The available land for commercial horticultural production is anticipated to decline globally; thus, the way to meet the growing demand of vegetables would be by increasing the produce in greenhouses, with the aid of new technologies and the use of improved cultivars. In asparagus, hybrid cultivars have been released for the last 40 years, mainly due to its dioecious nature and the possibility to micropropagate selected genotypes; however, information regarding combining abilities and heterosis among materials or genetics stocks is limited, and no heterotic pattern has been identified so far. In this sense, we advanced the study of hybrid performance among 12 accessions from 11 different origins as a way to reveal to what degree heterosis is prevalent, and to what extent it is associated to different origins or genetic distance. Overall, we found highly significant variation for market yield among materials (parents and experimental hybrids) in the two sets of crosses (
Table 2 and
Table 3). Two of the parents included were collected as growing wild (5 and 10, see
Table 1), however the mean market yield did no significant vary from some cultivated accessions (
Table 4 and
Table 5), this may reflect either a lack of differentiation between some cultivated and wild populations or that the supposedly collected wild are really escapees from cultivation. Some experimental hybrids showed a very good performance: in Set 1, (4 × 13) significantly exceeded the parental 13 (UC157), considered a commercial check (
Table 4). In Set 2, the hybrids (12 × 13), (1 × 12), and (3 × 13) significantly surpassed the UC157, and surprisingly (12 × 13) did not differ from the Atticus F1 all-male check (
Table 5). The heterosis test (contrast crosses vs. parents) was highly significant for Set 2 (
Table 3); and the best parent heteroses expressed for market yield were remarkably high, in one case being over 300% (12 × 13) and in two cases over 100%, (1 × 12) and (3 × 13) (
Table 6). In an evaluation of 16 crosses between only an inbred used as the staminate parent (originating from the Netherlands) and a group of inbred and commercial open pollinated varieties used as pistillate parents, the inbreds mostly derived from the varieties KBF (UK) and Mary Washington (USA); in [
