Morphological and Physiological Responses of In Vitro-Grown Cucurbita sp. Landraces Seedlings under Osmotic Stress by Mannitol and PEG
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Condition, Experimental Design, and Treatments
2.2. Hypocotyl Length, Shoot and Root Fresh and Dry Weight
2.3. Photosynthetic Pigments
2.4. Total Proline Content
2.5. Malondialdehyde (MDA) Content
2.6. Total Soluble Protein Content
2.7. Total Phenol Content (TPC)
2.8. Total Flavonoids Content (TFC)
2.9. H2O2 Content
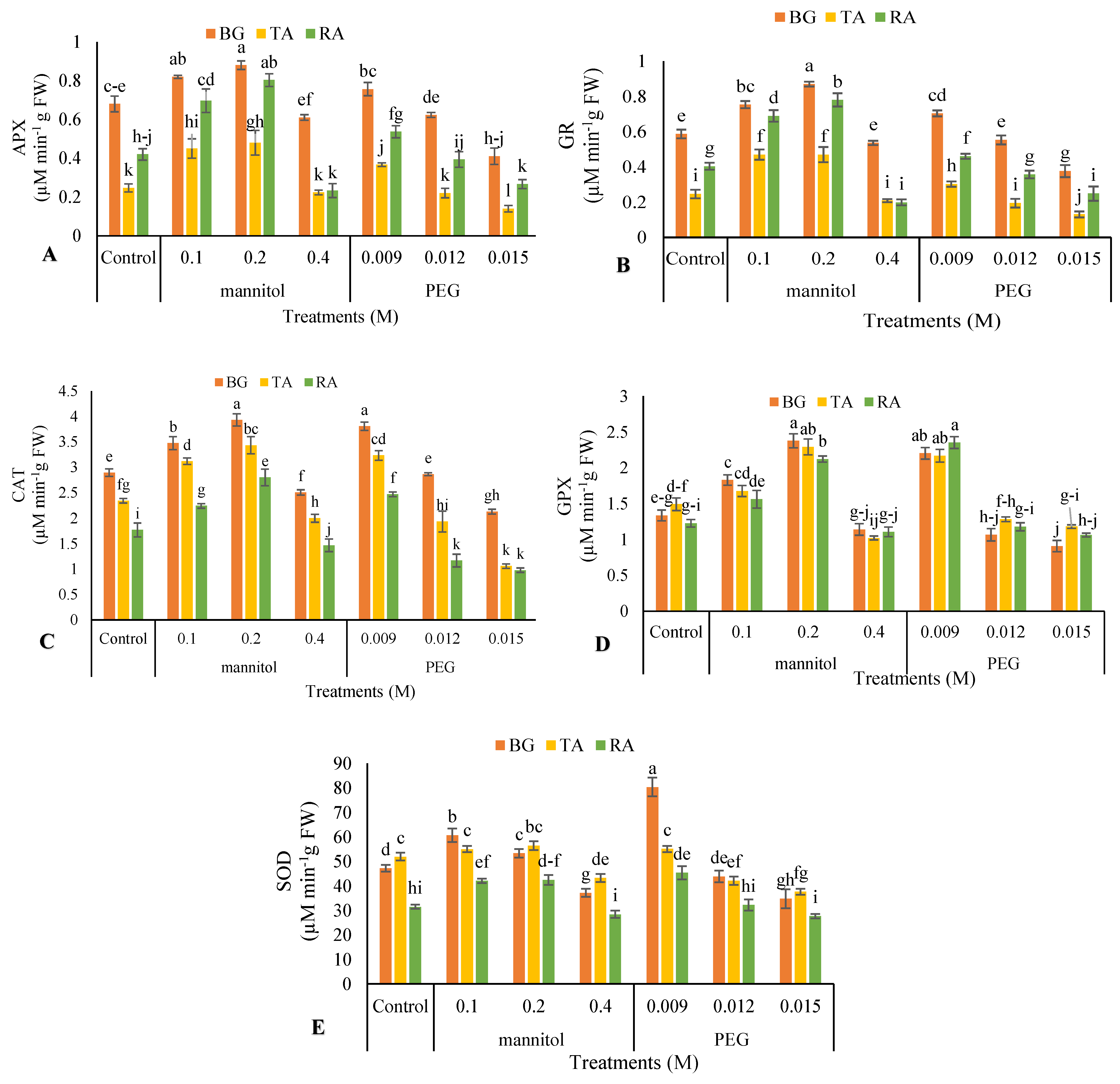
2.10. Enzymatic Antioxidants Activity
2.10.1. Ascorbate Peroxidase Activity (APX)
2.10.2. Guaiacol Peroxidase Activity (GPX)
2.10.3. Superoxide Dismutase Activity (SOD)
2.10.4. Glutathione Reductase (GR) Activity
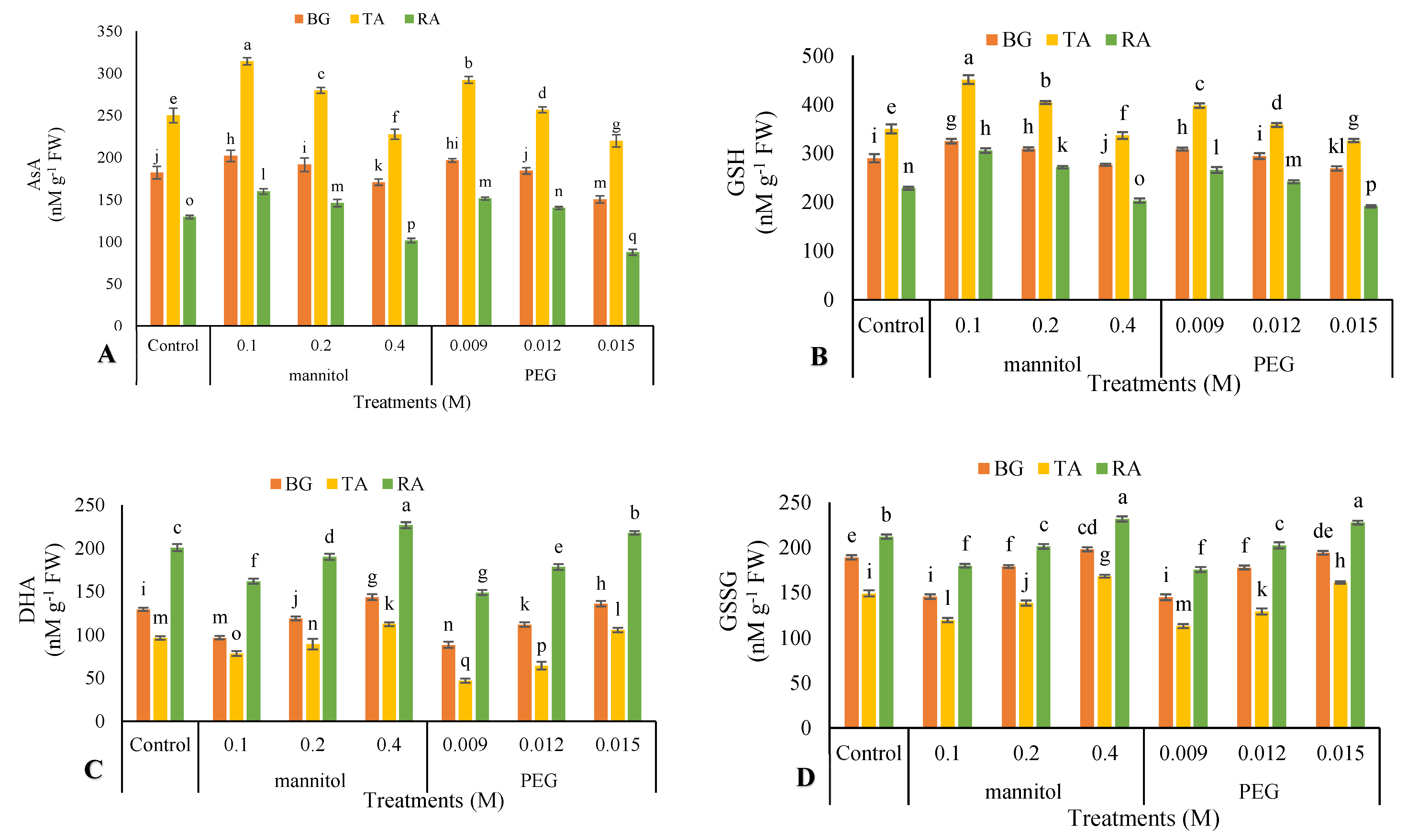
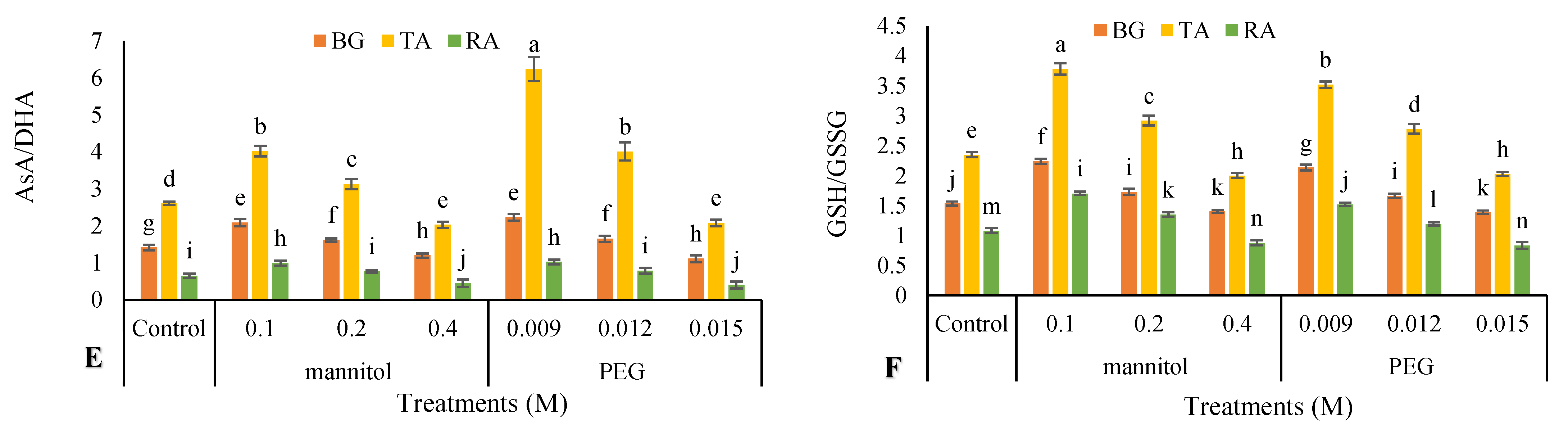
2.11. Non-Enzymatic Antioxidants Activity
2.12. Statistical Analysis
3. Results
3.1. Hypocotyl Length
3.2. Shoot and Root FW and DW
3.3. Photosynthesis Pigments
3.4. Total Proline Content
3.5. Total Soluble Protein Content
3.6. Total Phenol and Flavonoid Content
3.7. Enzymatic Antioxidant Activity
3.8. Malondialdehyde Content
3.9. Hydrogen Peroxide Content
3.10. Nonenzymatic Antioxidants
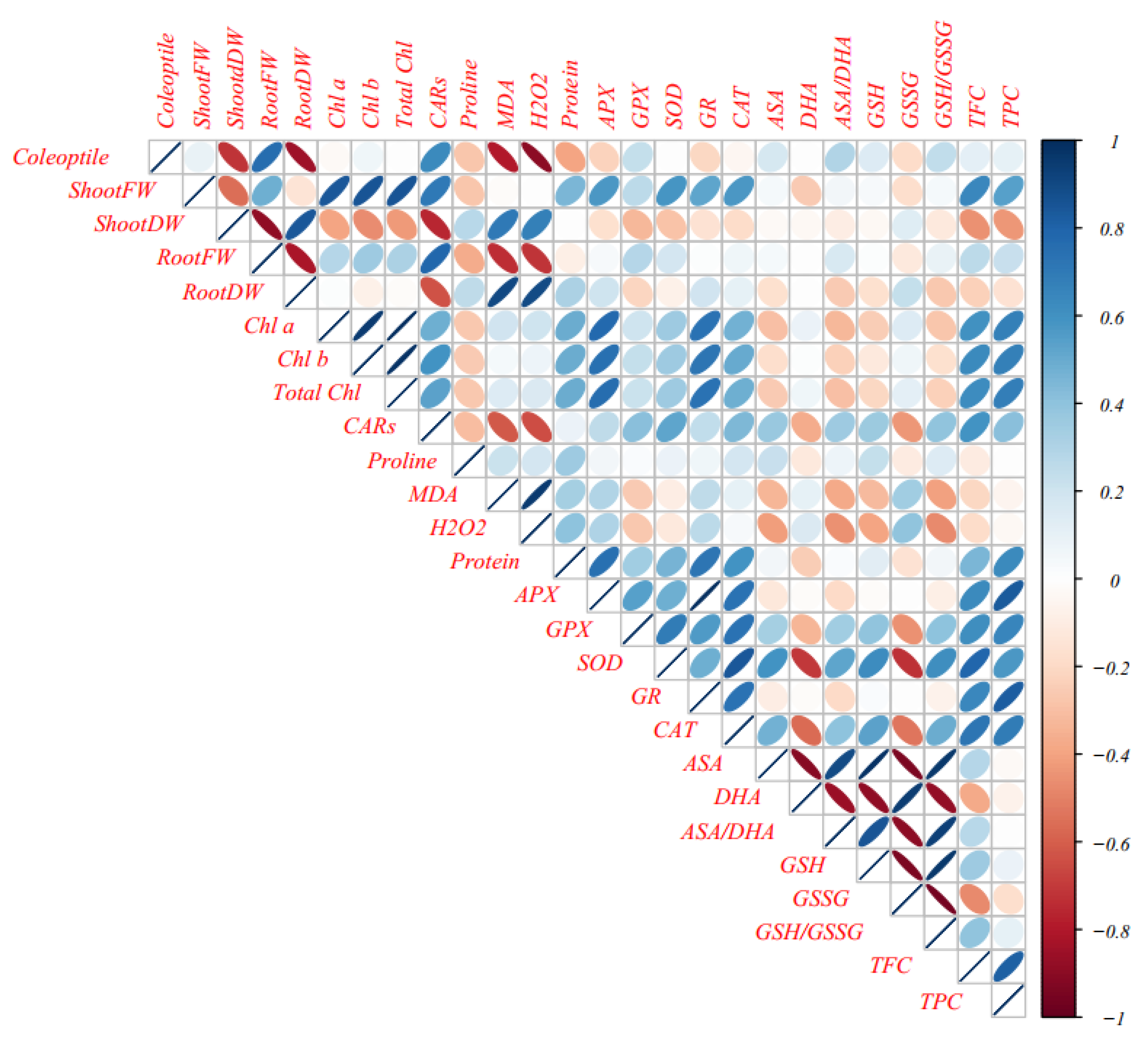
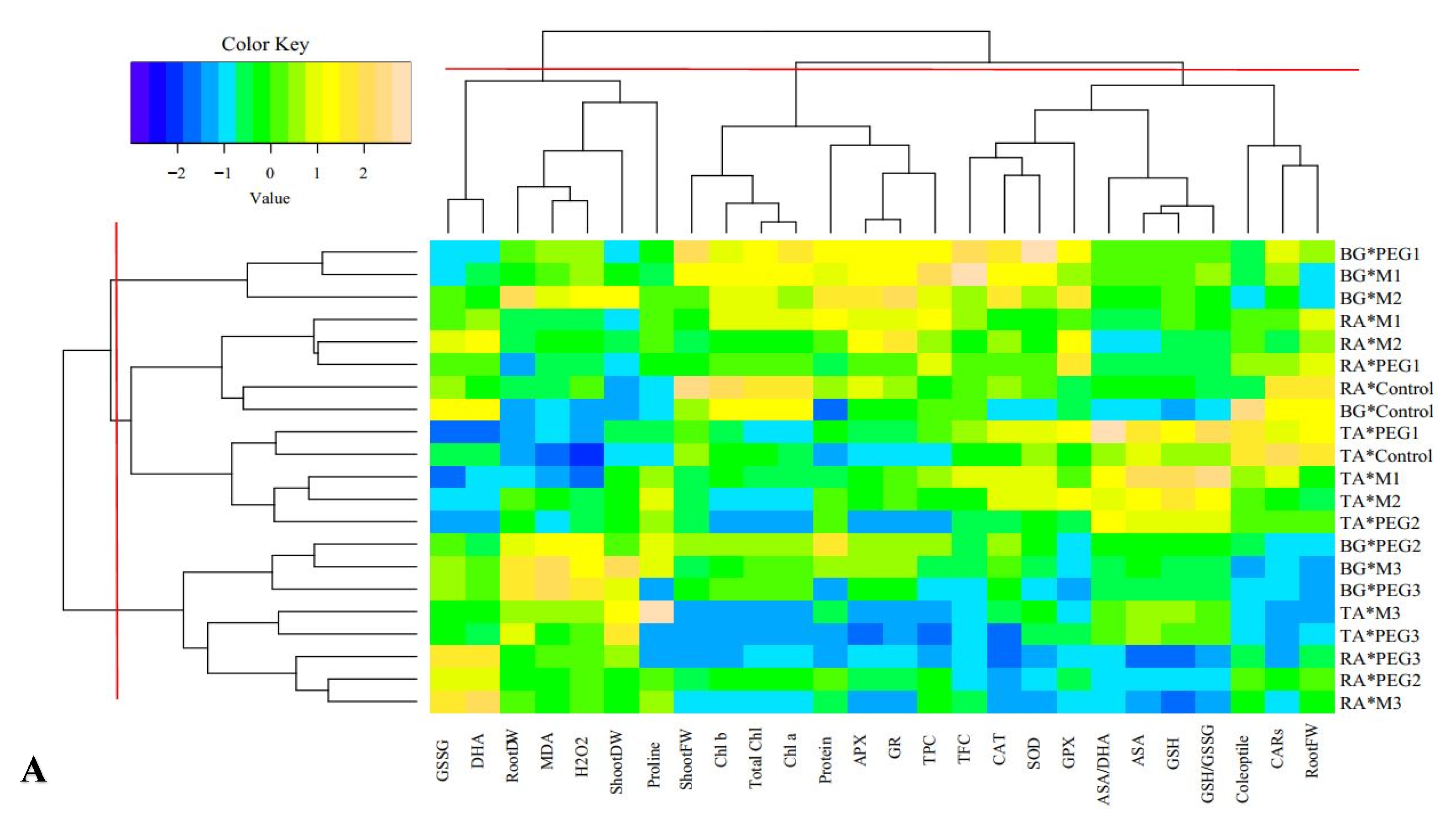
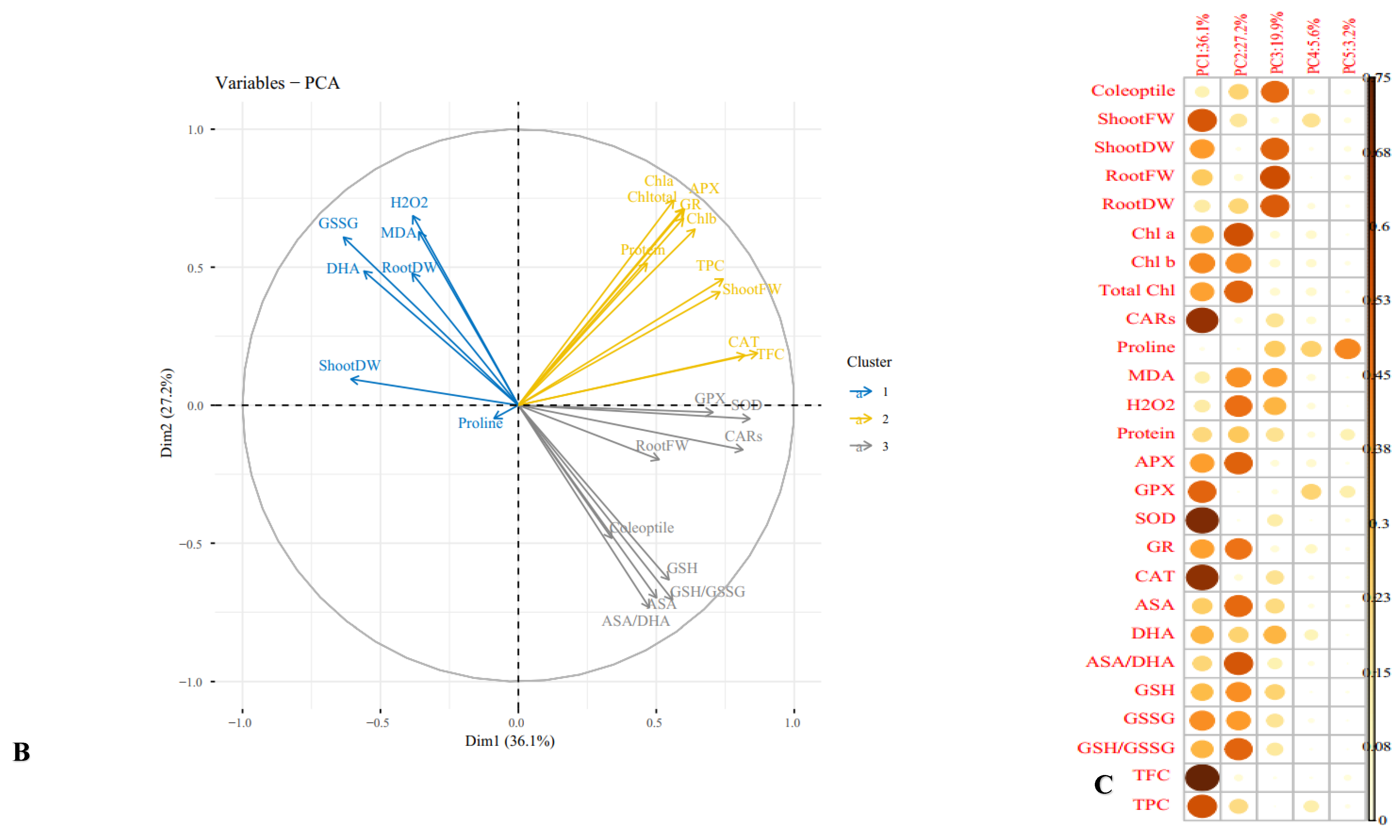
3.11. Multivariate Analysis of Cucurbits Landraces under Normal and Mannitol and PEG Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, F.A.; Golovina, E.A.; Buitink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Rahdari, P.; Hoseini, S. Drought stress: A review. Int. J. Agron. Plant Prod. 2012, 3, 443–446. [Google Scholar]
- Zayova, E.; Philipov, P.; Nedev, T.; Stoeva, D. Response of in vitro cultivated eggplant (Solanum melongena L.) to salt and drought stress. AgroLife Sci. J. 2017, 6, 276–282. [Google Scholar]
- Parry, M.A.; Andralojc, P.J.; Khan, S.; Lea, P.J.; Keys, A.J. Rubisco activity: Effects of drought stress. Ann. Bot. 2002, 89, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.J.; Oliveira, D.S.; Oliveira, M.T.; Willadino, L.; Houllou, L.; Santos, M.G. Ecophysiological, anatomical and biochemical aspects of in vitro culture of zygotic Syagrus coronata embryos and of young plants under drought stress. Trees 2015, 29, 1219–1233. [Google Scholar] [CrossRef]
- Li, J.; Cang, Z.; Jiao, F.; Bai, X.; Zhang, D.; Zhai, R. Influence of drought stress on photosynthetic characteristics and protective enzymes of potato at seedling stage. J. Saudi Soc. Agric. Sci. 2017, 16, 82–88. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Farhadi, N.; Nikpour-Rashidabad, N. Responses of in vitro-cultured Allium hirtifolium to exogenous sodium nitroprusside under PEG-imposed drought stress. Plant Cell Tissue Organ Cult. PCTOC 2018, 133, 237–248. [Google Scholar] [CrossRef]
