Development of BC3F2 Tomato Genotypes with Arthropod Resistance Introgressed from Solanum habrochaites var. hirsutum (PI127826)
Abstract
1. Introduction
2. Materials and Methods
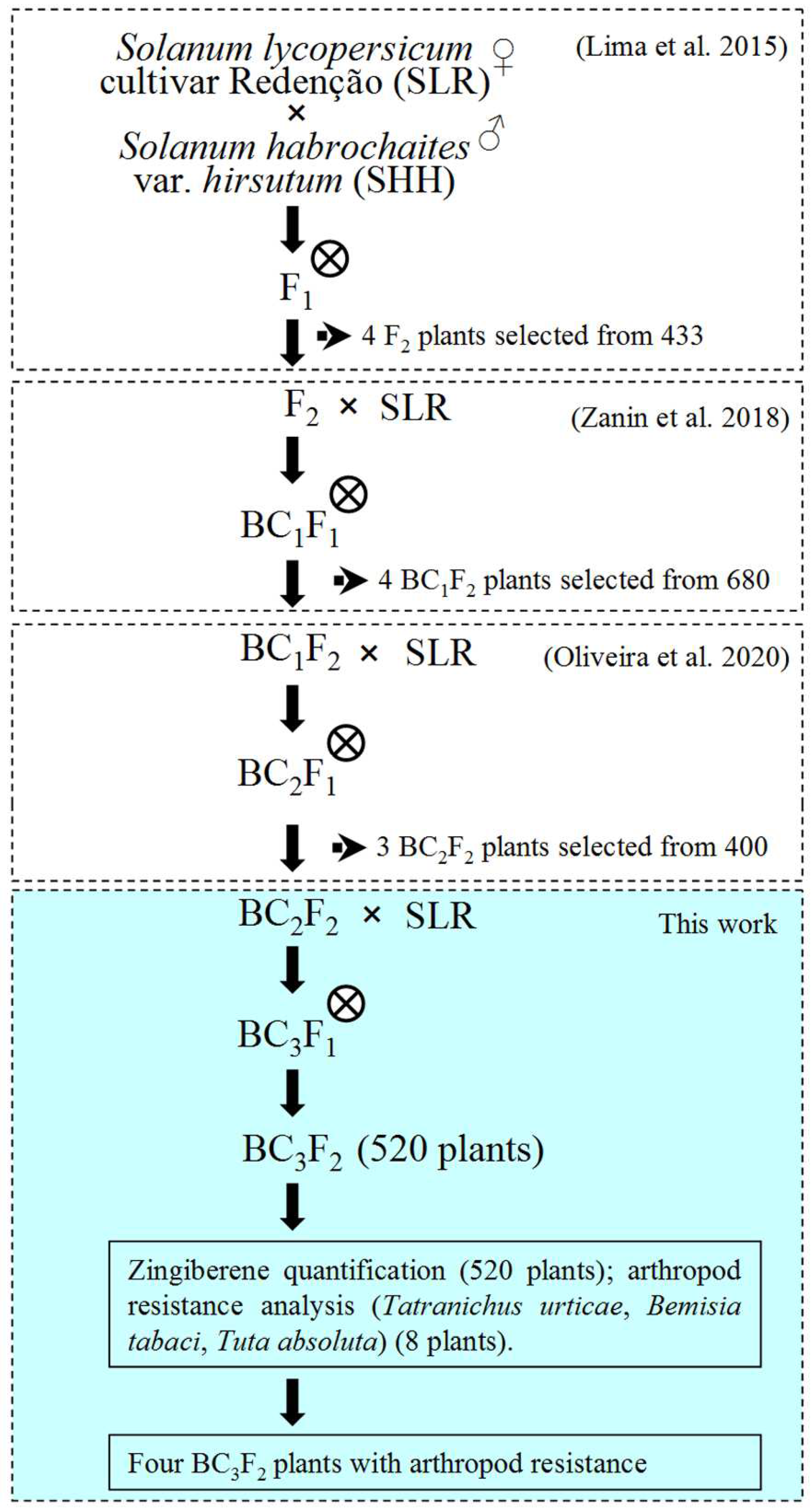
2.1. Plant Materials and Breeding Strategy
2.2. Zingiberene Quantification
2.3. Trichome Analysis
2.4. Bamesia tabaci Resistance Test
2.5. Tuta absoluta Risistance Test
2.6. Tatranichus Urticae Resistance Test
2.7. Statistical Analyses
2.8. Evaluation of Recurrent Genome Recovery
3. Results
3.1. Zingiberene Quantification
3.2. Trichome Analysis
3.3. Bamesia tabaci Resistance Test
3.4. Tuta absoluta Risistance Test
3.5. Tatranichus urticae Resistance Test
3.6. Evaluation of Recurrent Genome Recovery
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Askari-khorasgani, O.; Pessarakli, M. Tomato (Solanum lycopersicum) culture in vermi-aquaponic systems: II. Strategies for sustainable and economic development: Fertilization practices in vermi-ponic unit. J. Plant Nutr. 2020, 43, 1726–1739. [Google Scholar] [CrossRef]
- Mehta, K.; Rajesh, K.T.; Guleria, J.S. Socio-economic impact of protected cultivation on tomato growers of Himachal Pradesh. Econ. Aff. 2020, 65, 1–7. [Google Scholar] [CrossRef]
- Rahman, M.S.; Manjira, S.; Majumder, M.K.; Rahman, S. Socio-economic determinants of off-season summer tomato cultivation. Int. J. Veg. Sci. 2020, 27, 252–259. [Google Scholar] [CrossRef]
- Wohner, B.; Gabriel, V.H.; Krenn, B.; Krauter, V.; Tacker, M. Environmental and economic assessment of food-packaging systems with a focus on food waste. Case study on tomato ketchup. Sci. Total Environ. 2020, 738, 139–846. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Peng, Z.; Tong, H.; Yng, F.; Xing, G.; Wang, L.; Zheng, J.; Zhang, Y.; Su, Q. Tomato plant flavonoids increase whitefly resistance and reduce spread of Tomato Yellow Leaf Curl Virus. J. Econ. Entomol. 2019, 112, 2790–2796. [Google Scholar] [CrossRef]
- Dias, D.M.; Resende, J.T.V.; Zeist, A.R.; Gabriel, A.; Santos, M.H.; Vilela, N.C. Resistance of processing tomato genotypes to leafminer (Tuta absoluta). Hortic. Bras. 2019, 37, 1040–1046. [Google Scholar] [CrossRef]
- Vidyarthi, S.; Simmons, C. Characterization and management strategies for process discharge streams in California industrial tomato processing. Sci. Total Environ. 2020, 723, 137976. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food and Agriculture Data: Production: Crops. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 15 March 2021).
- Mkonyi, L.; Rubanga, D.; Richard, M.; Zekeya, N.; Sawahiko, S.; Maiseli, B.; Machuve, D. Early identification of Tuta absoluta in tomato plants using deep learning. Sci. Afr. 2020, 10, e00590. [Google Scholar] [CrossRef]
- Johnston, N.; Martini, X. The influence of visual and olfactory cues in host selection for Bemisia tabaci Biotype B in the presence or absence of Tomato Yellow Leaf Curl Virus. Insects 2020, 2, 115. [Google Scholar] [CrossRef]
