Seaweed Extract Improves Lagenaria siceraria Young Shoot Production, Mineral Profile and Functional Quality
Abstract
:1. Introduction
2. Materials and Methods
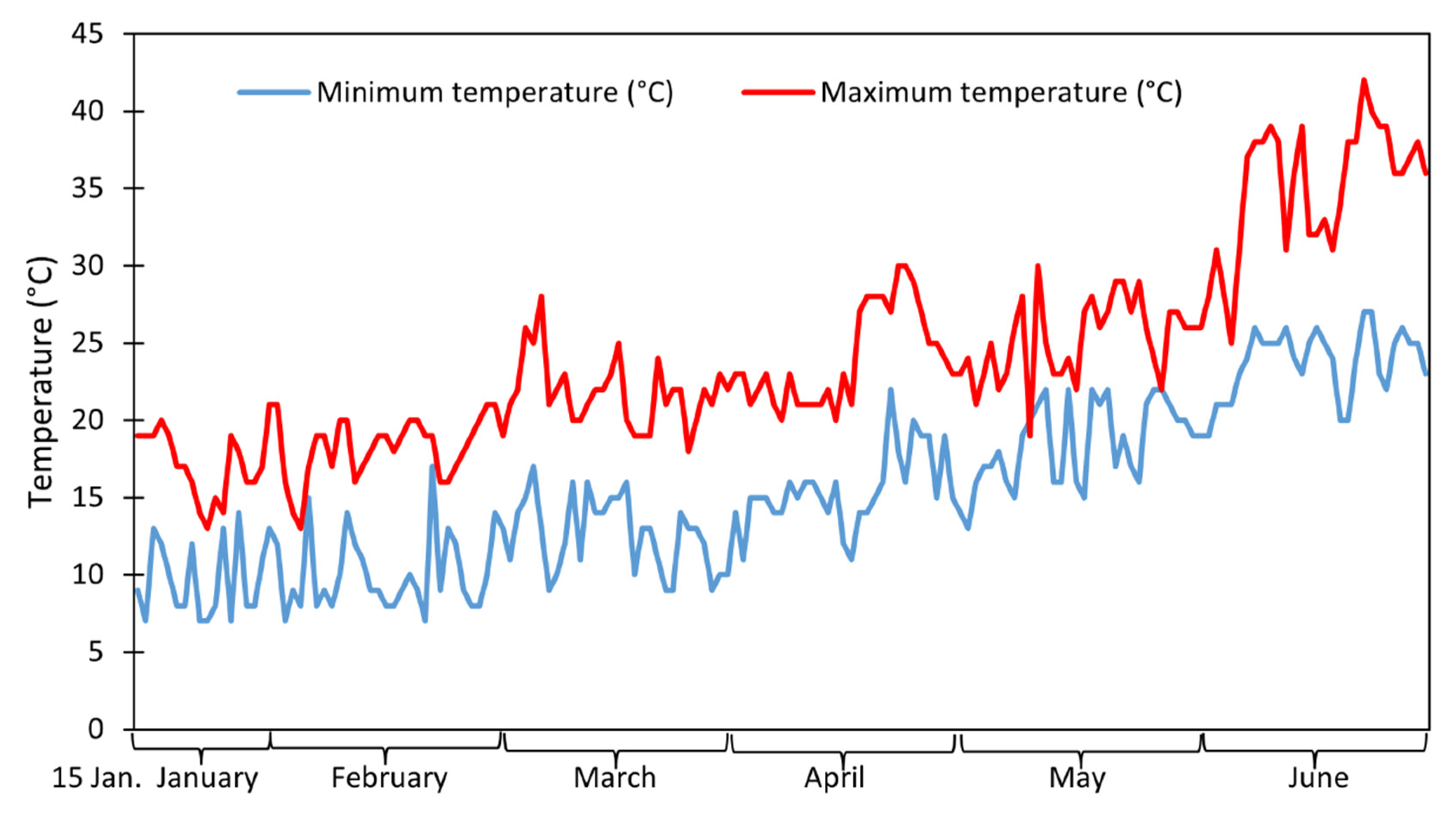
2.1. Experimental Field and Treatments
2.2. Plant Growth, Fruit Yield and Firmness
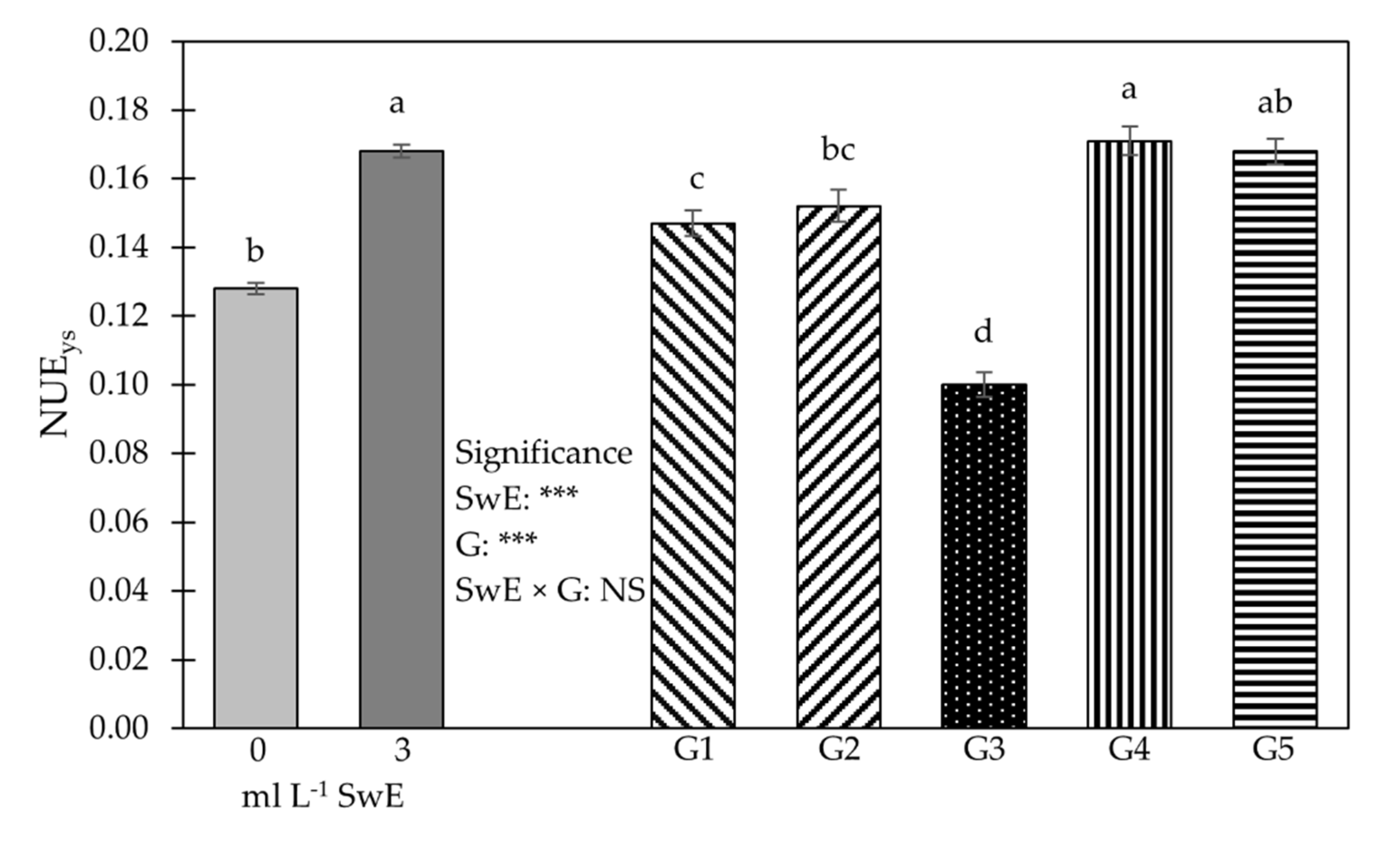
2.3. Young Shoot Yield, Nutritional and Functional Components and NUEys
2.4. Statistics
3. Results
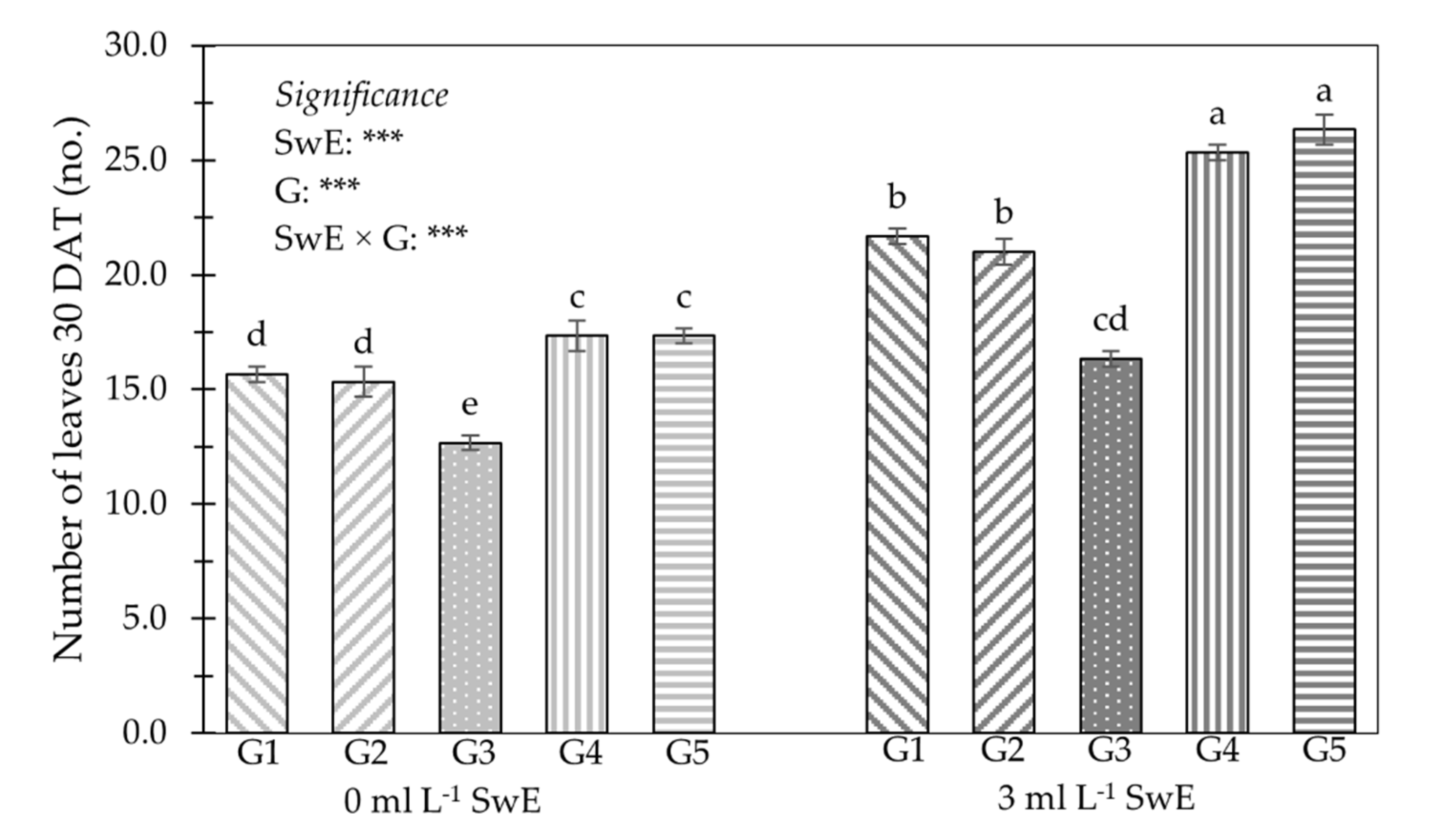
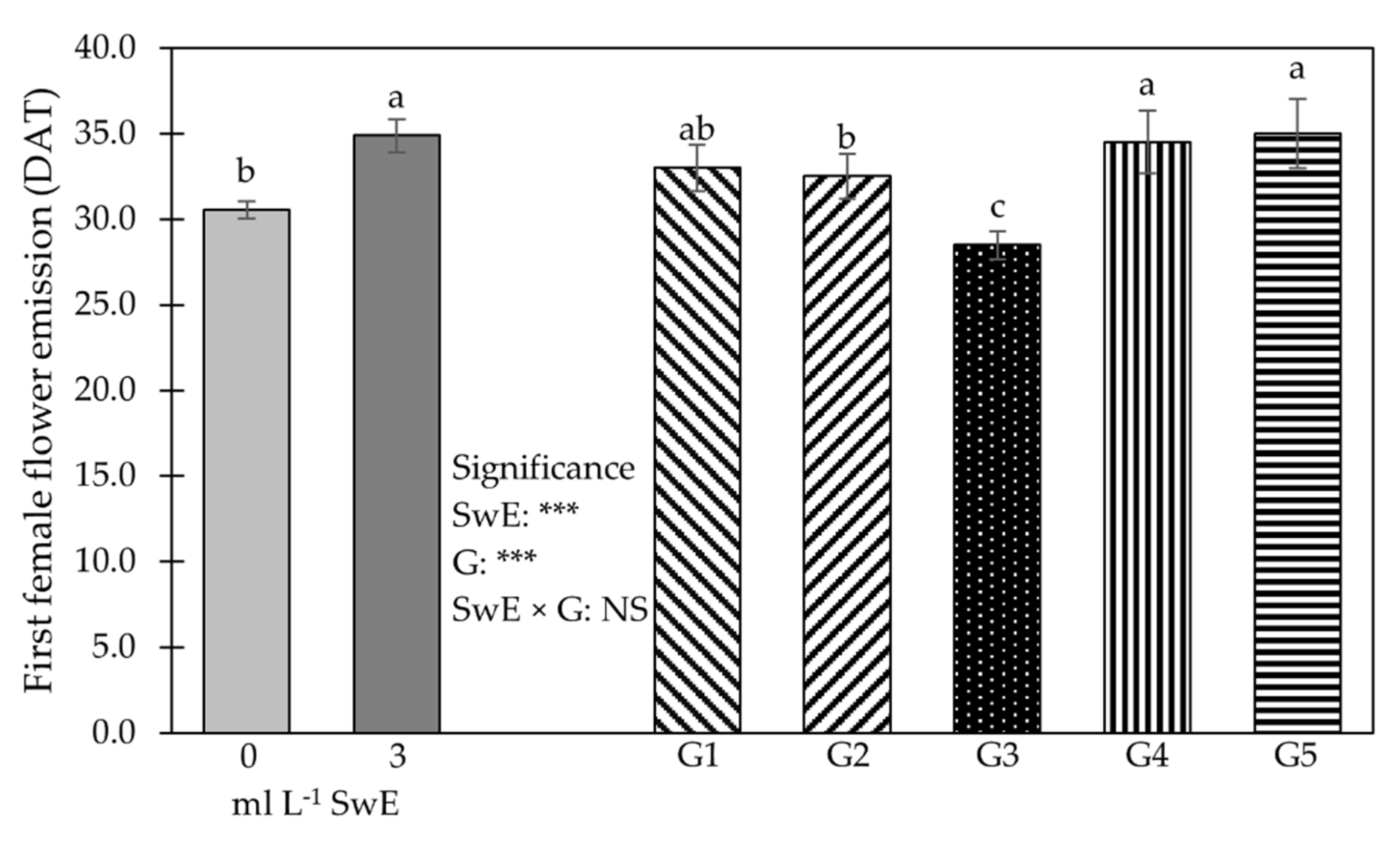
3.1. Plant Growth Traits, Production Features, NUE and Fruit Firmness
3.2. Young Shoot Nutritional Properties, Mineral Profile and Functional Components
3.3. Heat Map Analysis of the Whole Data Set
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Choudhary, B.L.; Katewa, S.S.; Galav, P.K. Plants in material culture of tribals and rural communities of Rajsamand district of Rajasthan. Indian J. Trad. Knowl. 2008, 7, 11–22. [Google Scholar]
- Ghule, B.V.; Ghante, M.H.; Upaganlawar, A.B.; Yeole, P.G. Analgesic and anti-inflammatory activities of Lagenaria siceraria Stand. fruit juice extract in rats and mice. Pharmacogn. Mag. 2006, 2, 232–238. [Google Scholar]
- Ghule, B.V.; Ghante, M.H.; Yeole, P.G.; Saoji, A.N. Diuretic activity of Lagenaria siceraria fruit extracts in rats. Indian J. Pharm. Sci. 2007, 69, 817–819. [Google Scholar] [CrossRef]
- Ghule, B.V.; Ghante, M.H.; Saoji, A.N.; Yeole, P.G. Antihyperlipidemic effect of the methanolic extract from Lagenaria siceraria Stand. fruit in hyperlipidemic rats. J. Ethnopharmacol. 2009, 124, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, F.M.; Bazan, G.; Troia, A. Taxa a rischio nella flora vascolare della Sicilia. Biogeogr.-J. Integr. Biogeogr. 2011, 30. [Google Scholar] [CrossRef] [Green Version]
- D’Anna, F.; Sabatino, L. Morphological and agronomical haracterisation of eggplant genetic resources from the Sicily area. J. Food Agric. Environ. 2013, 11, 401–404. [Google Scholar]
