Does Curing Moisture Content Affect Black Garlic Physiochemical Quality?
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Physicochemical Analyses
2.2.1. Texture
2.2.2. pH and Colour
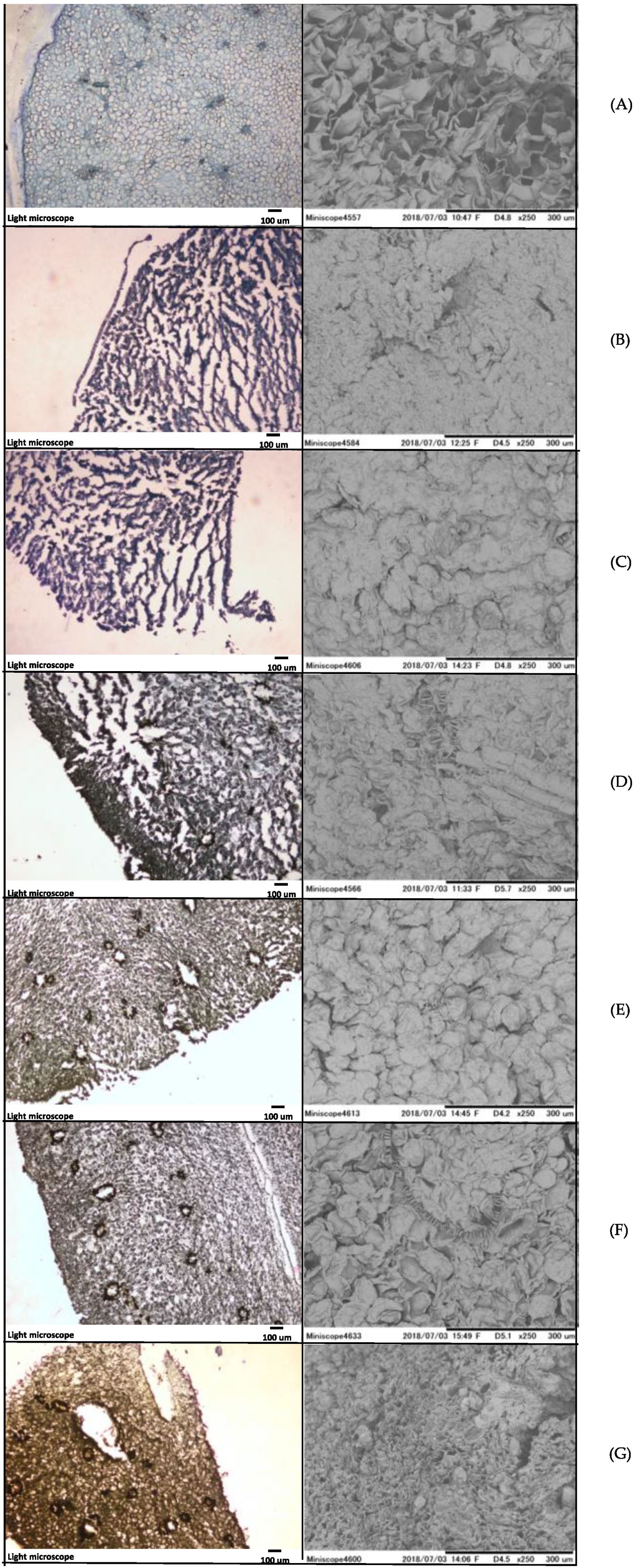
2.3. Light Microscope and Electron Microscope Observation
2.4. Chemical Properties
2.4.1. Extraction of Soluble Metabolites
2.4.2. Sugar Content
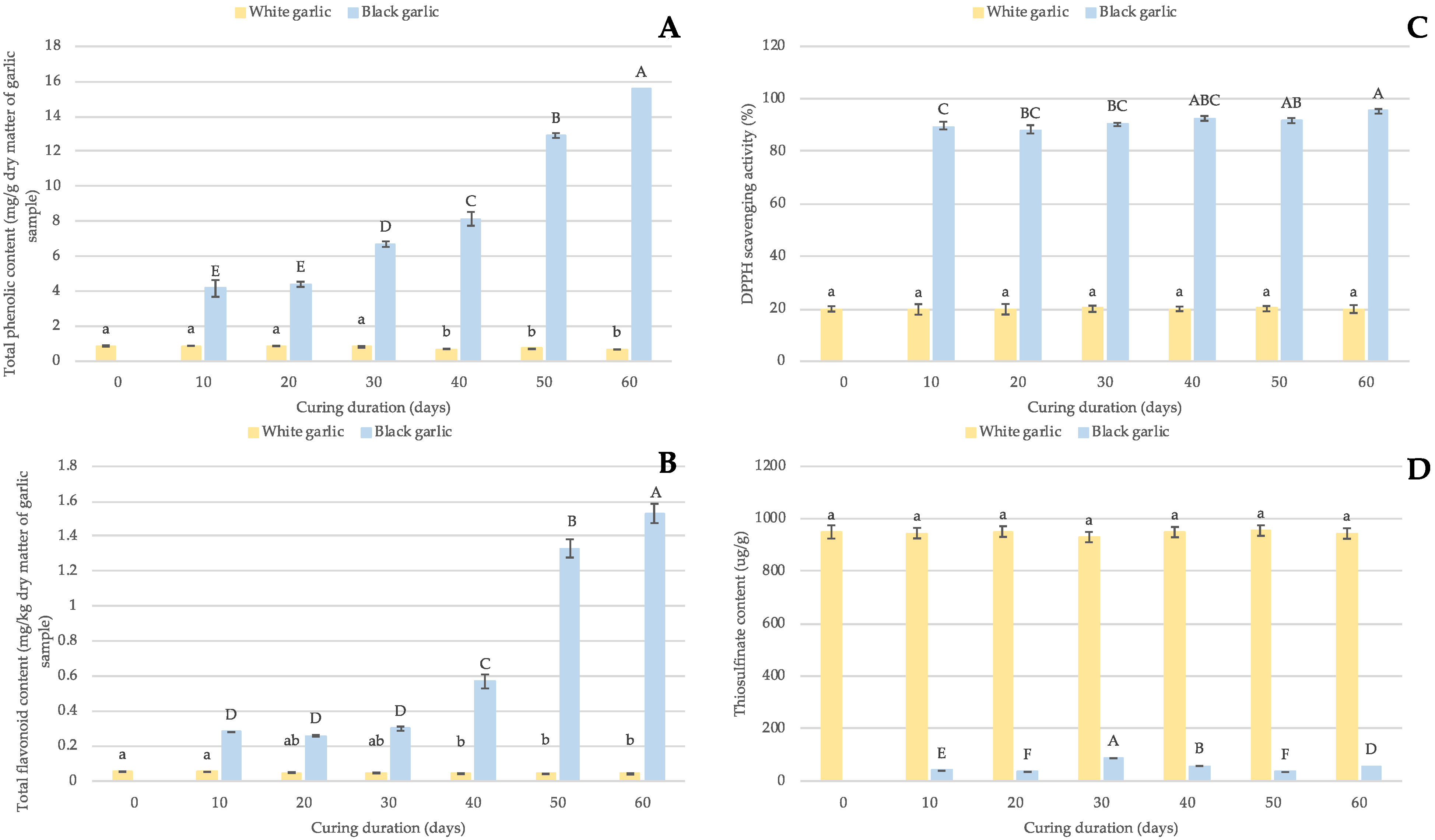
2.4.3. Total Phenolic Content
2.4.4. Total Flavonoid Content
2.4.5. DPPH Scavenging Radical Activity
2.4.6. Thiosulfinate Content
2.4.7. Identification of Soluble Metabolites Using LCMS and FTIR
2.5. Statistical Analysis
3. Result and Discussion
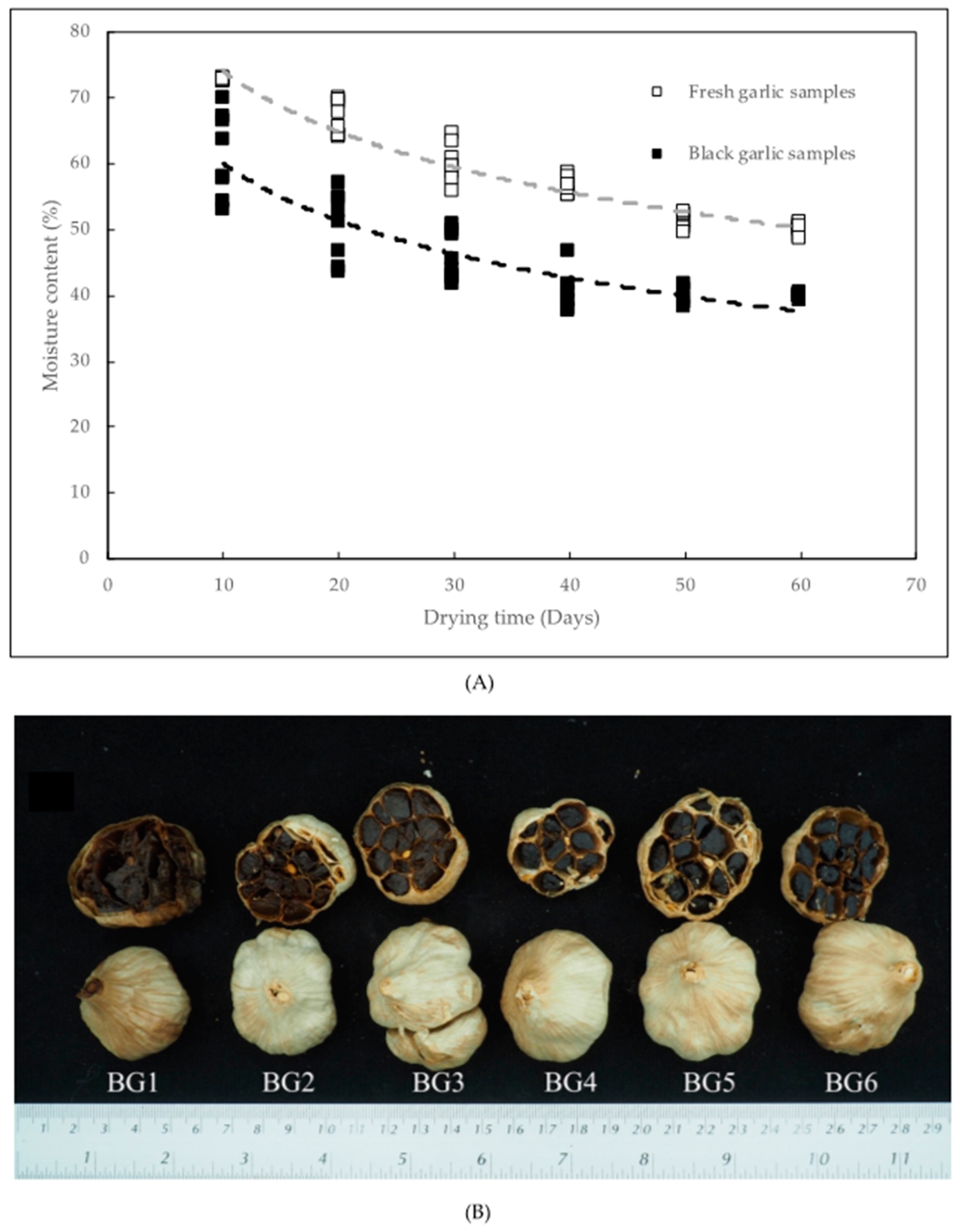
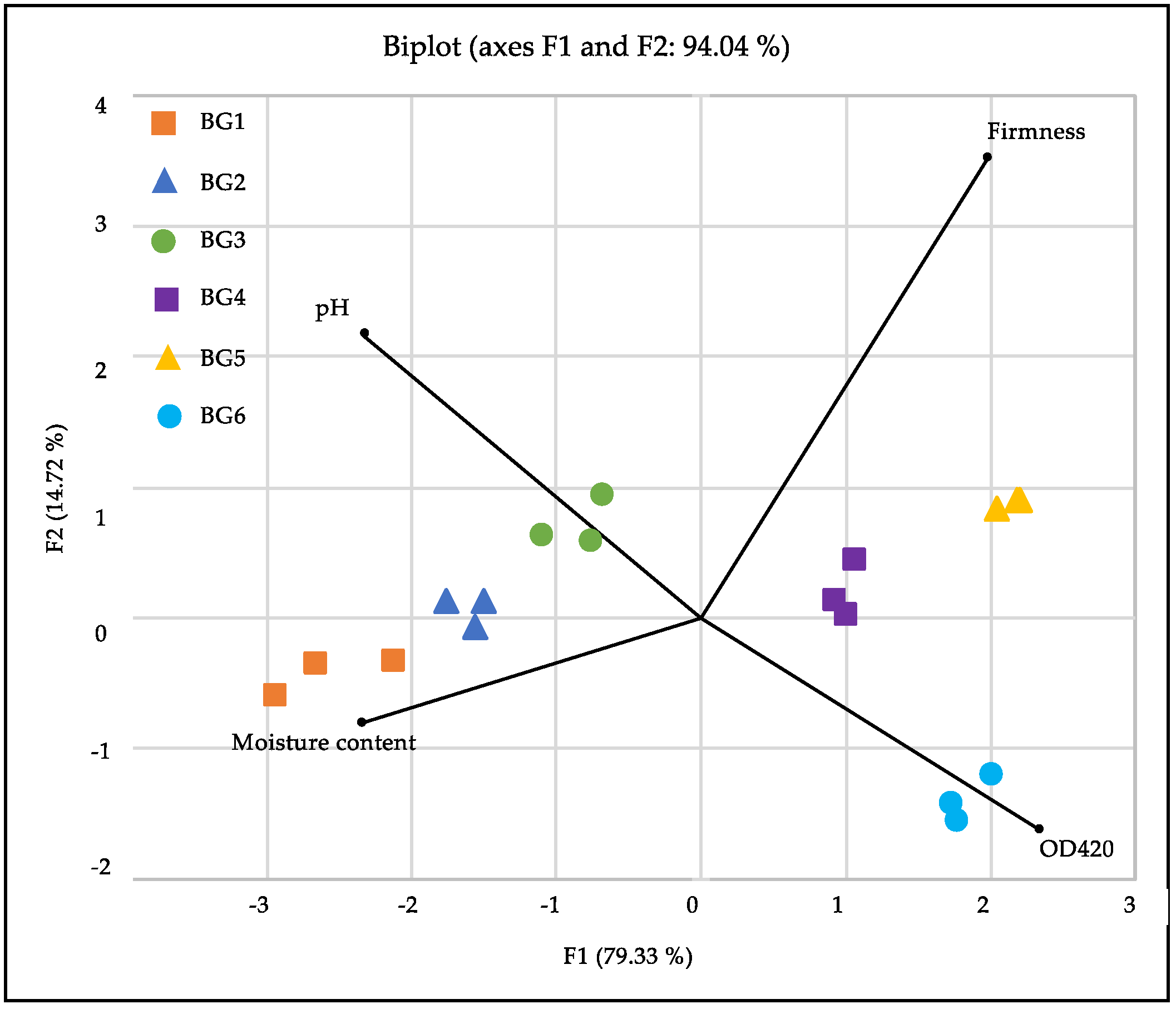
3.1. Physicochemical Properties
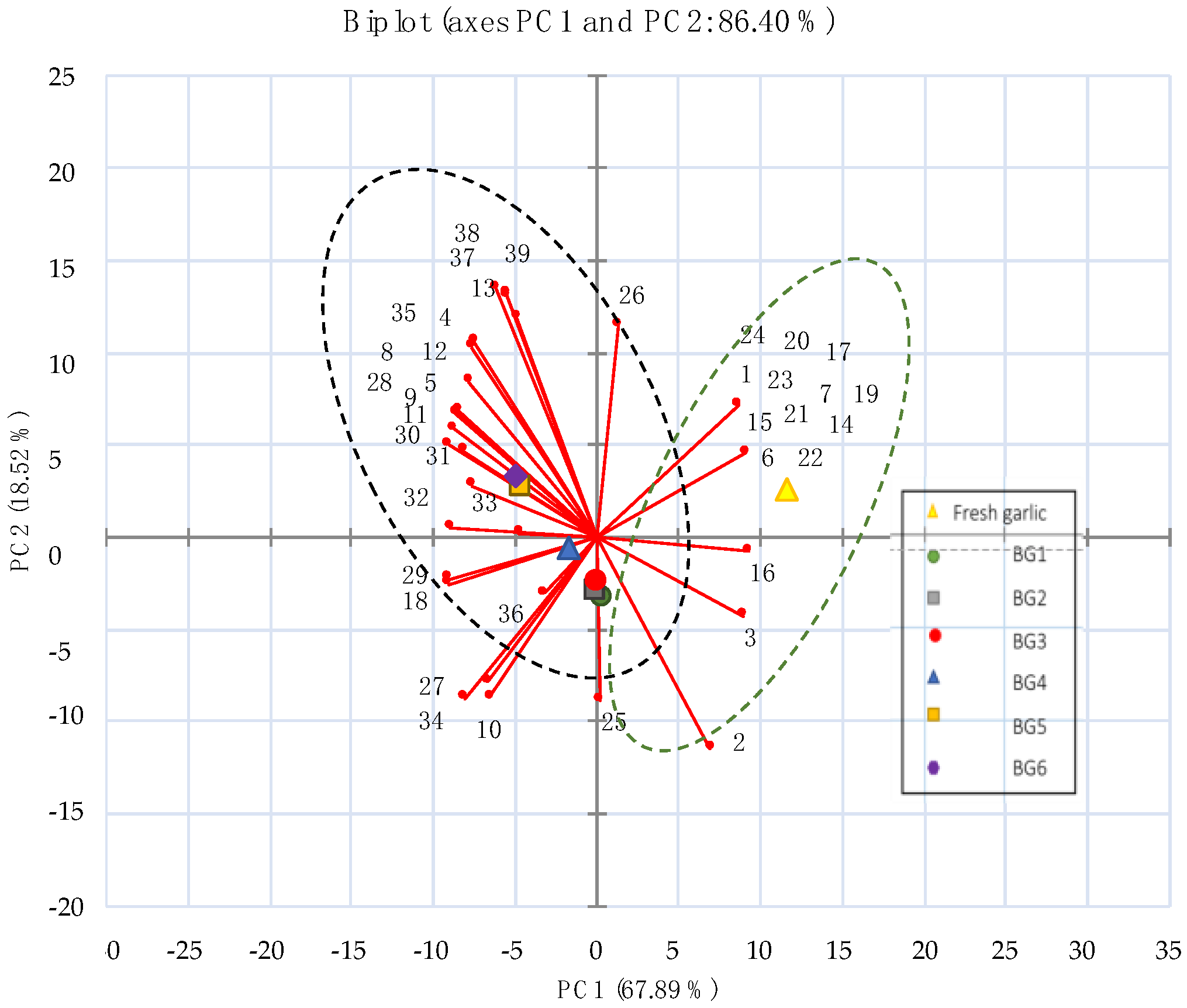
3.2. Light Microscope and Electron Microscope Observation
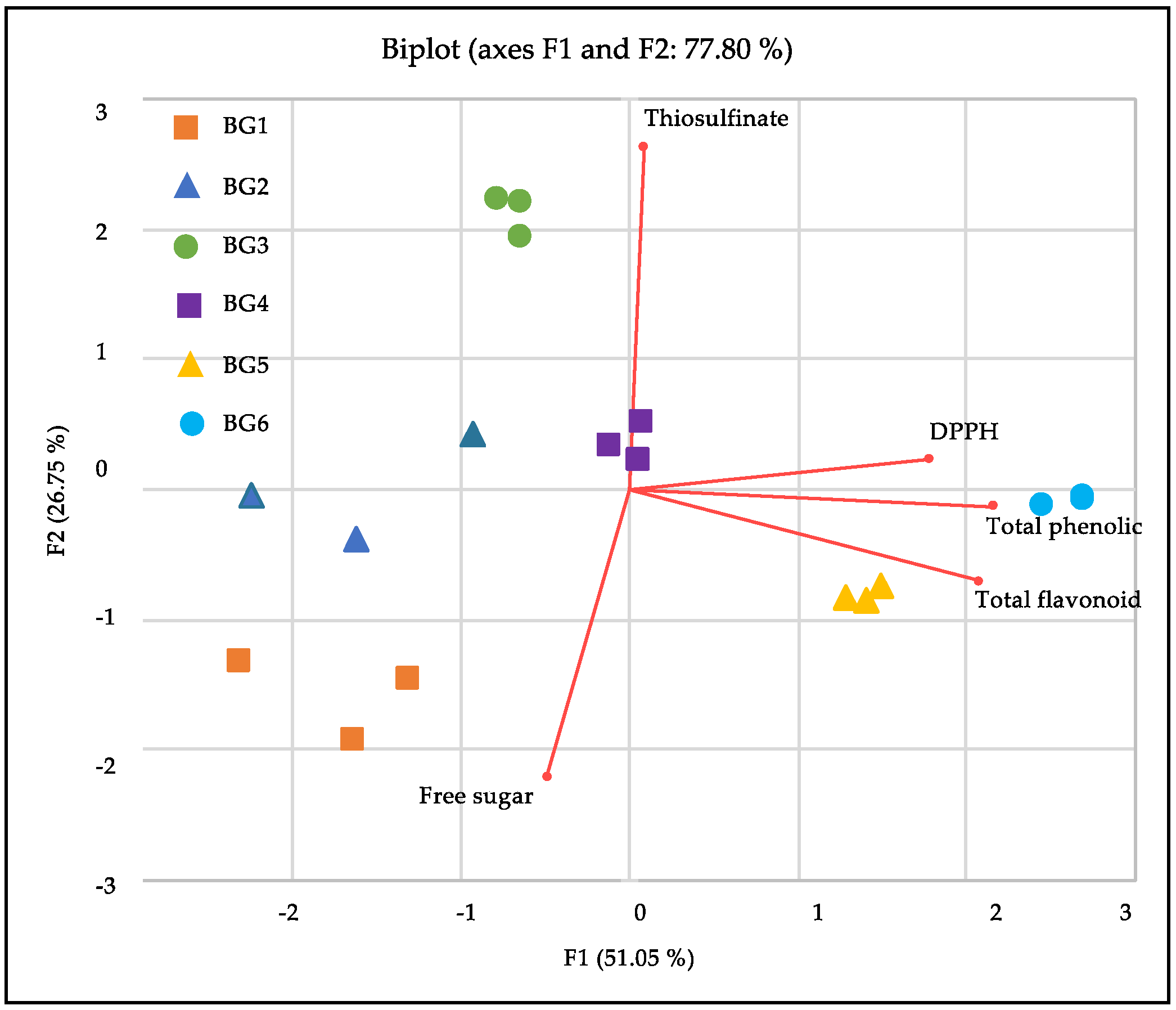
3.3. Chemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bae, S.E.; Cho, S.Y.; Won, Y.D.; Lee, S.H.; Park, H.J. Changes in S-allyl cysteine contents and physicochemical properties of black garlic during heat treatment. LWT-Food Sci. Technol. 2014, 55, 397–402. [Google Scholar] [CrossRef]
- Dufoo-Hurtado, M.D.; Huerta-Ocampo, J.Á.; Barrera-Pacheco, A.; Barba de la Rosa, A.P.; Mercado-Silva, E.M. Low temperature conditioning of garlic (Allium sativum L.) “seed” cloves induces alterations in sprouts proteome. Front. Plant Sci. 2015, 6, 332. [Google Scholar] [CrossRef]
- Butt, M.S.; Sultan, M.T.; Butt, M.S.; Iqbal, J. Garlic: Nature’s Protection against Physiological Threats. Crit. Rev. Food Sci. Nutr. 2009, 49, 538–551. [Google Scholar] [CrossRef]
- Block, E. The Chemistry of Garlic and Onions; Scientific American: New York, NY, USA, 1985. [Google Scholar] [CrossRef]
- Sierpina, V.; Devries, S.; Prasad, A.; Eisenberg, D.; McKee, J.; Kreitzer, M. Nutritional deficiency in healthcare education. Explore 2013, 9, 192–195. [Google Scholar] [CrossRef]