- Soares, M.A.; Carvalho, G.A.; Campos, M.R.; Passos, L.C.; Haro, M.M.; Lavoir, A.V.; Desneux, N. Detrimental sublethal effects hamper the effective use of natural and chemical pesticides in combination with a key natural enemy of Bemisia tabaci on tomato. Pest Manag. Sci. 2020, 76, 3551–3559. [Google Scholar] [CrossRef]
- Tarusikirwa, V.L.; Mutamiswa, R.; English, S.; Chidawanyika, F.; Nyamukondiwa, C. Thermal plasticity in the invasive south American tomato pinworm Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). J. Biol. 2020, 20, 102598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.F.; Wang, Y.S.; Gao, Y.H.; Liu, W.X.; Zhang, R.; Fu, W.J.; Wan, F.H. First report of the South American tomato leafminer, Tuta absoluta (Meyrick), in China. J. Integr. Agric. 2020, 19, 1912–1917. [Google Scholar] [CrossRef]
- Pulga, P.S.; Henshel, J.M.; Resende, J.T.V.; Zeist, A.R.; Moreira, A.F.P.; Gabriel, A.; Gonçalves, L.S.A. Salicylic acid treatments induce resistance to Tuta absoluta and Tetranychus urticae on tomato plants. Hortic. Bras. 2020, 38, 288–294. [Google Scholar] [CrossRef]
- Tiftikçi, P.; Kök, Ş.; Kasap, I. Biological control of twospotted spider mites [Tetranychus urticae Koch (Acari: Tetranychidae)] using Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseidae) at different ratios of release on field-grown tomatos. Biol. Control 2020, 151, 104404. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X. Tomato diseases and pests detection based on improved yolo v3 convolutional neural network. Front. Plant Sci. 2020, 11, 898. [Google Scholar] [CrossRef]
- Mulugeta, T.; Muhinyuza, J.B.; Gouws-Meyer, R.; Matsaunyane, L.; Andreasson, E.; Alexandersson, E. Botanicals and plant strengtheners for potato and tomato cultivation in Africa. J. Integr. Agric. 2020, 19, 406–427. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, X.; Bao, E.; Li, J.; Zou, Z.; Cao, K. Bio-organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci. Rep. 2020, 10, 177. [Google Scholar] [CrossRef]
- Knegt, B.; Meijer, T.T.; Kant, M.R.; Kiers, E.T.; Egas, M. Tetranychus evansi spider mite populations suppress tomato defenses to varying degrees. Ecol. Evol. 2020, 10, 4375–4390. [Google Scholar] [CrossRef]
- Sene, S.O.; Tendeng, E.; Diatte, M.; Sylla, S.; Labou, B.; Diallo, A.W.; Diarra, K. Insecticide resistance in field populations of the tomato fruitworm, Helicoverpa armigera, from Senegal. Int. J. Biol. Chem. Sci. 2020, 14, 181–191. [Google Scholar] [CrossRef]
- Oliveira, J.R.F.; Resende, J.T.V.D.; Roberto, S.R.; Da-Silva, P.R.; Rech, C.; Nardi, C. Tomato Breeding for Sustainable Crop Systems: High Levels of Zingiberene Providing Resistance to Multiple Arthropods. Horticulturae 2020, 6, 34. [Google Scholar] [CrossRef]
