Combined Dairy Manure-Food Waste Digestate as a Medium for Pleurotus djamor—Mineral Composition in Substrate and Bioaccumulation of Elements in Fruiting Bodies
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Mushroom Substrate
2.2. Mushroom Cultivation
2.2.1. Inoculation
2.2.2. Pinning
2.2.3. Harvesting
2.3. Analysis of Raw Materials, Experimental Mushroom Substrate (EMS), Spent Mushroom Substrate (SMS) and Mushroom Tissues
2.3.1. Sampling and Homogenisation
2.3.2. Analytical Methods
Sample Collection and Preparation Procedure
Element Contents in Substrate and Mushroom
Analytical Method Validation
2.4. Yield and Biological Efficiency
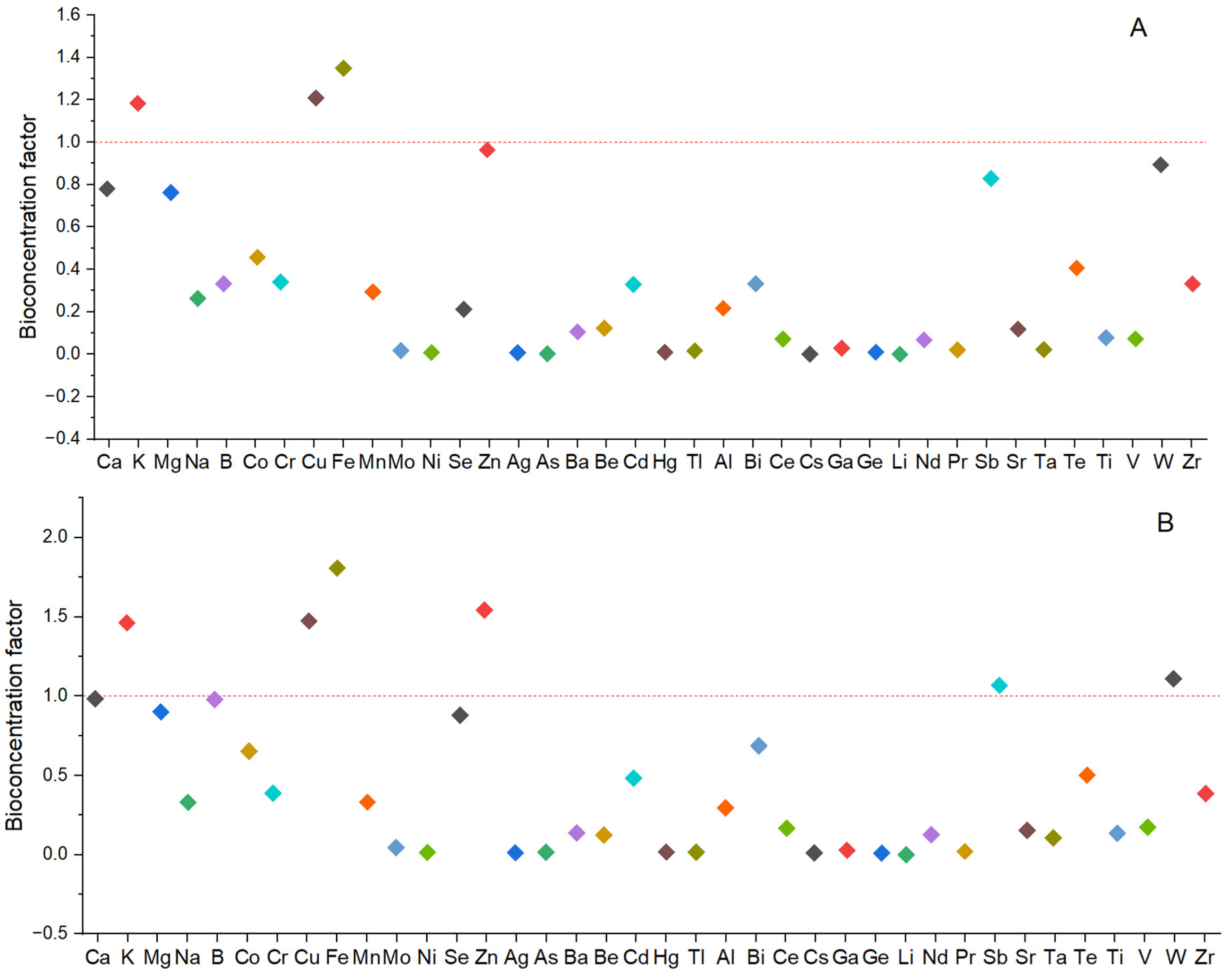
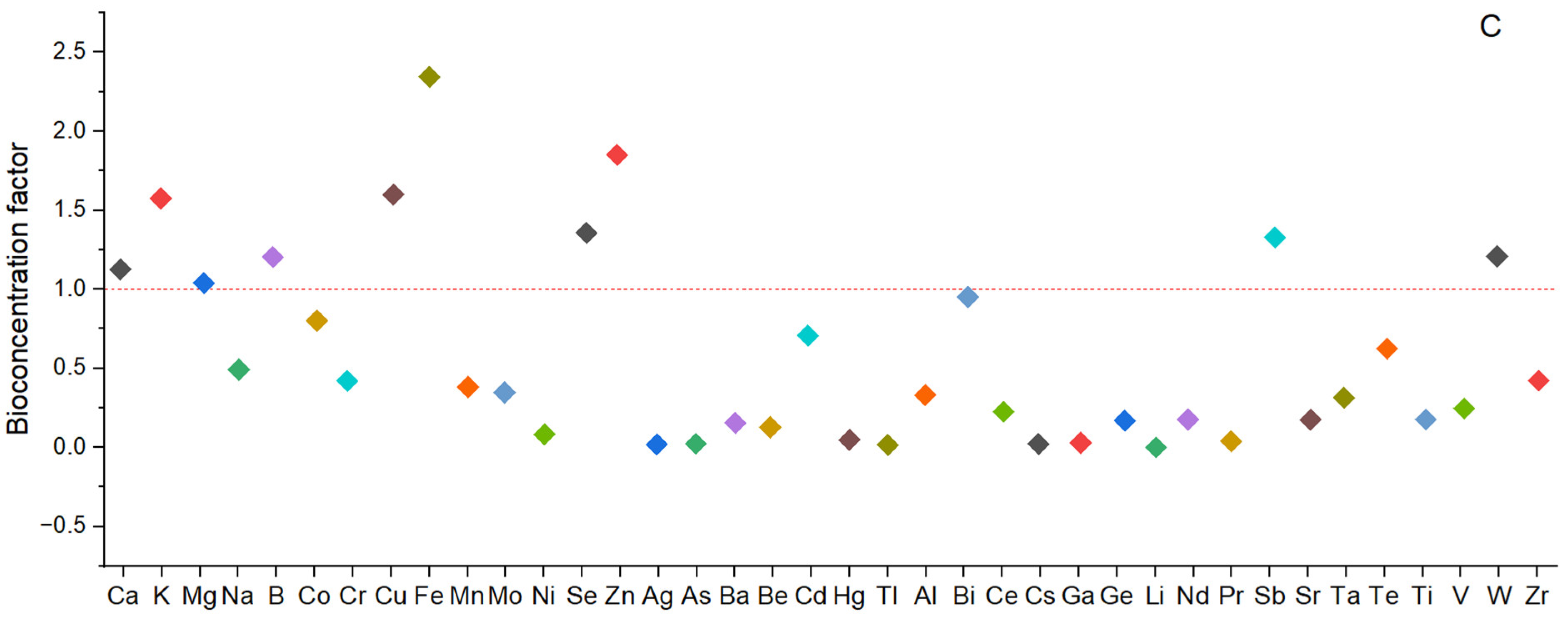
2.5. Bioconcentration Factors (BCFs)
2.6. Dietary Intake and Health Risk Assessment of P. djamor Consumption
2.7. Statistical Analysis
3. Results
3.1. Yield, Dry Matter and Biological Efficiency of P. djamor
3.2. Elemental Concentration in the EMS (Experimental Mushroom Substrate) Used for the Cultivation of P. djamor
3.3. Elemental Concentration in the SMS (Spent Mushroom Substrate) after P. djamor Cultivation
| Element (mg∙kg−1 DM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Major Essential Elements (MEEs) | ||||||||||
| Ca | K | Mg | Na | |||||||
| EMS | 1420 | 11,000 | 1270 | 1950 | ||||||
| SMS | 2130 | 17,000 | 2520 | 2910 | ||||||
| Essential trace elements (ETEs) | ||||||||||
| B | Co | Cr | Cu | Fe | Mn | Mo | Ni | Se | Zn | |
| EMS | 2.67 | 0.39 | 35.2 | 1.99 | 28.8 | 51.5 | 0.47 | 0.06 | 1.10 | 47.3 |
| SMS | 5.43 | 0.47 | 47.4 | 3.78 | 130 | 79.1 | 0.59 | 3.03 | 1.99 | 82.0 |
| Trace elements with detrimental health effects (TEWDHE) | ||||||||||
| Ag | As | Ba | Be | Cd | Hg | Tl | ||||
| EMS | <0.01 | <0.01 | 40.85 | 0.05 | 0.62 | 0.04 | 0.29 | |||
| SMS | 0.01 | 0.2 | 60.39 | 0.06 | 0.66 | 0.04 | 1.63 | |||
| Nutritionally nonessential elements (NNEs) | ||||||||||
| Al | Bi | Ce | Cs | Ga | Ge | Li | Nd | Pr | Sb | |
| EMS | 59.3 | 0.53 | 0.45 | <0.01 | <0.01 | 0.35 | <0.01 | 0.38 | 1.35 | 1.85 |
| SMS | 250 | 1.13 | 0.21 | 6.15 | 0.01 | 0.77 | 0.25 | 0.26 | 2.07 | 3.38 |
| Sr | Ta | Te | Ti | V | W | Zr | ||||
| EMS | 60.4 | 0.52 | 12.05 | 2.05 | 0.31 | 3.61 | 0.25 | |||
| SMS | 85.3 | 0.56 | 17.42 | 12.6 | 0.76 | 3.30 | 0.53 | |||
3.4. Elemental Concentration in Fruiting Bodies of P. djamor
3.4.1. Content of Major Essential Elements (MEEs) in Fruiting Bodies of P. djamor
