Postharvest Exogenous Melatonin Treatment of Table Grape Berry Enhances Quality and Maintains Bioactive Compounds during Refrigerated Storage
Abstract
:1. Introduction
2. Materials and Methods
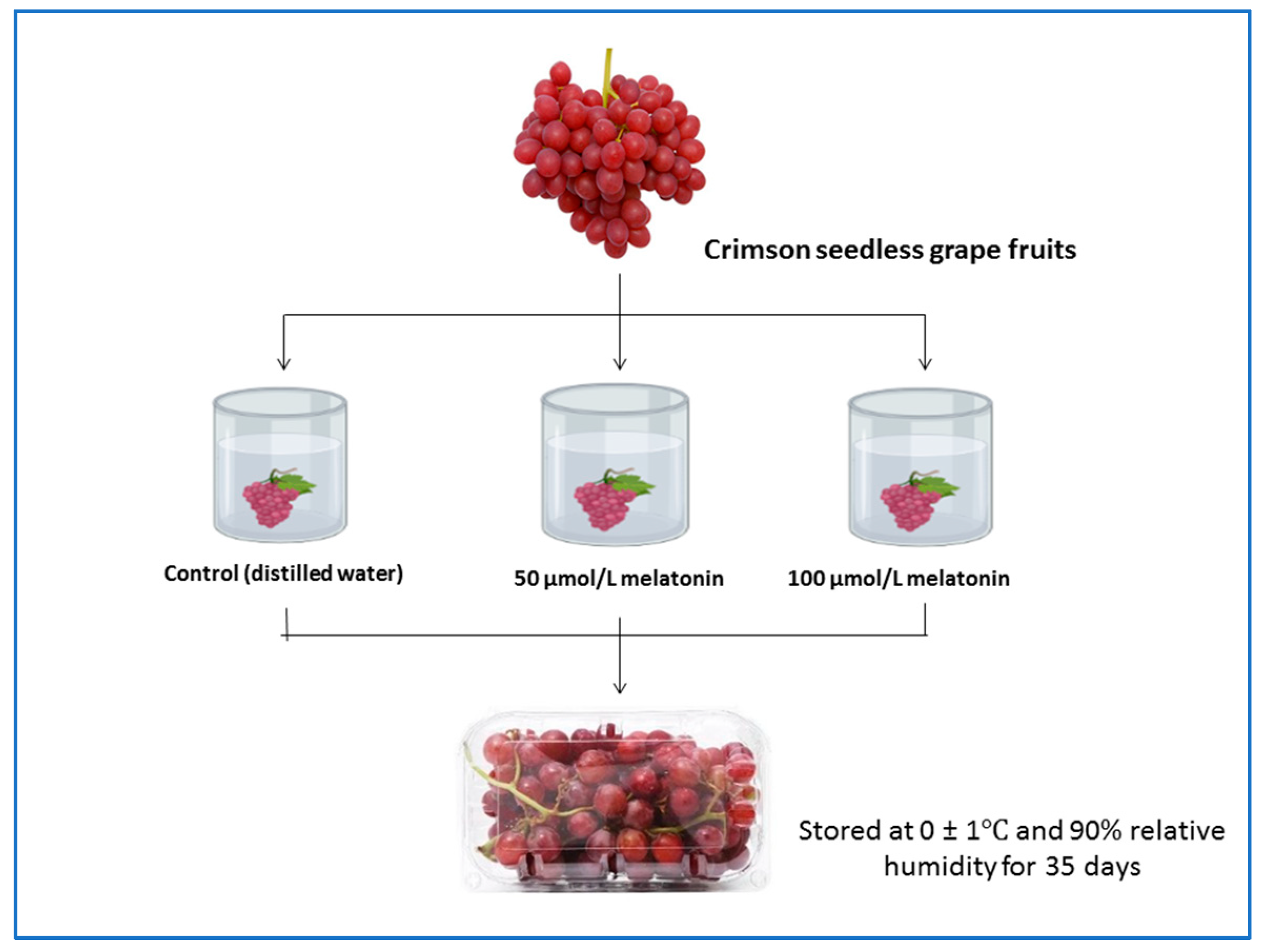
2.1. Experiment Design and Treatments
2.2. Fruit Quality
2.3. Enzyme Activity
2.4. Determination of O2•− Production Rate
2.5. H2O2 Measurement
2.6. Statistical Analysis
3. Results
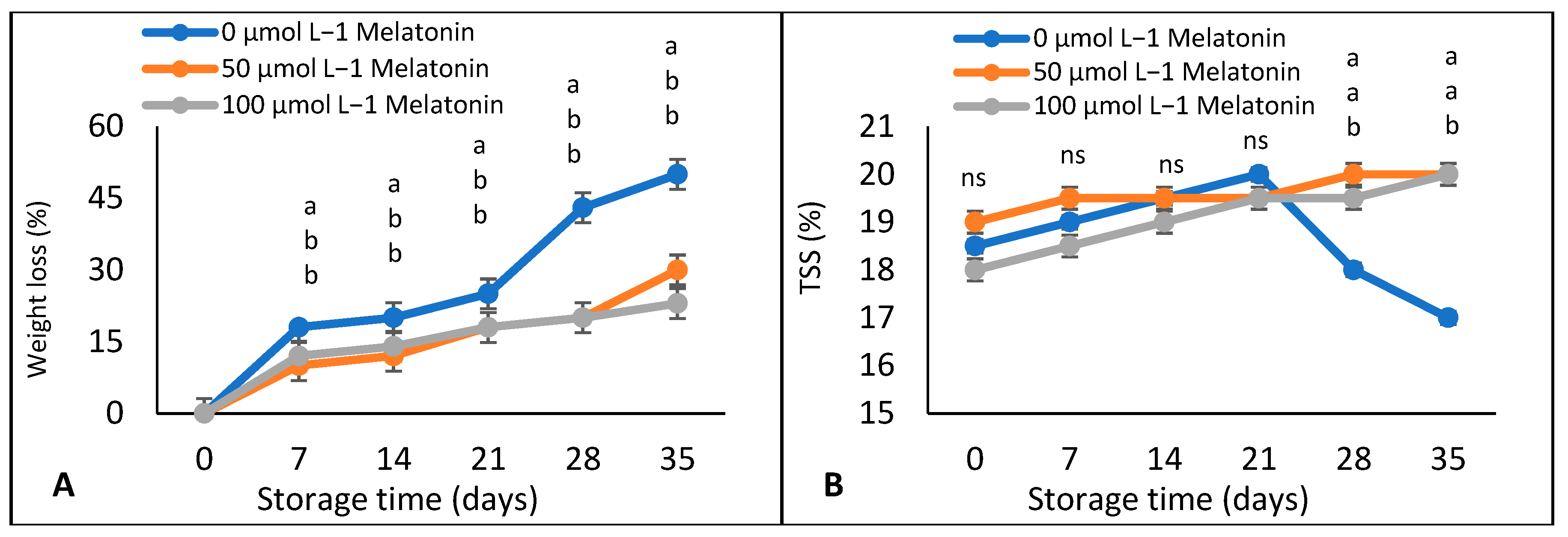
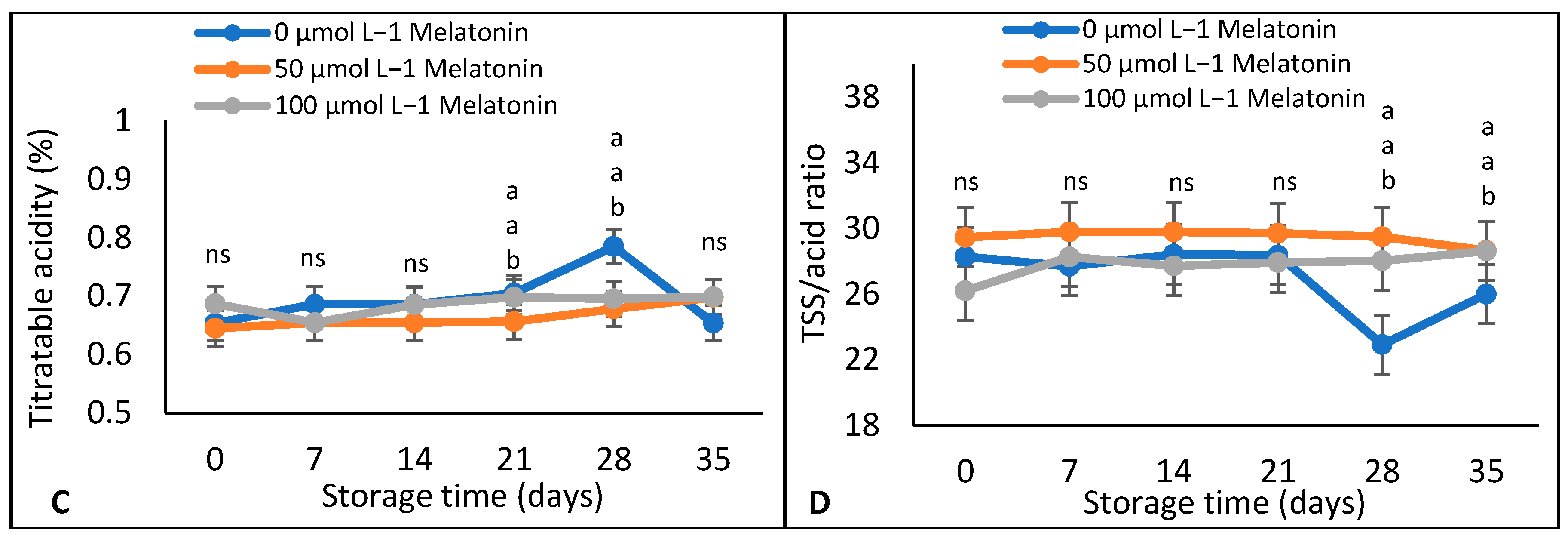
Weight Loss, TSS, Titratable Acidity, and TSS: Acids Ratio
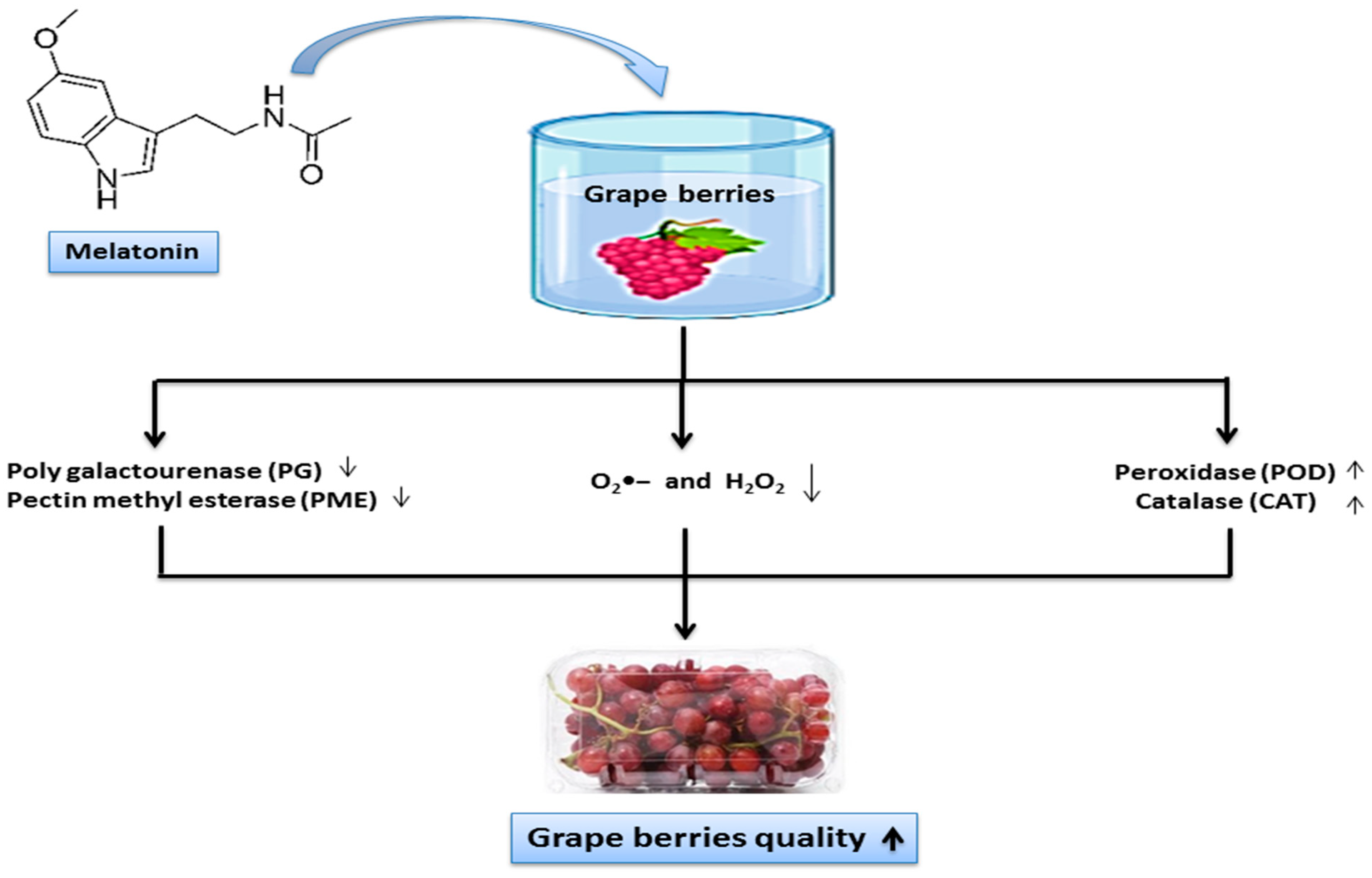
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraige, K.; Pereira-Filho, E.R.; Carrilho, E. Fingerprinting of anthocyanins from grapes produced in Brazil using HPLC-DAD-MS and exploratory analysis by principal component analysis. Food Chem. 2014, 145, 395–403. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Rabie, M.M.; Alhaithloul, H.A.S.; Alghanem, S.M.S.; Ibrahim, A.M.; Abdein, M.A.; Abdelgawad, Z.A. On the Biochemical and PhysiologicalResponses of ‘Crimson Seedless’ Grapes Coated with an Edible Composite of Pectin, Polyphenylene Alcohol, and Salicylic Acid. Horticulturae 2021, 7, 498. [Google Scholar] [CrossRef]
- Ramming, D.; Tarailo, R.; Badr, S.A. ‘Crimson Seedless’: A New Late-maturing, Red Seedless Grape. HortScience 1995, 30, 1473–1474. [Google Scholar] [CrossRef]
- Samaan, M.S.F.; Nasser, M.A. Effect of Spraying Paclobutrazol (PP333) on Yield and Fruit Quality of Crimson Seedless Grape. J. Plant Prod. Mansoura Univ. 2020, 11, 1031–1034. [Google Scholar] [CrossRef]
- Xu, T.; Chen, Y.; Kang, H. Melatonin Is a Potential Target for Improving Post-Harvest Preservation of Fruits and Vegetables. Front. Plant Sci. 2019, 10, 1388. [Google Scholar] [CrossRef]