- Dutta, P.; Hazari, S.; Karak, C.; Talukdar, S. Study on genetic variability of different tomato (Solanum lycopersicum) cultivars grown under open field condition. Int. J. Chem. Stud. 2018, 6, 1706–1709. [Google Scholar]
- Dawood, M.H.; Snyder, J.C. The alcohol and epoxy alcohol of zingiberene, produced in trichomes of wild tomato, are more repellent to spider mites than zingiberene. Front. Plant Sci. 2020, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.A.; Abbas, M.M.; Amjad, M.; Ziaf, K.; Ali, B.; Shaheen, T.; Awan, F.S.; Khan, A.N. Production and characterisation of tomato derived from interspecific hybridisation between cultivated tomato and its wild relatives. J. Hortic. Sci. Biotechnol. 2020, 95, 506–520. [Google Scholar] [CrossRef]
- Zörb, C.; Piepho, H.P.; Zikeli, S.; Horneburg, B. Heritability and variability of quality parameters of tomatoes in outdoor production. Research 2020, 2020, 6707529. [Google Scholar] [CrossRef] [PubMed]
- Resende, N.C.V.; Silva, A.A.; Maluf, W.R.; Resende, J.T.V.; Zeist, A.R.; Gabriel, A. Selection of lines and populations of tomato for fruit shape and resistance to tomato leafminer. Hortic. Bras. 2020, 38, 117–125. [Google Scholar] [CrossRef]
- Muigai, S.G.; Basset, M.J.; Schuster, D.J.; Scott, J.W. Greenhouse and field screening of wild Lycopersicon germplasm for resistance to the whitefly Bemisia argentifolii. Phytoparasitica 2003, 31, 27. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Andrade Júnior, V.C.; Maluf, W.R.; Neiva, I.P.; Maciel, G.M. Resistance of tomato strains to the moth Tuta absoluta imparted by allelochemicals and trichome density. Ciênc. Agrotecnol. 2012, 36, 45–52. [Google Scholar] [CrossRef]
- Rakha, M.; Zekeya, N.; Sevgan, S.; Musembi, M.; Ramasamy, S.; Hanson, P. Screening recently identified whitefly/spider mite-resistant wild tomato accessions for resistance to Tuta absoluta. Plant Breed 2017, 136, 562–568. [Google Scholar] [CrossRef]
- Zanin, D.S.; Resende, J.T.V.; Zeist, A.R.; Oliveira, J.R.; Henschel, J.M.; Lima Filho, R.B. Selection of processing tomato genotypes resistant to two spotted spider mite. Hortic. Bras. 2018, 36, 253258. [Google Scholar] [CrossRef]
- Marchant, W.G.; Legarrea, S.; Smeda, J.R.; Mutschler, M.A.; Srinivasa, N.R. Evaluating acylsugars-mediated resistance in tomato against Bemisia tabaci and transmission of Tomato Yellow Leaf Curl Virus. Insects 2020, 11, 842. [Google Scholar] [CrossRef]
- Santos, N.C.; Marquez, G.R.; Maciel, G.M.; Pereira, L.M.; Peres, H.G.; Peixoto, J.V.M. Resistance of round tomato genotypes wifh determinate growth habit to two-spotted spider mites and silverleaf whitefly. Bioagro 2020, 32, 15–22. [Google Scholar]
