Use of Biotechnological Methods to Support the Production of New Peach Hybrids
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Establishment of Embryoculture In Vitro
2.3. Adventitious Shoot Regeneration
2.4. Acclimatization of Plantlets
2.5. Leaf Morphological and Anatomical Examination
2.6. Methods for Assessing the Functional State of Plants
2.7. Statistical Analysis
3. Results
3.1. Embryoculture
3.2. Shoot Regeneration
3.3. Plantlet Acclimatization
3.4. Leaf Micromorphological and Anatomical Characteristics
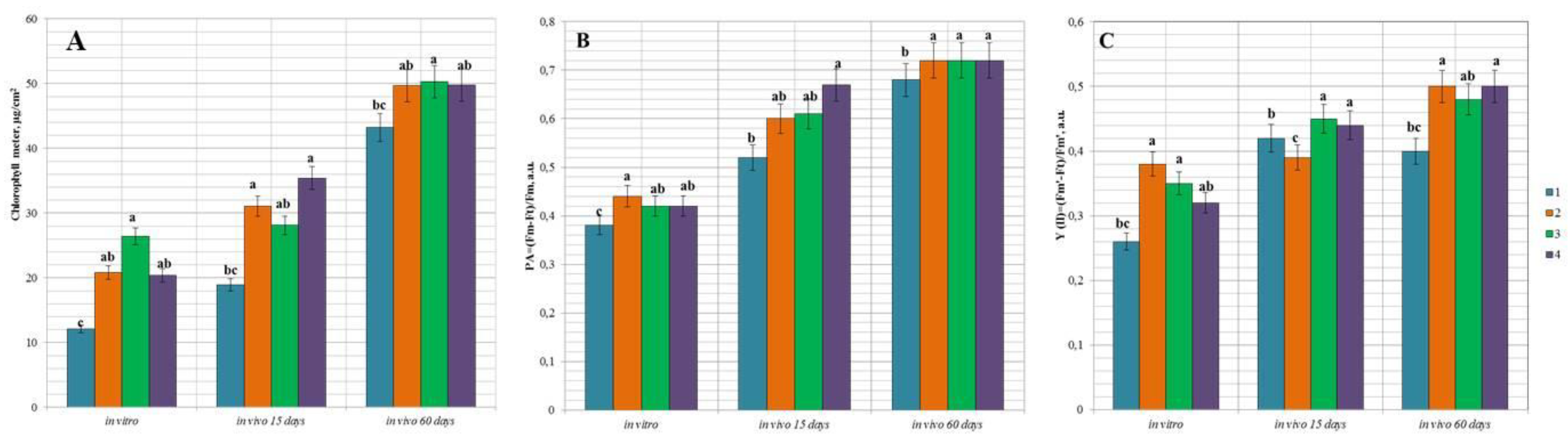
3.5. Functional State of Peach Hybrids In Vitro and during the Adaptation In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization the United Nations, FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 8 September 2021).
- Auden, A.B.; Comlekcioglu, S.; Sarier, K.; Imrak, B.; Kuden, A. Peach Breeding Studies in Turkey and the Evaluation of Peach and Nectarine Hybrids. In Breeding and Health Benefits of Fruit and Nut Crops; Nageswara-Rao, J.S.M., Ed.; IntechOpen Book: Frankfurt, Germany, 2018; pp. 47–62. [Google Scholar] [CrossRef]
- Dragavtseva, I.A.; Efimova, I.L.; Kuznetsova, A.P.; Morenets, A.S.; Dragavtsev, V.A. Effects of the “genotype-environment” interaction for fruit crops in the changing weather conditions of the South of Russia (in time and in space). Proc. Kuban State Agrar. Univ. 2017, 67, 36–43. [Google Scholar] [CrossRef]
- Smykov, A.V.; Ivashchenko, I.A.; Fedorova, O.S. The influence of climatic factors of the environment of the Southern Coast of the Crimea on the productivity of hybrid forms of peach. Bull. State Nikita Bot. Gard. 2018, 126, 76–81. [Google Scholar] [CrossRef]
- Umer, M.; Liu, J.; You, H.; Xu, C.; Dong, K.; Luo, N.; Kong, L.; Li, X.; Hong, N.; Wang, G.; et al. Genomic, Morphological and Biological Traits of the Viruses Infecting Major Fruit Trees. Viruses 2019, 11, 515. [Google Scholar] [CrossRef] [PubMed]
- Cambra, M.; Capote, N.; Myrta, A.; Llбcer, G. Plum pox virus and the estimated costs associated with sharka disease. EPPO Bull. 2006, 36, 202–204. [Google Scholar] [CrossRef]
- Osman, F.; Rwahnih, M.A.; Golino, D.; Pitman, T.; Cordero, F.; Preece, J.E.; Rowhani, A. Short communication evaluation of the phytosanitary status of the Prunus species in the Natiional clonal germplasm repository in California: Survey of viruses and viroids. J. Plant Pathol. 2012, 94, 249–253. [Google Scholar]
- Sochor, J.; Babula, P.; Adam, V.; Krska, B.; Kizek, R. Sharka: The Past, The Present and The Future. Viruses 2012, 4, 2853–2901. [Google Scholar] [CrossRef] [PubMed]
- Barba, M.; Ilardi, V.; Pasguini, G. Control of Pome and Stone Fruit Diseases. Adv. Virus Res. 2015, 91, 47–83. [Google Scholar] [CrossRef]
- Mitrofanova, I.; Mitrofanova, O.; Chirkov, S.; Lesnikova-Sedoshenko, N.; Chelombit, S. Detection and inditification of Plum pox virus on Prunus species in Crimea. Agric. For. 2015, 61, 197–204. [Google Scholar] [CrossRef]
