Abstract
Sugar content is a primary determinant of taste and quality in tomato (Solanum lycopersicum) fruit. Sugar allocation from source to sink is dependent on the activity of plasma membrane sugar transporters and is a critical process in plant development. Sugar will eventually be exported transporters (SWEETs) are sugar transporters that play key roles in plant biology, including growth and development. However, few studies have been conducted on the tomato SWEET protein family to date. Through gene expression analysis, we found that SlSWEET12c had the highest expression during the red ripening stage of tomato fruits. Yeast functional complementation, subcellular localization, and GUS activity assays showed that SlSWEET12c is a plasma membrane-localized sugar transporter that accumulates in the vascular bundles, carpel, and sarcocarp. Silencing SlSWEET12c increased sucrose accumulation and reduced the number of hexoses in tomato fruits; the opposite effects were observed under SISWEET12c overexpression. Invertase activity was also decreased after silencing SISWEET12c. These results suggest that SlSWEET12c is a sugar transporter that promotes sucrose unloading and metabolism in ripening tomato fruits, offering a new target for improving tomato quality and production.
1. Introduction
Sugars are essential for the subsistence of plants as major sources of carbon and energy; plants acquire carbon from the atmosphere and fix it in the form of sugars [1]. Tomato is one of the most important fleshy fruit crops globally and is a major horticultural crop; thus, tomato is widely used as a research model for the development of fleshy fruits [2,3]. In tomato fruits, sugar content is a primary determinant of taste and quality [4]. For example, sucrose serves as a building block for cell walls and is a key component of signaling for the maintenance of osmotic homeostasis under certain abiotic stress conditions and various other purposes [3]. Sugar allocation from source to sink is critical for plant development [5]. This movement from source to sink organs is controlled by the loading and unloading of transport tissues [6].
During the development of tomato fruit, sugar for fruit metabolism and storage is supplied via the phloem. After unloading from the phloem, sugars are distributed within the fruit via both apoplastic and symplastic pathways [7,8]. During the ripening stage, because of the combined activity of cell wall-bound invertases and vacuolar invertases, tomatoes mostly contain glucose and fructose at an equimolar concentration, along with a small amount of sucrose [9,10,11]. Sucrose is the principal end product of photosynthesis in most higher plants; it is translocated through the phloem from the source leaves to the sink organs where it is hydrolyzed by invertase into glucose and fructose or cleaved by sucrose synthase into UDP-Glc and fructose [12]. Sucrose is loaded into the phloem for translocation to the sink organs via the conversion of photosynthetic assimilates, which are not required for leaf function [13].
Sugar will eventually be exported transporters (SWEETs) are a newly discovered family of transporter proteins that play an important role in surface loading, especially members of clade III SWEETs [14,15,16]. Early studies have indicated that the absence of SWEET exporters can significantly suppress sucrose loading and plant growth, resulting in the accumulation of sugars and starch in the source leaves [5,17]. The loading is mediated by sucrose transporter 1 (SUT1)/sucrose symporter 2 (SUC2)-type transporters in the plasma membrane of phloem cells, which actively import apoplasmic sucrose into companion cell (CC)–sieve element (SE) complexes against a concentration gradient [18]. During unloading, part of the sucrose can be unloaded from the CCs to the apoplast along the SE–CC unloading pathway [19]. This process is likely motivated by simple or facilitated diffusion [18]. Sucrose in the apoplast can then be imported into nearby parenchyma cells with the help of an SUT, which is also hydrolyzed by a cell wall invertase into hexoses [18,20,21]. SUT family members are well-established sucrose/H+ symporters [22]. During the development of tomato (Lycopersicon esculentum) plants, LeSUT1 and LeSUT2 were reported to participate in sugar allocation from the source to sink [21]. Furthermore, SWEETs can also act as unloaders to mediate sugar efflux and import [23,24,25]. The transport of monosaccharides and/or disaccharides by SWEET proteins requires energy but nevertheless follows a concentration gradient [26].
Early studies have shown that the SWEET protein family can be divided into four clades. Clade I and II members transport hexoses, clade III members mainly transport sucrose, and clade IV members are involved in fructose transport [15,16]. Clade III SWEETs are expressed in many different organs during different developmental stages, such as the leaves, roots, and seeds [2,17,24,25,26,27,28,29,30,31]. For example, clade III SWEETs catalyze sucrose efflux and import, supporting embryo development in the maternal cells of developing seeds in Arabidopsis [24].
Tomato ripening is a highly coordinated developmental process that coincides with seed maturation. In addition, this stage is characterized by the increased accumulation of sugars, acids, and volatile compounds [9]. Among all 31 members of tomato Solanum lycopersicum SWEETs (SlSWEETs), only SlSWEET1a, SlSWEET7a, SlSWEET14, and SlSWEET15 have been experimentally studied to date. SlSWEET1a was shown to be involved in the uptake of glucose into unloading cells as part of the sugar unloading mechanism in the sink leaves of tomatoes [5]. Thus, altering the expression of SlSWEET7a and SlSWEET14 could be a potential strategy to enhance the sugar content of tomato fruits [31]. In addition, SlSWEET15 acts as a sucrose transporter, unloading sucrose from the phloem and seed coat for fruit and seed development in tomatoes [32]. Therefore, it is necessary to study other members of the SlSWEET family as they may provide targets to improve the quality and taste of tomato fruits, as well as to identify key sugar transporters in the mature green (MG) to red ripening (RR) stage. Based on quantitative analysis of SWEET genes in S. lycopersicum, we found that SlSWEET12c (Solyc05g024260) had the highest expression level in RR fruits. Therefore, we further focused on the function and distribution of SlSWEET12c through analyses of sugar transport activity, subcellular localization, and tissue localization. Moreover, we established transgenic lines with the overexpression or silencing of SlSWEET12 to better understand its roles in sugar transport and the genetic regulation mechanism.
2. Materials and Methods
2.1. Expression of SlSWEET during Tomato Development
To identify the expression levels of SlSWEET genes at different developmental stages, the tomato database website (Tomato Functional Genomics Database, http://ted.bti.cornell.edu/, accessed on 17 March 2022) was first used to obtain data for cultivated tomato ‘Heinz 1706’ (S. lycopersicum) and wild tomato Solanum pimpinellifolium.