3.4.2. Content of Essential Trace Elements (ETEs) in Fruiting Bodies of P. djamor
3.4.3. Content of Trace Elements with Detrimental Health Effects (TEWDHE) in the Fruiting Bodies of P. djamor
3.4.4. Content of Nutritionally Nonessential Elements (NNEs) in the Fruiting Bodies of P. djamor
3.5. Bioconcentration Factor (BCF) of Elements from EMS into the Fruiting Bodies of P. djamor
4. Discussion
4.1. Effect of the EMS (Experimental Mushroom Substrate) on the Cultivation of P. djamor
4.2. Value of Spent Mushroom Substrate (SMS)
4.3. Chemistry and Safety of Mushrooms
4.3.1. Effect of EMS (Experimental Mushroom Substrate) on the Content of Elements in P. djamor Fruiting Bodies
4.3.2. Consumption of P. djamor—Dietary Intake
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zervakis, G.I.; Koutrotsios, G. Solid-State Fermentation of Plant Residues and Agro-industrial Wastes for the Production of Medicinal Mushrooms. In Medicinal Plants and Fungi: Recent Advances in Research and Development; Springer: Berlin/Heidelberg, Germany, 2017; pp. 365–396. [Google Scholar] [CrossRef]
- Roncero-Ramos, I.; Delgado-Andrade, C. The beneficial role of edible mushrooms in human health. Curr. Opin. Food Sci. 2017, 14, 122–128. [Google Scholar] [CrossRef]
- Chaturvedi, V.K.; Agarwal, S.; Gupta, K.K.; Ramteke, P.W.; Singh, M.P. Medicinal mushroom: Boon for therapeutic applications. 3 Biotech 2018, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal Mushrooms: Bioactive Compounds, Use, and Clinical Trials. Int. J. Mol. Sci. 2021, 22, 634. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-González, J.A.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Nutritional composition and nutraceutical properties of the Pleurotus fruiting bodies: Potential use as food ingredient. J. Food Compos. Anal. 2017, 58, 69–81. [Google Scholar] [CrossRef]
- Ma, G.; Yang, W.; Zhao, L.; Pei, F.; Fang, D.; Hu, Q. A critical review on the health promoting effects of mushrooms nutraceuticals. Food Sci. Hum. Wellness 2018, 7, 125–133. [Google Scholar] [CrossRef]
- Mushroom Cultivation Market by Type (Button Mushroom, Oyster Mushroom, Shiitake Mushroom, Other Types), by Phase, by Region (North America, Europe, Asia Pacific, South America, Rest of the World)—Global Forecast to 2025. Available online: https://www.researchandmarkets.com/reports/5018601/mushroom-cultivation-market-by-type-button (accessed on 20 July 2022).