- Capasso, A. Antioxidant Action and Therapeutic Efficacy of Allium sativum L. Molecules 2013, 18, 690. [Google Scholar] [CrossRef] [PubMed]
- Morihara, N.; Nishihama, T.; Ushijima, M.; Ide, N.; Takeda, H.; Hayama, M. Garlic as an anti-fatigue agent. Mol. Nutr. Food Res. 2007, 51, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, H.L.; Ross, S.A.; Milner, J.A. Garlic and onions: Their cancer prevention properties. Cancer Prev. Res. 2015, 8, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Gafar, M.K.; Itodo, A.U.; Warra, A.A.; Abdullahi, L. Extraction and Physicochemical Determination of Garlic (Allium sativum L.) Oil. Int. J. Food Sci. 2012, 1, 4–7. [Google Scholar]
- Rahman, K. Garlic and aging: New insights into an old remedy. Ageing Res. Rev. 2003, 2, 39–56. [Google Scholar] [CrossRef]
- Zakarova, A.; Seo, J.Y.; Kim, H.Y.; Kim, J.H.; Shin, J.-H.; Cho, K.M.; Lee, C.H.; Kim, J.-S. Garlic Sprouting Is Associated with Increased Antioxidant Activity and Concomitant Changes in the Metabolite Profile. J. Agric. Food Chem. 2014, 62, 1875–1880. [Google Scholar] [CrossRef]
- Choi, I.; Cha, H.; Lee, Y. Physicochemical and antioxidant properties of black garlic. Molecules 2014, 19, 16811–16823. [Google Scholar] [CrossRef]
- Rahman, K.; Lowe, G.M. Garlic and cardiovascular disease: A critical review. J. Nutr. 2006, 136, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lei, M.; Liu, R.; Gao, Y.; Xu, M.; Zhang, M. Evaluation of Alliin, Saccharide Contents and Antioxidant Activities of Black Garlic during Thermal Processing. J. Food Biochem. 2015, 39, 39–47. [Google Scholar] [CrossRef]
- Sasaki, J.I. Overview of the Black Garlic Movement in the Fields of Research and Marketing. J. Life Sci. 2015, 9, 65–74. [Google Scholar] [CrossRef][Green Version]
- Kimura, S.; Tung, Y.-C.; Pan, M.-H.; Su, N.-W.; Lai, Y.-J.; Cheng, K.-C. Black garlic: A critical review of its production, bioactivity, and application. J. Food Drug Anal. 2017, 25, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.E.; Cho, S.Y.; Won, Y.D.; Lee, S.H.; Park, H.J. A comparative study of the different analytical methods for analysis of S-allyl cysteine in black garlic by HPLC. LWT-Food Sci. Technol. 2012, 46, 532–535. [Google Scholar] [CrossRef]