2.2. Plant Materials and Growth Conditions
Micro-Tom tomatoes were used as the subject materials for this study. The seeds were dipped in water at 55 °C for 15 min and then incubated at a constant temperature (25 °C) until they budded (3–4 days). All plants were grown in a controlled chamber (25–30 °C, 16/8 h light/dark illumination, and 70–75% humidity). Fully expanded leaves were collected 30 days after germination. The fruits, including MG and RR fruits, were collected at 35 and 55 days after anthesis, respectively.
2.3. RNA Extraction and Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
At 55 days after flowering, total RNA was extracted from the Micro-Tom tomato fruits using TRIzol reagent according to the manufacturer’s instructions. Total RNA transcripts were reverse transcribed as previously described [31]. Real-time qPCR was performed on each sample in triplicate using SYBR Green Real Master Mix (TIANGEN, Beijing, China) following the manufacturer’s instructions. The reaction was conducted using a CFX Manager v. 3.1 Real-Time PCR System (Bio-Rad Laboratories, Hercules, CA, USA). Gene-specific primers for all 31 SlSWEET genes and sucrose invertase-related genes (primer sequences are listed in Supplementary Table S1, S2) were used for qPCR as described previously [5,31]. The fold-change was calculated using the 2−ΔΔCT method, and β-actin was used as a quantitative internal control gene (actin-F: 5′-TGTCCCTATTTACGAGGGTTATGC-3′; actin-R: 5′-AGTTAAATCACGACCAGCAAGAT-3′). Data are reported as the mean of three replicates.
2.4. Construction of SlSWEET12c-GUS Fusion Protein and Tomato Transformation
To investigate spatial expression patterns and determine the tissue-specific localization of SlSWEET12c, we performed a β-glucuronidase (GUS) activity analysis. The upstream 2180-basepair sequence of SlSWEET12c was obtained by searching the tomato database (https://solgenomics.net/, accessed on 17 March 2022). The promoter sequence was amplified by PCR using SlSWEET12c-GUS primers (12c-GUS-F: 5′-CACCGATTGGGTGCATAGATAGTA-3′ and 12c-GUS-R: 5′-CTAAGACTCCAAAGACGAAG-3′) and introduced into a pBGWES7.0 vector (including a GUS gene-coding region). The fusion vector was transformed into Agrobacterium tumefaciens strain GV3101; then, the Micro-Tom tomatoes were infected with the transformed Agrobacterium. The leaves, flowers, green fruits, and red fruits were selected from T0 generation plants, stained with a GUS kit, and observed under a Nikon SMZ800 stereomicroscope.
2.5. Subcellular Localization
The coding sequence (CDS) of SlSWEET12c (1100 bp excluding stop codons) was obtained by RT-PCR using SlSWEET12c-GFP primers (12c-GFP-F: 5′-CCTCTAGATTCAAAGAACCAAATCAC-3′ and 12c-GFP-R: 5′-ACTGAGCTCGAGTAAAATTGCAGCACA-3′) and introduced into a pCAM35-GFP expression vector with XbaI and KpnI sites. The fusion vector was then transformed into A. tumefaciens strain GV3101 and injected into Nicotania benthamiana leaves and onion epidermis. FM4-64 dye (MedChemExpress, Shanghai, China) was used as a positive control. The fluorescence signal was observed at excitation wavelengths of 488 nm or 561 nm and emission wavelengths of 500–572 nm or 605–635 nm using a confocal laser-scanning microscope (Leica SP8, Weztlar, Germany).
2.6. RNA Interference (RNAi), Overexpression Vector Construction, and Plant Transformation
The complementary DNA (cDNA) of SlSWEET12c was cloned into a pCAMBIA3301 vector with BglII and BstEII sites (primer sequences were OE12c-F: 5′-CCCCCGGGCGTGAGAAAGAGAAAGAGCAAGG-3′ and OE12c-R: 5′-TGTAGGTAGGGTAGGATGACAT-3′). The recombinant plasmids were transformed into A. tumefaciens strain GV3101 as previously described [33]. Positive plants were selected based on resistance to the herbicide glufosinate-ammonium (PPT) and PCR analysis in the T1 generation. Three SlSWEET12c lines from the T4 generation were used for the functional study.
To create RNAi plants, a 300-basepair specific fragment of SlSWEET12c was amplified from tomato fruit cDNA using gene-specific primer pairs (12cRNAi-F: 5′-CACCGCATCGTGTTTCAAGTGGTTCG-3′ and 12cRNAi-R: 5′-TCTATCGCTGGCTTTGCGTT-3′). The resulting product was cloned into a TOPO Gateway entry vector (Invitrogen, Carlsbad, CA, USA) and recombined with the plant expression vector pB7GWIWG2 (Invitrogen). The constructs were individually introduced into A. tumefaciens strain GV3101 and further transformed into Micro-Tom tomatoes using the leaf disc method [33]. Positive plants were selected based on PPT resistance (60 μg/mL). Three T1 generation lines were selected to obtain non-segregating homozygous lines. Further analysis was based on the T4 generation plants.
2.7. Functional Complementary Characterization of SlSWEET12c in Yeast
To explore the function of SlSWEET12c, yeast functional complementation analysis was carried out using the pDR195-SlSWEET12c recombinant plasmid; the empty vector (pDR195), yeast sucrose transporter (AtSUT2), and pDR195-SlSWEET12c were loaded into yeast strains for heterologous expression. The full-length CDS of SlSWEET12c was cloned into the pDR195 vector with XhoI and BamHI sites (primer pairs Y-12c-F: 5′-CCTCGAGCGTGAGAAAGAGAAAGAGCAAGG-3′ and Y-12c-R: 5′-CGGATCCTGTAGGTAGGGTAGGATGACAT-3′). The fused construct was transformed into the hexose transport-deficient yeast strain EBY.VW4000 and the sucrose uptake-deficient yeast strain SUSY7/ura. The mutant yeast strain SUSY7/ura (Saccharomyces cerevisiae) can grow on synthetic deficient (SD)/uracil solid medium with glucose as the sole carbon source but cannot grow on SD/uracil medium (uracil-deficient type) with sucrose as the sole carbon source. EBY.VW4000 is a mutant yeast strain that can only grow with a defective hexose transport function [34]. Hexose transport activity was monitored in SD/-uracil medium containing 2% (w/v) maltose or glucose. Sucrose transport activity was verified in SD/-uracil medium containing 2% (w/v) glucose or sucrose. The growth of yeast cells was photographed after 3–4 days at 30 °C.