- Araus, J.L.; Slafer, G.A.; Royo, C.; Serret, M.D. Breeding for yield potential and stress adaptation in cereals. Crit. Rev. Plant Sci. 2008, 27, 377–412. [Google Scholar] [CrossRef]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Errabii, T.; Gandonou, C.B.; Essalmani, H.; Abrini, J.; Idaomar, M.; Skali-Senhaji, N. Growth, proline and ion accumulation in sugarcane callus cultures under drought-induced osmotic stress and its subsequent relief. Afr. J. Biotechnol. 2006, 5, 1488–1493. [Google Scholar]
- Gopal, J.; Iwama, K. In vitro screening of potato against water-stress mediated through sorbitol and polyethylene glycol. Plant Cell Rep. 2007, 26, 693–700. [Google Scholar] [CrossRef] [PubMed]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef] [PubMed]
- Siaga, E.; Maharijaya, A.; Rahayu, M.S. Plant Growth of Eggplant (Solanum melongena L.) In Vitro in Drought Stress Polyethylene Glycol (PEG). BIOVALENTIA Biol. Res. J. 2016, 2, 10–17. [Google Scholar] [CrossRef][Green Version]
- Godswill, A.C. Sugar alcohols: Chemistry, production, health concerns and nutritional importance of mannitol, sorbitol, xylitol, and erythritol. Int. J. Adv. Acad. Res. Sci. Technol. Eng. 2017, 3, 31–66. [Google Scholar]
- Mashilo, J.; Odindo, A.O.; Shimelis, H.A.; Musenge, P.; Tesfay, S.Z.; Magwaza, L.S. Photosynthetic response of bottle gourd [Lagenaria siceraria (Molina) Standl.] to drought stress: Relationship between cucurbitacins accumulation and drought tolerance. Sci. Hortic. 2018, 231, 133–143. [Google Scholar] [CrossRef]
- Kusvuran, S.; Dasgan, H.Y. Drought induced physiological and biochemical responses in Solanum lycopersicum genotypes differing to tolerance. Acta Sci. Pol. Hortorum Cultus 2017, 16, 2017. [Google Scholar] [CrossRef]
- Ouzounidou, G.; Giannakoula, A.; Ilias, I.; Zamanidis, P. Alleviation of drought and salinity stresses on growth, physiology, biochemistry and quality of two Cucumis sativus L. cultivars by Si application. Braz. J. Bot. 2016, 39, 531–539. [Google Scholar] [CrossRef]
- Reid, D.S. Water Activity. In Water Activity in Foods; Wiley: Hoboken, NJ, USA, 2020; pp. 13–26. [Google Scholar]
- Michel, B.E.; Kaufmann, M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar]
- Sinha, S.; Saxena, R.; Singh, S. Chromium induced lipid peroxidation in the plants of Pistia stratiotes L.: Role of antioxidants and antioxidant enzymes. Chemosphere 2005, 58, 595–604. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Kumar, K.; PA, K. Peroxidase & polyphenol oxidase in excised ragi (eleusine coracana cv pr 202) leaves during senescence. Indian J. Exp. Biol. 1982, 20, 412–416. [Google Scholar]
- Sairam, R.; Srivastava, G.; Saxena, D. Increased antioxidant activity under elevated temperatures: A mechanism of heat stress tolerance in wheat genotypes. Biol. Plant. 2000, 43, 245–251. [Google Scholar] [CrossRef]
