Analysis of Free Sugars, Organic Acids, and Fatty Acids of Wood Apple (Limonia acidissima L.) Fruit Pulp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Separation
2.2. Chemicals and Standards
2.3. Extraction of Oil from Wood Apple Fruit Pulp
2.4. Morphological and Proximate Characterization of Wood Apple Fruits
2.5. Extraction and Quantification of Total Phenolic Content
2.6. Fatty Acid Composition
Sample Preparation and Analysis of Fatty Acid Methyl Esters (FAMEs)
2.7. Sugars and Organic Acids
Sample Preparation Technique
2.8. LC Conditions for Sugars and Organic Acids
2.8.1. Investigation of Sugar Profile by UPLC-ELSD
2.8.2. Investigation of Organic Acids Profile by HPLC-PDA
2.9. Statistical Analysis
3. Results and Discussion
3.1. Morphological and Proximate Characterization of Wood Apple Fruits
3.2. Fatty Acid Composition
3.3. Investigation of Sugar Profile by UPLC-ELSD
3.4. Investigation of Organic Acid Profile by HPLC-PDA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murthy, H.N.; Bapat, V.A. Importance of underutilized fruits and nuts. In Bioactive Compounds in Underutilized Fruits and Nuts, Reference Series in Phytochemistry; Murthy, H.N., Bapat, V.A., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 3–19. [Google Scholar]
- Murthy, H.N.; Dalawai, D. Bioactive compounds of wood apple (Limonia acidissima L.). In Bioactive Compounds in Underutilized Fruits and Nuts, Reference Series in Phytochemistry; Murthy, H.N., Bapat, V.A., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 543–569. [Google Scholar]
- Murthy, H.N.; Dalawai, D.; Dewir, Y.H.; Ibrahim, A. Phytochemicals and biological activities of Garcinia morella (Gaertn.) Desr.: A review. Molecules 2020, 25, 5690. [Google Scholar] [CrossRef]
- Murthy, H.N.; Yadav, G.G.; Dewir, Y.H.; Ibrahim, A. Phytochemicals and biological activity of desert date (Belanites aegyptica (L.) Delile). Plants 2020, 10, 32. [Google Scholar] [CrossRef]
- Manohar, S.H.; Naik, P.M.; Patil, L.M.; Karikatti, S.I.; Murthy, H.N. Chemical composition of Garcinia xanthochymus seeds, seed oil, and evaluation of its antimicrobial and antioxidant activity. J. Herbs Spices Med. Plants 2014, 20, 148–155. [Google Scholar] [CrossRef]
- Joseph, K.S.; Bolla, S.; Joshi, K.; Bhat, M.; Naik, K.; Patil, S.; Bendre, S.; Gangappa, B.; Haibatti, V.; Payamalle, S.; et al. Determination of chemical composition and nutritive value with fatty acid compositions of African Mangosteen (Garcinia livingstonei). Erwerbs-Obstbau 2016, 59, 195–202. [Google Scholar] [CrossRef]
