Geographical Origin Affects the Nut Traits, Bioactive Compounds, and Fatty Acid Composition of Turkish Hazelnut Cultivars (Corylus avellana L. cvs. Çakıldak, Palaz, and Tombul)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
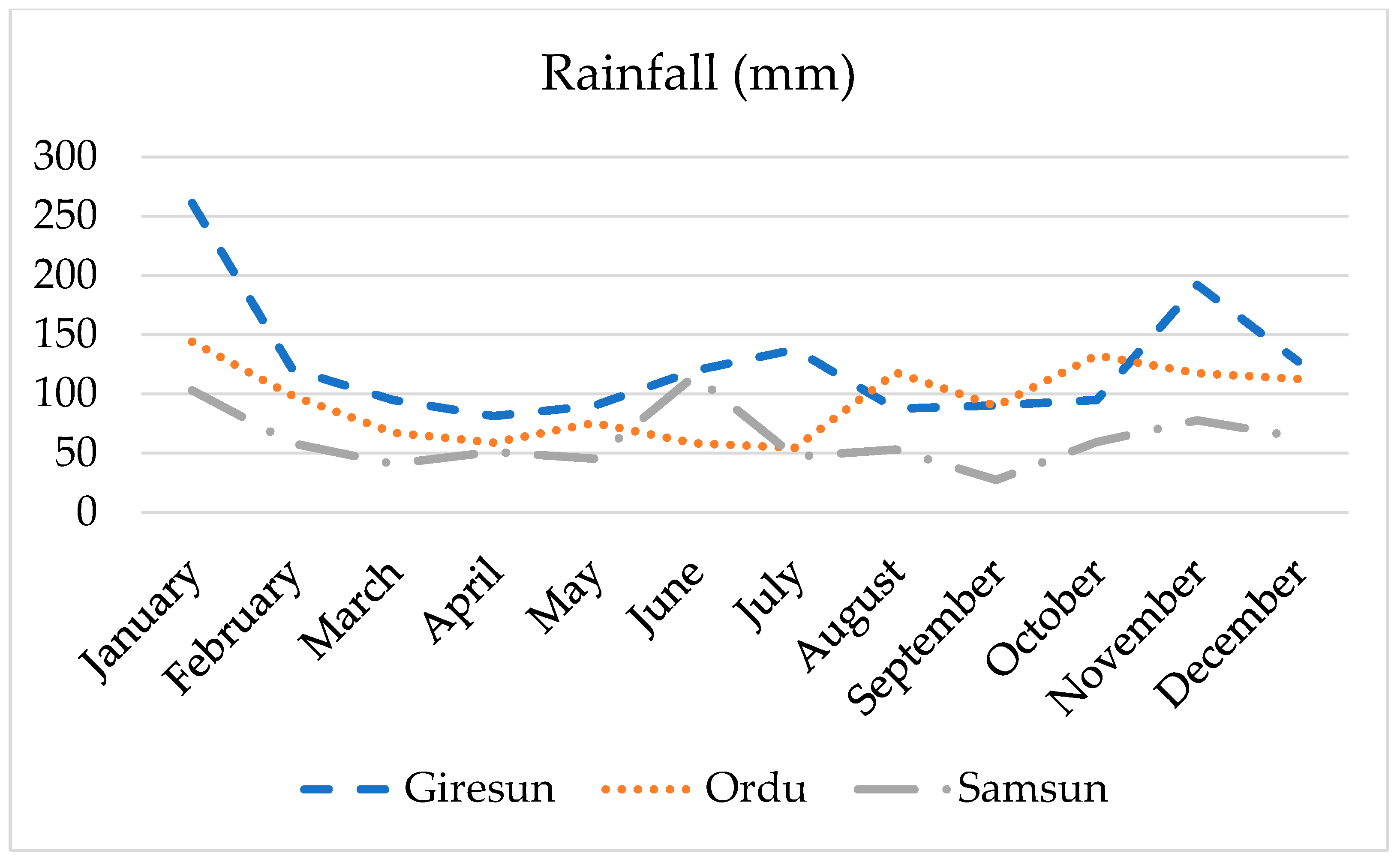
2.2. Experimental Design
2.3. Nut Traits
