An Early Calcium Loading during Cherry Tree Dormancy Improves Fruit Quality Features at Harvest
Abstract
1. Introduction
2. Materials and Methods
2.1. Tree Processing and Fruit Sampling
2.2. Fruit Set
2.3. Fruit Quality Characteristics
2.3.1. Skin Cracking Assessment
2.3.2. Fruit Water Absorption
2.3.3. Fruit Weight
2.3.4. Fruit Ripening Traits
2.3.5. Respiration Activity
2.4. Mineral Elements Analysis in Sweet Cherry Fruit, Buds, and Phloem Tissues
2.5. Total Phenols, Total Anthocyanins, Flavonols, and Hydroxycinnamic Acids of Sweet Cherries
2.6. Primary Polar Metabolite Analysis in Sweet Cherry Skin Tissue
2.7. Statistical Processing and Analysis
3. Results
3.1. Calcium Content in Sweet Cherry Tree Tissues during Dormancy and Its Effect on Fruit Set and Skin Cracking
3.2. The Effect of Calcium Application at Dormancy in the Nutrient Level of Sweet Cherry Fruit at Harvest
3.3. Factors Affecting the Quality Traits of Sweet Cherry Fruits at Harvest
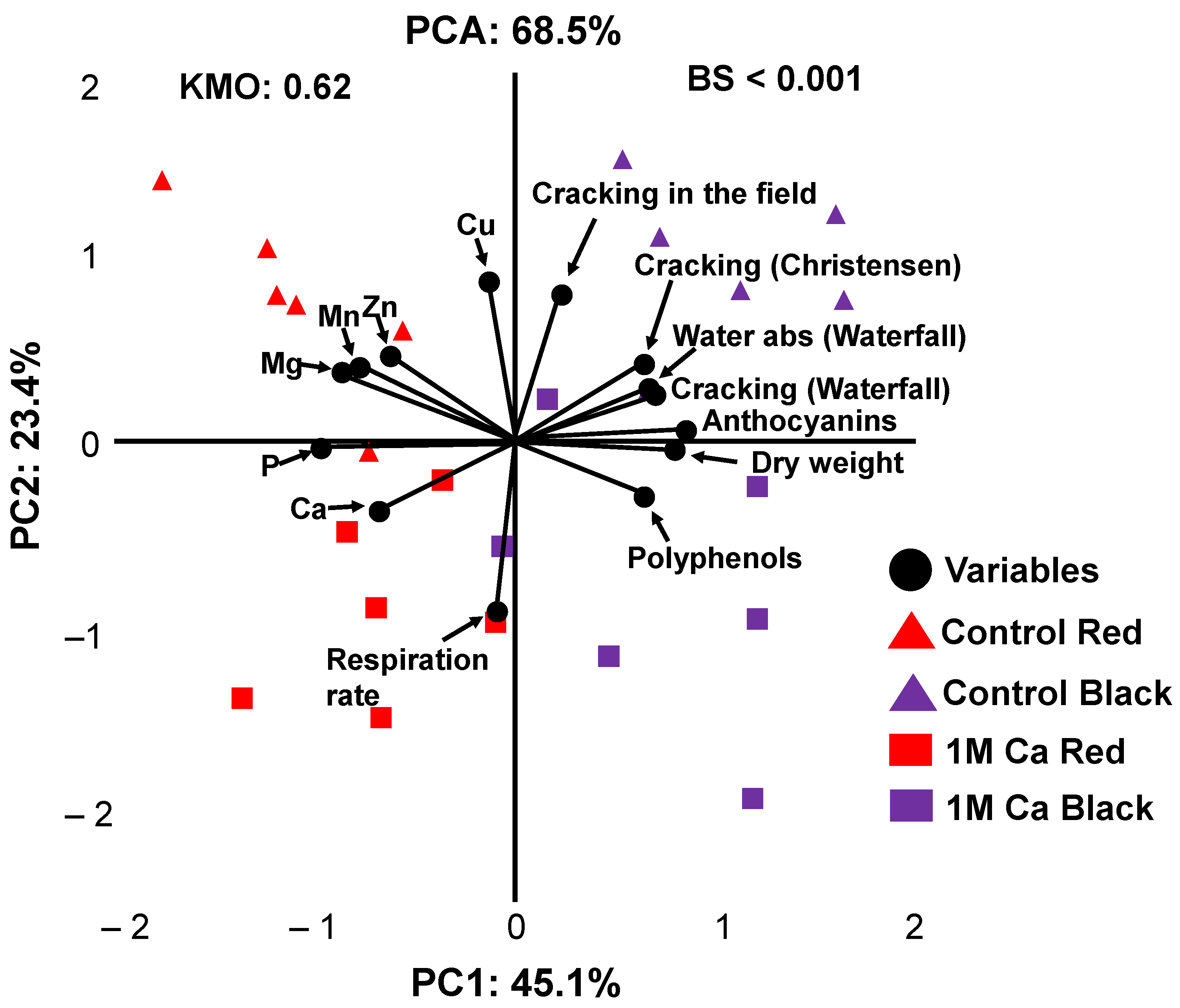
3.4. PCA Model of Quality Attributes and Nutrient Level of Cherry Fruits Treated with High Dose of Calcium
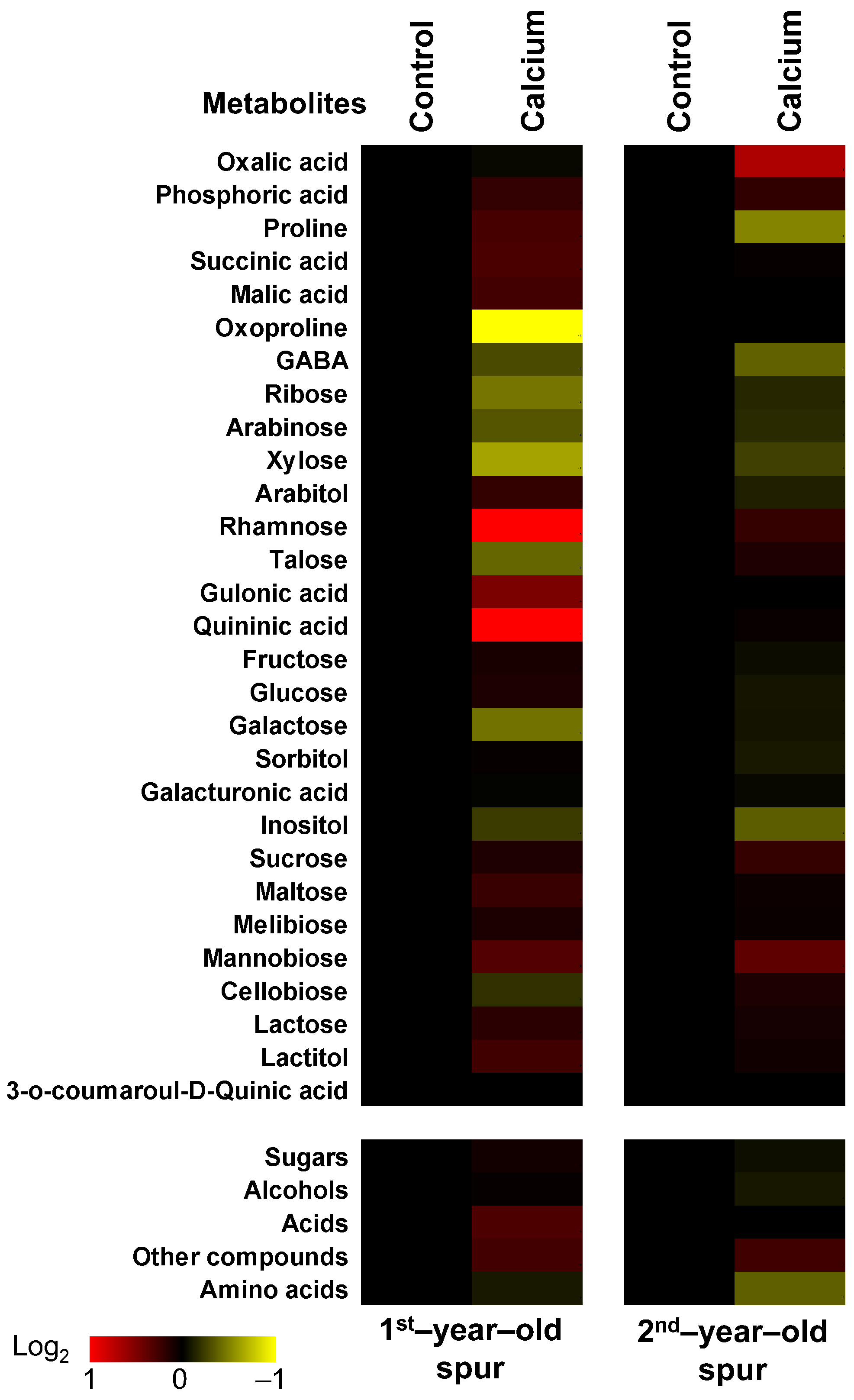
3.5. Changes in Sweet Cherry Skin Metabolites in Response to High Dose of Calcium Application
4. Discussion
4.1. External Calcium Feeding at Bud Dormancy Altered Nutrient Homeostasis and Ripening Physiology
4.2. Influence of Sweet Cherry Ripening in Nutrient Status and Quality Traits
4.3. Skin Primary Metabolism and Cracking Development Is Seriously Affected by the Early Calcium Application
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karagiannis, E.; Sarrou, E.; Michailidis, M.; Tanou, G.; Ganopoulos, I.; Bazakos, C.; Kazantzis, K.; Martens, S.; Xanthopoulou, A.; Molassiotis, A. Fruit quality trait discovery and metabolic profiling in sweet cherry genebank collection in Greece. Food Chem. 2021, 342, 128315. [Google Scholar] [CrossRef]
- Ceccarelli, D.; Antonucci, F.; Costa, C.; Talento, C.; Ciccoritti, R. An artificial class modelling approach to identify the most largely diffused cultivars of sweet cherry (Prunus avium L.) in Italy. Food Chem. 2020, 333, 127515. [Google Scholar] [CrossRef]