- Acharya, S.; Saha, A.K. Antimicrobial activity of Pleurotus djamor. Indian J. Mycopathol. Res. 2011, 2, 329–332. [Google Scholar]
- Maity, G.N.; Maity, P.; Khatua, S.; Acharya, K.; Dalai, S.; Mondal, S. Structural features and antioxidant activity of a new galactoglucan from edible mushroom Pleurotus djamor. Int. J. Biol. Macromol. 2021, 168, 743–749. [Google Scholar] [CrossRef]
- Waktola, G.; Temesgen, T. Pharmacological activities of Oyster mushroom (Pleurotus ostreatus). Novel Res. Microbiol. J. 2020, 4, 688–695. [Google Scholar] [CrossRef]
- Raman, J.; Jang, K.Y.; Oh, Y.L.; Oh, M.; Im, J.H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and Nutritional Value of Prominent Pleurotus spp.: An Overview. Mycobiology 2021, 49, 1–14. [Google Scholar] [CrossRef]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Ritota, M.; Manzi, P. Pleurotus spp. Cultivation on Different Agri-Food By-Products: Example of Biotechnological Application. Sustainability 2019, 11, 5049. [Google Scholar] [CrossRef]
- Melanouri, E.-M.; Dedousi, M.; Diamantopoulou, P. Cultivating Pleurotus ostreatus and Pleurotus eryngii mushroom strains on agro-industrial residues in solid-state fermentation. Part I: Screening for growth, endoglucanase, laccase and biomass production in the colonization phase. Carbon Resour. Convers. 2022, 5, 61–70. [Google Scholar] [CrossRef]
- Yohannes, B.; Abraham, M.; Bikila, G.; Robel, D.; Getahun, T.; Jale, M.; Malesu, A.; Tsehaynesh, F.; Lalise, D. Selection of appropriate substrate for production of oyster mushroom (Pleurotus ostreatus). J. Yeast Fungal Res. 2020, 11, 15–25. [Google Scholar] [CrossRef]
- Daba, A.S.; El nakieb, F.; Hafez, E.E. The effect of Recycling Different Wastes (as a substrate) on Mushroom (Pleurotus ostreatus) Fruit Bodies, Morphologically, Genetically and its Metabolites. Biosci. Res. 2018, 15, 2286–2294. [Google Scholar]
- Tesfay, T.; Godifey, T.; Mesfin, R.; Kalayu, G. Evaluation of waste paper for cultivation of oyster mushroom (Pleurotus ostreatus) with some added supplementary materials. AMB Express 2020, 10, 15. [Google Scholar] [CrossRef]
- Girmay, Z.; Gorems, W.; Birhanu, G.; Zewdie, S. Growth and yield performance of Pleurotus ostreatus (Jacq. Fr.) Kumm (oyster mushroom) on different substrates. AMB Express 2016, 6, 87. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Danezis, G.P.; Georgiou, C.A.; Zervakis, G.I. Rare earth elements concentration in mushroom cultivation substrates affects the production process and fruit-bodies content of Pleurotus ostreatus and Cyclocybe cylindracea. J. Sci. Food Agric. 2018, 98, 5418–5427. [Google Scholar] [CrossRef]
- Jasinska, A.; Wojciechowska, E.; Stoknes, K.; Roszak, M. Bioconversion of Agricultural Wastes into a Value-Added Product: Straw of Norwegian Grains Composted with Dairy Manure Food Waste Digestate in Mushroom Cultivation. Horticulturae 2022, 8, 331. [Google Scholar] [CrossRef]
- Lisiecka, J.; Prasad, R.; Jasinska, A. The Utilisation of Pholiota nameko, Hypsizygus marmoreus, and Hericium erinaceus Spent Mushroom Substrates in Pleurotus ostreatus Cultivation. Horticulturae 2021, 7, 396. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Patsou, M.; Mitsou, E.K.; Bekiaris, G.; Kotsou, M.; Tarantilis, P.A.; Pletsa, V.; Kyriacou, A.; Zervakis, G.I. Valorization of Olive By-Products as Substrates for the Cultivation of Ganoderma lucidum and Pleurotus ostreatus Mushrooms with Enhanced Functional and Prebiotic Properties. Catalysts 2019, 9, 537. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Tagkouli, D.; Bekiaris, G.; Kaliora, A.; Tsiaka, T.; Tsiantas, K.; Chatzipavlidis, I.; Zoumpoulakis, P.; Kalogeropoulos, N.; Zervakis, G.I. Enhancing the nutritional and functional properties of Pleurotus citrinopileatus mushrooms through the exploitation of winery and olive mill wastes. Food Chem. 2022, 370, 131022. [Google Scholar] [CrossRef]
- Chae, H.J.; Ahn, J.H. Optimization of rice bran and food waste compost contents in mushroom culture medium to maximize mycelial growth rate and fruit body yield of Pleurotus ostreatus. Int. Biodeter. Biodegr. 2013, 80, 66–70. [Google Scholar] [CrossRef]
- Chang, S.T.; Lau, O.W.; Cho, K.Y. The cultivation and nutritional value of Pleurotus sajor-caju. Eur. J. Appl. Microbiol. Biotechnol. 1981, 12, 58–62. [Google Scholar] [CrossRef]
- Chanakya, H.N.; Malayil, S.; Vijayalakshmi, C. Cultivation of Pleurotus spp. on a combination of anaerobically digested plant material and various agro-residues. Energy Sustain. Dev. 2015, 27, 84–92. [Google Scholar] [CrossRef]
- Garuba, T.; Abdukkareem, K.A.; Ibrahim, I.A.; Oyebamiji, O.I.; Shoyooye, O.A.; Ajibade, T.D. Influence of substrates on the nutritional quality of Pleurotus pulmonarius and Pleurotus ostreatus. Ceylon J. Sci. 2017, 46, 67. [Google Scholar] [CrossRef]
- Jin, Z.; Li, Y.; Ren, J.; Qin, N. Yield, Nutritional Content, and Antioxidant Activity of Pleurotus ostreatus on Corncobs Supplemented with Herb Residues. Mycobiology 2018, 46, 24–32. [Google Scholar] [CrossRef]
- Lin, L.; Xu, F.; Ge, X.; Li, Y. Improving the sustainability of organic waste management practices in the food-energy-water nexus: A comparative review of anaerobic digestion and composting. Renew. Sust. Energy Rev. 2018, 89, 151–167. [Google Scholar] [CrossRef]
- Li, Y.; Park, S.Y.; Zhu, J. Solid-state anaerobic digestion for methane production from organic waste. Renew. Sust. Energ. Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Dosta, J.; Romero-Güiza, M.S.; Fonoll, X.; Peces, M.; Astals, S. A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew. Sust. Energ. Rev. 2014, 36, 412–427. [Google Scholar] [CrossRef]
- O’Brien, B.J.; Milligan, E.; Carver, J.; Roy, E.D. Integrating anaerobic co-digestion of dairy manure and food waste with cultivation of edible mushrooms for nutrient recovery. Bioresour. Technol. 2019, 285, 121312. [Google Scholar] [CrossRef] [PubMed]