- Sabatino, L.; Palazzolo, E.; D’Anna, F. Grafting suitability of Sicilian eggplant ecotypes onto Solanum torvum: Fruit composition, production and phenology. J. Food Agric. Environ. 2013, 11, 1195–1200. [Google Scholar]
- Sabatino, L.; Iapichino, G.; Vetrano, F.; D’Anna, F. Morphological and agronomical characterisation of Sicilian bottle gourd Lagenaria siceraria (Mol.) Standley. J. Food Agric. Environ. 2014, 12, 587–590. [Google Scholar]
- Sabatino, L.; Iapichino, G.; Maggio, A.; D’anna, E.; Bruno, M.; D’Anna, F. Grafting affects yield and phenolic profile of Solanum melongena L. landraces. J. Integr. Agric. 2016, 15, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Virga, G.; Licata, M.; Consentino, B.B.; Tuttolomondo, T.; Sabatino, L.; Leto, C.; La Bella, S. Agro-morphological characterization of sicilian chili pepper accessions for ornamental purposes. Plants 2020, 9, 1400. [Google Scholar] [CrossRef]
- Mauro, R.; Portis, E.; Acquadro, A.; Lombardo, S.; Mauromicale, G.; Lanteri, S. Genetic diversity of globe artichoke landraces from Sicilian small-holdings: Implications for evolution and domestication of the species. Conserv. Genet. 2009, 10, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Kyriacou, M.C.; Colla, G. Vegetable grafting: A toolbox for securing yield stability under multiple stress conditions. Front. Plant Sci. 2018, 8, 2255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauro, R.P.; Agnello, M.; Distefano, M.; Sabatino, L.; San Bautista Primo, A.; Leonardi, C.; Giuffrida, F. Chlorophyll fluorescence, photosynthesis and growth of tomato plants as affected by long-term oxygen root zone deprivation and grafting. Agronomy 2020, 10, 137. [Google Scholar] [CrossRef] [Green Version]
- Expósito, A.; Pujolà, M.; Achaerandio, I.; Giné, A.; Escudero, N.; Fullana, A.M.; Conquero, M.; Loza-Alvarez, P.; Sorribas, F.J. Tomato and melon Meloidogyne resistant rootstocks improve crop yield but melon fruit quality is influenced by the cropping season. Front. Plant Sci. 2020, 11, 560024. [Google Scholar] [CrossRef]