- Shehata, S.A.; El-Mogy, M.M.; Mohamed, H.F.Y. Postharvest quality and nutrient contents of long sweet pepper enhanced by supplementary potassium foliar application. Int. J. Veg. Sci. 2019, 25, 196–209. [Google Scholar] [CrossRef]
- Barrett, D.M.; Lloyd, B. Advanced preservation methods and nutrient retention in fruits and vegetables. J. Sci. Food Agric. 2012, 92, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Liangjie, B.A.; Sen, C.A.O.; Ning, J.I.; Chao, M.A.; Rui, W.A.N.G.; Donglan, L.U.O. Exogenous melatonin treatment in the postharvest storage of pitaya fruits delays senescence and regulates reactive oxygen species metabolism. Food Sci. Technol. 2022, 42, e15221. [Google Scholar] [CrossRef]
- EL-Bauome, H.A.; Abdeldaym, E.A.; Abd El-Hady, M.A.M.; Darwish, D.B.E.; Alsubeie, M.S.; El-Mogy, M.M.; Basahi, M.A.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Alzuaibr, F.M.; et al. Exogenous Proline, Methionine, and Melatonin Stimulate Growth, Quality, and Drought Tolerance in Cauliflower Plants. Agriculture 2022, 12, 1301. [Google Scholar] [CrossRef]
- Cano, A.; Giraldo-Acosta, M.; García-Sánchez, S.; Hernández-Ruiz, J.; Arnao, B.M. Effect of Melatonin in Broccoli Postharvest and Possible Melatonin Ingestion Level. Plants 2022, 11, 2000. [Google Scholar] [CrossRef] [PubMed]
- Ze, Y.; Gao, H.; Li, T.; Yang, B.; Jiang, Y. Insights into the roles of melatonin in maintaining quality and extending shelf life of postharvest fruits. Trends Food Sci. Technol. 2021, 109, 569–578. [Google Scholar] [CrossRef]