- Pereira, S.; Silva, V.; Bacelar, E.; Guedes, F.; Silva, A.P.; Ribeiro, C.; Gonçalves, B. Cracking in sweet cherry cultivars early bigi and lapins: Correlation with quality attributes. Plants 2020, 9, 1557. [Google Scholar] [CrossRef]
- Knoche, M.; Winkler, A. Rain-induced cracking of sweet cherries. In Cherries: Botany, Production and Uses; CABI: Wallingford, UK, 2017; pp. 140–165. ISBN 9781780648378. [Google Scholar]
- Winkler, A.; Knoche, M. Calcium and the physiology of sweet cherries: A review. Sci. Hortic. 2019, 245, 107–115. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit Calcium: Transport and Physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef]
- Saure, M.C. Calcium translocation to fleshy fruit: Its mechanism and endogenous control. Sci. Hortic. 2005, 105, 65–89. [Google Scholar] [CrossRef]
- Arrington, M.; DeVetter, L.W. Foliar applications of calcium and boron do not increase fruit set or yield in northern highbush blueberry (Vaccinium corymbosum). HortScience 2017, 52, 1259–1264. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Day, K.R.; Johnson, R.S.; Garner, D. Influence of in-season foliar calcium sprays on fruit quality and surface discoloration incidence of peaches and nectarines. Fruit Var. J. 2000, 54, 118–122. [Google Scholar]
- Wojcik, P. “Jonagold” apple fruit quality as influenced by fall sprays with calcium chloride at high rates. J. Plant Nutr. 2001, 24, 1925–1936. [Google Scholar] [CrossRef]
- Peryea, F.J. Comparison of dormant and circum-bloom zinc spray programs for washington apple orchards. J. Plant Nutr. 2007, 30, 1903–1920. [Google Scholar] [CrossRef]
- Scortichini, M. Field efficacy of a zinc-copper-hydracid of citric acid biocomplex compound to reduce oozing from winter cankers caused by Pseudomonas syringae pv. Actinidiae to Actinidia spp. J. Plant Pathol. 