2.4. Sample Preparation of Bioactive Compounds
2.5. Total Phenolics
2.6. Total Flavonoids
2.7. Antioxidant Activity (AA)
2.7.1. DPPH
2.7.2. FRAP
2.8. Fatty Acid Composition
2.9. Statistical Analysis
3. Results and Discussion
3.1. Nut Traits
3.2. Bioactive Compounds
3.3. Fatty Acid Composition
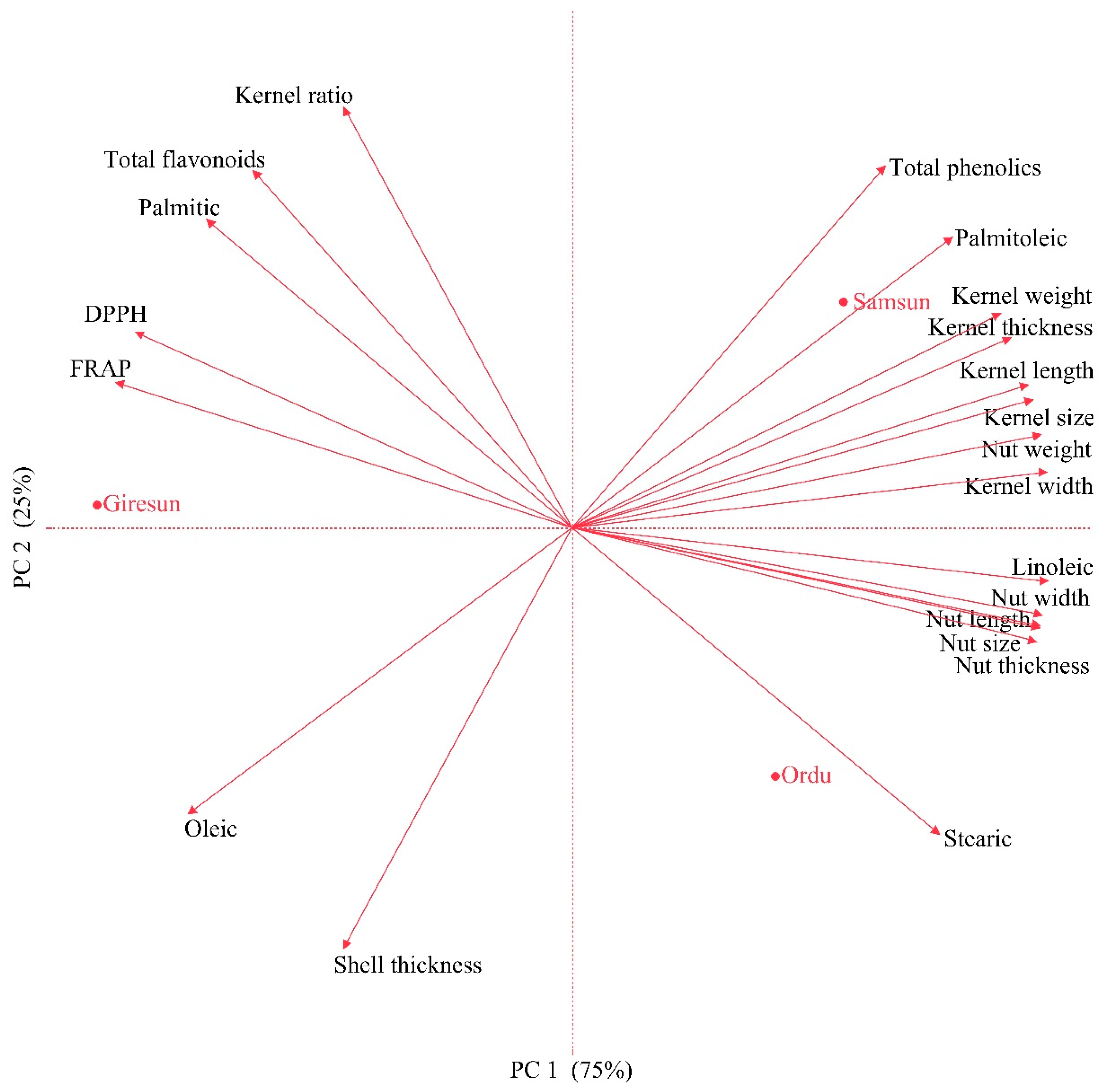
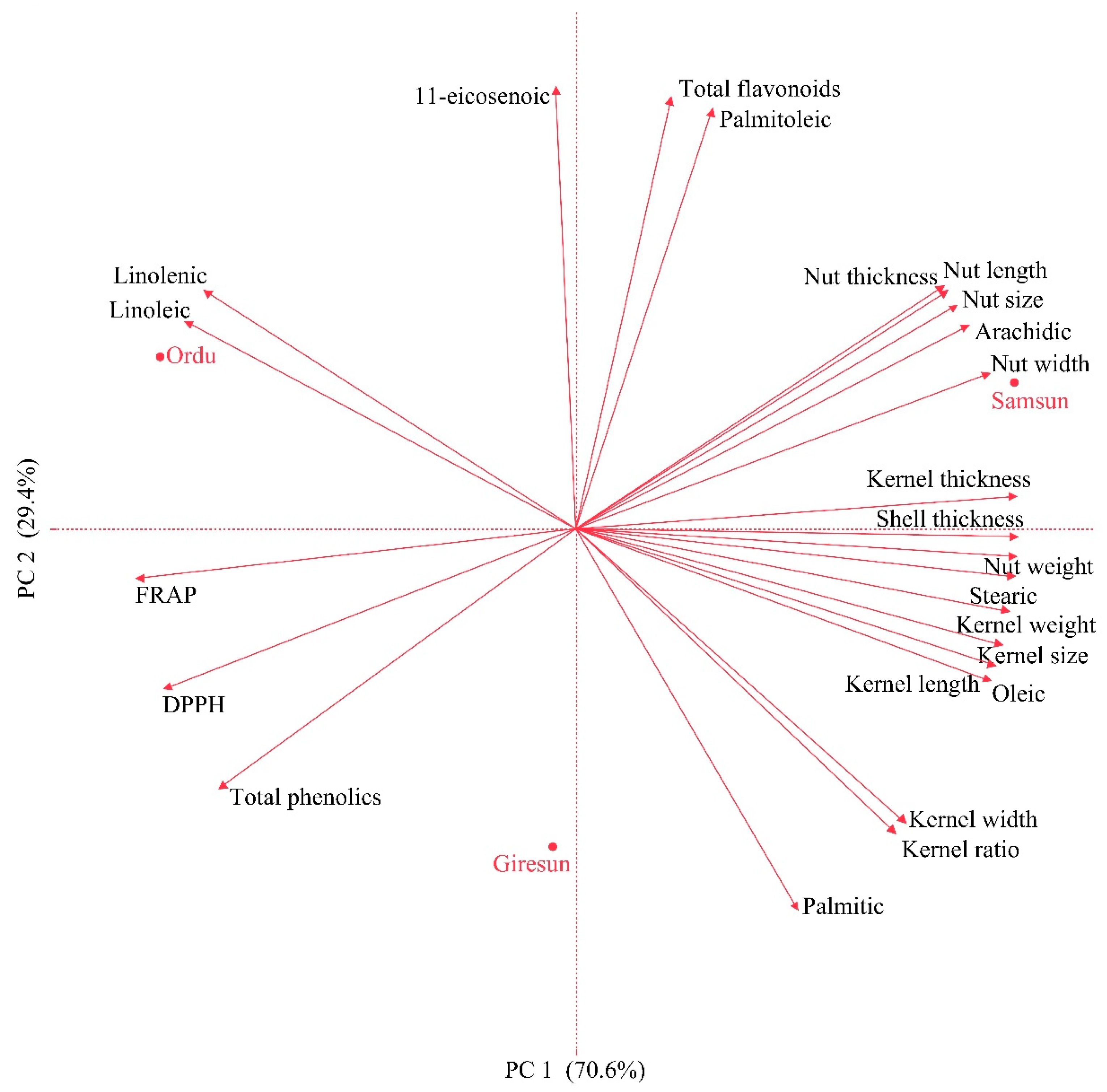
3.4. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| cvs. | Cultivars |
| LSD | Least Significant Difference |
| RH | Relative Humidity |
References
- Food and Agriculture Organization Statistics (FAO). Hazelnut Production Statistics. 2025. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 11 June 2025).