- Hee Kim, J.; Hyun Nam, S.; Rico, C.; Young Kang, M. A comparative study on the antioxidative and anti-allergic activities of fresh and aged black garlic extracts. J. Food Sci. Technol. 2012, 47, 1176–1182. [Google Scholar] [CrossRef]
- Kang, O.J. Physicochemical Characteristics of Black Garlic after Different Thermal Processing Steps. Prev. Nutr. Food Sci. 2016, 21, 348–354. [Google Scholar] [CrossRef]
- Wang, L.F.; Rhim, J.W. Isolation and characterization of melanin from black garlic and sepia ink. LWT-Food Sci. Technol. 2019, 99, 17–23. [Google Scholar] [CrossRef]
- Shin, J.-H.; Choi, D.-J.; Lee, S.-J.; Cha, J.-Y.; Kim, J.-G.; Sung, N.-J. Changes of Physicochemical Components and Antioxidant Activity of Garlic During its Processing. J. Life Sci. 2008, 18, 1123–1131. [Google Scholar] [CrossRef]
- Ide, N.; Lau, B.H. Aged garlic extract attenuates intracellular oxidative stress. Phytomedicine 1999, 6, 125–131. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kang, O.-J.; Gweon, O.-C. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J. Funct. Foods 2013, 5, 80–86. [Google Scholar] [CrossRef]
- Corzo-Martínez, M.; Corzo, N.; Villamiel, M. Biological properties of onions and garlic. Trends Food Sci. Technol. 2007, 18, 609–625. [Google Scholar] [CrossRef]
- Queiroz, Y.S.; Ishimoto, E.Y.; Bastos, D.H.; Sampaio, G.R.; Torres, E.A. Garlic (Allium sativum L.) and ready-to-eat garlic products: In vitro antioxidant activity. Food Chem. 2009, 115, 371–374. [Google Scholar] [CrossRef]
- Sommano, S.R.; Saratan, N.; Suksathan, R.; Pusadee, T. Chemical composition and comparison of genetic variation of commonly available Thai garlic used as food supplement. J. Appl. Bot. Food Qual. 2016, 89, 235–242. [Google Scholar] [CrossRef]
- Mushobozi, W.L. Good Agricultural Practices on Horticultural Production for Extension Staff in Tanzania; FAO: Rome, Italy, 2010. [Google Scholar]