- Janick, J. Origin, domestication, and early culture of fruit crops. In Plant Breeding Reviews. The Origins of Fruits, Fruit Growing, and Fruit Breeding; Janick, J., Ed.; Department of Horticulture and Landcape Purdue University: West Lafayette, IN, USA, 2005; Volume 25, pp. 255–321. [Google Scholar]
- Romano, A.; Martins-Loução, M.A. Water loss and morphological modifications in leaves during acclimatization of cork oak micropropagated plantlets. Acta Hortic. 2003, 616, 439–442. [Google Scholar] [CrossRef]
- Apostolo, N.M.; Brutti, C.B.; Llorente, B.E. Leaf anatomy of Cynara scolymus L. in successive micropropagation stages. Vitr. Cell. Dev. Biol. Plant 2005, 41, 307–313. [Google Scholar] [CrossRef]
- Werner, E.T.; Milanez, C.R.D.; Gontijo, A.B.P.L.; Soares, T.C.B.; Amaral, J.A.T. Do Leaf anatomy changes related to cultivate in vivo and in vitro and during pre-acclimatization of Crambe abyssinica Hochst. Plant Cell Cult. Micropropag. 2018, 14, 10–17. [Google Scholar]
- Mitrofanova, O.V.; Brailko, V.A.; Zhdanova, I.V.; Borkuta, A.I.; Andreev, M.S.; Mitrofanova, I.V. Ex vitro morphological and anatomical features of lavender and lavandin microplants. Acta Hortic. 2020, 1285, 23–30. [Google Scholar] [CrossRef]
- Jagiełło-Kubiec, K.; Nowakowska, K.; Łukaszewska, A.J.; Pacholczak, A. Acclimation to Ex Vitro Conditions in Ninebark. Agronomy 2021, 11, 612. [Google Scholar] [CrossRef]
- Kozai, T. Micropropagation under photoautotrophic conditions. In Micropropagation; Debergh, P.C., Zimmerman, R.H., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 447–469. [Google Scholar] [CrossRef]
- Serret, M.D.; Trillas, M.I. Effects of light and sucrose levels on the anatomy, ultrastructure, and photosynthesis of Gardenia jasminoides Ellis leaflets cultured in vitro. Int. J. Plant Sci. 2000, 161, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Mathur, A.K.; Verma, P.; Yadav, S.; Gupta, M.L.; Darokar, M.P. Biological hardening and genetic fidelity testing of micro-cloned progeny of Chlorophytum borivilianum Sant. et Fernand. Afr. J. Biotechnol. 2008, 7, 1046–1053. [Google Scholar]
- Chandra, S.; Bandopadhyay, R.; Kumar, V.; Chandra, R. Acclimatization of tissue cultured plantlets: From laboratory to land. Biotechnol. Lett. 2010, 32, 1199–1205. [Google Scholar] [CrossRef]
- Dousseau, S.; Alvarenga, A.A.D.; Castro, E.M.D.; Soares, R.P.; Emrich, E.B.; Melo, L.A.D. Leaf anatomy of Tabebuia serratifolia (Vahl) Nich.(Bignoniaceae) propagated in vitro, in vivo and during the acclimatization. Ciência e Agrotecnologia 2008, 32, 1694–1700. [Google Scholar] [CrossRef]
- Mayer, J.L.S.; Ribas, L.L.F.; Bona, C.; Quoirin, M. Anatomia comparada das folhas e raízes de Cymbidium Hort. (Orchidaceae) cultivadas ex vitro e in vitro. Acta Bot. Bras. 2008, 22, 323–332. [Google Scholar] [CrossRef]
- Braga, F.T.; Pasqual, M.; Castro, E.M.D.; Rafael, G.C.; Favero, A.C.; Valente, T.C.T. Alterações morfofisiolócias de plantas de abacaxizeiro influenciadas por diferentes substratos durante o processo de aclimatização. Ciência Agrotecnol. 2011, 35, 863–868. [Google Scholar] [CrossRef][Green Version]