- Murthy, H.N.; Dalawai, D.; Mamatha, U.; Angadi, N.B.; Dewir, Y.H.; Al-Suhaibani, N.A.; El-Hendawy, S.; Al-Ali, A.M. Bioactive constituents and nutritional composition of Bridelia stipularis L. Blume fruits. Int. J. Food Prop. 2021, 24, 796–805. [Google Scholar] [CrossRef]
- Murthy, H.N.; Dandin, V.S.; Dalawai, D.; Park, S.Y.; Paek, K.Y. Bioactive compounds from Garcinia fruits of high economic value for food and health. In Bioactive Molecules in Food, Reference Series in Phytochemistry; Merllion, J.M., Ramawat, K.G., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 1–26. [Google Scholar]
- Yoshida, H.; Hirakawa, Y.; Abe, S. Roasting influences on molecular species of triacylglycerols in sunflower seeds (Helianthus annuus L.). Food Res. Int. 2001, 34, 613–619. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Vongsvivut, J.; Adhikari, R.; Adhikari, B. Physicochemical and thermal characteristics of Australian chia seed oil. Food Chem. 2017, 228, 394–402. [Google Scholar] [CrossRef]
- German, J.B. Dietary lipids from an evolutionary perspective: Sources, structures and functions. Matern. Child Nutr. 2011, 7, 2–16. [Google Scholar] [CrossRef]
- Westman, E.C. Is dietary carbohydrate essential for human nutrition? Am. J. Clin. Nutr. 2002, 75, 951–953. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.M. Cellular and genetic responses of plants to sugar starvation. Plant Physiol. 1999, 121, 687–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkle, B.J.; Zavala, M.E.; Ulrich, J.M. Cryoprotective compounds in the viable freezing of plant tissues. In Cryopreservation of Plant Cells and Organs; Kartha, K.K., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1985; pp. 75–115. [Google Scholar]
- Bekers, M.; Marauska, M.; Grube, M.; Karklina, D.; Duma, M. New prebiotics for functional food. Acta Aliment. 2004, 33, 31–37. [Google Scholar] [CrossRef]
- Rai, A.K.; Prakash, M.; Anu Appaiah, K.A. Production of Garcinia wine: Changes in biochemical parameters, organic acids and free sugars during fermentation of Garcinia must. Int. J. Food Sci. Technol. 2010, 45, 1330–1336. [Google Scholar] [CrossRef]
- Orwa, C.; Mutua, A.; Kindt, R.; Jamnadass, R.; Anthony, S. Agroforestry Database: A Tree Reference and Selection Guide Version 4.0; World Agroforestry Centre: Nairobi, Kenya, 2009. [Google Scholar]
- Singh, A.K.; Singh, S.; Singh, R.S.; Joshi, H.K.; Sharma, S.K. Characterization of bael (Aegle marmelos) varieties under rainfed hot semi-arid environment of western India. Indian J. Agric. Sci. 2014, 84, 1236–1242. [Google Scholar]