6], for the first season’s total yield, a significantly higher parent heterosis over 200% was found in three cases, which is in concordance to the magnitude found in our study; in general, the best experimental hybrids were those derived from a Mary Washington pistillate origin, and they did not significantly differ from the best variety. In other vegetable crops, the percentage of heterosis reported for yield, in general, is lower than that present in asparagus (e.g., melon, 8%; squash, 44%; watermelon, 10%; broccoli, 65%; carrot, 28%; onion, 40%) [
25]. Interestingly, in a recent review regarding heterosis in vegetable crops [
26], the overdominance effects were involved in the largest proportion of the quantitative trait loci related to heterosis; and for many vegetable species (tomato, cabbage, eggplant, cucumber, etc.), heterotic groups were defined and associated to molecular markers.
In relation to the combining abilities, in our study both were highly significant for market yield; this means that the GCA of the parents (related to additive gene actions), and the SCA (related to non-additive gene actions) are important components of the hybrid output (
Table 7). When comparing the relative contribution along the Baker´s ratio, the SCA tended to be of a greater importance than the GCA for both sets of crosses (
Table 8). A similar contribution of the GCA and SCA to the total genetic variance was found in [
7]; however, the fact that they evaluated a set of diallel crosses of genotypes selected from an Argenteuil population could have reduced the contribution of the SCA, which is associated to dissimilar gene frequencies, most expected to be found in crosses derived from different populations. The SREG biplot applied to diallel crosses (
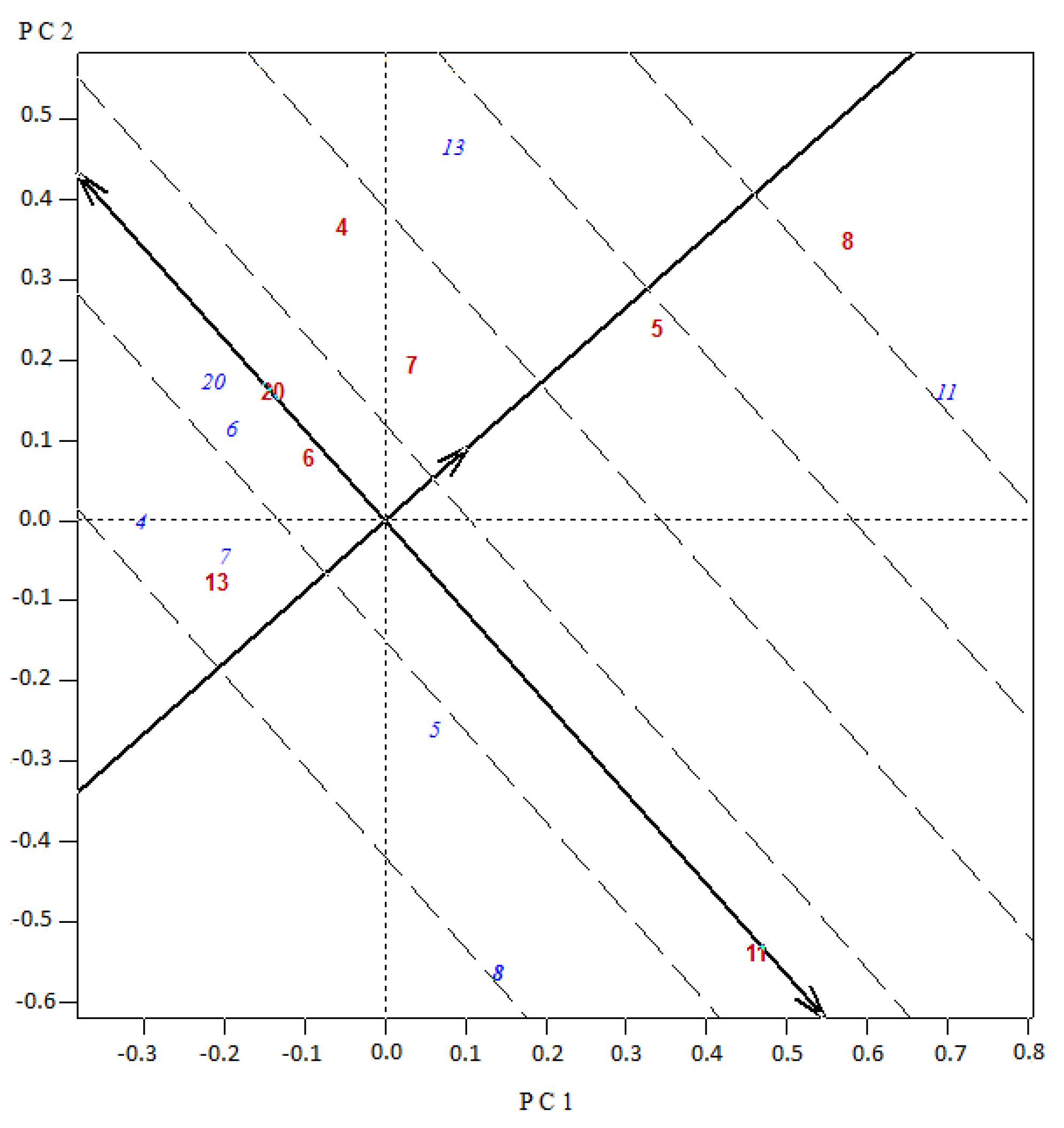
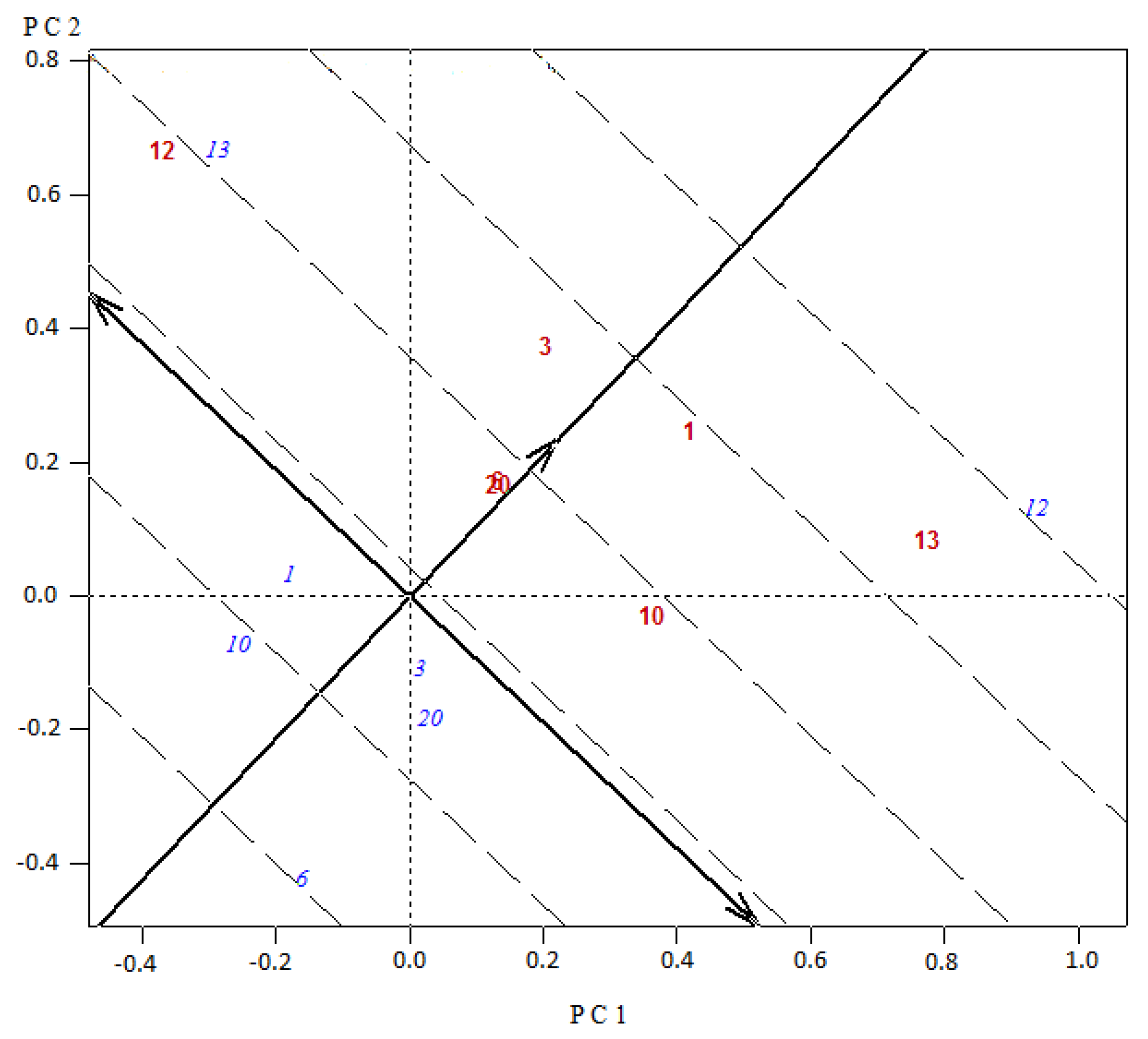
Figure 3 and
Figure 4) proved to be an appropriate tool for the graphical identification of the entries with the highest GCA effects (projections of the blue labeled codes onto the average tester abscissa) and the superior SCA combination effects, accessions as testers (red labeled, with a higher projection onto the average tester ordinate) placed close to entries (blue labeled). In this sense, in Set 1, for the experimental hybrid 4 × 13, the parental 4 as tester presented the highest ordinate projection and interacted with the parental 13 as variety (blue labeled) being closely positioned in the biplot (
Figure 3). In the other hybrid (8 × 11) with a significant heterosis and best mean marketable yield for the set (