- Kutas, E.; Ogorodnyk, L. Acclimatization results of micropropagated plantlets. Afr. J. Plant Sci. 2015, 9, 124–127. [Google Scholar] [CrossRef]
- Godoy, S.; Tapia, E.; Seit, P.; Andrade, D.; Sánchez, E.; Andrade, P.; Prieto, H. Temporary immersion systems for the mass propagation of sweet cherry cultivars and cherry rootstocks: Development of a micropropagation procedure and effect of culture conditions on plant quality. Vitr. Cell. Dev. Biol. Plant 2017, 53, 494–504. [Google Scholar] [CrossRef]
- Copetta, A.; Bazzicalupo, M.; Cassetti, A.; Marchioni, I.; Mascarello, C.; Cornara, L.; Pistelli, L.; Ruffoni, B. Plant Production and Leaf Anatomy of Mertensia maritima (L.) Gray: Comparison of In Vitro Culture Methods to Improve Acclimatization. Horticulturae 2021, 7, 111. [Google Scholar] [CrossRef]
- Shin, K.S.; Park, S.Y.; Paek, K.Y. Sugar metabolism, photosynthesis, and growth of in vitro plantlets of Doritaenopsis under controlled microenvironmental conditions. Vitr. Cell. Dev. Biol. Plant 2013, 49, 445–454. [Google Scholar] [CrossRef]
- Pérez, L.; Padrón, Y.; González, J.; Rodríguez, R.; Norman, O.; Barbón, R.; Hurtado, O.; Rodríguez, R.; Daniels, D.; Gómez-Kosky, R. Effects of different culture conditions (photoautotrophic, photomixotrophic) and the auxin indole-butyric acid on the in vitro acclimatization of papaya (Carica papaya L. var. Red Maradol) plants using zeolite as support. Afr. J. Biotech. 2015, 14, 2622–2635. [Google Scholar] [CrossRef]
- Mitrofanova, O.V.; Mitrofanova, I.V.; Smykov, A.V.; Lesnikova, N.P. Biotechnological methods in subtropical and stone fruit crops breeding and reproduction. Work. State Nikita Bot. Gard. 1999, 118, 189–199. (In Russian) [Google Scholar]
- Zdruykovskaya-Richter, A.I. Embryo Culture of Isolated Embryos, Generative Structures and Obtaining New Plant Forms, 1st ed.; KrymFarm-Trejding: Yalta, Ukraine, 2003; 368p. (In Russian) [Google Scholar]
- Kyte, L.; Kleyn, J.; Scoggins, H.; Bridgen, M. Plants from Test Tubes: An Introduction of Micropropagation, 4th ed.; Timber Press: Portland, OR, USA, 2013; 274p. [Google Scholar]
- Monnier, M. Croissance et dйveloppement des embryons globulaires de Capsella Bursa-pastoris cultivйs in vitro dans un milieu а base d’une nouvelle solution minйrale. Bull. Soc. Bot. Fr. Mem. Coll. Morphol. 1973, 120, 179–194. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Eveleigh, D.E. Culture methods and detection of glucanases in cultures of wheat and barley. Can. J. Biochem. 1968, 46, 417–421. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Pausheva, Z.P. Practicum in Plant Cytology, 4th ed; Kolos: Moscow, Russia, 1988; 271p. (In Russian) [Google Scholar]
- Stirbet, A.; Govindjee, J. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 2011, 104, 236–257. [Google Scholar] [CrossRef] [PubMed]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Casa, R.; Castaldi, F.; Pascucci, S.; Pignatti, S. Chlorophyll estimation in field crops: An assessment of handheld leaf meters and spectral reflectance measurements. J. Agric. Sci. 2015, 153, 876–890. [Google Scholar] [CrossRef]