2016, 98, 651–655. [Google Scholar] [CrossRef]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Sarrou, E.; Karamanoli, K.; Lazaridou, A.; Martens, S.; Molassiotis, A. Sweet cherry fruit cracking: Follow-up testing methods and cultivar-metabolic screening. Plant Methods 2020, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.V. Cracking in Cherries: III. Determination of Cracking Susceptibility. Acta Agric. Scand. 1972, 22, 128–136. [Google Scholar] [CrossRef]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Sarrou, E.; Stavridou, E.; Ganopoulos, I.; Karamanoli, K.; Madesis, P.; Martens, S.; Molassiotis, A. An integrated metabolomic and gene expression analysis identifies heat and calcium metabolic networks underlying postharvest sweet cherry fruit senescence. Planta 2019, 250, 2009–2022. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Karamanoli, K.; Lazaridou, A.; Matsi, T.; Molassiotis, A. Metabolomic and physico-chemical approach unravel dynamic regulation of calcium in sweet cherry fruit physiology. Plant Physiol. Biochem. 2017, 116, 68–79. [Google Scholar] [CrossRef]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Sarrou, E.; Adamakis, I.D.; Karamanoli, K.; Martens, S.; Molassiotis, A. Metabolic mechanisms underpinning vegetative bud dormancy release and shoot development in sweet cherry. Environ. Exp. Bot. 2018, 155, 1–11. [Google Scholar] [CrossRef]
- Asami, D.K.; Hong, Y.J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef]
- Obied, H.K.; Allen, M.S.; Bedgood, D.R.; Prenzler, P.D.; Robards, K.; Stockmann, R. Bioactivity and analysis of biophenols recovered from olive mill waste. J. Agric. Food Chem. 2005, 53, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, M.; Karagiannis, E.; Polychroniadou, C.; Tanou, G.; Karamanoli, K.; Molassiotis, A. Metabolic features underlying the response of sweet cherry fruit to postharvest UV-C irradiation. Plant Physiol. Biochem. 2019, 144, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, E.; Michailidis, M.; Karamanoli, K.; Lazaridou, A.; Minas, I.S.; Molassiotis, A. Postharvest responses of sweet cherry fruit and stem tissues revealed by metabolomic profiling. Plant Physiol. Biochem. 