2.8. Sugar Content Measurement
Fruits (0.5 g) were placed in a test tube with 5 mL 80% ethanol 55 days after flowering. Sucrose, glucose, and fructose were extracted and analyzed using liquid chromatography as previously described [35]. The measurement conditions for liquid chromatography (Waters e2695, Milford, MA, USA) were as follows: injection temperature, 35 °C; flow rate, 1.0 mL/min; column, Prevail Carbohydrate ES 5u; evaporative light-scattering detector (Alltech ELSD2000ES).
2.9. Determination of Enzyme Activity Related to Sugar Metabolism
The enzyme activities of invertase, sucrose phosphate synthase (SPS), and sucrose synthase (SS) were analyzed in the fruit samples (1 g) using the extraction and analysis methods described previously [36,37]. Invertase enzyme activity was expressed as μmol glucose ·h−1·g−1 fresh weight (FW), and SPS and SS enzyme activities were expressed as μmol sucrose·h−1·g−1 FW.
2.10. Statistical Analysis
Three biological and three technical replicates were used for each experiment. Significant differences were determined according to a one-way analysis of variance using the SPSS Statistics software (version 17.0; IBM Corp., Armonk, NY, USA). Error bars represent standard errors. A p-value < 0.05 was considered to be statistically significant.
3. Results
3.1. SlSWEET12c Is Highly Expressed in the RR Stage of Tomato Fruit
Based on gene expression data from the tomato database (Tomato Functional Genomics Database, http://ted.bti.cornell.edu/, accessed on 17 March 2022), SlSWEET12c was found to be the most highly expressed SlSWEET gene at the RR stage in both S. lycopersicum (‘Heinz tomato’) and S. pimpinellifolium (wild tomato) (Supplementary Figure S1). The RT-qPCR analysis of 31 SlSWEET genes showed that SlSWEET12c was also highly expressed in the RR stage of Micro-Tom tomatoes (Figure 1a). Therefore, we chose SlSWEET12c for further studies.
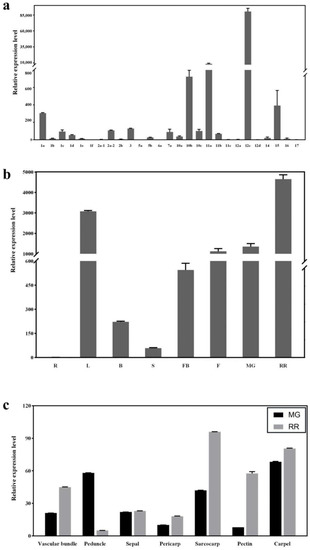
Figure 1.
Expression and distribution of SlSWEET genes in Micro-Tom tomato tissues. (a) Relative expression levels of SlSWEET genes analyzed using RT-qPCR. cDNA was isolated from fruits in the RR stage. The SlSWEET12d expression data were normalized to 1, and the beta-actin gene was used as an internal reference. (b) Expression of SlSWEET12c in different tomato organs and at different developmental stages (R—root; L—leaves; B—branch; S—stem; FB—flower bud; F—flower; MG—mature green; RR—red ripening). The expression data for SlSWEET12c in the roots were normalized to 1, and the beta-actin gene was used as an internal reference. (c) Expression of SlSWEET12c in different tissues at different developmental stages. The wild type (WT) expression data were normalized to 1, and the beta-actin gene was used as an internal reference. Values represent the means ± SD of three biological replicates.
To determine the effect of SlSWEET12c during tomato development, we measured its expression levels in different tissues at different developmental stages in Micro-Tom tomatoes (Figure 1b). Consistently, SlSWEET12c expression was the highest in RR fruit, suggesting that SlSWEET12c may play an important role in tomato fruit ripening. To further explore the function of SlSWEET12c, we refined the tomato fruit tissue and measured the expression level again (Figure 1c). With the exception of the peduncle, the transcript levels of SlSWEET12c at the RR stage in all other tissues were higher than those in the MG stage. At the MG stage, the expression levels of SlSWEET12c in the peduncle, sarcocarp, and carpel were higher than those in other tissues, especially in the carpel. The distribution of SlSWEET12c in the RR stage was similar to that in the MG stage: the expression level was significantly higher in the pectin, vascular bundles, sarcocarp, and carpel than in other tissues. Overall, SlSWEET12c was specifically and highly expressed in RR fruits.
3.2. Histochemical Localization of SlSWEET12c
Blue staining (indicating GUS activity) was observed in the leaves and MG fruits (Figure 2a,b). The blue-stained tissue was abundant in the carpel, sarcocarp, and vascular bundles. Compared to MG fruits, more intense blue staining was detected in RR fruits (Figure 2c). The intensity of blue staining was higher in the pectin, vascular bundles, carpels, and sarcocarps. In the flowers of the T0 transgenic tomato plants, the pollen grains were stained blue (Figure 2d). These results indicated that SlSWEET12c was abundantly expressed in the vascular bundles of RR fruit, suggesting a potential role in sugar allocation to the sink tissue.
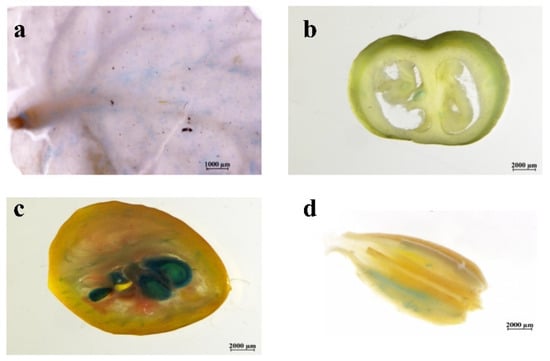
Figure 2.