- Mitrofanova, I.V.; Smykov, A.V.; Mitrofanova, O.V.; Lesnikova-Sedoshenko, N.P.; Chirkov, S.N.; Zhdanova, I.V. Using in vitro embryo culture for obtaining new breeding forms of peach. Acta Hortic. 2020, 1289, 159–165. [Google Scholar] [CrossRef]
- Rout, G.R.; Mohapatra, A.; Jain, S.M. Tissue culture of ornamental pot plant: A critical review on present scenario and future prospects. Biotechnol. Adv. 2006, 24, 531–560. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.-J. (Eds.) Plant Propagation by Tissue Culture, 3rd ed.; Springer: Dordrecht, The Netherlands, 2008; Volume 1, 501p. [Google Scholar] [CrossRef]
- Goncalves, S.; Romano, A. Micropropagation of Lavandula spp. In Methods in Molecular Biology; Lambardi, M., Ozudogru, E.A., Jain, S.M., Eds.; Springer Science+Business Media: New York, NY, USA, 2013; Volume 994, pp. 189–198. [Google Scholar]
- Lambardi, M.; Ozudogru, E.A.; Jain, S.M. (Eds.) Protocols for Micropropagation of Selected Economically-Important Horticultural Plants (Methods in Molecular Biology); Springer Science+Business Media: New York, NY, USA, 2013; Volume 994, 490p. [Google Scholar]
- Mitrofanova, I.V. Somatic Embryogenesis and Organogenesis as a Base of Biotechnology Perennial Horticultural Plants Obtaining and Conservation, 1st ed.; Agrarnanauka: Kiev, Ukraina, 2011; 344p. [Google Scholar]
- Bidabadi, S.S.; Jain, S.M. Cellular, Molecular, and Physiological Aspects of In Vitro Plant Regeneration (Review). Plants 2020, 9, 702. [Google Scholar] [CrossRef] [PubMed]
- Mitrofanova, I.V.; Lesnikova-Sedoshenko, N.P.; Brailko, V.A.; Chelombit, S.V.; Zhdanova, I.V.; Mitrofanova, O.V. Biotechnological methods of propagation for some rare endemic plant species of the Southern Russian flora. Acta Hortic. 2020, 1285, 177–184. [Google Scholar] [CrossRef]
- Al Ghasheem, N.; Stănică, F.; Peticilă, A.G. Overview of studies on in vitro propagation of peach. Acta Hortic. 2021, 1304, 147–154. [Google Scholar] [CrossRef]
- Anderson, N.; Byrne, D.H.; Sinclair, J.; Burrell, A.M. Cooler Temperature During Germination Improves the Survival of Embryo Cultured Peach Seed. HortScience 2002, 37, 402–403. [Google Scholar] [CrossRef]
- Ghayyad, M.A. Effects of gibberellic acid and low temperature on germination of some Prunus species embryos (without cotyledons) under laboratory conditions. Acta Hortic. Regiotect. 2018, 2, 42–47. [Google Scholar] [CrossRef]
- Devi, I.; Singh, H.; Thakur, A. Effect of developmental stage and medium on embryo culture of low chill peach hybrids. Curr. Sci. 2017, 113, 1771–1775. [Google Scholar] [CrossRef]
- Daorden, M.E.; Marín, J.A.; Arbeloa, A. Stratification temperature affects the in vitro germination of immature Prunus embryos. Acta Hortic. 2004, 658, 135–140. [Google Scholar] [CrossRef]
- Ramming, D.W. The Use of Embryo Culture in Fruit Breeding. HortScience 1990, 25, 393–398. [Google Scholar] [CrossRef]
- Damiano, C.; Della, S.G.; Quarta, R. Studies on nutrient media for in vitro culture of early peach cultivars. Ann. De ll’ Ist. Sper. per la Fruttic. 1976, 7, 47–50. [Google Scholar]