- Lim, T.K. Limonia acidissima . In Edible Medicinal and Non-Medicinal Plants: Fruits; Lim, T.K., Ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 4, pp. 884–889. [Google Scholar]
- Chatterjee, T.K. Herbal Options, 3rd ed.; Books and Allied (P) Ltd.: Calcutta, India, 2003; pp. 203–256. [Google Scholar]
- Joshi, R.K.; Badakar, V.M.; Kholkute, S.D.; Khatib, N. Chemical composition and antimicrobial activity of the essential oil of the leaves of Feronia elephantum (Rutaceae) from North West Karnataka. Nat. Prod. Commun. 2011, 6, 141–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.; Johri, S.; Saxena, A.M. Effect of ethanolic extract of Feronia elephantum Correa fruits on blood glucose levels in normal and streptozotocin induced diabetic rats. NPR 2009, 8, 32–36. [Google Scholar]
- Ilango, K.; Chitra, V. Wound healing and antioxidant activities of the fruit pulp of Limonia acidissima Linn (Rutaceae) in rats. Trop. J. Pharm. Res. 2010, 9, 223–230. [Google Scholar] [CrossRef]
- Ilaiyaraja, N.; Likhith, K.R.; Sharath Babu, G.R.; Khanum, F. Optimization of extraction of bioactive compounds from Feronia limonia (wood apple) fruit using response surface methodology (RSM). Food Chem. 2015, 173, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Lamani, S.; Anu-Appaiah, K.A.; Murthy, H.N.; Dewir, Y.H.; Rihan, H.Z. Fatty acid profile, tocopherol content of seed oil, and nutritional analysis of seed cake of wood-apple (Limonia acidissima L.), an underutilized fruit-yielding tree species. Horticulturae 2021, 7, 275. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Sadasivam, S.; Manickam, A. Biochemical Methods, 3rd ed.; New Age International (P) Limited, Publishers: New Delhi, India, 2008. [Google Scholar]
- AOAC. Official Methods of Analysis of Association of Analytical Chemists, 15th ed.; AOAC: Washington, DC, USA, 1998. [Google Scholar]
- Anonymous. Manual of Methods of Analysis of Foods: Fruit and Vegetable Products. Lab. Manual 5; Food Safety and Standards Authority of India; Ministry of Health and Family Welfare: New Delhi, India, 2015; Volume 40, p. 11. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.S. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-ciocalteau Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Morison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride-methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Ghfar, A.A.; Wabaidur, S.M.; Ahmed, A.Y.B.H.; Alothman, Z.A.; Khan, M.R.; Al-Shaalan, N.H. Simultaneous determination of monosaccharides and oligosaccharides in dates using liquid chromatography—Electrospray