- Nishizawa, T.; Nagasawa, S.; Retamales, J.; Lavin, A.; Motomura, Y. Comparison of cell wall components between Fragaria × ananassa and Fragaria chiloensis grown in Chile. J. Hortic. Sci. 2002, 77, 404–410. [Google Scholar] [CrossRef]
- De Ancos, B.; Cano, M.P.; Hernandez, A.; Monreal, M. Effects of microwave heating on pigment composition and colour of fruit purees. J. Sci. Food Agric. 1999, 79, 663–670. [Google Scholar] [CrossRef]
- Palou, E.; López-Malo, A.; Barbosa-Cánovas, G.; Welti-Chanes, J.; Swanson, B. Polyphenoloxidase activity and color of blanched and high hydrostatic pressure treated banana puree. J. Food Sci. 1999, 64, 42–45. [Google Scholar] [CrossRef]
- Lee, J.-W.; Lee, S.-K.; Do, J.-H.; Shim, K.-H. Characteristics of the water soluble browning reaction of Korean red ginseng as affected by heating treatment. J. Ginseng Res. 1998, 22, 193–199. [Google Scholar] [CrossRef][Green Version]
- Sunanta, P.; Chung, H.H.; Kunasakdakul, K.; Ruksiriwanich, W.; Jantrawut, P.; Hongsibsong, S.; Sommano, S.R. Genomic relationship and physiochemical properties among raw materials used for Thai black garlic processing. Food Sci. Nutr. 2020, 8, 4534–4545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, N.; Lu, X.; Liu, P.; Qiao, X. Effects of temperature on the quality of black garlic. Int. Food Res. J. 2016, 96, 2366–2372. [Google Scholar] [CrossRef]
- Li, F.; Cao, J.; Liu, Q.; Hu, X.; Liao, X.; Zhang, Y. Acceleration of the Maillard reaction and achievement of product quality by high pressure pretreatment during black garlic processing. Food Chem. 2000, 318, 126517. [Google Scholar] [CrossRef]
- Lee, E.N.; Choi, Y.W.; Kim, H.K.; Park, J.K.; Kim, H.J.; Kim, M.J.; Lee, H.W.; Kim, K.H.; Bae, S.S.; Kim, B.S. Chloroform extract of aged black garlic attenuates TNF-α-induced ROS generation, VCAM-1 expression, NF-κB activation and adhesiveness for monocytes in human umbilical vein endothelial cells. Phytother. Res. 2011, 25, 92–100. [Google Scholar] [CrossRef]