Histochemical localization of GUS expression patterns in SlSWEET12c-GUS transgenic tomato: (a) leaves; (b) MG fruits; (c) RR fruits; (d) flower. The scale bars correspond to 2 mm in (b–d) and 1 mm in (a). Every experiment was repeated at least three times.
3.3. Subcellular Localization of SlSWEET12c
To confirm the subcellular localization of SlSWEET12c, the Pro35S::SlSWEET12c::GFP fusion construct was used for transformation in onion skin. Green fluorescence signals were observed in the cell walls of the onion epidermal cells (Supplementary Figure S2). Since there is no chloroplast in the onion epidermis, the accuracy of subcellular localization could be limited, and it is also impossible to determine whether SlSWEET12c was located in the chloroplast or on the chloroplast membrane. Therefore, tobacco leaves were also used for this analysis, with FM4-64 as a positive control owing to its specific plasma membrane localization. Green and red fluorescence signals were observed on the plasma membrane (Figure 3). These results demonstrated that SlSWEET12c was located on the plasmalemma.
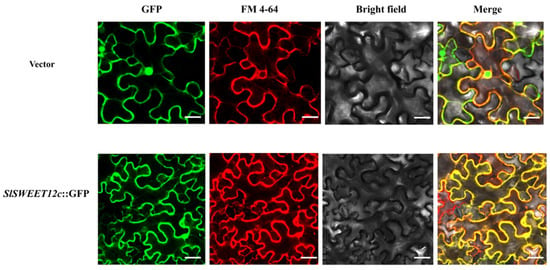
Figure 3.
Subcellular localization of SlSWEET12c in tobacco leaves. FM4-64 was used as a positive control. Scale bars correspond to 25 μm. Results are representative of experiments repeated at least three times.
3.4. Transport Activity of SlSWEET12c in Yeast
On SD/-uracil solid medium containing 2% glucose as the sole carbon source, the mutant yeast strains pDR195, AtSUT2, and pDR195-SlSWEET12c all grew well at different dilution concentrations. However, on SD/-uracil solid medium containing 2% sucrose as the sole carbon source, the growth of pDR195-SlSWEET12c was much better than that of the empty vector; when the dilution concentration reached 1000 times, the difference between the two was particularly obvious (Figure 4). In addition, the growth of pDR195-SlSWEET12c was similar to that of the yeast sucrose transporter AtSUT2. This result further suggested that SlSWEET12c functions as a sucrose transporter. Moreover, since SWEET clade III family members also have glucose and fructose transport activities, we fused the pDR195-SlSWEET12c recombinant plasmid into the yeast strain EBY.VW4000 for heterologous expression (Supplementary Figure S3). These results confirmed that the SlSWEET12c protein also functions as a hexose transporter.
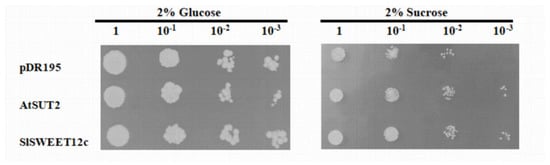
Figure 4.
Transport activity of SlSWEET12c in yeast strain SUSY7/ura. SlSWEET12c, yeast sucrose transporter (AtSUT2), or empty vector (pDR195) were grown on synthetic deficient (SD)/-uracil solid medium containing 2% glucose or 2% sucrose as the sole carbon source. Representative results of experiments repeated at least three times are shown.
3.5. Construction and Identification of Transgenic Plants
To further study the function of SlSWEET12c during tomato development, we constructed RNAi and overexpression transgenic plants (Supplementary Figure S4A,B). RT-qPCR confirmed the silencing or overexpression of SlSWEET12c in the transfected plant lines (Figure 5). Nine SlSWEET12c overexpression (OE) transgenic plants were obtained. The gene expression levels in the RR tomato fruits of the OE plants were approximately three times higher than those in the wild type (WT); OE5, OE6, and OE8 were chosen for further studies because of their high expression levels (Figure 5a). The expression levels in the seven RNAi lines were decreased by approximately 75% compared to those of the WT plants. We chose the RNAi lines i3, i5, and i6 for further experimentation because of their low expression levels (Figure 5b). The overexpression (OE-12c) and SlSWEET12c RNAi (RNAi-12c) lines showed no significant phenotypic variation (Supplementary Figure S5). However, the time of anthesis in the RNAi-12c plants was later than that in the WT and OE-12c lines.
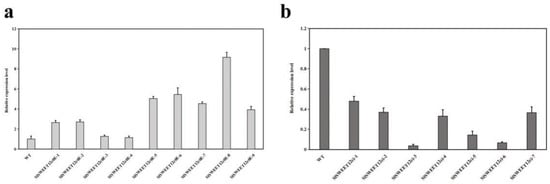
Figure 5.
Relative expression level of SlSWEET12c in transgenic plants. (a) Relative expression level of OE-12c lines. (b) Relative expression level of RNAi-12c lines. The wild type (WT) expression data were normalized to 1, and the beta-actin gene was used as an internal control. The primers are shown in Supplementary Table S1. Results are representative of at least three experiments.
3.6. Soluble Sugar Content of SlSWEET12c Transgenic Plants in the RR Stage
Because SlSWEET12c exhibits sucrose transport activities, we measured the soluble sugar concentrations of the WT, OE-12c, and RNAi-12c fruits (Figure 6). In the OE12c-5 and OE12c-6 lines, the fructose and glucose concentrations were increased compared to those of the WT. The OE12c-8 lines also showed an increase in these sugars, but to a lesser extent than found for the other OE-12c lines. Fructose and glucose concentrations increased by approximately 20% and 40%, respectively, in OE-12c fruits compared to those of the WT. Conversely, in the RNAi-12c lines, both fructose and glucose concentrations were much lower than those in the WT, with a reduction of more than 17% and approximately 25%, respectively. There was no significant difference in sucrose concentration between the OE-12c lines and WT. However, the sucrose concentration increased remarkably by approximately 66% in the RNAi-12c lines compared to that of the WT.