- Oliveira, J.R.F.; Resende, J.T.V.; Maluf, W.R.; Lucini, T.; Lima-Filho, R.B.; Lima, I.P.; Nardi, C. Trichomes and allelochemicals in tomato genotypes have antagonistic effects upon behavior and biology of Tetranychus urticae. Front. Plant Sci. 2018, 9, 1132. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, W.I.; Toscano, L.C.; Boiça Júnior, A.L.; Barbosa, J.C. Resistance of tomato genotypes to spider mite. Hortic. Bras. 2002, 20, 480–484. [Google Scholar] [CrossRef][Green Version]
- Suinaga, F.A.; Picanço, M.C.; Moreira, M.D.; Semeão, A.A.; Magalhães, S.T.V. Antibiosis resistance of Lycopersicon peruvianum to tomato leafminer. Hortic. Bras. 2004, 22, 281–285. [Google Scholar] [CrossRef][Green Version]
- Gonçalves Neto, A.C.; Silva, V.F.; Maluf, W.R.; Maciel, G.M.; Nizio, D.A.C.; Gomes, L.A.A.; Azevedo, S.M. Resistance to the South American tomato pinworm in tomato plants with high foliar acylsugar contentes. Hortic. Bras. 2010, 28, 203–208. [Google Scholar] [CrossRef][Green Version]
- Maciel, G.M.; Maluf, W.R.; Silva, V.F.; Gonçalves Neto, A.C.; Gomes, L.A.A. Pre-commercial hybrids obtained from an acylsugar-rich tomato inbred line, resistant to Tuta absoluta. Hortic. Bras. 2011, 29, 151–156. [Google Scholar] [CrossRef][Green Version]
- Albaladejo, I.; Meco, V.; Plasencia, F.; Flores, F.B.; Bolarin, M.C.; Egea, I. Unravelling the strategies used by the wild tomato species Solanum pennellii to confront salt stress: From leaf anatomical adaptations to molecular responses. Environ. Exp. Bot. 2017, 135, 1–12. [Google Scholar] [CrossRef]
- Yan, Z.; Pérez De Castro, A.; Díez, M.J.; Hutton, S.F.; Visser, R.G.; Wolters, A.M.A.; Li, J. Resistance to Tomato Yellow Leaf Curl Virus in tomato germplasm. Front. Plant Sci. 2018, 9, 1198. [Google Scholar] [CrossRef]
- Sade, D.; Sade, N.; Brotman, Y.; Czosnek, H. Tomato yellow leaf curl virus (TYLCV)-resistant tomatoes share molecular mechanisms sustaining resistance with their wild progenitor Solanum habrochaites but not with TYLCV-susceptible tomatoes. Plant Sci. 2020, 295, 110439. [Google Scholar] [CrossRef]
- Iftekharuddaula, K.M.; Newaz, M.A.; Salam, M.A. Rapid and high-precision marker assisted backcrossing to introgress the SUB1 QTL into BR11, the rainfed lowland rice mega variety of Bangladesh. Euphytica 2011, 178, 83–97. [Google Scholar] [CrossRef]
- Ellur, R.K.; Khanna, A.; Yadav, A.; Pathania, S.; Rajashekara, H.; Singh, V.K.; Gopala Krishnan, S.; Bhowmick, P.K.; Nagarajan, M.; Vinod, K.K.; et al. Improvement of Basmati rice varieties for resistance to blast and bacterial blight diseases using marker assisted backcross breeding. Plant Sci. 2016, 242, 330–341. [Google Scholar] [CrossRef]
- Lee, T.G.; Shekasteband, R.; Menda, N.; Mueller, L.A.; Hutton, S.F. Molecular markers to select for the j-2–mediated jointless pedicel in tomato. HortScience 2018, 53, 153–158. [Google Scholar] [CrossRef]
- Lima, I.P.; Resende, J.T.V.; Oliveira, J.R.F.; Faria, M.V.; Resende, N.C.V.; Lima Filho, R.B. Indirect selection of industrial tomato genotypes rich in zingiberene and resistant to Tuta absoluta Meyrick. Genet. Mol. Res. 2015, 14, 15081–15089. [Google Scholar] [CrossRef] [PubMed]