- Law, M.; Charles, S.A.; Halliwell, B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. The effect of hydrogen peroxide and of paraquat. Biochem. J. 1983, 210, 899–903. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Hellal, F.; El-Shabrawi, H.; Abd El-Hady, M.; Khatab, I.; El-Sayed, S.; Abdelly, C. Influence of PEG induced drought stress on molecular and biochemical constituents and seedling growth of Egyptian barley cultivars. J. Genet. Eng. Biotechnol. 2018, 16, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Kavas, M.; Baloğlu, M.C.; Akca, O.; Köse, F.S.; Gökçay, D. Effect of drought stress on oxidative damage and antioxidant enzyme activity in melon seedlings. Turk. J. Biol. 2013, 37, 491–498. [Google Scholar] [CrossRef]
- Hughes, S.G.; Bryant, J.A.; Smirnoff, N. Molecular biology, application to studies of stress tolerance. In Plants under Stress. Biochemistry, Physiology and Ecology and Their Application to Plant Improvement; Cambridge University Press: Cambridge, UK, 1989; pp. 131–155. [Google Scholar]
- Saeidi, M.; Abdoli, M. Effect of drought stress during grain filling on yield and its components, gas exchange variables, and some physiological traits of wheat cultivars. J. Agric. Sci. Technol. 2015, 17, 885–898. [Google Scholar]
- Shivakrishna, P.; Reddy, K.A.; Rao, D.M. Effect of PEG-6000 imposed drought stress on RNA content, relative water content (RWC), and chlorophyll content in peanut leaves and roots. Saudi J. Biol. Sci. 2018, 25, 285–289. [Google Scholar]
- Agastian, P.; Kingsley, S.; Vivekanandan, M. Effect of salinity on photosynthesis and biochemical characteristics in mulberry genotypes. Photosynthetica 2000, 38, 287–290. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Murali, P.; Marimuthu, G. Impact of salicylic acid on growth, photosynthesis and compatible solute accumulation in Allium cepa L. subjected to drought stress. Int. J. Agric. Food Sci. 2014, 4, 22–30. [Google Scholar]
- Cechin, I.; Cardoso, G.S.; Fumis, T.d.F.; Corniani, N. Nitric oxide reduces oxidative damage induced by water stress in sunflower plants. Bragantia 2015, 74, 200–206. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Sankar, B.; Kishorekumar, A.; Panneerselvam, R. Calcium chloride effects on salinity-induced oxidative stress, proline metabolism and indole alkaloid accumulation in Catharanthus roseus. Comptes Rendus Biol. 2007, 330, 674–683. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Jariteh, M.; Ebrahimzadeh, H.; Niknam, V.; Mirmasoumi, M.; Vahdati, K. Developmental changes of protein, proline and some antioxidant enzymes activities in somatic and zygotic embryos of Persian walnut (Juglans regia L.). Plant Cell Tissue Organ Cult. PCTOC 2015, 122, 101–115. [Google Scholar] [CrossRef]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Ni, F.; Rizwan, M.; Fahad, S.; Hu, L. Morpho-physiological and biochemical responses of tolerant and sensitive rapeseed cultivars to drought stress during early seedling growth stage. Acta Physiol. Plant. 2019, 41, 25. [Google Scholar] [CrossRef]