2018, 127, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Hummel, J.; Strehmel, N.; Selbig, J.; Walther, D.; Kopka, J. Decision tree supported substructure prediction of metabolites from GC-MS profiles. Metabolomics 2010, 6, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Samiotaki, M.; Tsiolas, G.; Sarrou, E.; Stamatakis, G.; Ganopoulos, I.; Martens, S.; Argiriou, A.; et al. Novel insights into the calcium action in cherry fruit development revealed by high-throughput mapping. Plant Mol. Biol. 2020, 104, 597–614. [Google Scholar] [CrossRef]
- Luo, H.; Dai, S.J.; Ren, J.; Zhang, C.X.; Ding, Y.; Li, Z.; Sun, Y.; Ji, K.; Wang, Y.; Li, Q.; et al. The Role of ABA in the Maturation and Postharvest Life of a Nonclimacteric Sweet Cherry Fruit. J. Plant Growth Regul. 2014, 33, 373–383. [Google Scholar] [CrossRef]
- Falchi, R.; D’Agostin, E.; Mattiello, A.; Coronica, L.; Spinelli, F.; Costa, G.; Vizzotto, G. ABA regulation of calcium-related genes and bitter pit in apple. Postharvest Biol. Technol. 2017, 132, 1–6. [Google Scholar] [CrossRef]
- Martins, V.; Garcia, A.; Costa, C.; Sottomayor, M.; Gerós, H. Calcium- and hormone-driven regulation of secondary metabolism and cell wall enzymes in grape berry cells. J. Plant Physiol. 2018, 231, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Knight, H.; Trewavas, A.J.; Knight, M.R. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 1997, 12, 1067–1078. [Google Scholar] [CrossRef]
- Knight, H. Calcium signaling during abiotic stress in plants. Int. Rev. Cytol. 1999, 195, 269–324. [Google Scholar] [CrossRef]
- Knight, H.; Brandt, S.; Knight, M.R. A history of stress alters drought calcium signalling pathways in Arabidopsis. Plant J. 1998, 16, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Tanou, G.; Minas, I.S.; Karagiannis, E.; Tsikou, D.; Audebert, S.; Papadopoulou, K.K.; Molassiotis, A. The impact of sodium nitroprusside and ozone in kiwifruit ripening physiology: A combined gene and protein expression profiling approach. Ann. Bot. 2015, 116, 649–662. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jajo, A.; Rahim, M.A.; Serra, S.; Gagliardi, F.; Jajo, N.K.; Musacchi, S.; Costa, G.; Bonghi, C.; Trainotti, L. Impact of tree training system, branch type and position in the canopy on the ripening homogeneity of ȁabbé fétel’ pear fruit. Tree Genet. Genomes 2014, 10, 1477–1488. [Google Scholar] [CrossRef]