- Stoknes, K.; Scholwin, F.; Krzesiński, W.; Wojciechowska, E.; Jasińska, A. Efficiency of a novel “Food to waste to food” system including anaerobic digestion of food waste and cultivation of vegetables on digestate in a bubble-insulated greenhouse. Waste Manag. 2016, 56, 466–476. [Google Scholar] [CrossRef]
- Jasinska, A.J.; Wojciechowska, E.; Krzesiński, W.; Spiżewski, T.; Stoknes, K. and Krajewska, K. Mushroom cultivation on substrates with addition of anaerobically digested food waste. Acta Hortic. 2016, 1123, 199–206. [Google Scholar] [CrossRef]
- De Groot, L.M.; Bogdanski, A.K. Bioslurry = Brown Gold? A Review of Scientific Literature on the Co-Product of Biogas Production; FAO: Rome, Italy, 2013. [Google Scholar]
- Spielmeyer, A.; Ahlborn, J.; Hamscher, G. Simultaneous determination of 14 sulfonamides and tetracyclines in biogas plants by liquid-liquid-extraction and liquid chromatography tandem mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 2513–2524. [Google Scholar] [CrossRef]
- Zirkler, D.; Peters, A.; Kaupenjohann, M. Elemental composition of biogas residues: Variability and alteration during anaerobic digestion. Biomass Bioenerg. 2014, 67, 89–98. [Google Scholar] [CrossRef]
- Ali, S.S.; Nessem, A.A.; Sun, J.; Li, X. The effects of water hyacinth pretreated digestate on Lupinus termis L. seedlings under salinity stress: A complementary study. J. Environ. Chem. Eng. 2019, 7, 103159. [Google Scholar] [CrossRef]
- Lehmann, L.; Bloem, E. Antibiotic residues in substrates and output materials from biogas plants–Implications for agriculture. Chemosphere 2021, 278, 130425. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Sun, X.; Wang, X.; Su, W.; Song, N. A Study on Recycling of Spent Mushroom Substrate to Prepare Chars and Activated Carbon. BioResources 2014, 9, 3939–3954. [Google Scholar] [CrossRef]
- Hanafi, F.H.M.; Rezania, S.; Mat Taib, S.; Md Din, M.F.; Yamauchi, M.; Sakamoto, M.; Hara, H.; Park, J.; Ebrahimi, S.S. Environmentally sustainable applications of agro-based spent mushroom substrate (SMS): An overview. J. Mater. Cycles Waste Manag. 2018, 20, 1383–1396. [Google Scholar] [CrossRef]
- Phan, C.W.; Sabaratnam, V. Potential uses of spent mushroom substrate and its associated lignocellulosic enzymes. Appl. Microbiol. Biotechnol. 2012, 96, 863–873. [Google Scholar] [CrossRef]
- Rinker, D.L. Spent mushroom substrate uses: Technology and applications. In Edible and Medicinal Mushrooms: Technology and Applications; Zied, D.C., Pardo-Giménez, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 427–454. [Google Scholar] [CrossRef]
- Zied, D.C.; Sánchez, J.E.; Noble, R.; Pardo-Giménez, A. Use of Spent Mushroom Substrate in New Mushroom Crops to Promote the Transition towards A Circular Economy. Agronomy 2020, 10, 1239. [Google Scholar] [CrossRef]
- Gern, R.M.M.; Libardi Junior, N.; Patrício, G.N.; Wisbeck, E.; Chaves, M.B.; Furlan, S.A. Cultivation of Agaricus blazei on Pleurotus spp. spent substrate. Braz. Arch. Biol. Technol. 2010, 53, 939–944. [Google Scholar] [CrossRef]
- Ashrafi, R.; Mian, M.H.; Rahman, M.; Jahiruddin, M. Recycling of Spent Mushroom Substrate for the Production of Oyster Mushroom. Res. Biotechnol. 2014, 5, 13–21. [Google Scholar]
- Noonsong, V.; Puttakun, N.; Tinsirisuk, M.; Seephueak, P. Recycling of spent Pleurotus compost for production of the Agrocybe cylindracea. Mycosphere 2016, 7, 36–43. [Google Scholar] [CrossRef]
- Mleczek, M.; Siwulski, M.; Stuper-Szablewska, K.; Rissmann, I.; Sobieralski, K.; Goliński, P. Accumulation of elements by edible mushroom species: Part, I. Problem of trace element toxicity in mushrooms. J. Environ. Sci. Health Part B 2013, 48, 69–81. [Google Scholar] [CrossRef]
- Cocchi, L.; Vescovi, L.; Petrini, L.E.; Petrini, O. Heavy metals in edible mushrooms in Italy. Food Chem. 2006, 98, 277–284. [Google Scholar] [CrossRef]
- Györfi, J.; Geösel, A.; Vetter, J. Mineral composition of different strains of edible medicinal mushroom Agaricus subrufescens Peck. J. Med. Food 2010, 13, 1510–1514. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Q.; Cai, H.; Guo, X.; Wang, T.; Gui, M. Study of heavy metal concentrations in wild edible mushrooms in Yunnan Province, China. Food Chem. 2015, 188, 294–300. [Google Scholar] [CrossRef]
- Largeteau, M.L.; Llarena-Hernández, R.C.; Regnault-Roger, C.; Savoie, J.M. The medicinal Agaricus mushroom cultivated in Brazil: Biology, cultivation and non-medicinal valorisation. Appl. Microbiol. Biot. 2011, 92, 897–907. [Google Scholar] [CrossRef]
- Mleczek, M.; Magdziak, Z.; Gąsecka, M.; Niedzielski, P.; Kalač, P.; Siwulski, M.; Sobieralski, K. Content of selected elements and low-molecular-weight organic acids in fruiting bodies of edible mushroom Boletus badius (Fr.) Fr. from unpolluted and polluted areas. Environ. Sci. Pollut. R. 2016, 23, 20609–20618. [Google Scholar] [CrossRef]
- Muñoz, A.H.S.; Corona, F.G.; Wrobel, K.; Soto, G.M.; Wrobel, K. Subcellular distribution of aluminum, bismuth, cadmium, chromium, copper, iron, manganese, nickel, and lead in cultivated mushrooms (Agaricus bisporus and Pleurotus ostreatus). Biol. Trace Elem. Res. 2005, 106, 265–277. [Google Scholar] [CrossRef]
- Rzymski, P.; Mleczek, M.; Niedzielski, P.; Siwulski, M.; Gąsecka, M. Cultivation of Agaricus bisporus enriched with selenium, zinc and copper. J. Sci. Food Agr. 2017, 97, 923–928. [Google Scholar] [CrossRef]
- Sun, L.; Liu, G.; Yang, M.; Zhuang, Y. Bioaccessibility of cadmium in fresh and cooked Agaricus blazei Murill assessed by in vitro biomimetic digestion system. Food Chem. Toxicol. 2012, 50, 1729–1733. [Google Scholar] [CrossRef]
- Schlecht, M.T.; Säumel, I. Wild growing mushrooms for the Edible City? Cadmium and lead content in edible mushrooms harvested within the urban agglomeration of Berlin, Germany. Environ. Pollut. 2015, 204, 298–305. [Google Scholar] [CrossRef]
- Vetter, J. Chemical composition of fresh and conserved Agaricus bisporus mushroom. Eur. Food Res. Technol. 2003, 217, 10–12. [Google Scholar] [CrossRef]