- Yae, B.W.; Cho, H.M.; Yim, Y.J.; Kang, S.J. Studies on embryo culture of peaches (Prunus persica). The influence of medium, fruit harvest day, low temperature storage and pre treatments on growth and development of embryos in vitro. J. Korean Soc. Hort. Sci. 1986, 27, 167–173. [Google Scholar]
- Srivastav, M.; Singh, S.K.; Arora, R.L.; Krishna, B. Embryo Culture Studies in Subtropical Peach (Prunus persica Batsch.). Acta Hortic. 2004, 662, 297–301. [Google Scholar] [CrossRef]
- Şan, B.; Yildirim, A.N.; Yildirim, F. An In Vitro Germination Technique for Some Stone Fruit Species: The Embryo Isolated from Cotyledons Successfully Germinated without Cold Pre-treatment of Seeds. HortScience 2014, 49, 294–296. [Google Scholar] [CrossRef]
- Nagaty, M.A. Establishment of Regeneration system for Taif peach (Prunus persica L. Batsch) cultivar (Balady cultivar) in Taif, KSA. J. Am. Sci. 2012, 8, 232–239. [Google Scholar]
- Monnier, M. Culture of zygotic embryos. In Frontiers of Plant Tissue Culture; Thorpe, T.A., Ed.; University of Calgary Press: Calgary, AB, Canada, 1978; pp. 277–286. [Google Scholar]
- Bridgen, M.P. A Review of Plant Embryo Culture. HortScience 1994, 29, 1243–1246. [Google Scholar] [CrossRef]
- Marchi, S.; Tognetti, R.; Minnocci, A.; Borghi, M.; Sebastiani, L. Variation in mesophyll anatomy and photosynthetic capacity during leaf development in a deciduous mesophyte fruit tree (Prunus persica) and an evergreen sclerophyllous Mediterranean shrub (Olea europaea). Trees 2008, 22, 559–571. [Google Scholar] [CrossRef]
- Golubkova, I.M. Micromorphological structure in some Persica Mill. species in the M.M. Hryshko National Botanical Garden. Bull. Probl. Biol. Med. 2016, 2, 38–41. (In Ukraine) [Google Scholar]
- Shoferistova, E.G.; Smykov, A.V. Anatomical features of peach leaves after the effect of chemical mutagens. Bull. State Nikitsk. Bot. Gard. 2007, 95, 30–35. (In Russian) [Google Scholar]
- Calvete, E.O.; Azevedo, M.; Bordignon, M.H.; Suzin, M. Análises anatômicas e da biomassa em plantas de morangueiro cultivadas in vitro e ex vitro. Hortic. Bras. 2002, 20, 649–653. [Google Scholar] [CrossRef][Green Version]
- Yang, S.H.; Yeh, D.M. In vitro leaf anatomy, ex vitro photosynthetic behaviors and growth of Calathea orbifolia (Linden) Kennedy plants obtained from semi-solid medium and temporary immersion systems. Plant Cell Tissue Organ Cult. 2008, 93, 201–207. [Google Scholar] [CrossRef]
- Batagin-Piotto, K.D.; Almeida, C.V.D.; Piotto, F.A.; Almeida, M.D. Anatomical analysis of peach palm (Bactris gasipaes) leaves cultivated in vitro, ex vitro and in vivo. Braz. J. Bot. 2012, 35, 71–78. [Google Scholar] [CrossRef]
- Mitrofanova, I.V.; Tevfik, A.S.; Mitrofanova, O.V.; Brailko, V.A.; Lesnikova-Sedoshenko, N.P. Features of Canna regeneration in vitro and plantlets adaptation in vivo. Acta Hortic. 2017, 1155, 447–454. [Google Scholar] [CrossRef]
- Sarikhani, H.; Sarikhani-Khorami, H. Effect of light quality on micropropagation and some morphological properties of cadaman avimag (Prunus persica × P. davidiana) rootstock. Int. J. Hortic. Sci. Technol. 2021, 8, 51–65. [Google Scholar] [CrossRef]