- Brüggenwirth, M.; Winkler, A.; Knoche, M. Xylem, phloem, and transpiration flows in developing sweet cherry fruit. Trees-Struct. Funct. 2016, 30, 1821–1830. [Google Scholar] [CrossRef]
- Brummell, D.S. Fruit growth, ripening and post-harvest physiology. In Plants in Action: Adaptation in Nature, Performance in Cultivation; Atwell, B.J., Kriedemann, P.E., Turnbull, C.G., Eds.; Macmillan Education AU: New York, NY, USA, 1999; p. 650. [Google Scholar]
- Grimm, E.; Pflugfelder, D.; van Dusschoten, D.; Winkler, A.; Knoche, M. Physical rupture of the xylem in developing sweet cherry fruit causes progressive decline in xylem sap inflow rate. Planta 2017, 246, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.V. Cracking in Cherries. VI. Cracking Susceptibility in Relation to the Growth Rhythm of the Fruit. Acta Agric. Scand. 1973, 23, 52–54. [Google Scholar] [CrossRef]
- Teribia, N.; Tijero, V.; Munné-Bosch, S. Linking hormonal profiles with variations in sugar and anthocyanin contents during the natural development and ripening of sweet cherries. N. Biotechnol. 2016, 33, 824–833. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, X.; Zhao, K.; Ben, Y.; Guo, X.; Zhang, X.; Li, T. Expression Analysis of Anthocyanin Biosynthetic Genes in Different Colored Sweet Cherries (Prunus avium L.) During Fruit Development. J. Plant Growth Regul. 2013, 32, 901–907. [Google Scholar] [CrossRef]
- Park, S.J. Dry weight and carbohydrate distribution in different tree parts as affected by various fruit-loads of young persimmon and their effect on new growth in the next season. Sci. Hortic. 2011, 130, 732–736. [Google Scholar] [CrossRef]
- Giné-Bordonaba, J.; Echeverria, G.; Ubach, D.; Aguiló-Aguayo, I.; López, M.L.; Larrigaudière, C. Biochemical and physiological changes during fruit development and ripening of two sweet cherry varieties with different levels of cracking tolerance. Plant Physiol. Biochem. 2017, 111, 216–225. [Google Scholar] [CrossRef]