- Karadeniz, T.; Bostan, S.Z.; Tuncer, C.; Tarakçıoğlu, C. Fındık Yetiştiriciliği; Ordu Ziraat Odası Başkanlığı Bilimsel Yayınlar Serisi: Ordu, Türkiye, 2009; pp. 1–154. [Google Scholar]
- İslam, A. Hazelnut culture in Turkey. Akad. Zir. Der. 2018, 7, 259–266. [Google Scholar]
- Bacchetta, L.; Aramini, M.; Procacci, S.; Zinni, A.; Di Giammatteo, V.; Battarelli, M.R.; Spera, D. Influence of genotype and geographical origin on lipid fraction of hazelnuts (Corylus avellana) in Europe. Acta Hortic. 2018, 1226, 333–338. [Google Scholar] [CrossRef]
- Gavilán-CuiCui, G.; Padilla-Contreras, D.; Manterola-Barroso, C.; Morina, F.; Meriño-Gergichevich, C. Antioxidant performance in hazelnut (Corylus avellana L.) cultivars shell is substantially influenced by season and locality. Agronomy 2024, 14, 1412. [Google Scholar] [CrossRef]
- Silvestri, C.; Bacchetta, L.; Bellincontro, A.; Cristofori, V. Advances in cultivar choice, hazelnut orchard management, and nut storage to enhance product quality and safety: An overview. J. Sci. Food Agric. 2021, 101, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, F.; Akıncı, I. Physical and nutritional properties of four major commercial Turkish hazelnut varieties. J. Food Eng. 2004, 63, 341–347. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M. Morphological traits and chemical composition of hazelnut from different geographical origins: A review. Agriculture 2020, 10, 375. [Google Scholar] [CrossRef]
- Yaman, M.; Balta, M.F.; Karakaya, O.; Kaya, T.; Necas, T.; Yildiz, E.; Dirim, E. Assessment of fatty acid composition, bioactive compounds, and mineral composition in hazelnut genetic resources: Implication. Horticulturae 2023, 9, 1008. [Google Scholar] [CrossRef]
- Cristofori, V.; Ferramondo, S.; Bertazza, G.; Bignami, C. Nut and kernel traits and chemical composition of hazelnut (Corylus avellana L.) cultivars. J. Sci. Food Agric. 2008, 88, 1091–1098. [Google Scholar] [CrossRef]
- Bacchetta, L.; Aramini, M.; Zini, A.; Di Giammatteo, V.; Spera, D.; Drogoudi, P.; Botta, R. Fatty acids and alpha-tocopherol composition in hazelnut (Corylus avellana L.): A chemometric approach to emphasize the quality of European germplasm. Euphytica 2013, 191, 57–73. [Google Scholar] [CrossRef]
- Çetin, N.; Yaman, M.; Karaman, K.; Demir, B. Determination of some physicomechanical and biochemical parameters of hazelnut (Corylus avellana L.) cultivars. Turk. J. Agric. For. 2020, 44, 439–450. [Google Scholar] [CrossRef]
- Di Nunzio, M. Hazelnuts as source of bioactive compounds and health value underestimated food. Curr. Res. Nutr. Food Sci. J. 2019, 7, 17–28. [Google Scholar] [CrossRef]
