Against Multiple Abiotic Stresses: Mission Possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, T.K.; Bashir, T.; Hashem, A.; Abd_Allah, E.F. Systems biology approach in plant abiotic stresses. Plant Physiol. Biochem. 2017, 121, 58–73. [Google Scholar] [CrossRef]
- Chikkaputtaiah, C.; Debbarma, J.; Baruah, I.; Havlickova, L.; Deka Boruah, H.P.; Curn, V. Molecular genetics and functional genomics of abiotic stress-responsive genes in oilseed rape (Brassica napus L.): A review of recent advances and future. Plant Biotechnol. Rep. 2017, 11, 365–384. [Google Scholar] [CrossRef]
- Kheirouri, S.; Alizadeh, M.; Abad, R.M.S.; Barkabi-Zanjani, S.; Mesgari-Abbasi, M. Effects of sulfur dioxide, ozone, and ambient air pollution on bone metabolism related biochemical parameters in a rat model. Environ. Anal. Health Toxicol. 2020, 35, e2020023-0. [Google Scholar] [CrossRef]
- Brychkova, G.; Xia, Z.; Yang, G.; Yesbergenova, Z.; Zhang, Z.; Davydov, O.; Fluhr, R.; Sagi, M. Sulfite oxidase protects plants against sulfur dioxide toxicity. Plant J. 2007, 50, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-H.; Yi, H.-L.; Liu, X.-P.; Qi, H.-X. Sulfur dioxide enhance drought tolerance of wheat seedlings through H2S signaling. Ecotoxicol. Environ. Saf. 2021, 207, 111248. [Google Scholar] [CrossRef]
- Leustek, T.; Martin, M.N.; Bick, J.A.; Davies, J.P. Pathways and Regulation of Sulfur Metabolism Revealed through Molecular and Genetic Studies. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 141–165. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Khaine, I.; Kwak, M.J.; Jang, J.H.; Lee, T.Y.; Lee, J.K.; Kim, I.R.; Kim, W.I.; Oh, K.S.; Woo, S.Y. The relationship between SO 2 exposure and plant physiology: A mini review. Hortic. Environ. Biotechnol. 2017, 58, 523–529. [Google Scholar] [CrossRef]
- Dvorakova, L.; Cvrckova, F.; Fischer, L. Analysis of the hybrid proline-rich protein families from seven plant species suggests rapid diversification of their sequences and expression patterns. BMC Genom. 2007, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Jose-Estanyol, M.; Perez, P.; Puigdomenech, P. Expression of the promoter of HyPRP, an embryo-specific gene from Zea mays in maize and tobacco transgenic plants. Gene 2005, 356, 146–152. [Google Scholar] [CrossRef]
- Blanco-Portales, R.; Lopez-Raez, J.A.; Bellido, M.L.; Moyano, E.; Dorado, G.; Gonzalez-Reyes, J.A.; Caballero, J.L.; Munoz-Blanco, J. A strawberry fruit-specific and ripening-related gene codes for a HyPRP protein involved in polyphenol anchoring. Plant Mol. Biol. 2004, 55, 763–780. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, L.; Srba, M.; Opatrny, Z.; Fischer, L. Hybrid proline-rich proteins: Novel players in plant cell elongation? Ann. Bot. 2012, 109, 453–462. [Google Scholar] [CrossRef]
- Tan, J.L.; Zhuo, C.L.; Guo, Z.F. Nitric oxide mediates cold- and dehydration-induced expression of a novel MfHyPRP that confers tolerance to abiotic stress. Physiol. Plant. 2013, 149, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Mellacheruvu, S.; Tamirisa, S.; Vudem, D.R.; Khareedu, V.R. Pigeonpea Hybrid-Proline-Rich Protein (CcHyPRP) Confers Biotic and Abiotic Stress Tolerance in Transgenic Rice. Front. Plant Sci. 2015, 6, 1167. [Google Scholar] [CrossRef]
- Liu, D.Q.; Han, Q.; Shah, T.; Chen, C.Y.; Wang, Q.; Tang, B.F.; Ge, F. A hybrid proline-rich cell-wall protein gene JsPRP1 from Juglans sigillata Dode confers both biotic and abiotic stresses in transgenic tobacco plants. Trees-Struct Funct. 2018, 32, 1199–1209. [Google Scholar] [CrossRef]