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. An analysis of the changes on intermediate products during the thermal processing of black garlic. Food Chem. 2018, 239, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Toledo, R. Antioxidant activity of water-soluble Maillard reaction products. Food Chem. 2005, 93, 273–278. [Google Scholar] [CrossRef]
- Kim, H.-K.; Jo, K.-S.; Kwon, D.-Y.; Park, M.-H. Effects of drying temperature and sulfiting on the qualities of dried garlic slices. J. Korean Chem. Soc. 1992, 35, 6–9. [Google Scholar]
- Baysal, T.; Icier, F.; Ersus, S.; Yıldız, H. Effects of microwave and infrared drying on the quality of carrot and garlic. Eur. Food Res. 2003, 218, 68–73. [Google Scholar] [CrossRef]
- Toledano-Medina, M.A.; Pérez-Aparicio, J.; Moreno-Rojas, R.; Merinas-Amo, T. Evolution of some physicochemical and antioxidant properties of black garlic whole bulbs and peeled cloves. Food Chem. 2016, 199, 135–139. [Google Scholar] [CrossRef]
- Peterson, B.I.; Tong, C.H.; Ho, C.T.; Welt, B.A. Effect of moisture content on Maillard browning kinetics of a model system during microwave heating. J. Sci. Food Agric. 1994, 42, 1884–1887. [Google Scholar] [CrossRef]
- Mann, L. Anatomy of the garlic bulb and factors affecting bulb development. Hilgardia 1952, 21, 195–251. [Google Scholar] [CrossRef][Green Version]
- Miryeganeh, M.; Movafeghi, A. Scape anatomy of Allium sect. Allium (Alliaceae) in Iran. Hilgardia 2009, 21, 195–251. [Google Scholar]
- Sterling, C. Effect of moisture and high temperature on cell walls in plant tissues. J. Food Sci. Technol. 1995, 20, 474–479. [Google Scholar] [CrossRef]
- Joardder, M.U.; Brown, R.J.; Kumar, C.; Karim, M.A. Effect of cell wall properties on porosity and shrinkage of dried apple. Int. J. Food Prop. 2015, 18, 2327–2337. [Google Scholar] [CrossRef]