ionization mass spectrometry. Food Chem. 2015, 176, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.; Park, J.; Lim, J.; Yea, M.; Bang, D. A rapid method for simultaneous quantification of 13 sugars and sugar alcohols in food products by UPLC-ELSD. Food Chem. 2018, 240, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Sakariah, K.K. Determination of organic acids in Garcinia cambogia (Desr.) by high-performance liquid chromatography. J. Chromatogr. A 1998, 806, 337–339. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, S.; Yadav, V.; Sharma, B.D. Genetic variability in wood apple (Feronia limonia) from Gujarat. Indian J. Agric. Sci. 2016, 86, 1504–1508. [Google Scholar]
- Poongodi Vijayakumar, T.; Punitha, K.; Banupriya, L. Drying characteristics and quality evaluation of wood apple (Limonia acidissima L.) fruit pulp powder. Int. J. Curr. Trends Res. 2013, 2, 147–150. [Google Scholar]
- Sonawane, S.K.; Bagul, M.B.; LeBlanc, J.G.; Arya, S.S. Nutritional, functional, thermal and structural characteristics of Citrullus lanatus and Limonia acidissima seed flours. J. Food Meas. Charact. 2016, 10, 72–79. [Google Scholar] [CrossRef]
- Abdualrahman, M.A.Y.; Ma, H.; Zhou, C.; Yagoub, A.E.A.; Ali, A.O.; Tahir, H.E.; Wali, A. Postharvest physicochemical properties of the pulp and seed oil from Annona squamosa L. (Gishta) fruit grown in Darfur region, Sudan. Arab. J. Chem. 2019, 12, 4514–4521. [Google Scholar] [CrossRef] [Green Version]
- Cheema, J.; Yadav, K.; Sharma, N.; Saini, I.; Aggarwal, A. Nutritional quality characteristics of different wild and underutilized fruits of Terai region, Uttarakhand (India). Int. J. Fruit Sci. 2016, 17, 72–81. [Google Scholar] [CrossRef]
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Sagar, V.R.; Kumar, R. Effect of drying treatments and storage stability on quality characteristics of bael powder. J. Food Sci. Technol. 2014, 51, 2162–2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddeeg, A.; Zeng, X.; Ammar, A.; Han, Z. Sugar profile, volatile compounds, composition and antioxidant activity of sukkari date palm fruit. J. Food Sci. Technol. 2019, 56, 754–762. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S.; Gubbuk, H.; Gunes, E. Comparative evaluation of volatiles, phenolics, sugars, organic acids and antioxidant properties of Sel-42 and Tainung papaya varieties. Food Chem. 2015, 173, 912–919. [Google Scholar] [CrossRef] [PubMed]