- Lee, J.M.; Oda, M. Grafting of herbaceous vegetable and ornamental crops. Hortic. Rev. 2003, 28, 61–124. [Google Scholar]
- Karaca, F.; Yetişir, H.; Solmaz, İ.; Candir, E.; Kurt, Ş.; Sari, N.; Güler, Z. Rootstock potential of Turkish Lagenaria siceraria germplasm for watermelon: Plant growth, yield and quality. Turk. J. Agric. For. 2012, 36, 167–177. [Google Scholar]
- Koike, S.T.; Gladders, P.; Paulus, A.O. Vegetable Diseases: A Color Handbook; Gulf Professional Publishing/Manson Publishing Ltd.: London, UK, 2007; pp. 220–228. [Google Scholar]
- Colla, G.; Rouphael, Y. Biostimulants in Horticulture; Elsevier B.V.: Amsterdam, The Netherlands, 2015; pp. 1–134. [Google Scholar]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Consentino, B.B.; Virga, G.; La Placa, G.G.; Sabatino, L.; Rouphael, Y.; Ntatsi, G.; Iapichino, G.; La Bella, S.; Mauro, R.P.; D’Anna, F.; et al. Celery (Apium graveolens L.) Performances as Subjected to Different Sources of Protein Hydrolysates. Plants 2020, 9, 1633. [Google Scholar] [CrossRef]
- Sabatino, L.; Iapichino, G.; Consentino, B.B.; D’Anna, F.; Rouphael, Y. Rootstock and Arbuscular Mycorrhiza Combinatorial Effects on Eggplant Crop Performance and Fruit Quality under Greenhouse Conditions. Agronomy 2020, 10, 693. [Google Scholar] [CrossRef]
- Sabatino, L.; Consentino, B.B.; Rouphael, Y.; De Pasquale, C.; Iapichino, G.; D’Anna, F.; La Bella, S. Protein Hydrolysates and Mo-Biofortification Interactively Modulate Plant Performance and Quality of ‘Canasta’ Lettuce Grown in a Protected Environment. Agronomy 2021, 11, 1023. [Google Scholar] [CrossRef]
- La Bella, S.; Consentino, B.B.; Rouphael, Y.; Ntatsi, G.; De Pasquale, C.; Iapichino, G.; Sabatino, L. Impact of Ecklonia maxima Seaweed Extract and Mo Foliar Treatments on Biofortification, Spinach Yield, Quality and NUE. Plants 2021, 10, 1139. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, I.; Conti, S.; Cozzolino, E.; Melchionna, G.; Ottaiano, L.; Testa, A.; Sabatino, L.; Rouphael, Y.; Mori, M. Plant-Based Protein Hydrolysate Improves Salinity Tolerance in Hemp: Agronomical and Physiological Aspects. Agronomy 2021, 11, 342. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [Green Version]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Branca, F.; La Malfa, G. Traditional vegetables of Sicily. Chron. Horticult. 2008, 48, 20–25. [Google Scholar]