- Bacelar, E.A.; Correia, C.M.; Moutinho-Pereira, J.M.; Gonçalves, B.C.; Lopes, J.I.; Torres-Pereira, J.M. Sclerophylly and leaf anatomical traits of five field-grown olive cultivars growing under drought conditions. Tree Physiol. 2004, 24, 233–239. [Google Scholar] [CrossRef]
- Oliveira, I.; Meyer, A.; Afonso, S.; Gonçalves, B. Compared leaf anatomy and water relations of commercial and traditional Prunus dulcis (Mill.) cultivars under rain-fed conditions. Sci. Hortic. 2018, 229, 226–232. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Bosabalidis, A.; Patakas, A.; Vemmos, S. Effects of water stress on water relations, gas exchange and leaf structure of olive tree. Acta Hortic. 2000, 537, 241–247. [Google Scholar] [CrossRef]
- Palasciano, M.; Camposeo, S.; Godini, A. Stomatal size and frequency in wild (A. webbii) and cultivated (A. communis) almonds. In XIII GREMPA Meeting on Almonds and Pistachios. Options Méditerranéennes; Série, A., Oliveira, M.M., Cordeiro, V., Eds.; CIHEAM: Zaragoza, Spain, 2005; Volume 63, pp. 305–310. [Google Scholar]
- Camposeo, S.; Palasciano, M.; Vivaldi, G.A.; Godini, A. Effect of increasing climatic water deficit on some leaf and stomatal parameters of wild and cultivated almonds under Mediterranean conditions. Sci. Hortic. 2011, 127, 234–241. [Google Scholar] [CrossRef]
- Park, S.Y.; Moon, H.K.; Murthy, H.N.; Kim, Y.W. Improved growth and acclimatization of somatic embryo-derived Oplopanax elatus plantlets by ventilated photoautotrophic culture. Biol. Plant. 2011, 55, 559–562. [Google Scholar] [CrossRef]
- Xiao, Y.; Niu, G.; Kozai, T. Development and application of photoautotrophic micropropagation plant system. Plant. Cell Tissue Organ. Cult. 2011, 105, 149–158. [Google Scholar] [CrossRef]
- Xi, Y.; Jiang, W.; Wen, Y.; Han, J.; Zhang, B.; Ma, R. Comparison of leaf chlorophyll fluorescence characteristics of different growth types of ornamental peach. J. Nanjing Agric. Univ. 2017, 40, 234–241. [Google Scholar]
- Scalisi, A.; Pelliccia, D.; O’Connell, M.G. Maturity Prediction in Yellow Peach (Prunus persica L.) Cultivars Using a Fluorescence Spectrometer. Sensors 2020, 20, 6555. [Google Scholar] [CrossRef]
- Li, Q.; Wei, Z.; Gao, D.; Si, P.; Yu, H.; Liu, J. Effects of herbicide drift on chlorophyll fluorescence and antioxidant enzyme levels of various types of fruit trees. Pak. J. Bot. 2021, 53, 847–857. [Google Scholar] [CrossRef]
- Wang, H.; Wang, F.; Wang, G.; Majourhat, K. The responses of photosynthetic capacity, chlorophyll fluorescence and chlorophyll content of nectarine (Prunus persica var. Nectarina Maxim) to greenhouse and field grown conditions. Sci. Hortic. 2007, 112, 66–72. [Google Scholar] [CrossRef]
- Serret, M.D.; Trillas, M.I.; Araus, J.L. The effect of in vitro culture conditions on the pattern of photoinhibition during acclimation of gardenia plantlets to ex vitro conditions. Photosynthetica 2001, 39, 67–73. [Google Scholar] [CrossRef]