- Freitas, J.A.; Maluf, W.R.; Cardoso, M.D.G.; Oliveira, A.C.B.D.; Seleção, D.E. Plantas de tomateiro visando à resistência à artrópodes-praga mediada por zingibereno. Acta Sci. 2000, 22, 919–923. [Google Scholar]
- Luckwill, L.C. The Genus Lycopersicon: An Historical, Biological, and Taxonomic Survey of the Wild and Cultivated Tomatoes; Aberdeen University Press: Aberdeen, UK, 1943. [Google Scholar]
- Labory, C.R.G.; Santa Cecília, L.V.C.; Maluf, W.R.; Cardoso, M.G.; Bearzotti, E.; Souza, J.C. Indirect selection to 2-tridecanone content and its relation to tomato pinworm resistance. Pesqui. Agropecuária Bras. 1999, 34, 733–740. [Google Scholar] [CrossRef]
- Weston, P.A.; Snyder, J.C. Thumb tack bioassay: A quick method of measuring plant resistance to two spotted spider mites (Acari: Tetranychidae). J. Econ. Entomol. 1990, 83, 500–504. [Google Scholar] [CrossRef]
- Sharma, K.; Ajay, K.M.; Raj, S.M. A simple and efficient method for extraction of genomic DNA from tropical tuber crops. Afr. J. Biotechnol. 2008, 7, 1018–1022. [Google Scholar]
- Rohlf, F.J. NTSYS-pc: Numerical Taxonomy System; Version 2.2; Exeter Software: New York, NY, USA, 2008. [Google Scholar]
- Suinaga, F.A.; Casali, V.W.D.; Silva, D.J.H.; Picanço, M.C. Genetic dissimilarity among soucers of resistance of Lycopersicon spp. To Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechidae). R. Bras. Agrociência 2003, 9, 371–376. [Google Scholar]
- Al-Bayati, A.S. Breeding for Tomato Resistance to Spider Mite Tetranychus Urticae Koch (Acari: Tetranychidae); University of Kentucky: Lexington, KY, USA, 2019. [Google Scholar]
- Freitas, J.A.; Maluf, W.R.; Cardoso, M.G.; Gomes, L.A.A.; Bearzotti, E. Inheritance of foliar zingiberene contents and their relationship to trichome densities and whitefly resistance in tomatoes. Euphytica 2002, 127, 275–287. [Google Scholar] [CrossRef]
- Maluf, W.R.; De Fátima Silva, V.; Das Graças Cardoso, M.; Gomes, L.A.A.; Neto, A.C.G.; Maciel, G.M.; Nízio, D.A.C. Resistance to the South American tomato pinworm Tuta absoluta in high acylsugar and/or high zingiberene tomato genotypes. Euphytica 2010, 176, 113–123. [Google Scholar] [CrossRef]
- Oriani, M.A.D.G.; Vendramim, J.D.; Vasconcelos, C.J. Biology of Bemisia tabaci (Genn.) B biotype (Hemiptera, Aleyrodidae) on tomato genotypes. Sci. Agric. 2011, 68, 37–41. [Google Scholar] [CrossRef]
- Neiva, I.P.; Andrade Júnior, V.C.; Maluf, W.R.; Oliveira, C.M.; Maciel, G.M. Role of allelochemicals and trichome density in the Resistance of tomato to whitefly. Ciênc. Agrotecnol. 2013, 37, 61–67. [Google Scholar] [CrossRef][Green Version]