- Vetter, J. Data on sodium content of common edible mushrooms. Food Chem. 2003, 81, 589–593. [Google Scholar] [CrossRef]
- Vetter, J.; Lelley, J. Selenium level of the cultivated mushroom Agaricus bisporus. Acta Aliment. Hung. 2004, 33, 297–301. [Google Scholar] [CrossRef]
- Vetter, J. Arsenic content of some edible mushroom species. Eur. Food Res. Technol. 2004, 219, 71–74. [Google Scholar] [CrossRef]
- Vetter, J.; Hajdú, C.S.; Gyorfi, J.; Maszlavér, P. Mineral composition of the cultivated mushrooms Agaricus bisporus, Pleurotus ostreatus and Lentinula edodes. Acta Aliment. Hung. 2005, 34, 441–451. [Google Scholar] [CrossRef]
- Wisitrassameewong, K.; Karunarathna, S.C.; Thongklang, N.; Zhao, R.; Callac, P.; Moukha, S.; Ferandon, C.; Chukeatirote, E.; Hyde, K.D. Agaricus subrufescens: A review. Saudi J. Biol. Sci. 2012, 19, 131–146. [Google Scholar] [CrossRef]
- Zhu, F.; Qu, L.; Fan, W.; Qiao, M.; Hao, H.; Wang, X. Assessment of heavy metals in some wild edible mushrooms collected from Yunnan Province, China. Environ. Monit. Assess. 2011, 179, 191–199. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Danezis, G.; Georgiou, C.; Zervakis, G.I. Elemental Content in Pleurotus ostreatus and Cyclocybe cylindracea Mushrooms: Correlations with Concentrations in Cultivation Substrates and Effects on the Production Process. Molecules 2020, 25, 2179. [Google Scholar] [CrossRef]
- Yang, W.; Guo, F.; Wan, Z. Yield and size of oyster mushroom grown on rice/wheat straw basal substrate supplemented with cotton seed hull. Saudi J. Biol. Sci. 2013, 20, 333–338. [Google Scholar] [CrossRef]
- Siwulski, M.; Niedzielski, P.; Budka, A.; Budzyńska, S.; Kuczyńska-Kippen, N.; Kalač, P.; Sobieralski, K.; Mleczek, M. Patterns of changes in the mineral composition of Agaricus bisporus cultivated in Poland between 1977–2020. J. Food Compos. Anal. 2022, 112, 104660. [Google Scholar] [CrossRef]
- Aloupi, M.; Koutrotsios, G.; Koulousaris, M.; Kalogeropoulos, N. Trace metal contents in wild edible mushrooms growing on serpentine and volcanic soils on the island of Lesvos, Greece. Ecotoxicol. Environ. Saf. 2012, 78, 184–194. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking—Water Quality, 4th ed.; World Health Organization (WHO): Geneva, Switzerland, 2011; pp. 104–108. [Google Scholar]
- Bruce, R.M.; Odin, M.; World Health Organization. Beryllium and Beryllium Compounds; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- World Health Organization; Food and Agricultural Organization of the United Nations. Vitamin and Mineral Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Martone, G. Nutritional lithium. J. Clin. Psychiatry Neurosci. 2018, 1, 3–4. [Google Scholar]
- National Research Council (US) Subcommittee on the Tenth Edition of the Recommended Dietary Allowances. National Research Council Recommended Dietary Allowances; The National Academies Press: Washington, DC, USA, 1989. [Google Scholar]
- World Health Organization. Strontium and Strontium Compound. Concise International Chemical Assessment Document 77. 2010. Available online: https://apps.who.int/iris/bitstream/handle/10665/44280/9789241530774_?sequence=1 (accessed on 17 August 2022).
- Hoa, H.T.; Wang, C.L.; Wang, C.H. The Effects of Different Substrates on the Growth, Yield, and Nutritional Composition of Two Oyster Mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 423–434. [Google Scholar] [CrossRef]
- Atila, F. Evaluation of suitability of various agro-wastes for productivity of Pleurotus djamor, Pleurotus citrinopileatus and Pleurotus eryngii mushrooms. J. Exp. Agric. Int. 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Hasan, M.T.; Khatun, M.H.A.; Sajib, M.A.M.; Rahman, M.M.; Rahman, M.S.; Roy, M.; Miah, M.N.; Ahmed, K.U. Effect of Wheat Bran Supplement with Sugarcane Bagasse on Growth, Yield and Proximate Composition of Pink Oyster Mushroom (Pleurotus djamor). Am. J. Food Sci. Technol. 2015, 3, 150–157. [Google Scholar] [CrossRef]
- Selvakumar, P.; Rajasekar, S.; Babu, A.G.; Periasamy, K.; Raaman, N.; Reddy, M.S. Improving biological efficiency of Pleurotus strain through protoplast fusion between P. ostreatus var. florida and P. djamor var. roseus. Food Sci. Biotechnol. 2015, 24, 1741–1748. [Google Scholar] [CrossRef]
- Salmones, D.; Mata, G.; Waliszewski, K.N. Comparative culturing of Pleurotus spp. on coffee pulp and wheat straw: Biomass production and substrate biodegradation. Bioresour. Technol. 2005, 96, 537–544. [Google Scholar] [CrossRef]
- Hultberg, M.; Oskarsson, C.; Bergstrand, K.J.; Asp, H. Benefits and drawbacks of combined plant and mushroom production in substrate based on biogas digestate and peat. Environ. Technol. Innov. 2022, 28, 102740. [Google Scholar] [CrossRef]
- Stamets, P. Growing Gourmet and Medicinal Mushrooms, 3rd ed.; Ten Speed Press: Berkley, CA, USA, 2000. [Google Scholar]
- Kurtzman, R.H.; Zadrazil, F. Physiological and taxonomic considerations for cultivation of Pleurotus mushrooms. In Tropical Mushrooms: Biological Nature and Cultivation Methods; Chang, S.T., Quimio, T.H., Eds.; Chinese University Press: Hong Kong, China, 1982; pp. 299–348. ISBN1 9-62-201264-7. ISBN2 978-9-62201-264-6. [Google Scholar]
- Molena, O. O Moderno Cultivo de Cogumelos. Nobel: São Paulo, Brazil, 1986; p. 170. ISBN 852130377. [Google Scholar]
- Miles, P.G.; Chang, S.T. Mushrooms Biology: Concise Basics and Current Developments; Word Scientific: Singapore, 1997; p. 194. ISBN 9-81-022877-5. [Google Scholar]
- Chang, S.T.; Miles, P.G. Edible Mushrooms and Their Cultivation; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Sales-Campos, C.; Fereira da Eira, A.; Teixeira de Almeida Minhoni, M.; Nogueira de Andrade, M.C. Mineral composition of raw material, substrate and fruiting bodies of Pleurotus ostreatus in culture. Interciencia 2009, 34, 432–436. Available online: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0378-18442009000600013 (accessed on 6 July 2022).