- Atashi, S.; Akbarpour, V.; Mashayekhi, K.; Mousavizadeh, S.J. Garlic physiological characteristics from harvest to sprouting in response to low temperature. J. Stored Prod. 2011, 2, 285–291. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, N.; Lu, X.; Zheng, Z.; Zhang, M.; Qiao, X. Characterization of microbial community structure and metabolic potential using Illumina MiSeq platform during the black garlic processing. Int. Food Res. J. 2018, 106, 428–438. [Google Scholar] [CrossRef]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Sci. Food Agric. 2005, 53, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Jastrzebski, Z.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Zachwieja, Z.; Barton, H.; Pawelzik, E.; Arancibia-Avila, P.; Toledo, F.; Gorinstein, S. The bioactivity of processed garlic (Allium sativum L.) as shown in vitro and in vivo studies on rats. Food Chem. Toxicol. 2007, 45, 1626–1633. [Google Scholar] [CrossRef]
- Franco, D.; Sineiro, J.; Pinelo, M.; Núñez, M.J. Ethanolic extraction of Rosa rubiginosa soluble substances: Oil solubility equilibria and kinetic studies. J. Food Eng. 2007, 79, 150–157. [Google Scholar] [CrossRef]
- Ioannou, I.; Hafsa, I.; Hamdi, S.; Charbonnel, C.; Ghoul, M. Review of the effects of food processing and formulation on flavonol and anthocyanin behaviour. J. Food Eng. 2012, 111, 208–217. [Google Scholar] [CrossRef]
- Hof, K.H.v.h.; de Boer, B.C.; Tijburg, L.B.; Lucius, B.R.; Zijp, I.; West, C.E.; Hautvast, J.G.; Weststrate, J.A. Carotenoid bioavailability in humans from tomatoes processed in different ways determined from the carotenoid response in the triglyceride-rich lipoprotein fraction of plasma after a single consumption and in plasma after four days of consumption. J. Nutr. 2000, 130, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Leelarungrayub, N.; Rattanapanone, V.; Chanarat, N.; Gebicki, J.M. Quantitative evaluation of the antioxidant properties of garlic and shallot preparations. J. Nutr. 2006, 22, 266–274. [Google Scholar] [CrossRef]
- Eichner, K. Antioxidative Effect of Maillard Reaction Intermediates: Autoxidation in Food and Biological Systems; Springer: Berlin/Heidelberg, Germany, 1980; pp. 367–385. [Google Scholar]