- e Souza, C.S.; Anunciacao, P.C.; Della Lucia, C.M.; Rodrigues das Dores, R.G.; de Miranda Milagres, R.C.R.; Pinheiro Sant’Ana, H.M. Kumquat (Fortunella marginata): A good alternative for ingestion of nutrients and bioactive compounds. Proceedings 2021, 70, 105. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Dietary fiber as a carrier of dietary antioxidants: An essential physiological function. J. Agric. Food. Chem. 2011, 59, 43–49. [Google Scholar] [CrossRef]
- Ramful, D.; Bahorun, T.; Bourdon, E.; Tarnus, E.; Aruoma, O.I. Biactive phenolics and antioxidant propensity of flavedo extracts of Mauritian citrus fruits: Potential prophylactic ingredients for functional food application. Toxicology 2010, 278, 75–87. [Google Scholar] [CrossRef]
- San, B.; Yildirim, A.N. Phenolic, alpha-tocopherol, beta-carotene and fatty acid composition of four promising jujube (Ziziphus jujuba Miller) selections. J. Food Compos. Anal. 2010, 23, 706–710. [Google Scholar] [CrossRef]
- Reche, J.; Almansa, M.S.; Hernandez, F.; Carbonell-Barrachina, A.A.; Legua, P.; Amoros, A. Fatty acid profile of peel and pulp of Spanish jujube (Ziziphus jujuba Mill.) fruit. Food Chem. 2019, 295, 247–253. [Google Scholar] [CrossRef]
- Sanchez-Salcedo, E.M.; Sendra, E.; Carbonell-Barrachina, A.A.; Martínez, J.J.; Hernandez, F. Fatty acids composition of Spanish black (Morus nigra L.) and white (Morus alba L.) mulberries. Food Chem. 2016, 190, 566–571. [Google Scholar] [CrossRef]
- Elaloui, M.; Laamouri, A.; Albouchi, A.; Cerny, M.; Mathieu, C.; Vilarem, G.; Hasnaoui, B. Chemical compositions of the tunisian Ziziphus jujuba oil. Emir. J. Food Agric. 2014, 26, 602–608. [Google Scholar] [CrossRef] [Green Version]
- FAO/WHO. Fats and fatty acids in human nutrition. In Report of an Expert Consultation; FAO/WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Petukhov, I.; Malcolmson, L.J.; Przybylski, R.; Armstrong, L. Frying performance of genetically modified canola oils. JAOCS 1999, 76, 627–632. [Google Scholar] [CrossRef]
- Matthaus, B. Use of palm oil for frying in comparison with other high-stability oils. Eur. J. Lipid Sci. Technol. 2007, 109, 400–409. [Google Scholar] [CrossRef]
- Hu, W.; Sun, D.W.; Pu, H.; Pan, T. Recent developments in methods and techniques for rapid monitoring of sugar metabolism in fruits. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1067–1079. [Google Scholar] [CrossRef] [Green Version]
- Murrinie, E.D.; Yudono, P.; Purwantoro, A.; Sulistyaningsih, E. Morphological and physiological changes during growth and development of wood apple (Feronia limonia (L.) Swingle) fruit. Int. J. Bot. 2017, 13, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Seymour, G.B.; Taylor, J.E.; Tucker, C.A. Biochemistry of Fruit Ripening; Chapman and Hall: London, UK, 1993. [Google Scholar]