- Kutter, P.; Weissböck, A.D.; Leitner, V.; Jäger, A. Examination of commercial additives for biogas production. Agron. Res. 2015, 13, 337–347. Available online: https://agronomy.emu.ee/vol132/13_2_8_B5.pdf (accessed on 10 July 2022).
- Rajarathnam, S.; Bano, Z. Biopotentialities of basidiomacromycetes. Eur. J. Appl. Microbiol. Biotechnol. 1992, 37, 233–361. [Google Scholar] [CrossRef]
- Oliveira, H.C.B. Avaliação de Três Substratos com Diferentes Granulométricas, para o Cultivo de Duas Linhagens de Pleurotus ostreatus (Jacq.:Fr.) Kummer. Master’s Thesis, Universidade Federal do Ceará, Fortaleza, Brazil, 2000; 89p. [Google Scholar]
- Richard, T.L. Municipal solid waste composting: Physical and biological processing. Biomass Bioenerg. 1992, 3, 163–180. [Google Scholar] [CrossRef]
- Hanc, A.; Szakova, J.; Ochecova, P. Differences in the mobility of Cd, Cu, Pb and Zn during composting of two types of household bio-waste collected in four seasons. Bioresour. Technol. 2014, 168, 204–213. [Google Scholar] [CrossRef]
- Hanc, A.; Szakova, J.; Svehla, P. Effect of composting on the mobility of arsenic, chromium and nickel contained in kitchen and garden waste. Bioresour. Technol. 2012, 126, 444–452. [Google Scholar] [CrossRef]
- Zadrazil, F. Cultivation of Pleurotus. In The Biology and Cultivation of Edible Mushrooms; Ghang, S.T., Hayes, W.A., Eds.; Academic Press: New York, NY, USA, 1978; pp. 521–557. [Google Scholar] [CrossRef]
- Silva, S.O.; Costa, S.M.G.; Clemente, E. Chemical composition of Pleurotus pulmonarius (Fr.) Quél., substrates and residue after cultivation. Braz. Arch. Biol. Technol. 2002, 45, 531–535. [Google Scholar] [CrossRef]
- Stewart, D.P.C.; Cameron, K.C.; Cornforth, I.S. Effects of spent mushroom substrate on soil chemical conditions and plant growth in an intensive horticultural system: A comparison with inorganic fertiliser. Soil Res. 1998, 36, 185–198. [Google Scholar] [CrossRef]
- Medina, E.; Paredes, C.; Bustamante, M.A.; Moral, R.; Moreno-Caselles, J. Relationships between soil physico-chemical, chemical and biological properties in a soil amended with spent mushroom substrate. Geoderma 2012, 173, 152–161. [Google Scholar] [CrossRef]
- Pardo-Giménez, A.; Pardo-Gonzalez, J.E.; Cunha Zied, D. Evaluation of harvested mushrooms and viability of Agaricus bisporus growth using casing materials made from spent mushroom substrate. Int. J. Food Sci. Technol. 2011, 46, 787–792. [Google Scholar] [CrossRef]
- Pardo-Giménez, A.; Picornell Buendia, M.R.; de Juan Valero, J.A.; Pardo-Gonzalez, J.E.; Cunha Zied, D. Cultivation of Pleurotus ostreatus using supplemented spent oyster mushroom substrate. Acta Hortic. 2012, 933, 267–272. [Google Scholar] [CrossRef]
- Jasińska, A. Spent mushroom compost (SMC)–Retrieved added value product closing loop in agricultural production. Acta Agrar. Debr. 2018, 150, 185–202. [Google Scholar] [CrossRef]
- Aldoori, Z.T.; Al-Obaidi, A.S.; Abdulkareem, A.H.; Abdullah, M.K. Effect of dietary replacement of barley with mushroom cultivation on carcass characteristics of Awassi lambs. J. Anim. Health Prod. 2015, 3, 94–98. [Google Scholar] [CrossRef]
- Baek, Y.C.; Kim, M.S.; Reddy, K.E.; Oh, Y.K.; Jung, Y.H.; Yeo, J.M.; Choi, H. Rumen fermentation and digestibility of spent mush (Pleurotus ostreatus) substrate inoculated with Lactobacil brevis for Hanwoo steers. Revista Colombiana de Ciencias Pecuarias 2017, 30, 267–277. [Google Scholar] [CrossRef]
- Strmisková, G.; Strmiska, F.; Dubravicky, J. Mineral composition of oyster mushrooms. Nahrung 1992, 36, 210–212. [Google Scholar] [CrossRef]
- Vetter, J. Mineral element content of edible and poisonous macrofungi. Acta Alim. 1990, 19, 27–40. Available online: https://www.researchgate.net/profile/JanosVetter/publication/284105599_Mineral_element_content_of_edible_and_poisonous_macrofungi/links/5abdebe145851584fa6fdda0/Mineral-element-content-of-edible-and-poisonous-macrofungi.pdf (accessed on 15 August 2022).
- Vetter, J. Mineral elements in the important cultivated mushrooms Agaricus bisporus and Pleurotus ostreatus. Food Chem. 1994, 50, 277–279. [Google Scholar] [CrossRef]
- Wang, D.; Sakoda, A.; Suzuki, M. Biological efficiency and nutritional value of Pleurotus ostreatus cultivated on spent beer grain. Bioresour. Technol. 2001, 78, 293–333. [Google Scholar] [CrossRef]
- Bernás, B.; Jaworska, G.; Lisiewka, Z. Edible mushrooms as a source of valuable nutritive constituents. Acta Sci. Polon. Technol. Alim. 2006, 5, 5–20. Available online: https://agro.icm.edu.pl/agro/element/bwmeta1.element.agro-article-963ba13a-95a8-4bf1-ad64-74dcb80a7689 (accessed on 5 August 2022).