- Yeom, S.I.; Seo, E.; Oh, S.K.; Kim, K.W.; Choi, D. A common plant cell-wall protein HyPRP1 has dual roles as a positive regulator of cell death and a negative regulator of basal defense against pathogens. Plant J. 2012, 69, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Huang, X.; Xu, Z.Q.; Schlappi, M. The HyPRP gene EARLI1 has an auxiliary role for germinability and early seedling development under low temperature and salt stress conditions in Arabidopsis thaliana. Planta 2011, 234, 565–577. [Google Scholar] [CrossRef]
- Huang, G.; Gong, S.; Xu, W.; Li, P.; Zhang, D.; Qin, L.; Li, W.; Li, X. GhHyPRP4, a cotton gene encoding putative hybrid proline-rich protein, is preferentially expressed in leaves and involved in plant response to cold stress. Acta Biochim. Biophys. Sin. 2011, 43, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, B.; Sekhar, K.; Reddy, V.D.; Rao, K.V. Expression of pigeonpea hybrid-proline-rich protein encoding gene (CcHyPRP) in yeast and Arabidopsis affords multiple abiotic stress tolerance. Plant Biotechnol. J. 2010, 8, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, Y.; Wang, X.; Wang, W.; Li, Z.; Wu, J.; Wang, G.; Wu, L.; Zhang, G.; Ma, Z. HyPRP1 performs a role in negatively regulating cotton resistance to V. dahliae via the thickening of cell walls and ROS accumulation. BMC Plant Biol. 2018, 18, 339. [Google Scholar] [CrossRef]
- Sundaresan, S.; Philosoph-Hadas, S.; Ma, C.; Jiang, C.Z.; Riov, J.; Mugasimangalam, R.; Kochanek, B.; Salim, S.; Reid, M.S.; Meir, S. The Tomato Hybrid Proline-rich Protein regulates the abscission zone competence to respond to ethylene signals. Hortic. Res. 2018, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ouyang, B.; Wang, T.; Luo, Z.; Yang, C.; Li, H.; Sima, W.; Zhang, J.; Ye, Z. HyPRP1 Gene Suppressed by Multiple Stresses Plays a Negative Role in Abiotic Stress Tolerance in Tomato. Front. Plant Sci. 2016, 7, 967. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.T.; Doan, D.T.H.; Kim, J.; Song, Y.J.; Sung, Y.W.; Das, S.; Kim, E.J.; Son, G.H.; Kim, S.H.; Van Vu, T.; et al. CRISPR/Cas9-based precise excision of SlHyPRP1 domain(s) to obtain salt stress-tolerant tomato. Plant Cell Rep. 2021, 40, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Saikia, B.; Singh, S.; Debbarma, J.; Velmurugan, N.; Dekaboruah, H.; Arunkumar, K.P.; Chikkaputtaiah, C. Multigene CRISPR/Cas9 genome editing of hybrid proline rich proteins (HyPRPs) for sustainable multi-stress tolerance in crops: The review of a promising approach. Physiol. Mol. Biol. Plants 2020, 26, 857–869. [Google Scholar] [CrossRef]
- Gong, P.; Zhang, J.; Li, H.; Yang, C.; Zhang, C.; Zhang, X.; Khurram, Z.; Zhang, Y.; Wang, T.; Fei, Z.; et al. Transcriptional profiles of drought-responsive genes in modulating transcription signal transduction, and biochemical pathways in tomato. J. Exp. Bot. 2010, 61, 3563–3575. [Google Scholar] [CrossRef]
- Mundree, S.G.; Whittaker, A.; Thomson, J.A.; Farrant, J.M. An aldose reductase homolog from the resurrection plant Xerophyta viscosa Baker. Planta 2000, 211, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Garay-Arroyo, A.; Colmenero-Flores, J.M.; Garciarrubio, A.; Covarrubias, A.A. Highly hydrophilic proteins in prokaryotes and eukaryotes are common during conditions of water deficit. J. Biol. Chem. 2000, 275, 5668–5674. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Kim, I.S.; Kim, Y.H.; Park, H.M.; Lee, J.Y.; Kang, H.G.; Yoon, H.S. Scavenging reactive oxygen species by rice dehydroascorbate reductase alleviates oxidative stresses in Escherichia coli. Mol. Cells 2008, 26, 616–620. [Google Scholar] [PubMed]