- Richards, L.A. Diagnosis and improvement of saline and alkali soils. In Agricultural Hand Book; U.S. Dept. of Agriculture: Washington, DC, USA, 1954; p. 160. [Google Scholar]
- Baiamonte, G.; Crescimanno, G.; Parrino, F.; De Pasquale, C. Effect of biochar on the physical and structural properties of a sandy soil. Catena 2019, 175, 294–303. [Google Scholar] [CrossRef]
- Walkley, A.J.; Black, I.A. Estimation of soil organic carbon by the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Tesi, R. Orticoltura Mediterranea Sostenibile; Pàtron Editore: Bologna, Italy, 2010. [Google Scholar]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Morand, P.; Gullo, J.L. Mineralisation des tissus vegetaux en vue du dosage de P, Ca, Mg, Na, K. Ann. Agron. 1970, 21, 229–236. [Google Scholar]
- Fogg, D.N.; Wilkinson, A.N. The colorimetric determination of phosphorus. Analyst 1958, 83, 406–414. [Google Scholar] [CrossRef]
- Rouphael, Y.; De Micco, V.; Arena, C.; Raimondi, G.; Colla, G.; De Pascale, S. Effect of Ecklonia maxima seaweed extract on yield, mineral composition, gas exchange, and leaf anatomy of zucchini squash grown under saline conditions. J. Appl. Phycol. 2017, 29, 459–470. [Google Scholar] [CrossRef]
- Weiner, J.A.C.O.B. The influence of competition on plant reproduction. Plant Reprod. Ecol. Patterns Strateg. 1988, 11, 228–245. [Google Scholar]
- Ali, N.; Farrell, A.; Ramsubhag, A.; Jayaraman, J. The effect of Ascophyllum nodosum extract on the growth, yield and fruit quality of tomato grown under tropical conditions. J. Appl. Phycol. 2016, 28, 1353–1362. [Google Scholar] [CrossRef]