- Lewallen, K.S.; Marini, R.P. Relationship between Flesh Firmness and Ground Color in Peach as Influenced by Light and Canopy Position. J. Am. Soc. Hortic. Sci. 2003, 128, 163–170. [Google Scholar] [CrossRef]
- Rios, J.C.; Robledo, F.; Schreiber, L.; Zeisler, V.; Lang, E.; Carrasco, B.; Silva, H. Association between the concentration of n-alkanes and tolerance to cracking in commercial varieties of sweet cherry fruits. Sci. Hortic. 2015, 197, 57–65. [Google Scholar] [CrossRef]
- Moing, A.; Renaud, C.; Christmann, H.; Fouilhaux, L.; Tauzin, Y.; Zanetto, A.; Gaudillère, M.; Laigret, F.; Claverie, J. Is There a Relation between Changes in Osmolarity of Cherry Fruit Flesh or Skin and Fruit Cracking Susceptibility? J. Am. Soc. Hortic. Sci. 2019, 129, 635–641. [Google Scholar] [CrossRef]
- Durán-Soria, S.; Pott, D.M.; Osorio, S.; Vallarino, J.G. Sugar Signaling During Fruit Ripening. Front. Plant Sci. 2020, 11, 1329. [Google Scholar] [CrossRef] [PubMed]
- Basanta, M.F.; Ponce, N.M.A.; Salum, M.L.; Raffo, M.D.; Vicente, A.R.; Erra-Balsells, R.; Stortz, C.A. Compositional changes in cell wall polysaccharides from five sweet cherry (Prunus avium L.) cultivars during on-tree ripening. J. Agric. Food Chem. 2014, 62, 12418–12427. [Google Scholar] [CrossRef] [PubMed]
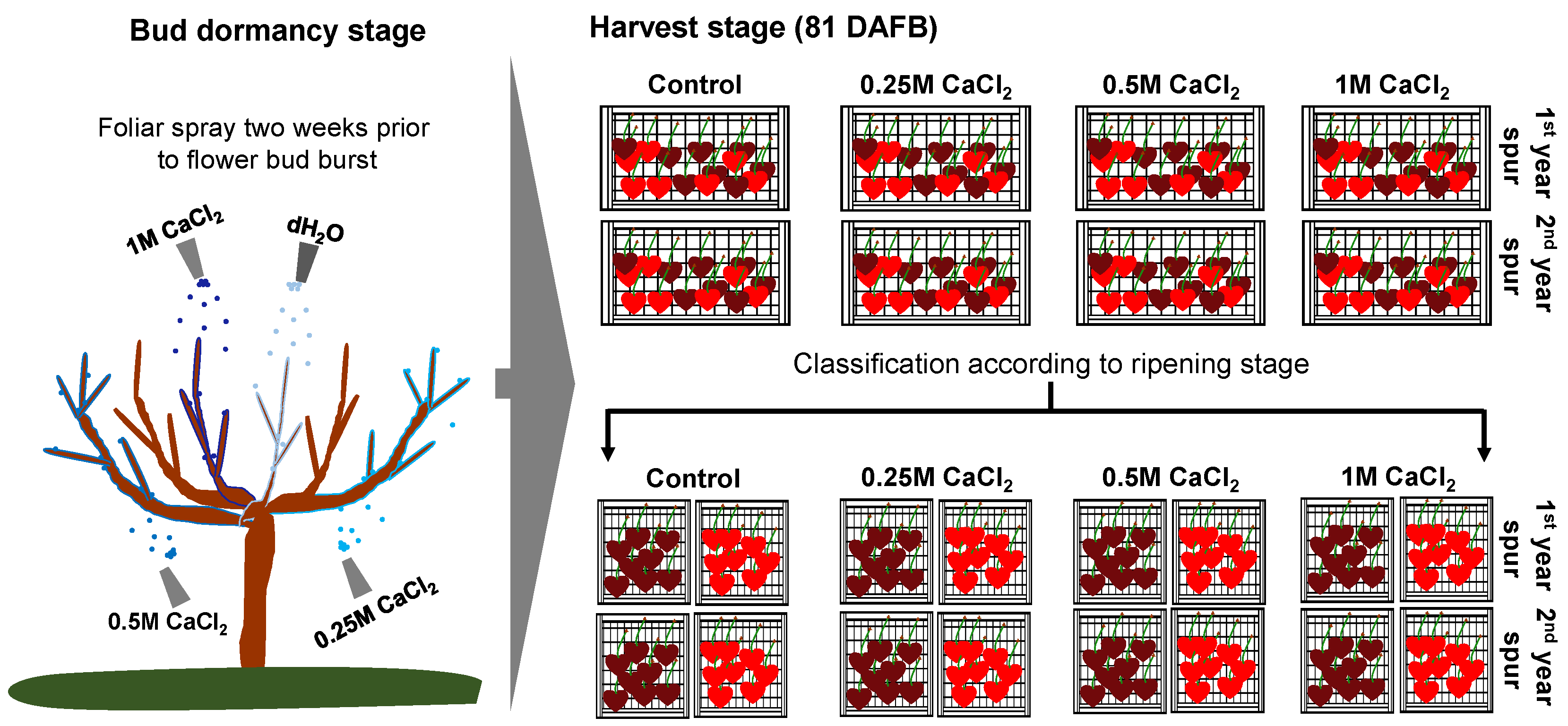
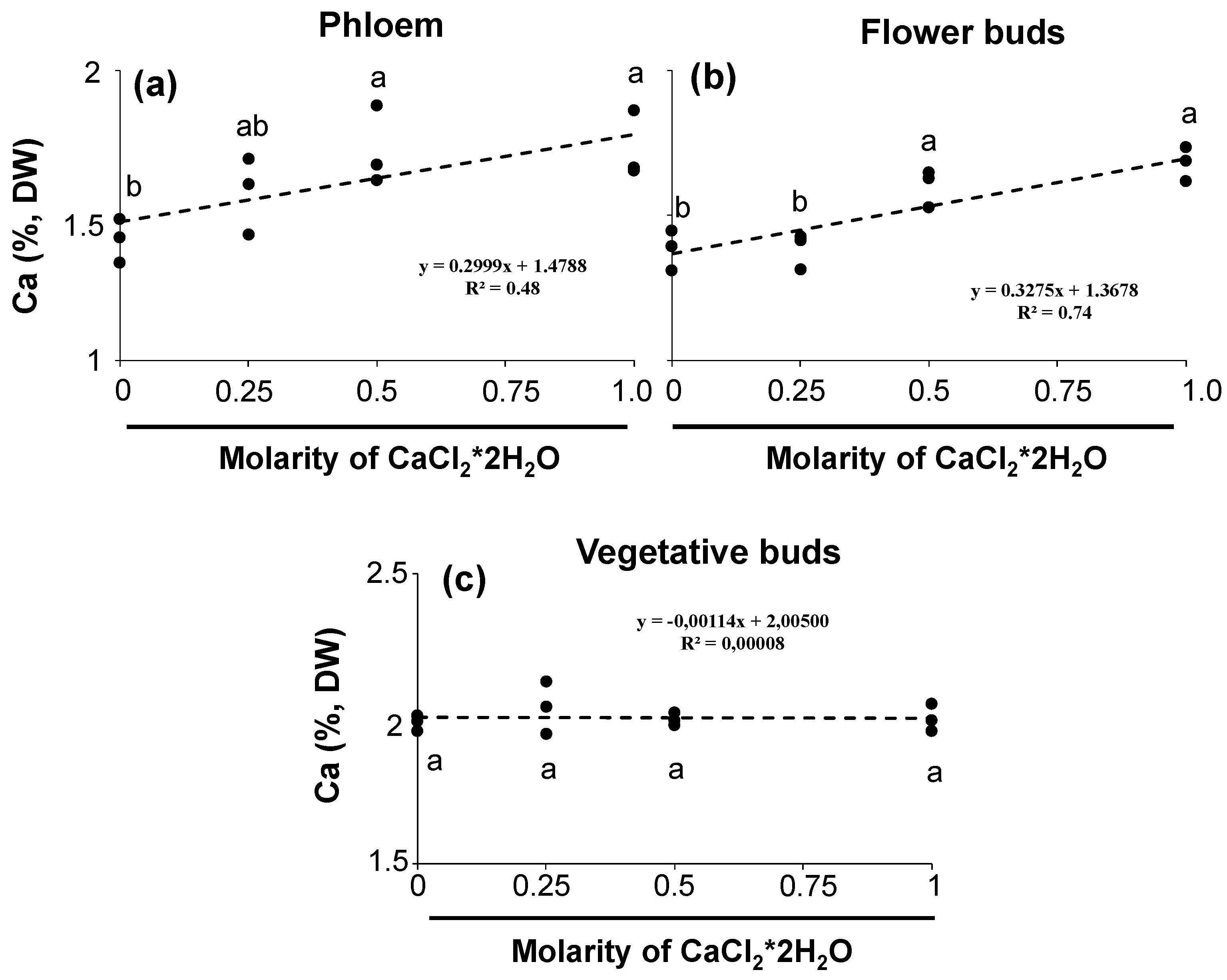
| Fruit Set (%) | On-Tree Fruit Cracking at Harvest (%) | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Treatment (T) | Control | 11.56 a | 6.24 | 28.71 a | 8.61 | ||
| 0.25M CaCl2 | 16.01 b | 8.92 | 23.84 ab | 11.29 | |||
| 0.5M CaCl2 | 14.55 ab | 5.76 | 21.18 b | 5.91 | |||
| 1M CaCl2 | 15.09 b | 4.49 | 19.09 b | 6.04 | |||
| Age of spurs (AS) | 1st | 14.54 | 6.14 | 25.86 | 9.07 | ||
| 2nd | 14.06 | 7.21 | 20.55 | 7.87 | |||
| Trees (Tr) | 1st | 16.91 a | 8.64 | Ripening (R) | Red | 21.48 | 7.29 |
| 2nd | 16.8 a | 3.82 | Black | 24.93 | 9.97 | ||
| 3rd | 13.23 b | 5.11 | |||||
| 4th | 10.27 b | 6.02 | |||||
| Factors | p-value | N | Factors | p-value | N | ||