- Boiça Júnior, A.L.; Bottega, D.B.; Lourenção, A.L.; Rodrigues, N.E.L. Resistance in tomato genotypes to attack of Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae): Non-preference for oviposition and feeding. Arq. Inst. Biol. 2012, 14, 541–548. [Google Scholar]
- Bitew, M.K. Significant role of wild genotypes of tomato trichomes for Tuta absoluta resistance. J. Plant Genet. Breed. 2018, 2, 104. [Google Scholar]
- Zanin, D.S.; Resende, J.T.V.; Zeist, A.R.; Lima Filho, R.B.; Gabriel, A.; Diniz, F.C.P.; Perrud, A.C.; Morales, R.G.F. Selection of F2BC1 tomato genotypes for processing containing high levels of zingiberene and resistant to tomato pinworms. Phytoparasitica 2021, 49, 265–274. [Google Scholar] [CrossRef]
- Antonious, G.F.; Snyder, J.C. Natural products: Repellency and toxicity of wild tomato leaf extracts to the two-spotted spider mite, Tetranychus urticae Koch. J. Environ. Sci. Health B 2006, 41, 43–55. [Google Scholar] [CrossRef]
- Lima, I.P.; Resende, J.T.V.D.; Oliveira, J.R.; Faria, M.V.; Dias, D.M.; Resende, N.C. Selection of tomato genotypes for processing with high zingiberene content, resistant to pests. Hortic Bras 2016, 34, 387–391. [Google Scholar] [CrossRef]
- Lawson, D.M.; Lunde, C.F.; Mutschler, M.A. Marker-assisted transfer of acylsugar-mediated pest resistance from the wild tomato, Lycopersicon pennellii, to the cultivated tomato, Lycopersicon esculentum. Mol. Breed. 1997, 3, 307–317. [Google Scholar] [CrossRef]
- Foolad, M.R.; Panthee, D.R. Marker-assisted selection in tomato breeding. Crit. Rev. Plant Sci. 2012, 31, 93–123. [Google Scholar] [CrossRef]



| Primer | Sequence * (5′–3′) | AT °C |
|---|---|---|
| UBC-807 | (AG)8T | 52 |
| UBC-808 | (AG)8C | 50 |
| UBC-809 | (AG)8G | 55 |
| UBC-810 | (GA)8T | 52 |
| UBC-811 | (GA)8C | 52 |
| UBC-815 | (CT)8G | 52 |
| UBC-827 | (AC)8G | 53 |
| UBC-835 | (AG)8YC | 52 |
| UBC-836 | (AG)8YA | 53 |
| UBC-848 | (CA)8AGG | 55 |
| Trichomes | B. tabaci | T. absoluta | T. urticae | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ZGB Abs & | GT IV & | GT VI & | NGT & | Number of Eggs & | Number of Nymphs & | Lesions & | DT & | ||||
| Genotype | AB | AD | AB | AD | AB | AD | 21 DAI | 21 DAI | 21 DAI | 60 min | |
| S. habrochaites var. hirsutum | 0.31 a | 5.00 a | 2.00 a | 0.00 e | 47.00 a | 0.00 c | 21.50 a | 4.75 h | 2.00 e | 1.60 d | 2.21 e |
| RVTZ pl#344 (high ZGB) | 0.19 abc | 1.00 c | 0.50 bc | 4.50 a | 1.50 ab | 30.00 bc | 20.50 a | 30.25 e | 6.00 cd | 2.26 bcd | 7.49 de |
| RVTZ pl#346 (high ZGB) | 0.17 abc | 1.25 bc | 0.75 abc | 1.00 cde | 0.75 abc | 20.00 cdefg | 12.00 cde | 20.75 f | 4.00 de | 1.70 d | 10.52 cd |
| RVTZ pl#348 (high ZGB) | 0.26 ab | 3.75 ab | 1.50 ab | 3.00 ab | 2.00 a | 12.25 g | 12.50 cde | 12.75 g | 4.00 de | 2.20 bcd | 8.01 d |
| RVTZ pl#361 (high ZGB) | 0.22 abc | 0.75 c | 0.25 bc | 2.75 abc | 1.25 ab | 24.00 cdef | 14.75 bc | 16.25 fg | 5.25 cd | 2.23 cd | 12.24 bcd |