- Rolland, F.B.; Moore, B.; Sheen, J. Sugar sensing and signaling in plants. Plant Cell 2002, 14 (Suppl. 1), S185–S205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, H.I.; Kasinadhuni, N.; Arioli, T. The effect of seaweed extract on tomato plant growth, productivity and soil. J. Appl. Phycol. 2021, 33, 1305–1314. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ashour, M.; Soliman, A.A.; Hassanien, H.A.; Alsanie, W.F.; Gaber, A.; Elshobary, M.E. The potential of a new commercial seaweed extract in stimulating morpho-agronomic and bioactive properties of Eruca vesicaria (L.) Cav. Sustainability 2021, 13, 4485. [Google Scholar] [CrossRef]
- Stirk, W.A.; Novak, M.S.; Van Staden, J. Cytokinins in macroalgae. Plant Growth Regul. 2003, 41, 13–24. [Google Scholar] [CrossRef]
- Wally, O.S.D.; Critchley, A.T.; Hiltz, D.; Craigie, J.S.; Han, X.; Zaharia, L.I.; Abrams, S.R.; Prithiviraj, B. Regulation of phytohormone biosynthesis and accumulation in Arabidopsis following treatment with commercial extract from the marine macroalga Ascophyllum nodosum. J. Plant Growth Regul. 2013, 32, 324–339. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Nocerino, S.; Rouphael, Y.; Colla, G.; El-Nakhel, C.; Mori, M. Nitrogen Use and Uptake Efficiency and Crop Performance of Baby Spinach (Spinacia oleracea L.) and Lamb’s Lettuce (Valerianella locusta L.) Grown under Variable Sub-Optimal N Regimes Combined with Plant-Based Biostimulant Application. Agronomy 2020, 10, 278. [Google Scholar] [CrossRef] [Green Version]
- Basile, B.; Brown, N.; Valdes, J.M.; Cardarelli, M.; Scognamiglio, P.; Mataffo, A.; Rouphael, Y.; Bonini, P.; Colla, G. Plant-Based Biostimulant as Sustainable Alternative to Synthetic Growth Regulators in Two Sweet Cherry Cultivars. Plants 2021, 10, 619. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, E.; Di Mola, I.; Ottaiano, L.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Foliar application of plant-based biostimulants improve yield and upgrade qualitative characteristics of processing tomato. Ital. J. Agron. 