- Zhang, M.; Lei, N.; Zhu, T.; Zhang, Z. Thermal processing effects on the chemical constituent and antioxidant activity of s-alk (en) ylcysteine s-oxides (alliin) extract. LWT-Food Sci. Technol. 2013, 51, 309–313. [Google Scholar] [CrossRef]
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of garlic and its bioactive components. J. Nutr. 2001, 131, 955–962. [Google Scholar] [CrossRef]
- Kinalski, T.; Noreña, C.P.Z. Effect of blanching treatments on antioxidant activity and thiosulfinate degradation of garlic (Allium sativum L.). Food Bioproc. Technol. 2014, 7, 2152–2157. [Google Scholar] [CrossRef]
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. The comparison of the contents of sugar, Amadori, and Heyns compounds in fresh and black garlic. J. Food Sci. Technol. 2016, 81, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
| Components | Samples | Curing Duration (Days) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 10 | 20 | 30 | 40 | 50 | 60 | ||
| Moisture content (%) | FG | 80.07 ± 0.13 a | 72.60 ± 0.00 b | 66.78 ± 2.53 c | 59.99 ± 2.88 d | 56.44 ± 1.23 e | 51.15 ± 1.15 f | 49.95 ± 0.85 f |
| BG | - | 61.20 ± 6.38 A | 50.56 ± 5.15 B | 45.72 ± 3.58 C | 40.40 ± 2.94 D | 40.04 ± 1.38 D | 39.82 ± 0.36 D | |
| Firmness (N) | FG | 1.77 ± 0.08 a | 1.76 ± 0.05 a | 1.73 ± 0.04 ab | 1.68 ± 0.02 ab | 1.61 ± 0.01 bc | 1.53 ± 0.02 cd | 1.47 ± 0.01 d |
| BG | - | 0.05 ± 0.01 A | 0.16 ± 0.02 B | 0.22 ± 0.02 C | 0.27 ± 0.02 D | 0.35 ± 0.03 D | 0.56 ± 0.01 E | |
| pH | FG | 6.47 ± 0.01 a | 6.48 ± 0.01 a | 6.47 ± 0.01 a | 6.27 ± 0.20 a | 6.47 ± 0.01 a | 6.46 ± 0.00 a | 6.46 ± 0.00 a |
| BG | - | 4.44 ± 0.01 A | 4.37 ± 0.01 B | 4.35 ± 0.00 C | 4.04 ± 0.00 D | 4.01 ± 0.00 E | 3.79 ± 0.00 F | |
| Browning pigments (OD420) | FG | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 0.05 ± 0.00 a | 0.05 ± 0.00 a |
| BG | 0.05 ± 0.00 D | 0.05 ± 0.00 D | 0.06 ± 0.00 D | 1.52 ± 0.07 C | 3.02 ± 0.04 B | 3.41 ± 0.09 A | ||
| Free sugar (g/kg dry weight) | ||||||||