- Jiménez, S.; Dridi, J.; Gutiérrez, D.; Moret, D.; Irigoyen, J.J.; Moreno, M.A.; Gogorcena, Y. Physiological, biochemical and molecular responses in four Prunus rootstocks submitted to drought stress. Tree Physiol. 2013, 33, 1061–1075. [Google Scholar] [CrossRef] [PubMed]





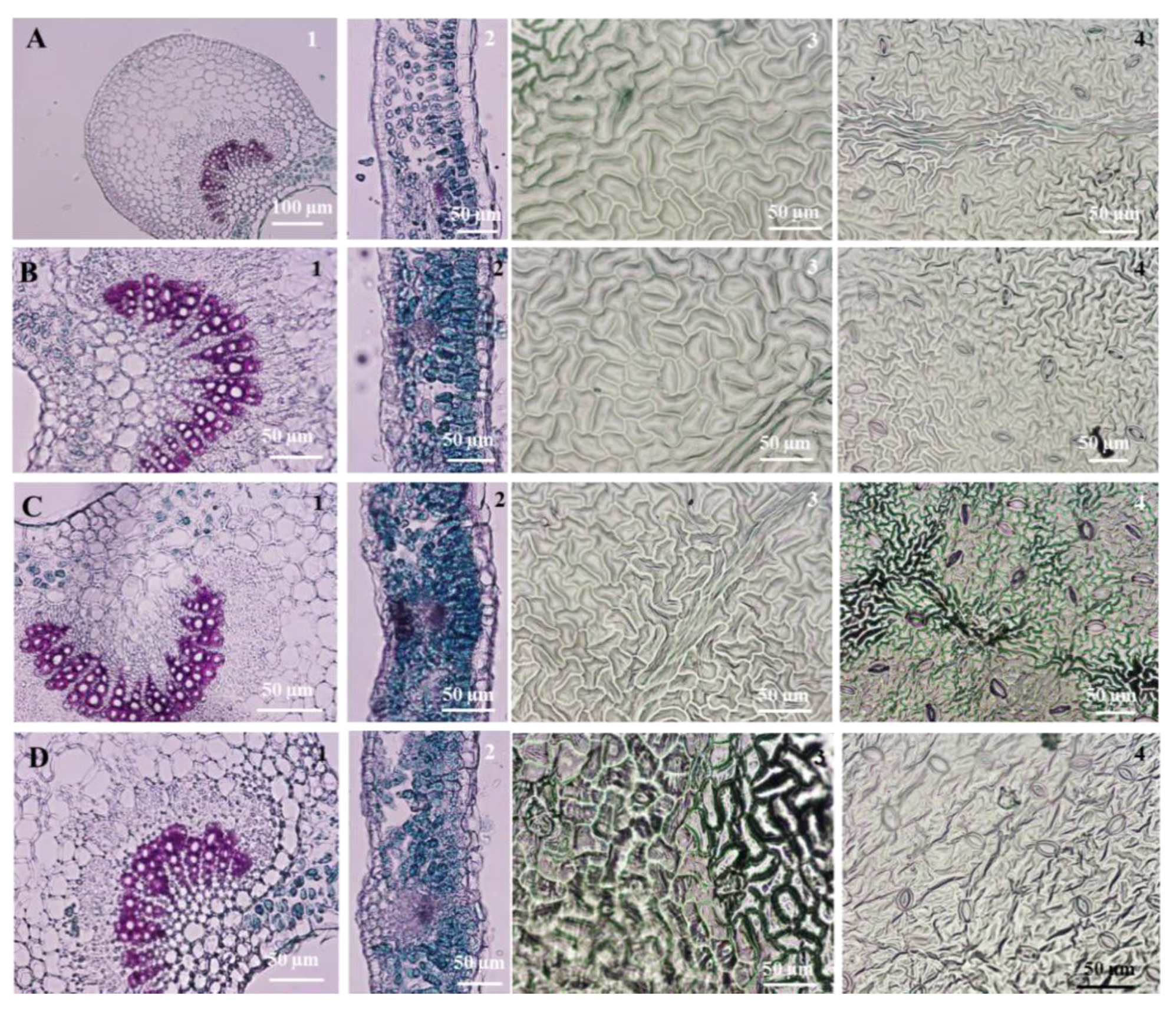

| Genotype | Days after Initial Culture | |||||
|---|---|---|---|---|---|---|
| 45 | 60 | |||||
| Length (cm) * | No. Leaves per Explant * | Length (cm) * | No. Leaves * per xplant | |||
| Shoot | Root | Shoot | Root | |||
| ‘Clyde Wilson’ × ‘Nikitskiy Podarok’ | 0 b,c | 0.82 ± 0.07 b,c | 0 c | 1.78 ± 0.18 b,c | 1.71 ± 0.18 b,c | 2.35 ± 0.31 b,c |
| ‘Jerseyglo’ × ‘Nikitskiy Podarok’ | 0.68 ± 0.07 a | 0.99 ± 0.30 a,b | 0.17 ± 0.09 a,b | 2.66 ± 0.28 a,b | 3.09 ± 0.30 a,b | 2.85 ± 0.18 a,b |
| ‘Loadel’ × ‘Nikitskiy Podarok’ | 0.89 ± 0.06 a | 1.34 ± 0.12 a | 0.35 ± 0.13 a | 3.06 ± 0.23 a,b | 4.42 ±0.59 a | 3.60 ± 0.29 a |
| ‘Summerglo’ × ‘Nikitskiy Podarok’ | 0.52 ± 0.07 a,b | 0.99 ± 0.14 a,b | 0.15 ± 0.11 a,b | 2.68 ± 0.25 a | 3.54 ± 0.29 a,b | 3.20 ± 0.19 a |
| Genotype | Length of the Embryo, cm | Regeneration Frequency *, % | |
|---|---|---|---|
| Culture Medium without PGRs | Culture Medium with 0.4 mg L−1 Kinetin and 0.1 mg L−1 GA3 | ||
| ‘Clyde Wilson’ × ‘Nikitskiy Podarok’ | 0.3–1.0 | 3.70 e,f | 11.11 e,f |
| 1.1–2.0 | 62.96 a,b | 51.85 a,b | |
| ‘Jerseyglo’ × ‘Nikitskiy Podarok’ | 0.3–1.0 | 7.41 c,d | 14.81 c,d |
| 1.1–2.0 | 85.19 a,b | 74.07 a,b | |
| ‘Loadel’ × ‘Nikitskiy Podarok’ | 0.3–1.0 | 18.51 b,c | 29.62 a,b |
| 1.1–2.0 | 96.30 a | 88.89 a | |
| ‘Summerglo’ × ‘Nikitskiy Podarok’ | 0.3–1.0 | 14.81 bc | 22.22 bc |
| 1.1–2.0 | 92.59 a | 81.48 a | |
| Culture Media | PGRs (mg L−1) | ‘Clyde Wilson’ × ‘Nikitskiy Podarok’ * | ‘Jerseyglo’ × ‘Nikitskiy Podarok’ * | ‘Loadel’ × ‘Nikitskiy Podarok’ * | ‘Summerglo’ × ‘Nikitskiy Podarok’ * | |||
|---|---|---|---|---|---|---|---|---|
| BAP | TDZ | IBA | IAA | |||||
| B5 (control) | 0 | 0 | 0 | 0 | 0.38 ± 0.12 c | 0.51 ± 0.11 b | 0.72 ± 0.12 a | 1.08 ± 0.12 a |
| B5 | 0.5 | 0 | 0.1 | 0 | 2.08 ± 0.15 c | 3.25 ± 0.09 a | 3.13 ± 0.23 a,b | 3.67 ± 0.20 a |
| 0.5 | 0 | 0.2 | 0 | 2.21 ± 0.13 c | 3.18 ± 0.09 a | 2.46 ± 0.19 a,b | 4.18 ± 0.13 a | |
| 0.75 | 0 | 0.1 | 0 | 3.03 ± 0.09 b,c | 3.72 ± 0.18 a,b | 4.95 ± 0.18 a | 5.18 ± 0.18 a | |
| 0.75 | 0 | 0.2 | 0 | 2.87 ± 0.12 c,d | 3.43 ± 0.09 b | 4.03 ± 0.11 a | 4.64 ± 0.19 a | |
| 1.0 | 0 | 0.1 | 0 | 2.79 ± 0.11 c | 3.79 ± 0.16 a | 3.18 ± 0.26 a,b | 4.31 ± 0.22 a | |
| 1.0 | 0 | 0.2 | 0 | 2.51 ± 0.12 bc | 2.95 ± 0.27 a,b | 3.59 ± 0.19 a | 3.54 ± 0.19 a | |
| MS (control) | 0 | 0 | 0 | 0 | 0.28 ± 0.09 c | 0.51 ± 0.14 b | 0.77 ± 0.14 a | 0.95 ± 0.13 a |
| MS | 0 | 1.27 | 0 | 0 | 1.82 ± 0.21 c | 2.33 ± 0.20 a,b | 2.92 ± 0.23 a | 3.03 ± 0.21 a |
| 1.0 | 0 | 0 | 1.0 | 2.67 ± 0.12 c | 2.82 ± 0.17 a,b | 3.26 ± 0.23 a | 4.51 ± 0.09 a | |
| 1.5 | 0 | 0 | 1.5 | 3.13 ± 0.08 c | 3.33 ± 0.21 ab | 4.33 ± 0.13 a | 4.79 ± 0.29 a | |
| 2.0 | 0 | 0 | 2.0 | 1.41 ± 0.18 c,d | 3.85 ± 0.22 a | 3.41 ± 0.23 a,b | 4.64 ± 0.21 a | |
| Parameters | Cross Combinations | In Vitro * | Ex Vitro * | Iv Vivo * |