2021, 16, 1825. [Google Scholar] [CrossRef]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit Calcium: Transport and Physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Schwarz, D.; Krumbein, A.; Colla, G. Impact of grafting on product quality of fruit vegetables. Sci Hortic. 2010, 127, 172–179. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bassal, A.; Leonardi, C.; Giuffrida, F.; Colla, G. Vegetable quality as affected by genetic, agronomic and environmental factors. J. Food Agric. Environ. 2012, 10, 680–688. [Google Scholar]
- Krouk, G.; Lacombe, B.; Bielach, A.; Perrine-Walker, F.; Malinska, K.; Mounier, E.; Hoyerova, K.; Tillard, P.; Leon, S.; Ljung, K.; et al. Nitrate-regulated auxin transport by NRT1. 1 defines a mechanism for nutrient sensing in plants. Dev. Cell 2010, 18, 927–937. [Google Scholar] [CrossRef]
- Castaings, L.; Marchive, C.; Meyer, C.; Krapp, A. Nitrogen signalling in Arabidopsis: How to obtain insights into a complex signalling network. J. Exp. Bot. 2011, 62, 1391–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Matteazzi, A.; Andreotti, C. Foliar Applications of Biostimulants Promote Growth, Yield and Fruit Quality of Strawberry Plants Grown under Nutrient Limitation. Agronomy 2019, 9, 483. [Google Scholar] [CrossRef] [Green Version]
- Di Matteo, A.; Sacco, A.; Anacleria, M.; Pezzotti, M.; Delledonne, M.; Ferrarini, A.; Frusciante, L.; Barone, A. The ascorbic acid content of tomato fruits is associated with the expression of genes involved in pectin degradation. BMC Plant Biol. 2010, 10, 163. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.; Anwar, J.; Zafar-ul-Hye, M.; Iqbal Khan, R.; Saleem, M.; Rahi, A.A.; Danish, S.; Datta, R. Effect of Seaweed Extract on Productivity and Quality Attributes of Four Onion Cultivars. Horticulturae 2020, 6, 28. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Kovalikova, Z.; Kubes, J.; Skalicky, M.; Kuchtickova, N.; Maskova, L.; Tuma, J.; Vachova, P.; Hejnak, V. Changes in Content of Polyphenols and Ascorbic Acid in Leaves of White Cabbage after Pest Infestation. Molecules 2019, 24, 2622. [Google Scholar] [CrossRef] [Green Version]