- Corpas, F.J.; Freschi, L.; Rodríguez-Ruiz, M.; Mioto, P.T.; González-Gordo, S.; Palma, J.M. Nitro-oxidative metabolism during fruit ripening. J. Exp. Bot. 2018, 69, 3449–3463. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Shi, X.-C.; Wang, R.; Wang, H.-L.; Liu, F.; Laborda, P. Melatonin in fruit production and postharvest preservation: A review. Food Chem. 2020, 320, 126642. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, J.; Fan, X.; Jiang, W. Applications of nitric oxide and melatonin in improving postharvest fruit quality and the separate and crosstalk biochemical mechanisms. Trends Food Sci. Technol. 2020, 99, 531–541. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous melatonin delays postharvest fruit senescence and maintains the quality of sweet cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef]
- Liu, S.; Huang, H.; Huber, D.J.; Pan, Y.; Shi, X.; Zhang, Z. Delay of ripening and softening in ‘Guifei’ mango fruit by postharvest application of melatonin. Postharvest Biol. Technol. 2020, 163, 111136. [Google Scholar] [CrossRef]
- Onik, J.C.; Wai, S.C.; Li, A.; Lin, Q.; Sun, Q.; Wang, Z.; Duan, Y. Melatonin treatment reduces ethylene production and maintains fruit quality in apple during postharvest storage. Food Chem. 2021, 337, 127753. [Google Scholar] [CrossRef]
- Yang, M.; Wang, L.; Belwal, T.; Zhang, X.; Lu, H.; Chen, C.; Li, L. Exogenous Melatonin and Abscisic Acid Expedite the Flavonoids Biosynthesis in Grape Berry of Vitis vinifera cv. Kyoho. Molecules 2020, 25, 12. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Fard, J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria × anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017, 221, 1650–1657. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; Horwitz, W., Ed.; AOAC International: Rockville, MD, USA, 2006. [Google Scholar]