- Hessini, K.; Martínez, J.P.; Gandour, M.; Albouchi, A.; Soltani, A.; Abdelly, C. Effect of water stress on growth, osmotic adjustment, cell wall elasticity and water-use efficiency in Spartina alterniflora. Environ. Exp. Bot. 2009, 67, 312–319. [Google Scholar] [CrossRef]
- Bartels, D.; Salamini, F. Desiccation tolerance in the resurrection plant Craterostigma plantagineum. A contribution to the study of drought tolerance at the molecular level. Plant Physiol. 2001, 127, 1346–1353. [Google Scholar] [CrossRef]
- Pareek, S.; Notterpek, L.; Snipes, G.J.; Naef, R.; Sossin, W.; Laliberte, J.; Iacampo, S.; Suter, U.; Shooter, E.M.; Murphy, R.A. Neurons promote the translocation of peripheral myelin protein 22 into myelin. J. Neurosci. 1997, 17, 7754–7762. [Google Scholar] [CrossRef] [PubMed]
- Razavizadeh, R.; Komatsu, S. Changes in essential oil and physiological parameters of callus and seedlings of Carum copticum L. under in vitro drought stress. J. Food Meas. Charact. 2018, 12, 1581–1592. [Google Scholar] [CrossRef]
- Abid, G.; Ouertani, R.N.; Muhovski, Y.; Jebara, S.H.; Hidri, Y.; Ghouili, E.; Abdelkarim, S.; Chaieb, O.; Souissi, F.; Zribi, F. Variation in antioxidant metabolism of faba bean (Vicia faba) under drought stress induced by polyethylene glycol reveals biochemical markers associated with antioxidant defense. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2021, 155, 797–806. [Google Scholar] [CrossRef]
- Quan, N.T.; Anh, L.H.; Khang, D.T.; Tuyen, P.T.; Toan, N.P.; Minh, T.N.; Minh, L.T.; Bach, D.T.; Ha, P.T.T.; Elzaawely, A.A. Involvement of secondary metabolites in response to drought stress of rice (Oryza sativa L.). Agriculture 2016, 6, 23. [Google Scholar] [CrossRef]
- Josipović, A.; Sudar, R.; Sudarić, A.; Jurković, V.; Matoša Kočar, M.; Markulj Kulundžić, A. Total phenolic and total flavonoid content variability of soybean genotypes in eastern Croatia. Croat. J. Food Sci. Technol. 2016, 8, 60–65. [Google Scholar] [CrossRef][Green Version]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd_Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of subcellular ROS and NO metabolism in higher plants: Multifunctional signaling molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumar, A.; Yadav, S.; Singh, I.K. Reactive oxygen species-mediated signaling during abiotic stress. Plant Gene 2019, 18, 100173. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Sahoo, M.R.; Devi, T.R.; Dasgupta, M.; Nongdam, P.; Prakash, N. Reactive oxygen species scavenging mechanisms associated with polyethylene glycol mediated osmotic stress tolerance in Chinese potato. Sci. Rep. 2020, 10, 5404. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, X.; Nawaz, G.; Yin, J.; Yang, J. Physiological and biochemical responses of four cassava cultivars to drought stress. Sci. Rep. 2020, 10, 6968. [Google Scholar] [CrossRef]