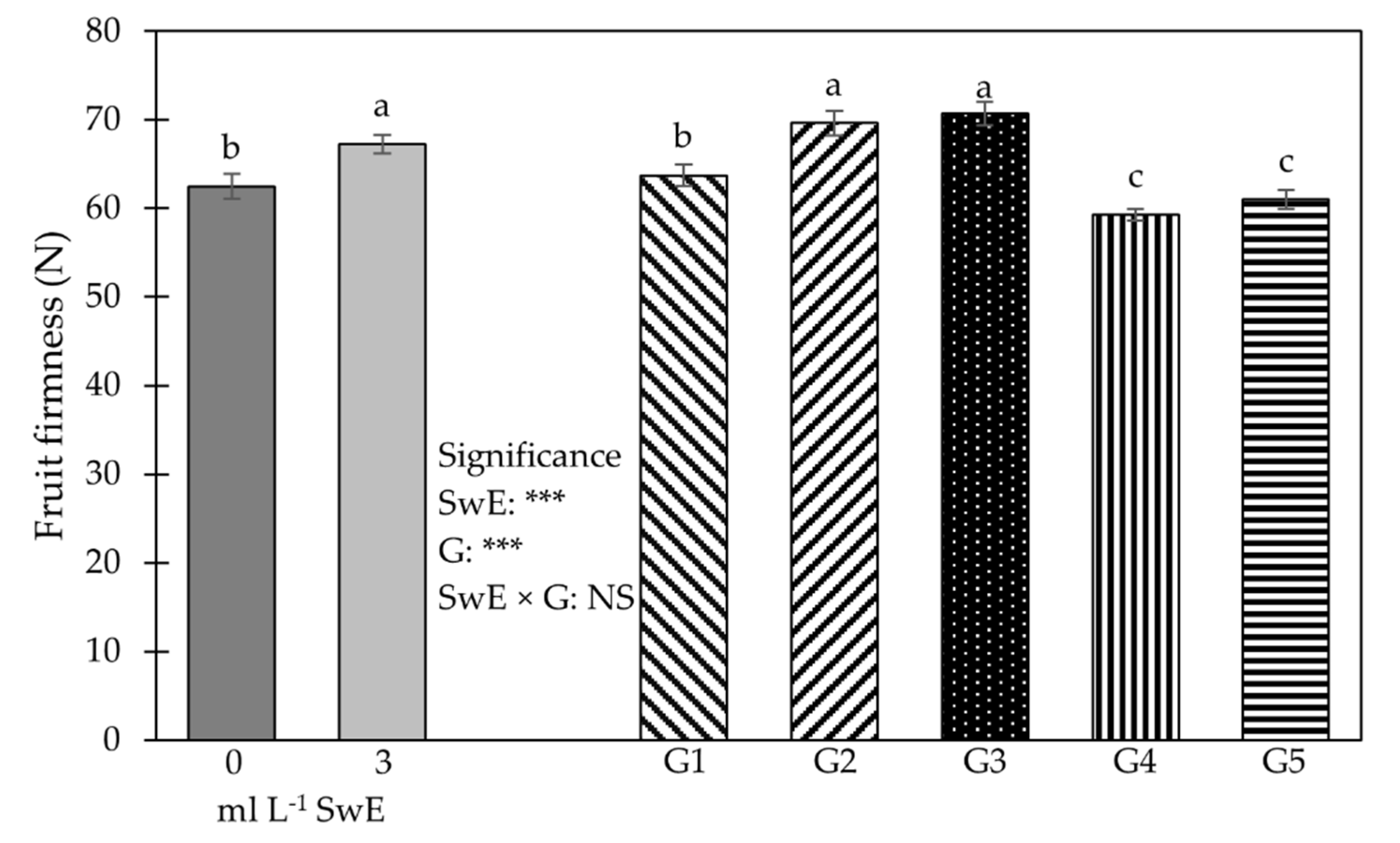


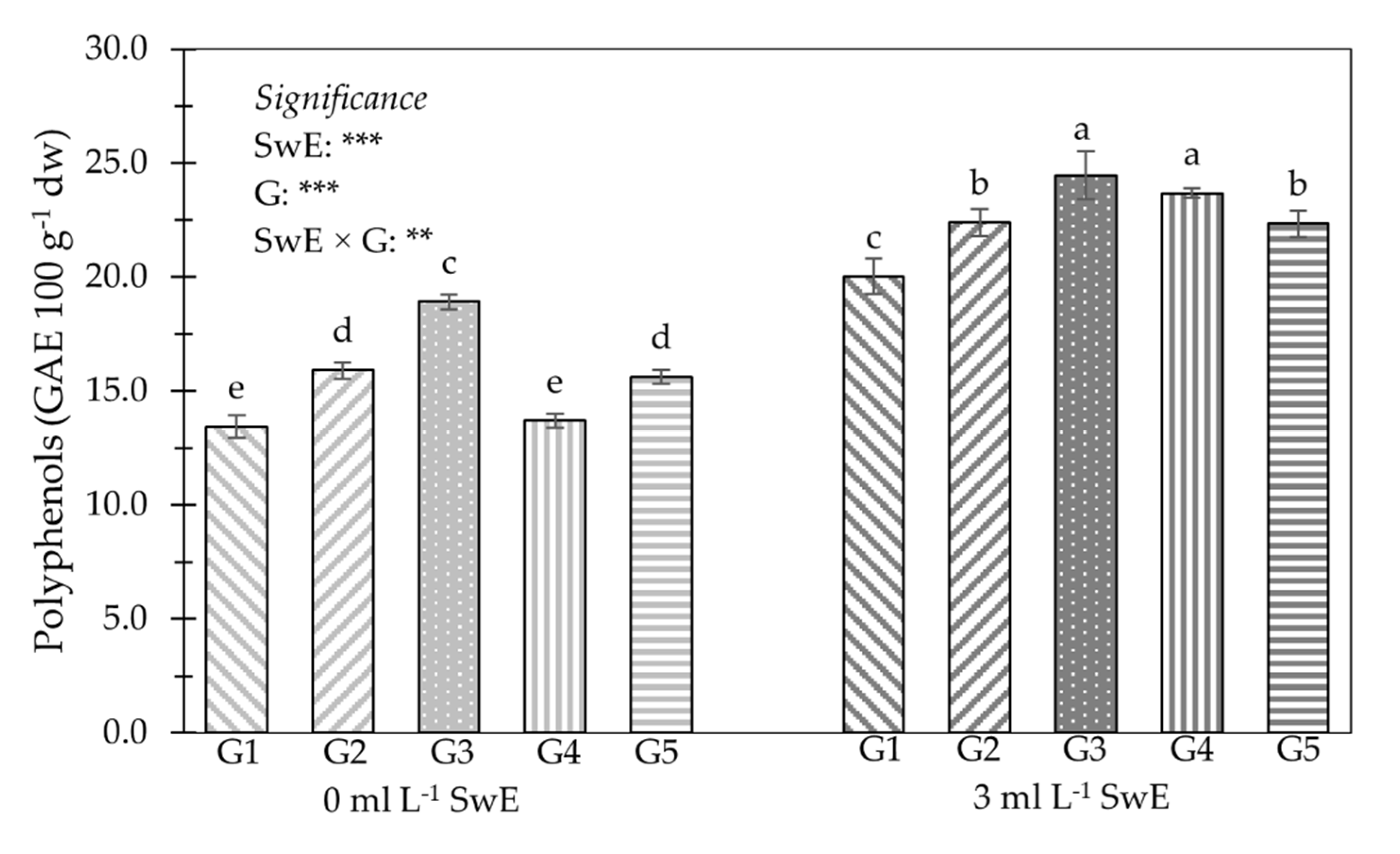
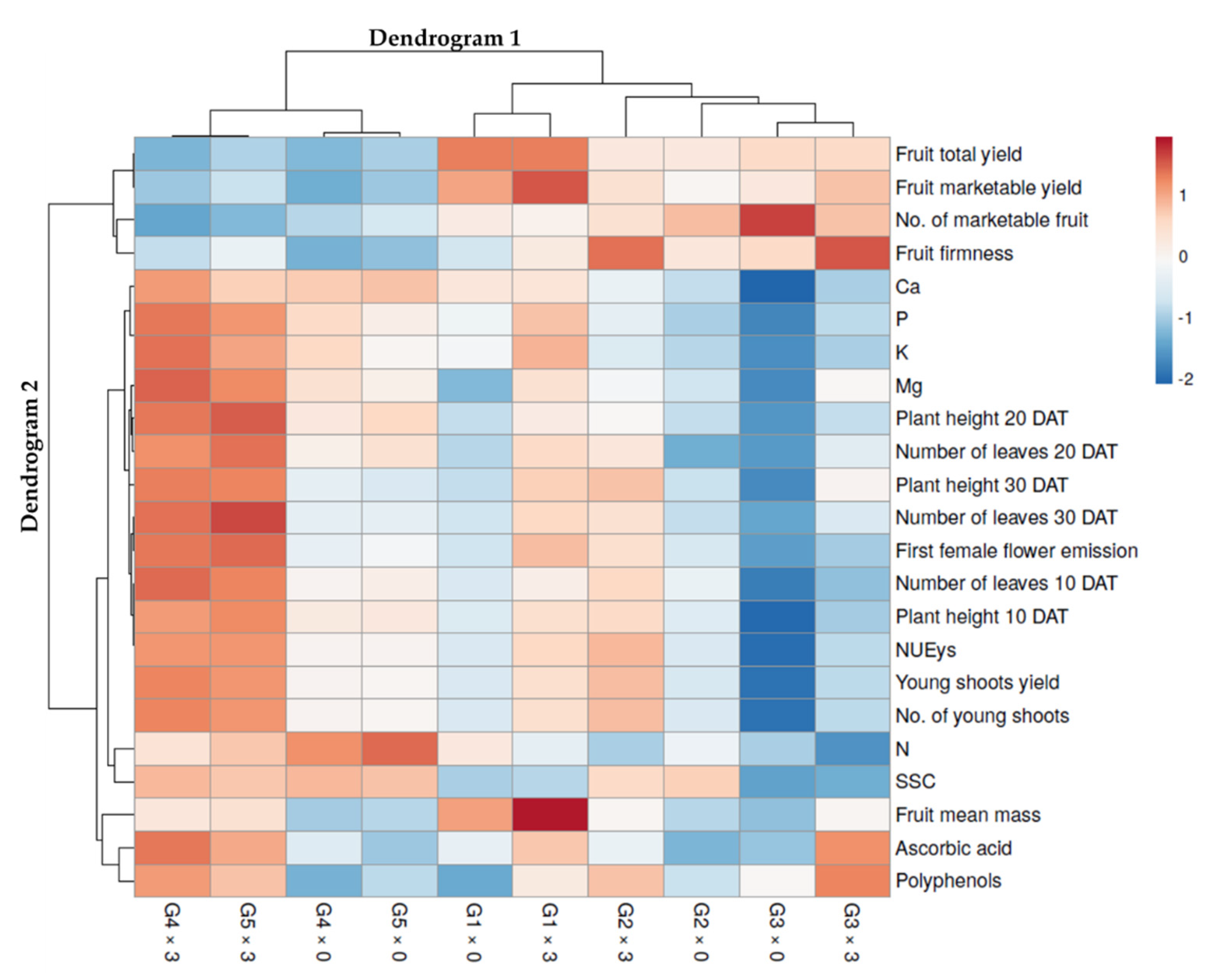
| Treatments | Plant Height 10 DAT (cm) | Plant Height 20 DAT (cm) | Plant Height 30 DAT (cm) | Number of Leaves 10 DAT (No.) | Number of Leaves 20 DAT (No.) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Seaweed extract dose (mL L−1) | ||||||||||
| 0 | 20.7 | b | 30.2 | b | 48.5 | b | 6.8 | b | 9.4 | b |
| 3 | 25.3 | a | 35.7 | a | 74.3 | a | 9.0 | a | 13.2 | a |
| Genotype | ||||||||||
| G1 | 22.9 | b | 31.1 | b | 60.4 | b | 7.5 | b | 10.8 | b |
| G2 | 23.2 | b | 30.5 | b | 61.9 | b | 8.3 | ab | 9.8 | b |
| G3 | 16.0 | c | 25.9 | c | 48.5 | c | 4.5 | c | 8.3 | c |
| G4 | 26.1 | a | 38.0 | a | 69.0 | a | 9.7 | a | 13.3 | a |
| G5 | 26.6 | a | 39.4 | a | 67.0 | a | 9.7 | a | 14.2 | a |
| Significance | ||||||||||
| SwE | *** | *** | *** | *** | *** | |||||
| G | *** | *** | *** | *** | *** | |||||
| SwE × G | NS | NS | NS | NS | NS | |||||
| Treatments | Fruits | Young Shoots | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Yield (kg plant−1) | Marketable Yield (kg plant−1) | Marketable Fruits (No. plant−1) | Mean Mass (kg) | Yield (kg plant−1) | Number (No. plant−1) | |||||||
| Seaweed extract dose (mL L−1) | ||||||||||||
| 0 | 4.86 | a | 4.0 | b | 4.5 | a | 0.9 | b | 2.04 | b | 51.0 | b |
| 3 | 4.89 | a | 4.6 | a | 4.0 | b | 1.1 | a | 2.70 | a | 67.3 | a |
| Genotype | ||||||||||||
| G1 | 6.80 | a | 6.0 | a | 4.4 | b | 1.4 | a | 2.35 | c | 58.8 | b |
| G2 | 5.30 | c | 4.6 | c | 4.9 | ab | 0.9 | b | 2.45 | bc | 61.0 | b |
| G3 | 5.67 | b | 5.0 | b | 5.6 | a | 0.9 | b | 1.60 | d | 40.2 | c |
| G4 | 3.12 | e | 2.8 | e | 3.0 | c | 1.0 | b | 2.73 | a | 68.3 | a |
| G5 | 3.50 | d | 3.1 | d | 3.2 | c | 1.0 | b | 2.70 | ab | 67.3 | b |
| Significance | ||||||||||||
| SwE | NS | *** | ** | *** | *** | *** | ||||||
| G | *** | *** | *** | *** | *** | *** | ||||||
| SwE × G | NS | NS | NS | NS | NS | NS | ||||||