- Abd Elwahab, W.A.; Abd Elwahab, S.M.; Kamel, O.T. Using Safe Alternatives for Controlling Postharvest Decay, Maintaining Quality of Crimson Seedless Grape. World Appl. Sci. J. 2014, 31, 1345–1357. [Google Scholar] [CrossRef]
- Ashraf, M.; Ali, Q. Relative membrane permeability and activities of some antioxidant enzymes as the key determinants of salt tolerance in canola (Brassica napus L.). Environ. Exp. Bot. 2008, 63, 266–273. [Google Scholar] [CrossRef]
- Lee, M.; Macmillan, J.D. Mode of action of pectic enzymes. I: Purification and certain properties of tomato pectin esterase. J. Biochem. 1968, 7, 4005. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Austin, P.J. Continuous spectrophotometric assay for plant pectin methyl esterase. J. Agric. Food Chem. 1986, 34, 440–444. [Google Scholar] [CrossRef]
- Tao, M.Q.; Jahan, M.S.; Hou, K.; Shu, S.; Wang, Y.; Sun, J.; Guo, S.R. Bitter melon (Momordica charantia L.) rootstock improves the heat tolerance of cucumber by regulating photosynthetic and antioxidant defense pathways. Plants 2020, 9, 692. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.L.; Reid, D.M. Leaf senescence and lipid peroxidation: Effects of some phytohormones, and scavengers of free radicals and singlet oxygen. Physiol. Plant 1982, 56, 453–457. [Google Scholar] [CrossRef]
- He, M.; Jahan, M.S.; Wang, Y.; Sun, J.; Shu, S.; Guo, S. Compost amendments based on vinegar residue promote tomato growth and suppress bacterial wilt caused by Ralstonia solanacearum. Pathogens 2020, 9, 227. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Parmar, A.; Ali, M.R.; Abdel-Aziz, M.E.; Abdeldaym, E.A. Improving postharvest storage of fresh artichoke bottoms by an edible coating of Cordia myxa gum. Postharvest Biol. Technol. 2020, 163, 111143. [Google Scholar] [CrossRef]