| RVTZ pl#448 (high ZGB) | 0.18 abc | 250 abc | 1.00 abc | 2.25 bcd | 1.25 ab | 22.25 cdefg | 12.50 cde | 33.75 e | 5.75 cd | 2.18 cd | 11.58 abcd |
| S. lycopersicum cv. Redenção | 0.06 bc | 1.00 c | 0.50 bc | 0.75 de | 0.50 abc | 26.25 bcde | 14.75 bc | 153.50 a | 25.00 b | 4.15 a | 19.52 ab |
| RVTZ pl#125 (low ZGB) | 0.05 c | 0.50 a | 0.25 bc | 0.25 e | 0.50 abc | 14.25 fg | 10.25 cde | 128.75 b | 47.50 a | 3.90 ab | 16.19 abc |
| RVTZ pl#126 (low ZGB) | 0.04 c | 0.25 c | 0.00 c | 0.50 de | 0.25 bc | 37.00 ab | 17.75 ab | 141.75 ab | 29.00 b | 3.65 ab | 21.26 a |
| RVTZ pl#128 (low ZGB) | 0.05 c | 0.50 c | 0.00 c | 0.75 de | 0.25 bc | 22.25 cdefg | 8.00 e | 106.50 c | 25.50 b | 4.40 a | 20.21 ab |
| Pearson correlation (r) | |||||||||||
| ZGB | - | 0.72 * | 0.73 * | 0.32 ns | 0.33 ns | 0.06 ns | 0.31 ns | −0.91 * | −0.87 * | −0.91 * | −0.94 * |
| GT IV AB leaflet side | 0.72 * | - | - | - | - | - | - | −0.58 * | −0.51 ns | −0.54 * | −0.78 * |
| GT IV AD leaflet side | 0.73 * | - | - | - | - | - | - | −0.60 * | −0.53* | −0.59 * | −0.83 * |
| GT VI AB leaflet side | 0.32 ns | - | - | - | - | - | - | −0.46 ns | −0.42 ns | −0.32 ns | −0.37 ns |
| GT VI AD leaflet side | 0.33 ns | - | - | - | - | - | - | −0.50 ns | −0.39 ns | −0.34 ns | −0.37 ns |
| NGT AB leaflet side | 0.06 ns | - | - | - | - | - | - | 0.05 ns | −0.05 ns | −0.03 ns | −0.22 ns |
| NGT AD leaflet side | 0.31 ns | - | - | - | - | - | - | −0.21 ns | −0.25 ns | −0.30 ns | −0.50 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panizzon Diniz, F.C.; Vilela de Resende, J.T.; Lima-Filho, R.B.d.; Pilati, L.; Gomes, G.C.; Roberto, S.R.; Da-Silva, P.R. Development of BC3F2 Tomato Genotypes with Arthropod Resistance Introgressed from Solanum habrochaites var. hirsutum (PI127826). Horticulturae 2022, 8, 1217. https://doi.org/10.3390/horticulturae8121217
Panizzon Diniz FC, Vilela de Resende JT, Lima-Filho RBd, Pilati L, Gomes GC, Roberto SR, Da-Silva PR. Development of BC3F2 Tomato Genotypes with Arthropod Resistance Introgressed from Solanum habrochaites var. hirsutum (PI127826). Horticulturae. 2022; 8(12):1217. https://doi.org/10.3390/horticulturae8121217
Chicago/Turabian StylePanizzon Diniz, Flávia Cristina, Juliano Tadeu Vilela de Resende, Renato Barros de Lima-Filho, Laura Pilati, Gabriella Correia Gomes, Sergio Ruffo Roberto, and Paulo Roberto Da-Silva. 2022. "Development of BC3F2 Tomato Genotypes with Arthropod Resistance Introgressed from Solanum habrochaites var. hirsutum (PI127826)" Horticulturae 8, no. 12: 1217. https://doi.org/10.3390/horticulturae8121217
APA StylePanizzon Diniz, F. C., Vilela de Resende, J. T., Lima-Filho, R. B. d., Pilati, L., Gomes, G. C., Roberto, S. R., & Da-Silva, P. R. (2022). Development of BC3F2 Tomato Genotypes with Arthropod Resistance Introgressed from Solanum habrochaites var. hirsutum (PI127826). Horticulturae, 8(12), 1217. https://doi.org/10.3390/horticulturae8121217