- Karuppanapandian, T.; Moon, J.-C.; Kim, C.; Manoharan, K.; Kim, W. Reactive oxygen species in plants: Their generation, signal transduction, and scavenging mechanisms. Aust. J. Crop Sci. 2011, 5, 709–725. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Cham, Switzerland, 2009; pp. 153–188. [Google Scholar]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar]
- Labudda, M.; Azam, F.M.S. Glutathione-dependent responses of plants to drought: A review. Acta Soc. Bot. Pol. 2014, 83, 3–12. [Google Scholar] [CrossRef]
- Young, S.-J.; Chu, Y.-L. Hydrothermal synthesis and improved CH3OH-sensing performance of ZnO nanorods with adsorbed Au NPs. IEEE Trans. Electron Devices 2021, 68, 1886–1891. [Google Scholar] [CrossRef]
- Dietz, K.-J.; Mittler, R.; Noctor, G. Recent progress in understanding the role of reactive oxygen species in plant cell signaling. Plant Physiol. 2016, 171, 1535–1539. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Buet, A.; Gergoff Grozeff, G.; Galatro, A.; Simontacchi, M. Ascorbate-glutathione cycle and abiotic stress tolerance in plants. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Springer: Berlin/Heidelberg, Germany, 2017; pp. 177–200. [Google Scholar]
- Musgrave, W.B.; Yi, H.; Kline, D.; Cameron, J.C.; Wignes, J.; Dey, S.; Pakrasi, H.B.; Jez, J.M. Probing the origins of glutathione biosynthesis through biochemical analysis of glutamate-cysteine ligase and glutathione synthetase from a model photosynthetic prokaryote. Biochem. J. 2013, 450, 63–72. [Google Scholar] [CrossRef]
- Rahman, A.; Hossain, M.; Mahmud, J.-A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Manganese-induced salt stress tolerance in rice seedlings: Regulation of ion homeostasis, antioxidant defense and glyoxalase systems. Physiol. Mol. Biol. Plants 2016, 22, 291–306. [Google Scholar] [CrossRef]
- Chakraborty, K.; Bishi, S.K.; Goswami, N.; Singh, A.L.; Zala, P.V. Differential fine-regulation of enzyme driven ROS detoxification network imparts salt tolerance in contrasting peanut genotypes. Environ. Exp. Bot. 2016, 128, 79–90. [Google Scholar] [CrossRef]


| Species | Osmotic Agent | Concentration (M) | Plant Characters | ||||
|---|---|---|---|---|---|---|---|
| Hypocotyl Length (mm) | Shoot FW (g Seedling−1) | Shoot DW (%) | Root FW (g Seedling−1) | Root DW (%) | |||
| BG | Control | 0 | 24.38 ± 0.33 gh | 2.71 ± 0.20 a | 7.78 ± 0.40 k | 0.71 ± 0.11 a | 7.85 ± 0.30 g–i |
| Mannitol | 0.1 | 18.05 ± 1.29 h–j | 2.02 ± 0.29 b | 12.57 ± 1.05 f | 0.20 ± 0.03 ij | 9.09 ± 0.50 ef | |
| 0.2 | 13.47 ± 1.32 jk | 1.45 ± 0.24 cd | 18.13 ± 0.47 bc | 0.15 ± 0.03 j | 14.18 ± 0.33 a | ||
| 0.4 | 7.20 ± 1.30 k | 1.12 ± 0.21 e–g | 20.66 ± 0.77 a | 0.13 ± 0.02 j | 13.52 ± 0.96 ab | ||
| PEG | 0.009 | 20.90 ± 0.88 hi | 2.50 ± 0.10 a | 9.23 ± 0.13 i–k | 0.52 ± 0.07 cd | 9.912 ± 0.45 de | |
| 0.012 | 17.69 ± 0.68 h–j | 1.75 ± 0.11 bc | 14.25 ± 1.17 de | 0.18 ± 0.04 ij | 11.31 ± 0.40 c | ||
| 0.015 | 15.06 ± 0.47 ij | 1.26 ± 0.12 d–f | 16.69 ± 0.33 c | 0.13 ± 0.02 j | 12.83 ± 0.42 b | ||
| TA | Control | 0 | 70.72 ± 2.30 b | 1.65 ± 0.09 c | 9.04 ± 0.80 jk | 0.70 ± 0.06 a | 5.68 ±0.19 l |