- Elsayed, N.; Hassan, A.A.-M.; Abdelaziz, S.M.; Abdeldaym, E.A.; Darwish, O.S. Effect of Whey Protein Edible Coating Incorporated with Mango Peel Extract on Postharvest Quality, Bioactive Compounds and Shelf Life of Broccoli. Horticulturae 2022, 8, 770. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Z.; Yang, M.; Li, D.; Qi, M.; Xu, Y.; Abdelshafy, A.M.; Ban, Z.; Wang, F.; Li, L. Role of exogenous melatonin in table grapes: First evidence on contribution to the phenolics-oriented response. Food Chem. 2020, 329, 127155. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yue, Q.; Xiang, G.; Bian, F.E.; Yao, Y. Melatonin promotes ripening of grape berry via increasing the levels of ABA, H2O2, and particularly ethylene. Hortic. Res. 2018, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, N.; Wang, J.; Zhang, H.; Li, D.; Shi, J.; Li, R.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes ripening and improves quality of tomato fruit during postharvest life. J. Exp. Bot. 2015, 66, 657–668. [Google Scholar] [CrossRef] [PubMed]
- El-Mogy, M.M.; Ludlow, R.A.; Roberts, C.; Müller, C.T.; Rogers, H.J. Postharvest exogenous melatonin treatment of strawberry reduces postharvest spoilage but affects components of the aroma profile. J. Berry Res. 2019, 9, 297–307. [Google Scholar] [CrossRef]
- Fan, S.; Xiong, T.; Lei, Q.; Tan, Q.; Cai, J.; Song, Z.; Yang, M.; Chen, W.; Li, X.; Zhu, X. Melatonin Treatment Improves Postharvest Preservation and Resistance of Guava Fruit (Psidium guajava L.). Foods 2022, 11, 262. [Google Scholar] [CrossRef]