- Yamada, A.; Saitoh, T.; Mimura, T.; Ozeki, Y. Expression of mangrove allene oxide cyclase enhances salt tolerance in Escherichia coli, yeast, and tobacco cells. Plant Cell Physiol. 2002, 43, 903–910. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Wei, J.; Pan, Y.; Su, C.; Zhang, X. SpPKE1, a Multiple Stress-Responsive Gene Confers Salt Tolerance in Tomato and Tobacco. Int. J. Mol. Sci. 2019, 20, 903–910. [Google Scholar] [CrossRef]
- Zhu, D.B.; Hu, K.D.; Guo, X.K.; Liu, Y.; Hu, L.Y.; Li, Y.H.; Wang, S.H.; Zhang, H. Sulfur Dioxide Enhances Endogenous Hydrogen Sulfide Accumulation and Alleviates Oxidative Stress Induced by Aluminum Stress in Germinating Wheat Seeds. Oxid. Med. Cell Longev. 2015, 2015, 612363. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yi, H. Effect of sulfur dioxide on ROS production, gene expression and antioxidant enzyme activity in Arabidopsis plants. Plant Physiol. Biochem. 2012, 58, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.; Popko, J.; Wirtz, M.; Hell, R.; Herschbach, C.; Kreuzwieser, J.; Rennenberg, H.; Mendel, R.R.; Hansch, R. Sulphite oxidase as key enzyme for protecting plants against sulphur dioxide. Plant Cell Environ. 2007, 30, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Tsakraklides, G.; Martin, M.; Chalam, R.; Tarczynski, M.C.; Schmidt, A.; Leustek, T. Sulfate reduction is increased in transgenic Arabidopsis thaliana expressing 5’-adenylylsulfate reductase from Pseudomonas aeruginosa. Plant J. 2002, 32, 879–889. [Google Scholar] [CrossRef] [PubMed]
- White, J.W.; McMaster, G.S.; Edmeades, G.O. Genomics and crop response to global change: What have we learned? Field. Crop Res. 2004, 90, 165–169. [Google Scholar] [CrossRef]
- Hirayama, T.; Shinozaki, K. Research on plant abiotic stress responses in the post-genome era: Past, present and future. Plant J. 2010, 61, 1041–1052. [Google Scholar] [CrossRef]
- Wurfel, M.; Haberlein, I.; Follmann, H. Inactivation of thioredoxin by sulfite ions. FEBS Lett. 1990, 268, 146–148. [Google Scholar] [CrossRef]
- Yonekura-Sakakibara, K.; Onda, Y.; Ashikari, T.; Tanaka, Y.; Kusumi, T.; Hase, T. Analysis of reductant supply systems for ferredoxin-dependent sulfite reductase in photosynthetic and nonphotosynthetic organs of maize. Plant Physiol. 2000, 122, 887–894. [Google Scholar] [CrossRef]
- Zhang, C.; Jia, P.; Jia, Y.; Weissbach, H.; Webster, K.A.; Huang, X.; Lemanski, S.L.; Achary, M.; Lemanski, L.F. Methionine sulfoxide reductase A (MsrA) protects cultured mouse embryonic stem cells from H2O2-mediated oxidative stress. J. Cell Biochem. 2010, 111, 94–103. [Google Scholar] [CrossRef]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Saikia, B.; Debbarma, J.; Maharana, J.; Singha, D.L.; Velmuruagan, N.; Dekaboruah, H.; Arunkumar, K.P.; Chikkaputtaiah, C. SlHyPRP1 and DEA1, the multiple stress responsive eight-cysteine motif family genes of tomato (Solanum lycopersicum L.) are expressed tissue specifically, localize and interact at cytoplasm and plasma membrane in vivo. Physiol. Mol. Biol. Plants 2020, 26, 2553–2568. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Kumar, G.; Arya, P.; Jaswal, R.; Jain, P.; Singh, K.; Sharma, T.R. Genome-Wide Analysis and Expression Profiling of Rice Hybrid Proline-Rich Proteins in Response to Biotic and Abiotic Stresses, and Hormone Treatment. Plants 2019, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.L.; Yu, Y.; Li, R.T.; Duan, X.B.; Zhu, D.; Sun, X.L.; Duanmu, H.Z.; Zhu, Y.M. A novel hybrid proline-rich type gene GsEARLI17 from Glycine soja participated in leaf cuticle synthesis and plant tolerance to salt and alkali stresses. Plant Cell Tiss. Org. 2015, 121, 633–646. [Google Scholar] [CrossRef]