| Glucose | FG | 0.61 ± 0.02 a | 0.65 ± 0.04 a | 0.59 ± 0.00 b | 0.57 ± 0.00 bc | 0.55 ± 0.00 bc | 0.51 ± 0.01 c | 0.45 ± 0.01 d |
| BG | - | 1.54 ± 0.11 a | 0.57 ± 0.03 c | 0.59 ± 0.03 c | 0.92 ± 0.04 b | 0.82 ± 0.07 b | 0.82 ± 0.07 b | |
| Fructose | FG | 0.04 ± 0.01 a | 0.02 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| BG | - | 3.71 ± 0.25 A | 3.22 ± 0.08 AB | 3.14 ± 0.12 B | 3.67 ± 0.13 A | 3.46 ± 0.06 AB | 3.58 ± 0.22 AB | |
| Sucrose | FG | 0.32 ± 0.01 a | 0.32 ± 0.00 a | 3.22 ± 0.08 ab | 0.29 ± 0.00 c | 0.29 ± 0.00 c | 0.27 ± 0.00 d | 0.26 ± 0.00 e |
| BG | - | 0.49 ± 0.01 A | 3.22 ± 0.08 AB | 0.44 ± 0.01 B | 0.40 ± 0.01 C | 0.32 ± 0.01 D | 0.42 ± 0.02 BC | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunanta, P.; Pankasemsuk, T.; Jantanasakulwong, K.; Chaiyaso, T.; Leksawasdi, N.; Phimolsiripol, Y.; Rachtanapun, P.; Seesuriyachan, P.; Sommano, S.R. Does Curing Moisture Content Affect Black Garlic Physiochemical Quality? Horticulturae 2021, 7, 535. https://doi.org/10.3390/horticulturae7120535
Sunanta P, Pankasemsuk T, Jantanasakulwong K, Chaiyaso T, Leksawasdi N, Phimolsiripol Y, Rachtanapun P, Seesuriyachan P, Sommano SR. Does Curing Moisture Content Affect Black Garlic Physiochemical Quality? Horticulturae. 2021; 7(12):535. https://doi.org/10.3390/horticulturae7120535
Chicago/Turabian StyleSunanta, Piyachat, Tanachai Pankasemsuk, Kittisak Jantanasakulwong, Thanongsak Chaiyaso, Noppol Leksawasdi, Yuthana Phimolsiripol, Pornchai Rachtanapun, Phisit Seesuriyachan, and Sarana Rose Sommano. 2021. "Does Curing Moisture Content Affect Black Garlic Physiochemical Quality?" Horticulturae 7, no. 12: 535. https://doi.org/10.3390/horticulturae7120535
APA StyleSunanta, P., Pankasemsuk, T., Jantanasakulwong, K., Chaiyaso, T., Leksawasdi, N., Phimolsiripol, Y., Rachtanapun, P., Seesuriyachan, P., & Sommano, S. R. (2021). Does Curing Moisture Content Affect Black Garlic Physiochemical Quality? Horticulturae, 7(12), 535. https://doi.org/10.3390/horticulturae7120535