- Mallikarjuna, S.E.; Ranjini, A.; Haware, D.J.; Vijayalakshmi, M.R.; Shashirekha, M.N.; Rajarathnam, S. Mineral composition of four edible mushrooms. J. Chem. 2013, 2013, 805284. [Google Scholar] [CrossRef]
- Siwulski, M.; Mleczek, M.; Rzymski, P.; Budka, A.; Jasińska, A.; Niedzielski, P.; Kalac, P.; Gąsecka, M.; Budzynska, S.; Mikołajczak, P. Screening the multi-element content of Pleurotus mushroom species using inductively coupled plasma optical emission spectrometer (ICP-OES). Food Anal. Method 2017, 10, 487–496. [Google Scholar] [CrossRef]
- Zsigmond, A.R.; Kántor, I.; May, Z.; Urák, I.; Héberger, K. Elemental composition of Russula cyanoxantha along an urbanization gradient in Cluj-Napoca (Romania). Chemosphere 2020, 238, 124566. [Google Scholar] [CrossRef]
- Yamaç, M.; Yildiz, D.; Sarikürkcü, C.; Çelikkollu, M.; Solak, M.H. Heavy metals in some edible mushrooms from the Central Anatolia, Turkey. Food Chem. 2007, 103, 263–267. [Google Scholar] [CrossRef]
- Ouzouni, P.K.; Petridis, D.; Koller, W.D.; Riganakos, K.A. Nutritional value and metal content of wild edible mushrooms collected from West Macedonia and Epirus, Greece. Food Chem. 2009, 115, 1575–1580. [Google Scholar] [CrossRef]
- Mustafa, S.; Sibal, S.; Mustafa, T.; Durali, M. Determination of trace metals in mushroom samples from Kayseri, Turkey. Food Chem. 2005, 92, 649–652. [Google Scholar] [CrossRef]
- Pei, D.; Xie, H.; Song, H.; Xu, H.; Wu, Y. Bioconcentration factors and potential human health risks of heavy metals in cultivated Lentinus edodes in Chengdu, People’s Republic of China. J. Food Prot. 2015, 78, 390–395. [Google Scholar] [CrossRef]
- Kalač, P. Trace element contents in European species of wild growing edible mushrooms: A review for the period 2000–2009. Food Chem. 2010, 122, 2–15. [Google Scholar] [CrossRef]
- Falandysz, J.; Borovička, J. Macro and trace mineral constituents and radionuclides in mushrooms: Health benefits and risks. Appl. Microbiol. Biotechnol. 2013, 97, 477–501. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific opinion on the risks to public health related to the presence of nickel in food and drinking water. Panel of contaminants in the food chain (CONTAM). EFSA J. 2015, 13, 4002. [Google Scholar] [CrossRef]
- EFSA. Dietary Reference Values for Nutrients. Summary Report; EFSA Supporting Publication; EFSA: Parma, Italy, 2017; p. e15121. [Google Scholar]
- Mleczek, M.; Siwulski, M.; Budka, A.; Mleczek, P.; Budzyńska, S.; Szostek, M.; Rzymski, P. Toxicological risks and nutritional value of wild edible mushroom species -a half-century monitoring study. Chemosphere 2021, 263, 128095. [Google Scholar] [CrossRef] [PubMed]
- EC. Commission Regulation (EC) No 629/2008 of 2 July 2008 Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; European Commission: Brussels, Belgium, 2008. [Google Scholar]
- IRIS (Integrated Risk Information System). Silver. Reference Dose for Oral Exposure. 1991. Available online: https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=99 (accessed on 15 July 2022).
- IRIS (Integrated Risk Information System). Barium and Compounds. Reference Dose for Oral Exposure. 2005. Available online: https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=10 (accessed on 15 July 2022).
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Safety of aluminium from dietary intake. Scientific opinion of the panel on food additives, flavourings, processing aids and food contact materials (AFC). EFSA J. 2008, 754, 1–34. [Google Scholar]
| Yield (g) | 196.5 ± 43.6 |
| Dry matter (g) | 24.20 ± 4.18 |
| Dry matter (%) | 12.52 ± 1.87 |
| Biological efficiency (%) | 39.30 ± 8.72 |
| Element (mg∙kg−1 DM) | Flush I | Flush II | Flush III |
|---|---|---|---|
| Ca | 880 ± 168 b * | 1110 ± 41.7 ab | 1270 ± 44.1 a |
| K | 11,000 ± 130 b | 13,000.20 ± 400 a | 14,000.20 ± = 300 a |
| Mg | 526 ± 35 c | 622 ± 293 b | 718 ± 38 a |
| Na | 218 ± 34 b | 274 ± 29 b | 406 ± 28 a |
| Element (mg∙kg−1 DM) | Flush I | Flush II | Flush III |
|---|---|---|---|
| B | 0.93 ± 0.92 b * | 2.74 ± 0.48 a | 3.37 ± 0.17 a |
| Co | 0.56 ± 0.05 a | 0.80 ± 0.07 a | 0.99 ± 0.08 a |
| Cr | 0.34 ± 2.6 a | 0.39 ± 1.3 a | 0.42 ± 0.5 a |
| Cu | 11.3 ± 0.7 a | 13.8 ± 0.8 a | 15.0 ± 0.4 a |
| Fe | 110 ± 22 c | 147 ± 15 b | 191 ± 7 a |
| Mn | 5.96 ± 0.68 a | 6.74 ± 0.21 a | 7.76 ± 0.45 a |
| Mo | 0.01 ± 0.00 a | 0.03 ± 0.03 a | 0.20 ± 0.11 a |
| Ni | 0.01 ± 0.00 a | 0.02 ± 0.01 a | 0.10 ± 0.03 a |
| Se | 0.38 ± 0.32 c | 1.58 ± 0.44 b | 2.44 ± 0.14 a |
| Zn | 48.7 ± 8.2 b | 78.0 ± 7.1 a | 93.5 ± 1.3 a |
| Element (mg∙kg−1 DM) | Flush I | Flush II | Flush III |
|---|---|---|---|
| Ag | 0.06 ± 0.04 a * | 0.12 ± 0.01 a | 0.16 ± 0.02 a |
| As | 0.14 ± 0.20 b | 0.62 ± 0.06 a | 0.88 ± 0.19 a |
| Ba | 1.58 ± 0.30 b | 2.06 ± 0.04 a | 2.32 ± 0.13 a |
| Be | 0.04 ± 0.00 a | 0.04 ± 0.00 a | 0.04 ± 0.00 a |