- Gao, Q.H.; Wu, C.S.; Wang, M.; Xu, B.N.; Du, L.J. Effect of drying of jujubes (Ziziphus jujuba Mill.) on the contents of sugars, organic acids, α-tocopherol, β-carotene, and phenolic compounds. J. Agric. Food Chem. 2012, 60, 9642–9648. [Google Scholar] [CrossRef] [PubMed]
- Slatnar, A.; Klancar, U.; Stampar, F.; Veberic, R. Effect of Drying of Figs (Ficus carica L.) on the contents of sugars, organic acids and phenolic compounds. J. Agric. Food Chem. 2011, 59, 11696–11702. [Google Scholar] [CrossRef] [PubMed]
- Albertini, M.; Carcouet, E.; Pailly, C.O.; Gambotti, C.; Luro, F.; Berti, L. Changes in organic acids and sugars during early stages of development of acidic and acidless citrus fruit. J. Agric. Food Chem. 2006, 54, 8335–8339. [Google Scholar] [CrossRef]


| Sl. No. | Parameters | Composition |
|---|---|---|
| 1 | Fruit size (cm) | |
| Length | 7.42 ± 0.19 | |
| Width | 7.43 ± 0.10 | |
| Circumference | 23.42 ± 0.29 | |
| 2 | Fruit weight (g) | 179.45 ± 1.42 |
| 3 | Moisture (% FW) | 58.89 ± 1.21 |
| 4 | Oil (% FW) | 0.99 ± 0.01 |
| 5 | TSS (°Brix) | 19.52 ± 0.17 |
| 6 | pH | 3.61 ± 0.09 |
| 7 | Titratable acidity (% FW) | 4.61 ± 0.13 |
| 8 | Ash (% FW) | 2.73 ± 0.12 |
| 9 | Crude fiber (% FW) | 3.32 ± 0.02 |
| 10 | Total protein (% FW) | 9.30 ± 0.16 |
| 11 | Total carbohydrate (% FW) | 24.74 ± 0.19 |
| 12 | Total phenolics (% DW) 2 | 0.50 ± 0.01 |
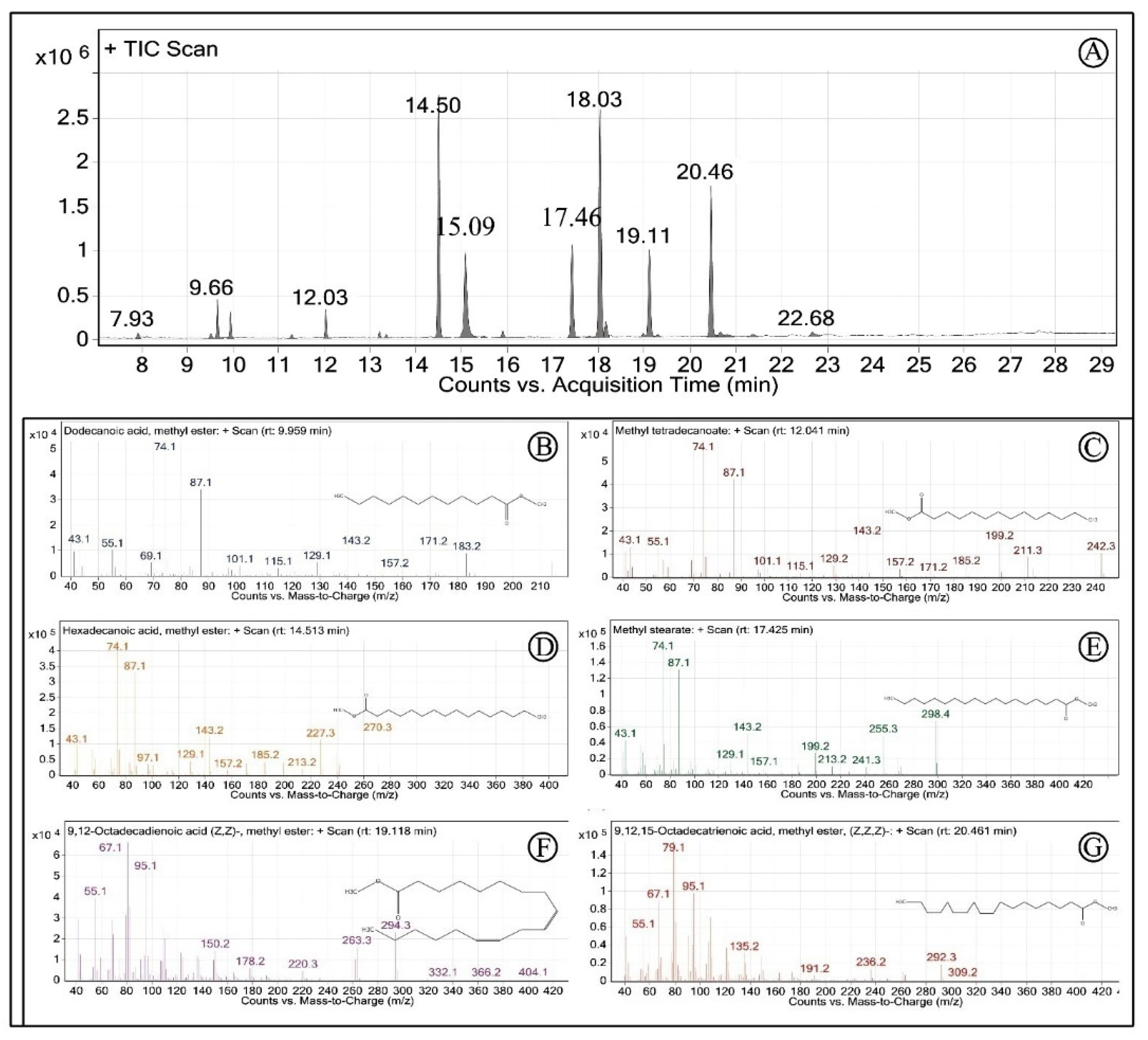
| Peak | tR (min) | Common Name | Fatty Acid Methyl Esters | Value (%) |
|---|---|---|---|---|
| 1 | 7.93 | Cyclooctasiloxane, hexadecamethyl- | 0.45 ± 0.05 | |
| 2 | 9.52 | Cyclononasiloxane, octadecamethyl- | 0.38 ± 0.05 | |
| 3 | 9.66 | Acetophenone | Acetophenone | 2.34 ± 0.06 |
| 4 | 9.95 | Lauric acid | Dodecanoic acid, methyl ester (C12:0) | 1.62 ± 0.02 |
| 5 | 12.03 | Myristic acid | Methyl tetradecanoate (C14:0) | 1.74 ± 0.04 |
| 6 | 13.21 | Pentadecylic acid | Pentadecanoic acid, methyl ester (C15:0) | 0.44 ± 0.02 |
| 7 | 14.5 | Palmitic acid | Hexadecanoic acid, methyl ester (C16:0) | 18.52 ± 0.12 |
| 8 | 15.09 | Phenol, 2,4-bis(1,1-dimethylethyl)- | 7.70 ± 0.06 | |
| 9 | 17.42 | Stearic acid | Methyl stearate (C18:0) | 9.02 ± 0.08 |
| 10 | 18.03 | Oleic acid | 9-Octadecenoic acid (Z)-, methyl ester (C18:1n9c) | 23.89 ± 0.06 |
| 11 | 18.16 | Vaccenic acid | 11-Octadecenoic acid, methyl ester (C18:1n7) | 1.78 ± 0.23 |
| 12 | 19.11 | Linoleic acid (ω- 6) | 9,12-Octadecadienoic acid (Z,Z)-, methyl ester (C18:2n6) | 9.23 ± 0.35 |