| T | 0.045 | * | 24 | T | 0.002 | ** | 16 |
| AS | 0.68 | 48 | AS | 0.003 | ** | 32 | |
| Tr | <0.001 | *** | 24 | R | 0.049 | * | 32 |
| T × AS | 0.459 | 12 | T × AS | 0.009 | ** | 8 | |
| T × Tr | 0.007 | ** | 6 | T × R | 0.027 | * | 8 |
| AS × Tr | 0.337 | 12 | AS × R | 0.12 | 16 | ||
| T × AS × Tr | 0.59 | 3 | T × AS × R | 0.969 | 4 | ||
| Calcium (Ca, % DW) | Zinc (Zn, ppm) | Copper (Cu, ppm) | Manganese (Mn, ppm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Treatment (T) | Control | 0.09 | 0.01 | 5.69 a | 0.98 | 4.04 a | 0.5 | 3.26 a | 0.43 |
| 0.25M CaCl2 | 0.1 | 0.02 | 5.86 a | 1 | 3.7 ab | 0.82 | 2.81 b | 0.42 | |
| 0.5M CaCl2 | 0.1 | 0.01 | 5.32 ab | 0.65 | 3.27 b | 0.75 | 3.03 ab | 0.49 | |
| 1M CaCl2 | 0.1 | 0.01 | 4.87 b | 1.09 | 3.2 b | 0.69 | 2.98 b | 0.37 | |
| Age of spurs (AS) | 1st | 0.1 | 0.01 | 5.56 | 1.04 | 3.66 | 0.77 | 2.91 | 0.45 |
| 2nd | 0.1 | 0.01 | 5.31 | 0.94 | 3.45 | 0.75 | 3.13 | 0.43 | |
| Ripening (R) | Red | 0.1 | 0.01 | 5.93 | 1 | 3.68 | 0.78 | 3.31 | 0.34 |
| Black | 0.09 | 0.01 | 4.94 | 0.71 | 3.42 | 0.73 | 2.73 | 0.34 | |
| Factors | p-value | N | p-value | N | p-value | N | p-value | N | |
| T | 0.263 | 12 | 0.026 | 12 | 0.009 | 12 | 0.013 | 12 | |
| AS | 0.145 | 24 | 0.299 | 24 | 0.266 | 24 | 0.019 | 24 | |
| R | <0.001 | 24 | <0.001 | 24 | 0.18 | 24 | <0.001 | 24 | |
| T × AS | 0.882 | 6 | 0.67 | 6 | 0.009 | 6 | 0.416 | 6 | |
| T × R | 0.035 | 6 | 0.546 | 6 | 0.677 | 6 | 0.708 | 6 | |
| AS × R | 0.54 | 12 | 0.158 | 12 | 0.464 | 12 | 0.759 | 12 | |
| T × AS × R | 0.993 | 3 | 0.426 | 3 | 0.721 | 3 | 0.921 | 3 | |
| TSS (%, Brix) | TA (%, Malic Acid) | Dry Weight (%) | Respiration Rate (mL CO2 kg−1 h−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Treatment (T) | Control | 15.75b | 0.69 | 0.71 a | 0.02 | 15.47 ab | 1.2 | 41.13 c | 3.11 |
| 0.25M CaCl2 | 15.91b | 0.41 | 0.68 ab | 0.03 | 14.94 b | 1.85 | 46.62 b | 2.31 | |
| 0.5M CaCl2 | 16.2a | 0.33 | 0.65 c | 0.03 | 15.99 a | 0.8 | 46.72 b | 3.1 | |
| 1M CaCl2 | 15.96ab | 0.31 | 0.66 bc | 0.04 | 15.68 a | 0.41 | 53.1 a | 2.62 | |