Table 4), the accession 8 as tester interacted with material 11 as variety, which in turn had the highest GCA for Set 1. In Set 2, for the best experimental hybrid 12 × 13, both parents 12 and 13 contributed with the highest GCA effects (projections of the blue labeled codes onto the average tester abscissa) and interacted with each other with the highest SCA effect (higher projection onto the average tester ordinate) (
Figure 4). In relation to origins and hybrid performance in asparagus, it was mentioned that there is no evidence of any clear heterotic pattern for yield, but some breeding programs intuitively included parents with some divergence [
27]. In our study, the top four experimental hybrids included in three cases UC157 (13) and in one case KBF (1) as one of the parents. The opposite parents involved were in two cases Argenteuil (12), and in one case Limburgia (3) and Espárrago de Navarra (4). It appears that good combinations of crosses arise from UC157 and KBF (origins from the USA and the UK) crossed to Argenteuil, Limburgia and Espárrago de Navarra (origins from France, the Netherlands and Spain). These results may be valuable for asparagus breeders in order to select specific origins when choosing parents to develop experimental hybrids, and with the same resources increase the combinations assessed.
The correlations between genetic distances and heterosis for market yield failed to be significant for the two sets of crosses (
Table 9). In maize, the authors of [
28] found a positive correlation between genetic distances and geographical diversity and the degree of heterosis for grain yield in a set of 15 crosses. In sunflower, it was found that the genetic distance of the parents was significantly correlated to hybrid seed yield; however, the genetic distance per se was not a good predictor of the heterosis of a cross [
29]. In this sense, we can recall the consideration in maize in [
30], where the presence of divergence solely between parents is not a guarantee of heterosis in their crosses. Some degree of divergence is necessary to exploit heterosis, but for the asparagus accessions included in both sets the relation was not straightforward. Moreover, according to a previous work, the genetic distances and genealogy or relationship between different accessions was not clearly related to the country of origin and breeding status [
11].