- Tucker, G.; Yin, X.; Zhang, A.; Wang, M.; Zhu, Q.; Liu, X.; Xie, X.; Chen, K.; Grierson, D. Ethylene† and fruit softening. Food Qual. Saf. 2017, 1, 253–267. [Google Scholar] [CrossRef]
- Zhai, R.; Liu, J.; Liu, F.; Zhao, Y.; Liu, L.; Fang, C.; Wang, H.; Li, X.; Wang, Z.; Ma, F.; et al. Melatonin limited ethylene production, softening and reduced physiology disorder in pear (Pyrus communis L.) fruit during senescence. Postharvest Biol. Technol. 2018, 139, 38–46. [Google Scholar] [CrossRef]
- Li, S.; Xu, Y.; Bi, Y.; Zhang, B.; Shen, S.; Jiang, T.; Zheng, X. Melatonin treatment inhibits gray mold and induces disease resistance in cherry tomato fruit during postharvest. Postharvest Biol. Technol. 2019, 157, 110962. [Google Scholar] [CrossRef]
- Zhu, L.L.; Hu, H.L.; Luo, S.F.; Wu, Z.X.; Li, P.X. Melatonin delaying senescence of postharvest broccoli by regulating respiratory metabolism and antioxidant activity. Trans. Chin. Soc. Agric. Eng. 2018, 34, 300–308. [Google Scholar]
- Watada, A.E.; Herner, R.C.; Kader, A.A.; Romani, R.J.; Staby, G.L. Terminology for the description of developmental stages of horticultural crops. Hort. Sci. 1984, 62, 217–254. [Google Scholar] [CrossRef]
- Tripathi, G.D.; Javed, Z.; Mishra, M.; Fasake, V.; Dashora, K. Phytomelatonin in stress management in agriculture. Heliyon 2021, 7, e06150. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, W. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, T.; Zhang, P.; Wang, Z.Y. Melatonin attenuates postharvest physiological deterioration of cassava storage roots. J. Pineal Res. 2016, 60, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a plant biostimulant in crops and during post-harvest: A new approach is needed. J. Sci. Food Agric. 