- Neto, L.B.; de Oliveira, R.R.; Wiebke-Strohm, B.; Bencke, M.; Weber, R.L.; Cabreira, C.; Abdelnoor, R.V.; Marcelino, F.C.; Zanettini, M.H.; Passaglia, L.M. Identification of the soybean HyPRP family and specific gene response to Asian soybean rust disease. Genet. Mol. Biol. 2013, 36, 214–224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Schlappi, M. Cold responsive EARLI1 type HyPRPs improve freezing survival of yeast cells and form higher order complexes in plants. Planta 2007, 227, 233–243. [Google Scholar] [CrossRef]
- Gao, C.X. Genome editing in crops: From bench to field. Natl. Sci. Rev. 2015, 2, 13–15. [Google Scholar] [CrossRef]
- Voytas, D.F.; Gao, C. Precision genome engineering and agriculture: Opportunities and regulatory challenges. PLoS Biol. 2014, 12, e1001877. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Datta, S.K. Micropropagation of gerbera: Lipid peroxidation and antioxidant enzyme activities during acclimatization process. Acta Physiol. Plant. 2008, 30, 325–331. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]




| M82-HyPRP1 | S. pennellii-HyPRP1 | Functions of cis-elements | Groups |
|---|---|---|---|
| AAGAA-motif | AAGAA-motif | ||
| ABRE | ABRE | ||
| ABRE3a | involved in the abscisic acid responsiveness | 1 | |
| ABRE4 | involved in the abscisic acid responsiveness | 1 | |
| ARE | ARE | ||
| AT-rich sequence | AT-rich sequence | ||
| AT1-motif | part of a light responsive module | 3 | |
| ATCT-motif | involved in light responsiveness | 3 | |
| AT~TATA-box | AT~TATA-box | ||
| Box 4 | Box 4 | ||
| CAAT-box | CAAT-box | ||
| CGTCA-motif | CGTCA-motif | ||
| DRE | involved in dehydration, low-temp, salt stresses | 2 | |
| ERE | ERE | ||
| G-box | G-box | ||
| GA-motif | part of a light responsive element | 3 | |
| GT1-motif | GT1-motif | ||
| MYB | MYB | ||
| MYB-like sequence | MYB-like sequence | ||
| MYC | MYC | ||
| P-box | O2-site | ||
| STRE | STRE | ||
| TATA | TATA | ||
| TATA-box | TATA-box | ||
| TATC-box | TATC-box | ||
| TGA-element | auxin-responsive element | 4 | |
| TGACG-motif | TGACG-motif | ||
| Unnamed__4 | Unnamed__4 | ||
| Unnamed__6 | Unnamed__6 | ||
| WUN-motif | WUN-motif | ||
| as-1 | as-1 | ||
| chs-CMA1a | part of a light responsive element | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wang, L.; Liang, Y.; Hu, X.; Pan, Q.; Ding, Y.; Li, J. HyPRP1, A Tomato Multipotent Regulator, Negatively Regulates Tomato Resistance to Sulfur Dioxide Toxicity and Can Also Reduce Abiotic Stress Tolerance of Escherichia coli and Tobacco. Horticulturae 2022, 8, 1118. https://doi.org/10.3390/horticulturae8121118
Chen X, Wang L, Liang Y, Hu X, Pan Q, Ding Y, Li J. HyPRP1, A Tomato Multipotent Regulator, Negatively Regulates Tomato Resistance to Sulfur Dioxide Toxicity and Can Also Reduce Abiotic Stress Tolerance of Escherichia coli and Tobacco. Horticulturae. 2022; 8(12):1118. https://doi.org/10.3390/horticulturae8121118
Chicago/Turabian StyleChen, Xueting, Lulu Wang, Yan Liang, Xiaomeng Hu, Qianqian Pan, Yin Ding, and Jinhua Li. 2022. "HyPRP1, A Tomato Multipotent Regulator, Negatively Regulates Tomato Resistance to Sulfur Dioxide Toxicity and Can Also Reduce Abiotic Stress Tolerance of Escherichia coli and Tobacco" Horticulturae 8, no. 12: 1118. https://doi.org/10.3390/horticulturae8121118
APA StyleChen, X., Wang, L., Liang, Y., Hu, X., Pan, Q., Ding, Y., & Li, J. (2022). HyPRP1, A Tomato Multipotent Regulator, Negatively Regulates Tomato Resistance to Sulfur Dioxide Toxicity and Can Also Reduce Abiotic Stress Tolerance of Escherichia coli and Tobacco. Horticulturae, 8(12), 1118. https://doi.org/10.3390/horticulturae8121118