| Cd | 0.24 ± 0.06 a | 0.35 ± 0.01 a | 0.51 ± 0.15 a |
| Hg | 0.01 ± 0.00 a | 0.02 ± 0.01 a | 0.05 ± 0.01 a |
| Tl | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| Element (mg∙kg−1 DM) | Flush I | Flush II | Flush III |
|---|---|---|---|
| Al | 5.41 ± 0.66 a * | 7.37 ± 0.99 a | 8.26 ± 0.23 a |
| Bi | 0.34 ± 0.14 a | 0.70 ± 0.18 a | 0.97 ± 0.03 a |
| Ce | 0.02 ± 0.01 a | 0.04 ± 0.00 a | 0.05 ± 0.01 a |
| Cs | 2.48 ± 4.29 c | 31.8 ± 14.9 b | 58.4 ± 17.4 a |
| Ga | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| Ge | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.16 ± 0.12 a |
| Li | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| Nd | 0.01 ± 0.00 a | 0.02 ± 0.00 a | 0.03 ± 0.00 a |
| Pr | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.02 ± 0.00 a |
| Sb | 1.52 ± 0.13 a | 1.96 ± 0.25 a | 2.43 ± 0.23 a |
| Sr | 2.66 ± 0.49 a | 3.45 ± 0.07 a | 3.91 ± 0.41 a |
| Ta | 0.01 ± 0.00 a | 0.05 ± 0.03 a | 0.13 ± 0.04 a |
| Te | 2.56 ± 0.42 a | 3.17 ± 0.27 a | 3.93 ± 0.26 a |
| Ti | 0.06 ± 0.03 a | 0.11 ± 0.01 a | 0.15 ± 0.01 a |
| V | 0.01 ± 0.00 a | 0.03 ± 0.00 a | 0.04 ± 0.00 a |
| W | 2.91 ± 0.53 a | 3.61 ± 0.16 a | 3.94 ± 0.06 a |
| Zr | 0.11 ± 0.02 a | 0.13 ± 0.01 a | 0.14 ± 0.00 a |
| Elements | EDI | Guideline Level | TDI | RDI | ||
|---|---|---|---|---|---|---|
| Flush I | Flush II | Flush III | ||||
| Major essential elements—MEEs | ||||||
| Ca | 367 | 462 | 530 | 950 [118] | Nr * | 1000 [71] |
| K | 4540 | 5610 | 6040 | 3500 [118] | nr | |
| Mg | 219 | 259 | 299 | 350 [118] | nr | 240 [71] |
| Na | 90.74 | 114 | 169 | 2000 [118] | nr | |
| Essential trace elements—ETEs | ||||||
| B | 0.39 | 1.14 | 1.41 | nr | nr | nr |
| Co | 0.23 | 0.33 | 0.41 | nr | nr | nr |
| Cu | 4.71 | 5.74 | 6.23 | 1.6 [118] | 10 [69] | 2.2 [69] |
| Fe | 45.9 | 61.5 | 79.7 | 11 [118] | 48 [69] | 10–50 [69] |
| Mn | 2.48 | 2.81 | 3.23 | 3.0 [118] | 11 [69] | 3 [69] |
| Mo | 0.01 | 0.01 | 0.08 | 0.065 [118] | nr | 0.1–0.3 [69] |
| Ni | 0.01 | 0.01 | 0.04 | 0.0195 [117] | 0.720 [69] | |
| Se | 0.16 | 0.66 | 1.02 | 0.070 [118] | 320–480 [69] | 0.026–0.035 [69] |
| Zn | 20.3 | 32.5 | 39.0 | 11.7 [118] | 60 [69] | 15-20 [69] |
| Trace elements with detrimental health effects—TEWDHE | ||||||
| Ag | 0.03 | 0.05 | 0.07 | nr | 0.005 [121] | nr |
| As | 0.06 | 0.26 | 0.37 | 0.5 [120] | nr | nr |
| Ba | 0.66 | 0.86 | 0.97 | nr | 0.2 [122] | nr |
| Be | 0.02 | 0.02 | 0.02 | 27.6 [70] | nr | |
| Cd | 0.10 | 0.15 | 0.22 | 1.0 [120] | nr | nr |
| Hg | 0.01 | 0.01 | 0.03 | 0.1 [120] | nr | nr |
| Tl | 0.01 | 0.01 | 0.01 | nr | nr | |
| Nutritionally nonessential elements—NNEs | ||||||
| Al | 2.25 | 3.08 | 3.44 | 1.0 [123] | 0.14 [118] | nr |
| Bi | 0.14 | 0.29 | 0.41 | nr | nr | nr |
| Ce | 0.01 | 0.02 | 0.03 | nr | nr | nr |
| Cs | 1.03 | 13.3 | 24.3 | nr | nr | nr |
| Ga | 0.01 | 0.01 | 0.01 | nr | nr | nr |
| Ge | 0.01 | 0.01 | 0.07 | nr | nr | nr |
| Li | 0.01 | 0.01 | 0.01 | nr | nr | 1 [72] |
| Nd | 0.01 | 0.01 | 0.02 | nr | nr | nr |
| Pr | 0.01 | 0.01 | 0.01 | nr | nr | nr |
| Sb | 0.63 | 0.82 | 1.02 | nr | 0.36 [69] | nr |
| Sr | 1.11 | 1.43 | 1.63 | nr | 0.13 [123] | nr |
| Ta | 0.01 | 0.02 | 0.06 | nr | nr | nr |
| Te | 1.07 | 1.33 | 1.63 | nr | nr | nr |
| Ti | 0.03 | 0.05 | 0.06 | nr | nr | nr |
| V | 0.01 | 0.02 | 0.02 | nr | nr | nr |
| W | 1.21 | 1.51 | 1.64 | nr | nr | nr |
| Zr | 0.05 | 0.06 | 0.06 | nr | nr | nr |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasinska, A.; Prasad, R.; Lisiecka, J.; Roszak, M.; Stoknes, K.; Mleczek, M.; Niedzielski, P. Combined Dairy Manure-Food Waste Digestate as a Medium for Pleurotus djamor—Mineral Composition in Substrate and Bioaccumulation of Elements in Fruiting Bodies. Horticulturae 2022, 8, 934. https://doi.org/10.3390/horticulturae8100934
Jasinska A, Prasad R, Lisiecka J, Roszak M, Stoknes K, Mleczek M, Niedzielski P. Combined Dairy Manure-Food Waste Digestate as a Medium for Pleurotus djamor—Mineral Composition in Substrate and Bioaccumulation of Elements in Fruiting Bodies. Horticulturae. 2022; 8(10):934. https://doi.org/10.3390/horticulturae8100934
Chicago/Turabian StyleJasinska, Agnieszka, Raghavendra Prasad, Jolanta Lisiecka, Michal Roszak, Ketil Stoknes, Miroslaw Mleczek, and Przemyslaw Niedzielski. 2022. "Combined Dairy Manure-Food Waste Digestate as a Medium for Pleurotus djamor—Mineral Composition in Substrate and Bioaccumulation of Elements in Fruiting Bodies" Horticulturae 8, no. 10: 934. https://doi.org/10.3390/horticulturae8100934
APA StyleJasinska, A., Prasad, R., Lisiecka, J., Roszak, M., Stoknes, K., Mleczek, M., & Niedzielski, P. (2022). Combined Dairy Manure-Food Waste Digestate as a Medium for Pleurotus djamor—Mineral Composition in Substrate and Bioaccumulation of Elements in Fruiting Bodies. Horticulturae, 8(10), 934. https://doi.org/10.3390/horticulturae8100934