| Mannitol | 0.1 | 43.73 ± 4.46 cd | 1.07 ± 0.07 e–g | 11.96 ± 0.32 fg | 0.30 ± 0.09 f–i | 7.04 ± 0.64 i–k | |
| 0.2 | 36.10 ± 5.83 ef | 1.00 ± 0.06 f–h | 12.71 ± 0.73 f | 0.25 ± 0.07 g–j | 9.17 ± 0.64 ef | ||
| 0.4 | 13.05 ± 3.71 jk | 0.69 ± 0.10 i | 17.50 ± 1.08 bc | 0.12 ± 0.03 j | 10.79 ± 0.59 cd | ||
| PEG | 0.009 | 69.83 ± 1.69 b | 1.45 ± 0.08 cd | 10.66 ± 0.34 g–i | 0.66 ± 0.11 ab | 6.25 ± 0.44 j–l | |
| 0.012 | 33.46 ± 6.56 ef | 1.11 ± 0.10 e–g | 12.92 ± 0.98 ef | 0.37 ± 0.05 e–g | 8.19 ± 0.63 f–i | ||
| 0.015 | 12.06 ± 1.70 jk | 0.63 ± 0.09 i | 18.82 ± 0.41 b | 0.21 ± 0.04 ij | 11.03 ± 0.96 c | ||
| RA | Control | 0 | 79.63 ± 8.64 a | 1.66 ± 0.13 c | 7.90 ± 0.42 k | 0.63 ± 0.02 a–c | 6.15 ± 0.61 kl |
| Mannitol | 0.1 | 39.52 ± 1.07 de | 1.22 ± 0.14 d–f | 9.79 ± 0.71 h–j | 0.54 ± 0.00 b–d | 7.31 ± 0.43 h–j | |
| 0.2 | 36.64 ± 2.32 d–f | 1.05 ± 0.13 e–g | 10.85 ± 1.03 gh | 0.47 ± 0.02 de | 8.12 ± 0.23 f–i | ||
| 0.4 | 29.43 ± 0.65 fg | 0.87 ± 0.06 g–i | 12.57 ± 0.16 f | 0.34 ± 0.03 f–h | 9.61 ± 0.39 e | ||
| PEG | 0.009 | 47.99 ± 3.47 c | 1.34 ± 0.07 de | 9.81 ± 0.43 h–j | 0.55 ± 0.04 b–d | 5.96 ± 0.38 kl | |
| 0.012 | 38.30 ± 1.93 de | 1.08 ± 0.12 e–g | 12.52 ±1.00 f | 0.39 ± 0.10 ef | 8.33 ± 0.19 f–h | ||
| 0.015 | 24.91 ± 2.22 gh | 0.73 ± 0.04 hi | 15.20 ± 0.56 d | 0.24 ± 0.04 h–j | 8.95 ± 0.33 e–g | ||
| S.O.V. | |||||||
| Species Osmotic agent Species × Osmotic agent Error | 4207.017 ** | 3.642 ** | 48.868 ** | 0.141 ** | 73.052 ** | ||
| 2035.468 ** | 1.614 ** | 98.948 ** | 0.312 ** | 31.913 ** | |||
| 368.631 * | 0.096 ** | 10.950 * | 0.025 ** | 2.185 ** | |||
| 16.472 | 0.028 | 0.753 | 0.005 | 0.406 | |||
| C.V. (%) | 12.31 | 12.26 | 6.71 | 19.08 | 7.00 | ||
| Species | Osmotic Agent | Concentration (M) | Character | |||
|---|---|---|---|---|---|---|
| Chl a (mg kg−1 FW) | Chl b (mg kg−1 FW) | Chl a + b (mg kg−1 FW) | CARs (mg kg−1 FW) | |||
| BG | Control | 0 | 34.43 ± 0.44 a | 21.86 ± 2.38 a | 56.29 ± 2.80 a | 8.68 ± 0.33 a |
| Mannitol | 0.1 | 31.53 ± 3.27 b | 17.98 ± 0.59 b | 49.51 ± 3.55 b | 5.88 ± 1.04 c–e | |
| 0.2 | 23.87 ± 0.87 c | 15.48 ± 0.61 c | 39.35 ± 1.26 c | 4.83 ± 0.33 ef | ||
| 0.4 | 20.17 ± 0.27 d | 7.94 ± 0.02 g | 28.12 ± 0.26 e | 3.30 ± 0.32 hi | ||
| PEG | 0.009 | 34.23 ± 0.53 a | 15.68 ± 0.99 c | 49.91 ± 0.56 b | 6.96 ± 0.69 bc | |
| 0.012 | 23.47 ± 0.93 c | 12.52 ± 0.99 d | 36.00 ± 0.91 d | 3.29 ± 0.15 hi | ||
| 0.015 | 19.79 ± 0.50 d | 9.71± 0.36 ef | 29.51 ± 0.76 e | 2.58 ± 0.29 ij | ||
| TA | Control | 0 | 13.44 ± 0.91 f | 8.39 ± 0.42 fg | 21.84 ± 0.54 fg | 9.25± 0.57 a |
| Mannitol | 0.1 | 10.99 ± 0.49 g | 8.09 ± 0.44 fg | 19.09 ± 0.93 g | 7.04 ± 0.64 bc | |
| 0.2 | 7.84 ± 0.76 h–j | 4.83 ± 0.56 hi | 12.68 ± 0.47 hi | 4.61 ± 0.99 e–g | ||
| 0.4 | 4.10 ± 0.68 kl | 2.67 ± 0.57 jk | 6.77 ± 1.10 kl | 1.94 ± 0.14 j | ||
| PEG | 0.009 | 8.78 ± 0.66 g–i | 6.03 ± 0.71 h | 14.81 ± 1.27 h | 6.82 ± 0.26 b–d | |