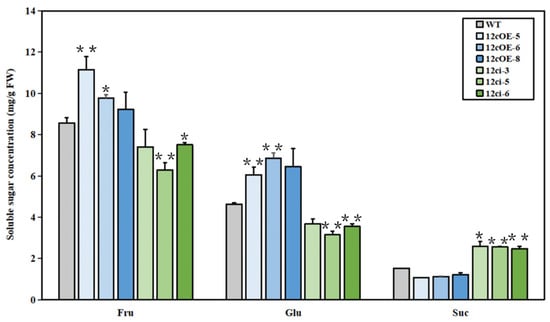
Figure 6.
Soluble sugar content in RR fruits of WT, SlSWEET12c OE, and RNAi lines. Suc—sucrose; Glu—glucose; Fru—fructose. Statistical significance is indicated by * (p < 0.05) and ** (p < 0.01); n ≥ 20. FW—fresh weight. Every experiment was repeated at least three times.
3.7. Enzyme Activity of Sucrose Metabolism in SlSWEET12c Transgenic Plants
To determine the cause of these differences in sugar content, we analyzed the invertase and sucrose synthase activity in the SlSWEET12c transgenic lines (OE-12c, RNAi-12c) and WT lines (Figure 7). The activities of three types of invertases, including cell wall invertase (CWIN), cytoplasmic invertase (CIN), and vacuolar invertase (VIN), were significantly lower in the RNAi-12c lines than those in the WT lines, with a decrease of approximately 30–41% for CIN (Figure 7a) and approximately 17–28% for CWIN (Figure 7b) and VIN (Figure 7c). In contrast, there were no significant differences in enzyme activities between the OE-12c and WT lines. There were no differences in the activities of SS and SPS in both the OE-12c lines and RNAi-12c lines when compared to those in the WT lines (Figure 7d,e). These results suggest that in the RNAi-12c lines, sucrose unloading from the phloem to the parenchyma cells was inhibited, resulting in a decrease in sucrose concentration in the intercellular space, which in turn caused a decrease in the activities of invertases.
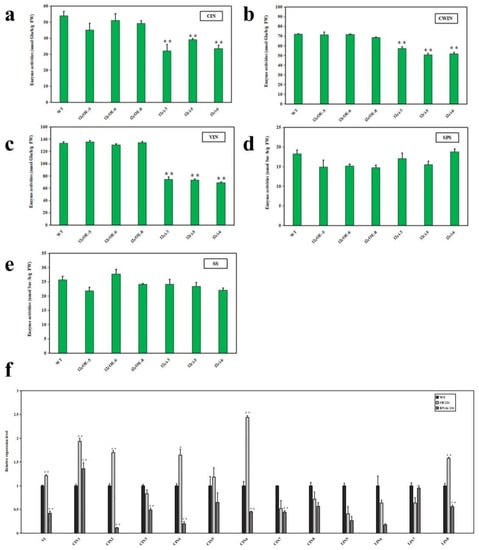
Figure 7.
Invertase and sucrose synthase enzyme activities and related gene expression levels in RR fruits of WT and SlSWEET12c OE and RNAi lines: (a–c) enzyme activities of invertases (CIN, CWIN, and VIN) and (d,e), enzyme activities of sucrose synthases (SS and SPS) in SlSWEET12c transgenic plants (OE-12c and RNAi-12c; n ≥ 20). (f) Transcript levels of sucrose invertase-related genes at the red ripening stage. The WT expression data were normalized to 1, and the actin gene was used as an internal reference. Statistical significance is indicated by * (p < 0.05) and ** (p < 0.01). FW—fresh weight. The experiments were repeated at least three times, with similar results.
We also analyzed the expression levels of specific genes related to sucrose invertases, including VI, LIN5-8, and CIN1-8, in the WT, OE-12c, and RNAi-12c lines during the RR stage. The expression of all sucrose invertase-related genes was downregulated in the RNAi-12c lines compared with that in the WT. In addition, the expression levels in the RNAi-12c lines were lower than those in the OE-12c lines. These findings suggested that SlSWEET12c regulates sucrose metabolism by modulating the expression of a specific set of genes associated with sucrose degradation.
4. Discussion
4.1. Plasma Membrane-Localized SlSWEET12c Can Transport Sucrose, Glucose, and Fructose
SWEET transporters can transport various monosaccharides and disaccharides and can simultaneously mediate the absorption and efflux of sugars by cells, as well as their typical low affinity for sugars. SWEET transporters may function mainly as one-way carriers, although this hypothesis has not yet been proven [24]. Phylogenetic analyses have indicated that SlSWEET12c belongs to clade III [2,5,16]. Our study showed that SlSWEET12c was located on the plasma membrane and had the ability to transport sucrose and glucose. This finding is similar to reports of AtSWEET11 and AtSWEET12 in Arabidopsis, which are located in the phloem parenchyma cytoplasmic membrane and participate in the outward transport of sucrose [38]. In addition, plasma membrane-localized OsSWEET11 and OsSWEET14 were previously reported to have a certain transport capacity for sucrose and glucose [14]. SlSWEET7a and SlSWEET14 were also reported to be localized on the plasma membrane of tomatoes and to transport hexoses (fructose and glucose) and sucrose [31]. In addition, another SlSWEET member from clade III, SlSWEET15, was reported to be located on the plasma membrane and to function as a sucrose-specific membrane transporter [32]. These results support our finding that SlSWEET12c transports hexoses (glucose and fructose) and sucrose.
4.2. SlSWEET12c May Be Involved in Sucrose Export during the RR Stage in Tomatoes
Recently, the role of sugar transporters in apoplasmic unloading during fruit development has been debated. The RR stage is the final step in fruit development and is important for the taste and quality of the fruit. In our study, the highest expression of SlSWEET12c among the other SlSWEET members was observed during the RR stage, suggesting that SlSWEET12c may play an important role in this stage of development. A further comparison of the expression of SlSWEET12c in different tissues and at different developmental stages also suggested that SlSWEET12c may mainly function in the RR stage in tomatoes. The GUS staining of the SlSWEET12c-GUS translational fusion fruits further demonstrated that SlSWEET12c was expressed in the vascular bundles, carpels, and sarcocarps.