| Treatments | N (g kg−1 DW) | P (g kg−1 DW) | K (g kg−1 DW) | Ca (g kg−1 DW) | Mg (g kg−1 DW) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Seaweed extract dose (mL L−1) | ||||||||||
| 0 | 1.86 | a | 5.14 | b | 19.30 | b | 25.77 | b | 9.54 | b |
| 3 | 1.74 | b | 5.30 | a | 19.75 | a | 26.00 | a | 10.00 | a |
| Genotype | ||||||||||
| G1 | 1.80 | c | 5.30 | b | 19.80 | a | 26.10 | a | 9.62 | b |
| G2 | 1.70 | d | 5.11 | c | 19.08 | b | 25.60 | b | 9.60 | b |
| G3 | 1.60 | e | 4.99 | d | 18.72 | b | 25.00 | c | 9.44 | b |
| G4 | 1.94 | b | 5.38 | a | 20.13 | a | 26.40 | a | 10.11 | a |
| G5 | 2.00 | a | 5.33 | ab | 19.82 | a | 26.30 | a | 10.02 | a |
| Significance | ||||||||||
| SwE | *** | *** | *** | *** | *** | |||||
| G | *** | *** | *** | * | *** | |||||
| SwE × G | NS | NS | NS | NS | NS | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Consentino, B.B.; Sabatino, L.; Mauro, R.P.; Nicoletto, C.; De Pasquale, C.; Iapichino, G.; La Bella, S. Seaweed Extract Improves Lagenaria siceraria Young Shoot Production, Mineral Profile and Functional Quality. Horticulturae 2021, 7, 549. https://doi.org/10.3390/horticulturae7120549
Consentino BB, Sabatino L, Mauro RP, Nicoletto C, De Pasquale C, Iapichino G, La Bella S. Seaweed Extract Improves Lagenaria siceraria Young Shoot Production, Mineral Profile and Functional Quality. Horticulturae. 2021; 7(12):549. https://doi.org/10.3390/horticulturae7120549
Chicago/Turabian StyleConsentino, Beppe Benedetto, Leo Sabatino, Rosario Paolo Mauro, Carlo Nicoletto, Claudio De Pasquale, Giovanni Iapichino, and Salvatore La Bella. 2021. "Seaweed Extract Improves Lagenaria siceraria Young Shoot Production, Mineral Profile and Functional Quality" Horticulturae 7, no. 12: 549. https://doi.org/10.3390/horticulturae7120549
APA StyleConsentino, B. B., Sabatino, L., Mauro, R. P., Nicoletto, C., De Pasquale, C., Iapichino, G., & La Bella, S. (2021). Seaweed Extract Improves Lagenaria siceraria Young Shoot Production, Mineral Profile and Functional Quality. Horticulturae, 7(12), 549. https://doi.org/10.3390/horticulturae7120549