|---|---|---|---|---|
| 15 Days | 60 Days | |||
| Leaf blade thickness, µm | ‘Clyde Wilson’ × ‘Nikitskiy Podarok’ | 97.2 ± 2.4 d,e | 100.3 ± 3.3 b,c | 105.5 ± 1.8 a,b |
| ‘Jerseyglo’ × ‘Nikitskiy Podarok’ | 103.3 ± 1.3 b,c | 113.5 ± 2.2 ab | 119.0 ± 1.4 a | |
| ‘Loadel’ × ‘Nikitskiy Podarok’ | 98.7 ± 2.6 c,d | 114.3 ± 2.5 ab | 127.2 ± 2.7 a | |
| ‘Summerglo’ × ‘Nikitskiy Podarok’ | 100.6 ± 1.4 b,c | 108.5 ± 1.2 ab | 116.3 ± 3.0 a | |
| Palisade mesophyll/thickness, µm | ‘Clyde Wilson’ × ‘Nikitskiy Podarok’ | 51.3 ± 1.8 f,g | 51.6 ± 2.0 e,f | 52.3 ± 1.3 c,d |
| ‘Jerseyglo’ × ‘Nikitskiy Podarok’ | 59.5 ± 1.1 a,b | 63.5 ± 1.3 a,b | 58.3 ± 1.2 a,b | |
| ‘Loadel’ × ‘Nikitskiy Podarok’ | 51.6 ± 0.8 e,f | 56.3 ± 2.4 b,c | 69.0 ± 1.5 a | |
| ‘Summerglo’ × ‘Nikitskiy Podarok’ | 52.4 ± 1.4 c,d | 53.9 ±1.3 b,c | 58.3 ± 1.1 a,b | |
| Adaxial epidermis thickness, µm | ‘Clyde Wilson’ × ‘Nikitskiy Podarok’ | 13.5 ± 0.6 c,d | 14.6 ± 0.9 bc | 14.9 ± 0.7 a,b |
| ‘Jerseyglo’ × ‘Nikitskiy Podarok’ | 13.6 ± 0.7 c,d | 16.1 ± 0.7 ab | 16.9 ± 0.7 a,b | |
| ‘Loadel’ × ‘Nikitskiy Podarok’ | 15.2 ± 0.6 a,b | 18.1 ± 0.5 a,b | 18.8 ± 0.7 a | |
| ‘Summerglo’ × ‘Nikitskiy Podarok’ | 18.1 ± 0.6 a,b | 20.0 ± 0.4 a | 19.5 ± 0.8 a | |
| Abaxial epidermis thickness, µm | ‘Clyde Wilson’ × ‘Nikitskiy Podarok’ | 7.5 ± 0.3 f,g | 8.7 ± 0.4 d,e | 11.7 ± 0.7 c,d |
| ‘Jerseyglo’ × ‘Nikitskiy Podarok’ | 16.9 ± 0.5 a | 19.1 ± 0.4 a | 17.1 ± 0.7 a | |
| ‘Loadel’ × ‘Nikitskiy Podarok’ | 12.1 ± 0.5 b,c | 14.3 ± 0.6 a,b | 15.1 ± 0.7 a,b | |
| ‘Summerglo’ × ‘Nikitskiy Podarok’ | 12.4 ± 0.5 b | 14.7 ± 0.6 a,b | 14.1 ± 0.7 a,b | |
| Stomata number per 1 mm2 surface on the abaxial leaf side | ‘Clyde Wilson’ × ‘Nikitskiy Podarok’ | 157 ± 5 a,b | 95 ± 3 c,d | 139 ± 2 a,b |
| ‘Jerseyglo’ × ‘Nikitskiy Podarok’ | 115 ± 4 b,c | 85 ± 4 f,g | 159 ± 2 a,b | |
| ‘Loadel’ × ‘Nikitskiy Podarok’ | 182 ± 5 a | 95 ± 2 c,d | 147 ± 3 a,b | |
| ‘Summerglo’ × ‘Nikitskiy Podarok’ | 207 ± 4 a | 93 ± 2 d,e | 133 ± 4 a,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitrofanova, I.; Lesnikova-Sedoshenko, N.; Tsiupka, V.; Smykov, A.; Mitrofanova, O. Use of Biotechnological Methods to Support the Production of New Peach Hybrids. Horticulturae 2021, 7, 533. https://doi.org/10.3390/horticulturae7120533
Mitrofanova I, Lesnikova-Sedoshenko N, Tsiupka V, Smykov A, Mitrofanova O. Use of Biotechnological Methods to Support the Production of New Peach Hybrids. Horticulturae. 2021; 7(12):533. https://doi.org/10.3390/horticulturae7120533
Chicago/Turabian StyleMitrofanova, Irina, Nina Lesnikova-Sedoshenko, Valentina Tsiupka, Anatoliy Smykov, and Olga Mitrofanova. 2021. "Use of Biotechnological Methods to Support the Production of New Peach Hybrids" Horticulturae 7, no. 12: 533. https://doi.org/10.3390/horticulturae7120533
APA StyleMitrofanova, I., Lesnikova-Sedoshenko, N., Tsiupka, V., Smykov, A., & Mitrofanova, O. (2021). Use of Biotechnological Methods to Support the Production of New Peach Hybrids. Horticulturae, 7(12), 533. https://doi.org/10.3390/horticulturae7120533