Among the different parts of the RR-stage fruit, the expression level of SlSWEET12c in the sarcocarp was the highest, followed by pectin and the vascular bundles. This may be related to the unloading of sugar in the phloem tissue of sink organs and the transport of sugar from pectin to the seed. Previous studies have shown that SWEET sugar transporters regulate the rational distribution and utilization of carbohydrates in plant source–sink tissues and can transport carbohydrates along a concentration gradient between cells and the environment, or between cells and cells. In other words, SWEETs clearly play an important role in sugar transportation [14,15,39].
At present, SWEET clade III members have been extensively studied in Arabidopsis, where most members have been found to be capable of transporting monosaccharides or disaccharides [14]. Similarly, regarding the regulation of disaccharides, AtSWEET11 and AtSWEET12 are low-affinity sucrose transport carriers. They are also expressed in the phloem parenchyma cells of the vascular bundles in the leaves of Arabidopsis and participate in sucrose transport to the apoplasts in the phloem parenchyma cells [39].
4.3. SlSWEET12c May Regulate Sugar Concentrations in the RR Fruit of Tomato
In our study, the sucrose concentration increased significantly during the RR stage in RNAi-12c fruits, which had SlSWEET12c expression silenced. In contrast, the hexose concentrations decreased during the same stage. However, in the OE-12c fruit, in which SlSWEET12c was overexpressed, the concentrations of glucose and fructose increased. The expression of SlSWEET12c during the RR stage in tomato fruit was significantly enriched in the pectin, sarcocarp, and vascular bundles, according to GUS activity analysis and RT-qPCR. Since SlSWEET12c transports sucrose, glucose, and fructose, more sucrose would be unloaded by the phloem of the fruit vascular bundle in OE-12c lines, and more fructose and glucose would be transported to the parenchyma cells of the sarcocarp via SlSWEET12c.
Sucrose is mainly hydrolyzed into hexose by two enzymes, SS and invertase. Invertase is a hydrolase that irreversibly hydrolyzes sucrose to fructose and glucose [40]; the low activity of cell wall, cytoplasmic, and vacuolar invertases supported the increase in sucrose concentration in RR RNAi-12c fruits. There were low levels of sucrose hydrolysis into glucose and fructose, which confirmed that there was less glucose and fructose in the SlSWEET12c-silenced line. Once sucrose is exported to the apoplasm of tomato pericarps, it is estimated that 70% of apoplasmic sucrose is hydrolyzed by cell wall inverses as hexoses [34,41]. The activities of SS and SPS were not significantly different among the WT, OE-12c, and RNAi-12c lines during the RR stage in tomato fruits. We further explored the decrease in invertase (VI, CWIN, and CIN) activities, and concluded that the variation in invertase activity might be caused by the downregulation of related genes. The low expression of SlSWEET12c in the RNAi-12c line caused the low transcript levels of CWIN-, CIN-, and VI-related genes and also reduced the enzyme activities of invertases. Consequently, less sucrose was hydrolyzed into fructose and glucose, ultimately resulting in higher sucrose concentrations in the tomato fruits during the RR stage.
A recent study showed that SlSWEET12c might facilitate sucrose unloading from the phloem down a concentration gradient maintained by cell wall invertase to support seed filling [2]. Our results further support that SlSWEET12c may be a key transporter involved in sucrose unloading during the RR stage in tomato fruit. Sugars are important carbon and energy sources, structural constituents of cells, and essential signaling molecules [42]. In the present study, RNAi-12c and OE-12c transgenic plants did not show differences in growth and development processes; however, there were obvious differences in the sugar content of the fruits. However, in previous studies, AtSWEET12 increased the starch content of leaves and limited the growth of plant roots [14]. Under low-temperature stress, the areas of the stem phloem and xylem of an atsweet11:12 double mutant plant were decreased, and the stem diameter was significantly reduced. However, the double mutant was resistant to low temperatures. This tendency indicated that the secondary xylem transports sugar to meet the nutritional requirements for the formation of secondary cell walls, thereby regulating the tolerance of Arabidopsis plants to low-temperature stress [43]. We found that the RNAi-12c lines had a later bloom time than the WT and OE-12c lines. This could be because SlSWEET12c is also expressed in the leaves, and silencing SlSWEET12c may affect sugar export. How sugar signaling in the presence of RNAi-12c induces later anthesis is an important question that remains to be explored.
5. Conclusions
In summary, we characterized one SlSWEET clade III family member gene, SlSWEET12c, which has sucrose and hexose transport activities. Silencing SlSWEET12c resulted in a large accumulation of sucrose in RR fruits, possibly because of the inhibition of sucrose unloading in the vascular bundles. Therefore, less sucrose was unloaded into the cells, which also led to a decrease in invertase activity, causing a reduction in fructose and glucose. In contrast, with the overexpression of SlSWEET12c, the ability to transport sucrose, fructose, and glucose was recovered, leading to increased fructose and glucose accumulation. These results show that plasma membrane-localized SlSWEET12c may play a key role in sucrose unloading and metabolism in the ripening of tomato fruits. Our characterization of the SlSWEET12c gene provides knowledge on the molecular basis of fruit quality control, and the utilization of SlSWEET12c could be a favorable option for improving the quality and quantity of tomatoes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8100935/s1, Figure S1: Transcriptome analysis of various tissues of SlSWEETs genes in tomatoes; Figure S2: Subcellular localization of SlSWEET12c in onion epidermal cells; Figure S3, Transport activity of SlSWEET12c in yeast; Figure S4, Construction of RNA inference and overexpressing transgenic plants; Figure S5, Morphological phenotypes and growth parameters of wild type (WT) and transgenic plants; Table S1, Primer sequences of the SlSWEET gene family; Table S2, Primer sequences of sucrose metabolism-related genes.
Author Contributions
J.S. and J.J. conceived this project and designed the study. J.S., C.F. and X.L. performed the research, analyzed data, and wrote the manuscript. All authors contributed critically to the drafts. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2019YFD1000300) and the National Natural Science Foundation of China (No. 31372054).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and supplementary information files.