2021, 101, 5297–5304. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasser, M.A.; El-Mogy, M.M.; Samaan, M.S.F.; Hassan, K.M.; El-Sayed, S.M.; Alsubeie, M.S.; Darwish, D.B.E.; Mahmoud, S.F.; Al-Harbi, N.A.; Al-Qahtani, S.M.; et al. Postharvest Exogenous Melatonin Treatment of Table Grape Berry Enhances Quality and Maintains Bioactive Compounds during Refrigerated Storage. Horticulturae 2022, 8, 860. https://doi.org/10.3390/horticulturae8100860
Nasser MA, El-Mogy MM, Samaan MSF, Hassan KM, El-Sayed SM, Alsubeie MS, Darwish DBE, Mahmoud SF, Al-Harbi NA, Al-Qahtani SM, et al. Postharvest Exogenous Melatonin Treatment of Table Grape Berry Enhances Quality and Maintains Bioactive Compounds during Refrigerated Storage. Horticulturae. 2022; 8(10):860. https://doi.org/10.3390/horticulturae8100860
Chicago/Turabian StyleNasser, Mohamed A., Mohamed M. El-Mogy, Mina S. F. Samaan, Karim M. Hassan, Salwa M. El-Sayed, Moodi Saham Alsubeie, Doaa Bahaa Eldin Darwish, Samy F. Mahmoud, Nadi Awad Al-Harbi, Salem Mesfir Al-Qahtani, and et al. 2022. "Postharvest Exogenous Melatonin Treatment of Table Grape Berry Enhances Quality and Maintains Bioactive Compounds during Refrigerated Storage" Horticulturae 8, no. 10: 860. https://doi.org/10.3390/horticulturae8100860
APA StyleNasser, M. A., El-Mogy, M. M., Samaan, M. S. F., Hassan, K. M., El-Sayed, S. M., Alsubeie, M. S., Darwish, D. B. E., Mahmoud, S. F., Al-Harbi, N. A., Al-Qahtani, S. M., Alzuaibr, F. M., & Abd El-Gawad, H. G. (2022). Postharvest Exogenous Melatonin Treatment of Table Grape Berry Enhances Quality and Maintains Bioactive Compounds during Refrigerated Storage. Horticulturae, 8(10), 860. https://doi.org/10.3390/horticulturae8100860