| Age of spurs (AS) | 1st | 15.95 | 0.56 | 0.68 | 0.04 | 15.67 | 1.35 | 45.11 | 4.95 |
| 2nd | 15.95 | 0.38 | 0.67 | 0.03 | 15.37 | 1.07 | 48.67 | 4.62 | |
| Ripening (R) | Red | 16.08 | 0.28 | 0.68 | 0.03 | 14.77 | 1.15 | 47.73 | 4.93 |
| Black | 15.83 | 0.59 | 0.67 | 0.04 | 16.27 | 0.72 | 46.05 | 5.17 | |
| Factors | p-value | N | p-value | N | p-value | N | p-value | N | |
| T | 0.022 | 12 | <0.001 | 12 | 0.024 | 12 | <0.001 | 12 | |
| AS | 1 | 24 | 0.592 | 24 | 0.216 | 24 | <0.001 | 24 | |
| R | 0.014 | 24 | 0.176 | 24 | <0.001 | 24 | 0.007 | 24 | |
| T × AS | 0.026 | 6 | 0.115 | 6 | 0.928 | 6 | 0.984 | 6 | |
| T × R | <0.001 | 6 | 0.432 | 6 | 0.013 | 6 | 0.393 | 6 | |
| AS × R | 0.127 | 12 | 0.114 | 12 | 0.031 | 12 | 0.449 | 12 | |
| T × AS × R | 0.038 | 3 | 0.079 | 3 | 0.289 | 3 | 0.26 | 3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michailidis, M.; Polychroniadou, C.; Kosmidou, M.-A.; Petraki-Katsoulaki, D.; Karagiannis, E.; Molassiotis, A.; Tanou, G. An Early Calcium Loading during Cherry Tree Dormancy Improves Fruit Quality Features at Harvest. Horticulturae 2021, 7, 135. https://doi.org/10.3390/horticulturae7060135
Michailidis M, Polychroniadou C, Kosmidou M-A, Petraki-Katsoulaki D, Karagiannis E, Molassiotis A, Tanou G. An Early Calcium Loading during Cherry Tree Dormancy Improves Fruit Quality Features at Harvest. Horticulturae. 2021; 7(6):135. https://doi.org/10.3390/horticulturae7060135
Chicago/Turabian StyleMichailidis, Michail, Chrysanthi Polychroniadou, Maria-Anastasia Kosmidou, Dafni Petraki-Katsoulaki, Evangelos Karagiannis, Athanassios Molassiotis, and Georgia Tanou. 2021. "An Early Calcium Loading during Cherry Tree Dormancy Improves Fruit Quality Features at Harvest" Horticulturae 7, no. 6: 135. https://doi.org/10.3390/horticulturae7060135
APA StyleMichailidis, M., Polychroniadou, C., Kosmidou, M.-A., Petraki-Katsoulaki, D., Karagiannis, E., Molassiotis, A., & Tanou, G. (2021). An Early Calcium Loading during Cherry Tree Dormancy Improves Fruit Quality Features at Harvest. Horticulturae, 7(6), 135. https://doi.org/10.3390/horticulturae7060135