Acknowledgments
We would like to thank Eckhard Boles from the University of Frankfurt, who provided us with the tested yeast strain EBY.VW4000.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Kohji, Y.; Yuriko, O. Sugar compartmentation as an environmental stress adaptation strategy in plants. Semin. Cell Dev. Biol. 2017, 83, 106–114. [Google Scholar]
- Ru, L.; He, Y.; Zhu, Z.; Patrick, J.W.; Ruan, Y.-L. Integrating Sugar Metabolism with Transport: Elevation of Endogenous Cell Wall Invertase Activity Up-Regulates SlHT2 and SlSWEET12c Expression for Early Fruit Development in Tomato. Front. Genet. 2020, 11, 592596. [Google Scholar] [CrossRef] [PubMed]
- Stefan, R.; Masahito, A.; Tomohide, Y.; Haruko, M.; Koh, A.; Daisuke, S.; Katsuhiro, S. The sugar transporter inventory of tomato: Genome-wide identification and expression analysis. Plant Cell Physiol. 2014, 55, 1123–1141. [Google Scholar]
- Shammai, A.; Petreikov, M.; Yeselson, Y.; Faigenboim, A.; Moy-Komemi, M.; Cohen, S.; Cohen, D.; Besaulov, E.; Efrati, A.; Houminer, N.; et al. Natural genetic variation for expression of a SWEET transporter among wild species of Solanum lycopersicum (tomato) determines the hexose composition of ripening tomato fruit. Plant J. 2018, 96, 343–357. [Google Scholar] [CrossRef]
- Ho, L.-H.; Klemens, P.A.W.; Neuhaus, H.E.; Ko, H.-Y.; Hsieh, S.-Y.; Guo, W.-J. SlSWEET1a is involved in glucose import to young leaves in tomato plants. J. Exp. Bot. 2019, 70, 3241–3254. [Google Scholar] [CrossRef] [PubMed]
- Bresinsky, A.; Körner, C.; Kadereit, J.W.; Neuhaus, G.; Sonnewald, U. Strasburger’s Plant Sciences, Including Prokaryotes and Fungi; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1065–1166. [Google Scholar]
- Ruan, Y.-L.; Patrick, J.W. The cellular pathway of postphloem sugar transport in developing tomato fruit. Planta 1995, 196, 434–444. [Google Scholar] [CrossRef]
- Patrick, J.W.; Offler, C.E. Post-sieve element transport of photoassimilates in sink regions. J. Exp. Bot. 1996, 47, 1165–1177. [Google Scholar] [CrossRef]
- Klee, H.J.; Giovannoni, J.J. Genetics and Control of Tomato Fruit Ripening and Quality Attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Zanor, M.I.; Osorio, S.; Nunes-Nesi, A.; Carrari, F.; Lohse, M.; Usadel, B.; Kühn, C.; Bleiss, W.; Giavalisco, P.; Willmitzer, L.; et al. RNA Interference of LIN5 in Tomato Confirms Its Role in Controlling Brix Content, Uncovers the Influence of Sugars on the Levels of Fruit Hormones, and Demonstrates the Importance of Sucrose Cleavage for Normal Fruit Development and Fertility. Plant Physiol. 2009, 150, 1204–1218. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ni, D.-A.; Ruan, Y.-L. Posttranslational Elevation of Cell Wall Invertase Activity by Silencing Its Inhibitor in Tomato Delays Leaf Senescence and Increases Seed Weight and Fruit Hexose Level. Plant Cell 2009, 21, 2072–2089. [Google Scholar] [CrossRef]
- Braun, D.M.; Wang, L.; Ruan, Y.-L. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J. Exp. Bot. 2014, 65, 1713–1735. [Google Scholar] [CrossRef] [PubMed]
- Tiessen, A.; Padilla, C.D. Subcellular compartmentation of sugar signaling: Links among carbon cellular status, route of sucrolysis, sink-source allocation, and metabolic partitioning. Front. Plant Sci. 2013, 3, 306. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Q.; Qu, X.-Q.; Hou, B.-H.; Sosso, D.; Osorio, S.; Fernie, A.R.; Frommer, W.B. Sucrose Efflux Mediated by SWEET Proteins as a Key Step for Phloem Transport. Science 2012, 335, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Eom, J.-S.; Chen, L.-Q.; Sosso, D.; Julius, B.T.; Lin, I.W.; Qu, X.-Q.; Braun, D.M.; Frommer, W.B. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr. Opin. Plant Biol. 2015, 25, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.-Y.; Han, J.-X.; Han, X.-X.; Jiang, J. Genome-wide identification, phylogeny, and expression analysis of the SWEET gene family in tomato. Gene 2015, 573, 261–272. [Google Scholar] [CrossRef]
- Bezrutczyk, M.; Hartwig, T.; Horschman, M.; Char, S.N.; Yang, J.L.; Yang, B.; Frommer, W.B.; Sosso, D. Impaired phloe m loading in zmsweet13a,b,c sucrose transporter triple knock-out mutants in Zea mays. New Phytol. 2018, 218, 594–603. [Google Scholar] [CrossRef]
- Julius, B.T.; Leach, K.A.; Tran, T.M.; Mertz, R.A.; Braun, D.M. Sugar Transporters in Plants: New Insights and Discoveries. Plant Cell Physiol. 2017, 58, 1442–1460. [Google Scholar] [CrossRef]
- Patrick, J.W. Phloem unloading: Sieve element unloading and post-sieve element transport. Annu. Rev. Plant Biol. 1997, 48, 191–222. [Google Scholar] [CrossRef]
- Ayre, B.G. Membrane-Transport Systems for Sucrose in Relation to Whole-Plant Carbon Partitioning. Mol. Plant 2011, 4, 377–394. [Google Scholar] [CrossRef]
- Osorio, S.; Ruan, Y.-L.; Fernie, A.R. An update on source-to-sink carbon partitioning in tomato. Front. Plant Sci. 2014, 5, 516. [Google Scholar] [CrossRef]