| 0.012 | 6.06 ± 0.46 jk | 3.02 ± 0.33 jk | 9.08 ± 0.79 jk | 4.96 ± 0.57 ef | ||
| 0.015 | 3.22 ± 0.17 l | 1.72 ± 0.23 k | 4.94 ± 0.41 l | 2.45 ± 0.16 ij | ||
| RA | Control | 0 | 30.44 ± 2.41 b | 17.77 ± 0.45 b | 48.21 ± 2.57 b | 7.50 ± 1.41 b |
| Mannitol | 0.1 | 25.28 ±0.51 c | 14.79 ± 0.55 c | 40.08 ± 0.53 c | 5.61 ± 0.28 de | |
| 0.2 | 16.17 ± 0.71 e | 8.10 ± 0.25 fg | 24.27 ± 0.95 f | 3.45 ± 0.05 g–i | ||
| 0.4 | 6.90 ± 0.35 ij | 3.83 ± 0.51 ij | 10.74 ± 0.84 ij | 2.49 ± 0.60 ij | ||
| PEG | 0.009 | 20.63 ± 0.56 d | 10.38 ± 0.88 e | 31.01 ± 1.39 e | 6.31 ± 0.53 b–d | |
| 0.012 | 13.96 ± 0.72 ef | 8.45 ± 0.80 fg | 22.41 ± 1.27 f | 4.21 ± 0.35 f–h | ||
| 0.015 | 9.69 ± 1.85 gh | 2.38 ± 0.06 jk | 12.08 ± 1.80 h–j | 1.98 ± 0.14 j | ||
| S.O.V. | ||||||
| Species | 1896.903 ** | 473.298 ** | 4262.121 ** | 3.489 ** | ||
| Osmotic agent | 328.683 ** | 163.292 ** | 946.994 ** | 45.801 ** | ||
| Species × Osmotic agent | 24.221 ** | 9.261 ** | 51.632 ** | 1.077 * | ||
| Error | 1.919 | 0.886 | 3.150 | 0.490 | ||
| C.V. (%) | 7.97 | 9.80 | 6.58 | 14.10 | ||
| Species | Osmotic Agent | Concentration (M) | Character | |||
|---|---|---|---|---|---|---|
| Proline (µm g−1 FW) | Total Protein (mg g−1 FW) | TPC (mg g−1 DW) | TFC (mg g−1 DW) | |||
| BG | Control | 0 | 6.75 ± 0.13 gh | 1.60 ± 0.12 b–e | 33.67 ± 1.25 gh | 5 ± 0.82 de |
| Mannitol | 0.1 | 8.21 ± 1.44 fg | 1.73 ± 0.22 a–d | 56 ± 2.45 a | 11.67 ± 1.25 a | |
| 0.2 | 12.26 ± 0.92 c–e | 1.97 ± 0.21 a | 46 ± 1.41 c | 5.333 ± 0.47 d | ||
| 0.4 | 16.17 ± 1.68 b | 1.56 ± 0.39 c–e | 29.67 ± 1.25 ij | 2.167 ± 0.45 g–i | ||
| PEG | 0.009 | 10.30 ± 0.71 ef | 1.80 ± 0.20 a–c | 51 ± 2.16 b | 9.667 ± 0.47 b | |
| 0.012 | 14.76 ± 1.91 b–d | 1.98 ± 0.26 a | 41.67 ± 1.25 de | 2.333 ± 0.47 gh | ||
| 0.015 | 4.23 ± 0.69 h | 0.81 ± 0.05 h–j | 25.67 ± 2.49 kl | 1.433 ± 0.17 h–j | ||
| TA | Control | 0 | 6.83 ± 1.83 gh | 0.81 ± 0.01 h–j | 27 ± 2.16 jk | 3.733 ± 0.38 f |
| Mannitol | 0.1 | 12.92 ± 0.54 b–e | 0.99 ± 0.01 g–i | 41 ± 1.41 de | 6.667 ± 0.47 c | |
| 0.2 | 15.45 ± 0.39 bc | 1.30 ± 0.05 e–g | 33.67 ± 1.70 gh | 4.067 ± 0.25 ef | ||
| 0.4 | 24.11 ± 4.71 a | 1.11 ± 0.09–h | 20 ± 0.82 mn | 1.533 ± 0.21 h–j | ||
| PEG | 0.009 | 8.00 ± 0.81 fg | 1.29 ± 0.17 e–g | 38.33 ± 0.94 ef | 5.400 ± 0.43 d | |
| 0.012 | 13.10 ± 0.89 b–e | 1.33 ± 0.15 e–g | 22.67 ± 1.70 lm | 2.633 ± 0.45 g | ||
| 0.015 | 4.52 ± 1.89 h | 0.82 ± 0.05 h–j | 17.67 ± 1.25 n | 1.167 ± 0.13 ij | ||
| RA | Control | 0 | 5.96 ± 0.71 gh | 0.61 ± 0.15 j | 36 ± 1.63 fg | 4.267 ± 0.38 ef |
| Mannitol | 0.1 | 11.57 ± 0.41 de | 1.34 ± 0.05 e–g | 49.67 ± 0.47 b | 5.7 ± 0.36 d | |
| 0.2 | 12.48 ± 0.33 c–e | 1.92 ± 0.12 ab | 43 ± c2.45 d | 3.8 ± 0.16 f | ||
| 0.4 | 14.46 ± 0.68 b–d | 1.07 ± 0.07 g–i | 32.33 ± 0.47 g–i | 2.1 ± 0.22 g–i | ||
| PEG | 0.009 | 10.05 ± 0.45 ef | 1.09 ± 0.15 gh | 45.67 ± 2.06 c | 4.3 ± 0.16 ef | |
| 0.012 | 11.87 ± 0.56 de | 1.45 ± 0.16 d–f | 32 ± 1.63 hi | 1.767 ± 0.21 g–j | ||