| 13 | 20.46 | α-Linolenic acid (ω-3) | 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)-(C18:3n3) | 16.55 ± 0.26 |
| 14 | 20.67 | Arachidic acid | Eicosanoic acid, methyl ester (C20:1) | 0.81 ± 0.06 |
| 15 | 21.38 | Paullinic acid (ω-7) | cis-13-Eicosenoic acid, methyl ester (C20:1n7) | 0.52 ± 0.03 |
| 16 | 25.60 | 2,5-di-tert-Butyl-1,4-dimethoxybenzene | 2.77 ± 0.07 | |
| ∑ Saturated fatty acids | 32.17 ± 0.35 | |||
| ∑ Monounsaturated fatty acids | 26.20 ± 0.33 | |||
| ∑ Polyunsaturated fatty acids | 25.78 ± 0.61 | |||
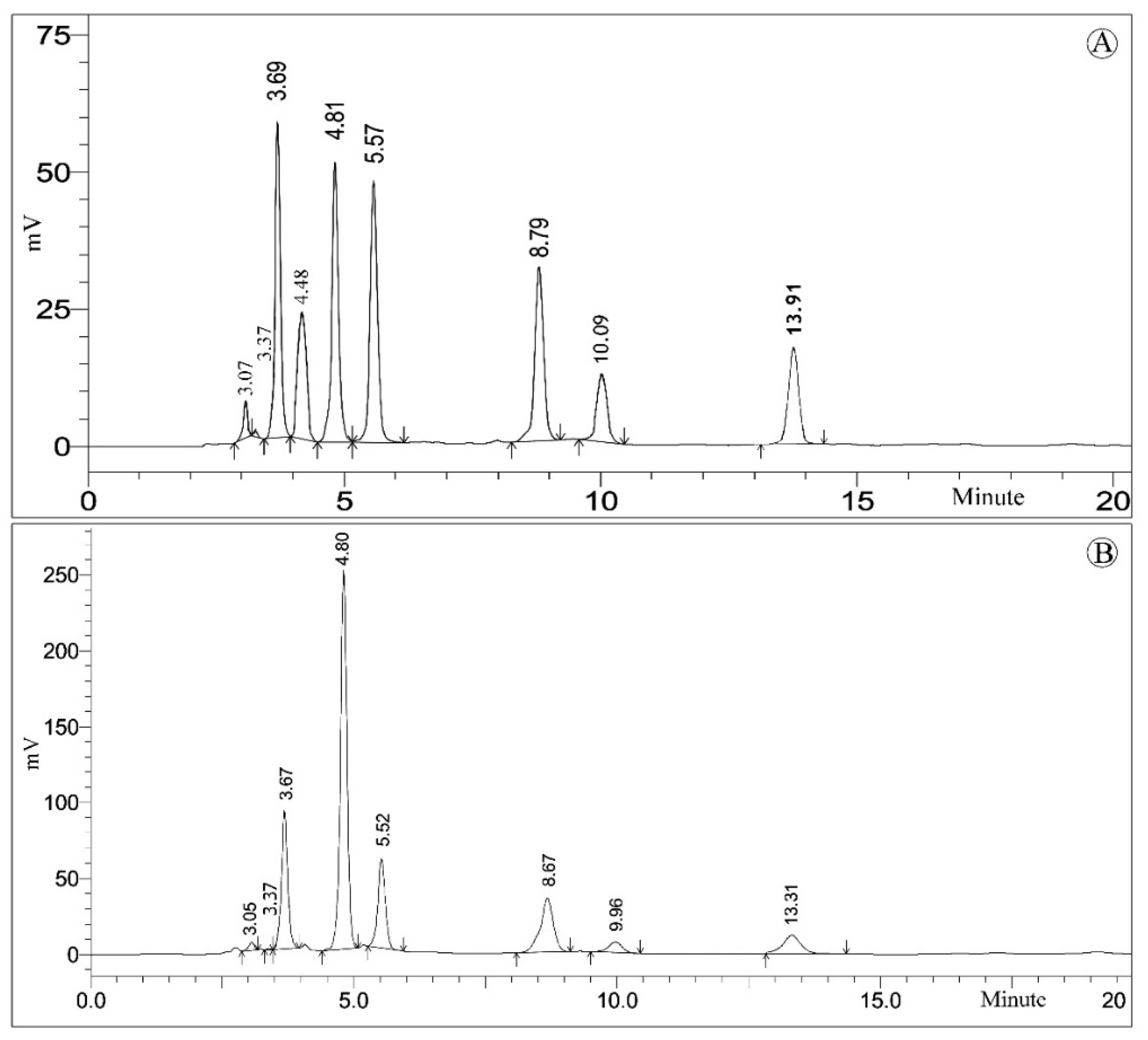
| Sl. No. | Retention Time | Free Sugars | Composition 2 |
|---|---|---|---|
| 1 | 3.32 | Rhamnose | 0.24 ± 0.01 |
| 2 | 4.93 | Fructose | 16.40 ± 0.23 |
| 3 | 5.89 | Glucose | 14.23 ± 0.10 |
| 4 | 7.71 | Sucrose | 0.13 ± 0.01 |
| 5 | 8.33 | Maltose | 0.57 ± 0.03 |
| Total sugars | 31.59 ± 0.17 | ||
| Sl. No. | tR (min) | Organic Acids | Composition 2 |
|---|---|---|---|
| 1 | 3.06 | D-Galacturonic acid | 0.93 ± 0.02 |
| 2 | 3.37 | Oxalic acid | 0.05 ± 0.01 |
| 3 | 3.55 | D-Tartaric acid | 4.01 ± 0.03 |
| 4 | 4.48 | Malic acid | 0.23 ± 0.01 |
| 5 | 4.74 | Ascorbic acid | 4.51 ± 0.05 |
| 6 | 5.59 | Acetic acid | 0.81 ± 0.06 |
| 7 | 8.74 | Citric acid | 4.27 ± 0.04 |
| 8 | 9.59 | Succinic acid | 1.83 ± 0.03 |
| 9 | 13.31 | Pyruvic acid | 0.79 ± 0.01 |
| Total organic acids | 17.46 ± 0.23 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamani, S.; Anu-Appaiah, K.A.; Murthy, H.N.; Dewir, Y.H.; Rikisahedew, J.J. Analysis of Free Sugars, Organic Acids, and Fatty Acids of Wood Apple (Limonia acidissima L.) Fruit Pulp. Horticulturae 2022, 8, 67. https://doi.org/10.3390/horticulturae8010067
Lamani S, Anu-Appaiah KA, Murthy HN, Dewir YH, Rikisahedew JJ. Analysis of Free Sugars, Organic Acids, and Fatty Acids of Wood Apple (Limonia acidissima L.) Fruit Pulp. Horticulturae. 2022; 8(1):67. https://doi.org/10.3390/horticulturae8010067
Chicago/Turabian StyleLamani, Shrinivas, Konerira Aiyappa Anu-Appaiah, Hosakatte Niranjana Murthy, Yaser Hassan Dewir, and Jesamine J. Rikisahedew. 2022. "Analysis of Free Sugars, Organic Acids, and Fatty Acids of Wood Apple (Limonia acidissima L.) Fruit Pulp" Horticulturae 8, no. 1: 67. https://doi.org/10.3390/horticulturae8010067
APA StyleLamani, S., Anu-Appaiah, K. A., Murthy, H. N., Dewir, Y. H., & Rikisahedew, J. J. (2022). Analysis of Free Sugars, Organic Acids, and Fatty Acids of Wood Apple (Limonia acidissima L.) Fruit Pulp. Horticulturae, 8(1), 67. https://doi.org/10.3390/horticulturae8010067