- Kühn, C.; Grof, C.P.L. Sucrose transporters of higher plants. Curr. Opin. Plant Biol. 2010, 13, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.W.; Sosso, D.; Chen, L.-Q.; Gase, K.; Kim, S.-G.; Kessler, D.; Klinkenberg, P.M.; Gorder, M.K.; Hou, B.-H.; Qu, X.-Q.; et al. Nectar secretion requires sucrose phosphate synthases and the sugar transporter SWEET9. Nature 2014, 508, 546–549. [Google Scholar] [CrossRef]
- Chen, L.-Q.; Lin, I.W.; Qu, X.-Q.; Sosso, D.; McFarlane, H.E.; Londoño, A.; Samuels, A.L.; Frommer, W.B. A Cascade of Sequentially Expressed Sucrose Transporters in the Seed Coat and Endosperm Provides Nutrition for the Arabidopsis Embryo. Plant Cell 2015, 27, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Luo, D.; Yang, B.; Frommer, W.B.; Eom, J.-S. SWEET 11 and 15 as key players in seed filling in rice. New Phytol. 2018, 218, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Krügel, U.; Kühn, C. Post-translational regulation of sucrose transporters by direct protein-protein interactions. Front. Plant Sci. 2013, 4, 237. [Google Scholar] [CrossRef]
- Rouina, H.; Tseng, Y.H.; Nataraja, K.N.; Uma, S.R.; Oelmüller, R. Arabidopsis restricts sugar loss to a colonizing trichoderma harzianum strain by downregulating SWEET11 and -12 and upregulation of SUC1 and SWEET2 in the roots. Microorganisms 2021, 9, 1246. [Google Scholar] [CrossRef]
- Carpenter, S.C.D.; Mishra, P.; Ghoshal, C.; Dash, P.K.; Wang, L.; Midha, S.; Laha, G.S.; Lore, J.S.; Kositratana, W.; Singh, N.K.; et al. A Strain of an Emerging Indian Xanthomonas oryzae pv. oryzae Pathotype Defeats the Rice Bacterial Blight Resistance Gene xa13 Without Inducing a Clade III SWEET Gene and Is Nearly Identical to a Recent Thai Isolate. Front. Microbiol. 2018, 9, 2703. [Google Scholar] [CrossRef]
- Pierre, G.; Martin, K.; Timo, E.; Uwe, S.; Christian, K.; Voll, L.M. Sugar accumulation in leaves of Arabidopsis sweet11/sweet12 double mutants enhances priming of the salicylic acid-mediated defense response. Front. Plant Sci. 2017, 8, 1378. [Google Scholar]
- Yao, L.; Ding, C.; Hao, X.; Zeng, J.; Yang, Y.; Wang, X.; Wang, L. CsSWEET1a and CsSWEET17 Mediate Growth and Freezing Tolerance by Promoting Sugar Transport across the Plasma Membrane. Plant Cell Physiol. 2020, 61, 1669–1682. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, C.; Wang, M.; Li, T.; Liu, X.; Jiang, J. Plasma membrane-localized SlSWEET7a and SlSWEET14 regulate sugar transport and storage in tomato fruits. Hortic. Res. 2021, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.-Y.; Ho, L.-H.; Neuhaus, H.E.; Guo, W.-J. Transporter SlSWEET15 unloads sucrose from phloem and seed coat for fruit and seed development in tomato. Plant Physiol. 2021, 187, 2230–2245. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, Y.L.; Meng, Z.J.; Jiang, J. Optimization of factors affecting Agrobacterium-mediated transformation of Micro-Tom tomatoes. Genet. Mol. Res. 2012, 11, 661–671. [Google Scholar] [CrossRef]
- McCurdy, D.W.; Dibley, S.; Cahyanegara, R.; Martin, A.; Patrick, J.W. Functional Characterization and RNAi-Mediated Suppression Reveals Roles for Hexose Transporters in Sugar Accumulation by Tomato Fruit. Mol. Plant 2010, 3, 1049–1063. [Google Scholar] [CrossRef]
- Hao, J.H.; Li, T.L.; Meng, S.D.; Zhao, B.; Sun, L.P. Effects of night low temperature on sugar accumulation and sugar-metabolizing enzyme activities in melon fruit. Sci. Agric. Sin. 2009, 42, 3592–3599. [Google Scholar]
- Martin, M.L.; Lechner, L.; Zabaleta, E.J.; Salerno, G.L. A mitochondrial alkaline/neutral invertase isoform (A/N-InvC) functions in developmental energy demanding processes in Arabidopsis. Planta 2013, 237, 813–822. [Google Scholar] [CrossRef]
- Kim, D.; Park, S.Y.; Chung, Y.; Park, J.; Lee, S.; Lee, T.-K. Biochemical Characterization of Soluble Acid and Alkaline Invertases from Shoots of Etiolated Pea Seedlings. J. Integr. Plant Biol. 2010, 52, 536–548. [Google Scholar] [CrossRef]
- Le Hir, R.; Spinner, L.; Klemens, P.A.W.; Chakraborti, D.; de Marco, F.; Vilaine, F.; Wolff, N.; Lemoine, R.; Porcheron, B.; Géry, C.; et al. Disruption of the Sugar Transporters AtSWEET11 and AtSWEET12 Affects Vascular Development and Freezing Tolerance in Arabidopsis. Mol. Plant 2015, 8, 1687–1690. [Google Scholar] [CrossRef]
- Chen, L.-Q.; Hou, B.-H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.-Q.; Guo, W.-J.; Kim, J.-G.; Underwood, W.; Chaudhuri, B.; et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef]
- Koch, K. Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.M.; Hall, J.L.; Ho, L.C. Sugar uptake by protoplasts isolated from tomato fruit tissues during various stages of fruit growth. Physiol. Plantarum. 2006, 101, 533–539. [Google Scholar] [CrossRef]
- Yu, S.M.; Lo, S.F.; Ho, T.H.D. Source-sink communication: Regulated by hormone, nutrient, and stress cross-signaling. Trends Plant Sci. 2015, 20, 844–857. [Google Scholar] [CrossRef]
- Chen, L. SWEET sugar transporters for phloem transport and pathogen nutrition. New Phytol. 2014, 201, 1150–1155. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).