| 0.015 | 3.81 ± 0.65 h | 0.72 ± 0.06 ij | 23 ± 2.16 lm | 1.033 ± 0.13 j | ||
| S.O.V. | ||||||
| Species | 26.633 ** | 1.815 ** | 799.302 ** | 26.634 ** | ||
| Osmotic agent | 198.032 ** | 0.865 ** | 850.106 ** | 56.207 ** | ||
| Species × Osmotic agent | 15.362 ** | 0.182 ** | 21.431 ** | 5.423 ** | ||
| Error | 3.113 | 0.036 | 4.270 | 0.316 | ||
| C.V. (%) | 16.26 | 14.60 | 5.82 | 13.76 | ||
| Mean Square | ||||||
|---|---|---|---|---|---|---|
| df | CAT Activity | GR Activity | APX Activity | GPX Activity | SOD Activity | |
| Species | 2 | 8.137 ** | 0.595 ** | 0.756 ** | 0.027 ns | 1449.242 ** |
| Osmotic agent | 6 | 4.809 ** | 0.252 ** | 0.237 ** | 2.464 ** | 850.888 ** |
| Species × Osmotic agent | 12 | 0.092 ** | 0.011 ** | 0.011 ** | 0.043 * | 111.711 ** |
| Error | 42 | 0.016 | 0.001 | 0.002 | 0.022 | 7.190 |
| C.V. (%) | 5.10 | 8.42 | 9.03 | 9.48 | 5.94 | |
| Mean Square | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| df | AsA | DHA | ASA/DHA | GSH | GSSG | GSH/GSSG | H2O2 | MDA | |
| Species | 2 | 120,832.68 ** | 56,691.48 | 36.608 ** | 91879 ** | 24,595.85 ** | 16.07 ** | 19.583 ** | 12.708 ** |
| Osmotic agent | 6 | 9104.22 ** | 15,851.95 | 9.536 ** | 10,168.21 ** | 5246.95 ** | 2.711 ** | 5.294 ** | 3.335 ** |
| Species × Osmotic agent | 12 | 756.35 ** | 896.86 | 2.373 ** | 682.037 ** | 240.63 ** | 0.095 ** | 0.314 ** | 0.323 ** |
| Error | 42 | 13.952 | 14.63 | 0.019 | 9.095 | 11.06 | 0.004 | 0.013 | 0.026 |
| C.V. (%) | 2 | 2.71 | 7.39 | 0.99 | 2.05 | 2.90 | 4.64 | 9.12 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajaragh, R.P.; Rasouli, F.; Giglou, M.T.; Zahedi, S.M.; Hassanpouraghdam, M.B.; Aazami, M.A.; Adámková, A.; Mlček, J. Morphological and Physiological Responses of In Vitro-Grown Cucurbita sp. Landraces Seedlings under Osmotic Stress by Mannitol and PEG. Horticulturae 2022, 8, 1117. https://doi.org/10.3390/horticulturae8121117
Tajaragh RP, Rasouli F, Giglou MT, Zahedi SM, Hassanpouraghdam MB, Aazami MA, Adámková A, Mlček J. Morphological and Physiological Responses of In Vitro-Grown Cucurbita sp. Landraces Seedlings under Osmotic Stress by Mannitol and PEG. Horticulturae. 2022; 8(12):1117. https://doi.org/10.3390/horticulturae8121117
Chicago/Turabian StyleTajaragh, Rana Panahi, Farzad Rasouli, Mousa Torabi Giglou, Seyed Morteza Zahedi, Mohammad Bagher Hassanpouraghdam, Mohammad Ali Aazami, Anna Adámková, and Jiří Mlček. 2022. "Morphological and Physiological Responses of In Vitro-Grown Cucurbita sp. Landraces Seedlings under Osmotic Stress by Mannitol and PEG" Horticulturae 8, no. 12: 1117. https://doi.org/10.3390/horticulturae8121117
APA StyleTajaragh, R. P., Rasouli, F., Giglou, M. T., Zahedi, S. M., Hassanpouraghdam, M. B., Aazami, M. A., Adámková, A., & Mlček, J. (2022). Morphological and Physiological Responses of In Vitro-Grown Cucurbita sp. Landraces Seedlings under Osmotic Stress by Mannitol and PEG. Horticulturae, 8(12), 1117. https://doi.org/10.3390/horticulturae8121117