- Pelvan, E.; Alasalvar, C.; Uzman, S. Effects of roasting on the antioxidant status and phenolic profiles of commercial Turkish hazelnut varieties (Corylus avellana L.). J. Agric. Food Chem. 2012, 60, 1218–1223. [Google Scholar] [CrossRef]
- Gülsoy, E.; Kaya, E.D.; Türkhan, A.; Bulut, M.; Koyuncu, M.; Güler, E.; Muradoğlu, F. The effect of altitude on phenolic, antioxidant and fatty acid compositions of some Turkish hazelnut (Corylus avellana L.) cultivars. Molecules 2023, 28, 5067. [Google Scholar] [CrossRef]
- Khavari, M.; Fatahi, R.; Zamani, Z. Salicylic acid and kaolin effects on pomological, physiological, and phytochemical characters of hazelnut (Corylus avellana) at warm summer condition. Sci. Rep. 2021, 11, 4568. [Google Scholar] [CrossRef]
- Balta, F.; Yılmaz, M.; Karakaya, O.; Çalışkan, K.; Yarılgaç, T.; Bostan, S.Z.; Uzun, S. Effect of Plant Density on Nut Traits, Nut Yield, Cluster Distribution and Chemical Components in Çakıldak (Corylus avellana L.) Hazelnut Cultivar. Appl. Fruit Sci. 2024, 66, 2295–2305. [Google Scholar] [CrossRef]
- Varol, D.; Bostan, S. Bioactive Components and Aflatoxins Changing According to the Farming Systems in Hazelnut. Appl. Fruit Sci. 2025, 67, 97. [Google Scholar] [CrossRef]
- Kodad, O.; Estopañán, G.; Juan, T.; Socias i Company, R. Protein content and oil composition of almond from Moroccan seedlings: Genetic diversity, oil quality and geographical origin. J. Am. Oil Chem. Soc. 2013, 90, 243–252. [Google Scholar] [CrossRef]
- Ma, X.; Wang, W.; Zheng, C.; Liu, C.; Huang, Y.; Zhao, W.; Du, J. Quality evaluation of walnuts from different regions in China. Foods 2023, 12, 4123. [Google Scholar] [CrossRef]
- Matin, A.; Brandić, I.; Gubor, M.; Pezo, L.; Krička, T.; Matin, B.; Antonović, A. Effect of conduction drying on nutrient and fatty acid profiles: A comparative analysis of hazelnuts and walnuts. Front. Sustain. Food Syst. 2024, 8, 1351309. [Google Scholar] [CrossRef]
- Karakaya, O. The Effect of region on nut and biochemical traits of Mincane hazelnut cultivar. Black Sea J. Agric. 2023, 6, 134–139. [Google Scholar] [CrossRef]
- Ay, A.; Kızılkaya, R. Ordu ve Giresun illerindeki fındık bahçelerinin toprak özellikleri ile biyolojik özellikleri arasındaki ilişkiler. Top. Bil. Bit. Bes. Derg. 2021, 9, 71–78. [Google Scholar] [CrossRef]
- Güler, E.; Balta, F. Determination of yield and quality characteristics of hazelnut populations of Taskesti district (Mudurnu-Bolu). Int. J. Agric. Wildl. Sci. 2020, 6, 115–128. [Google Scholar]
- Firestone, D. (Ed.) Physical and Chemical Characteristics of Oils, Fats and Waxes; AOCS Press: Champaign, IL, USA, 1997. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Altun, M.; Çelik, S.E.; Güçlü, K.; Özyürek, M.; Erçağ, E.; Apak, R. Total antioxidant capacity and phenolic contents of Turkish hazelnut (Corylus avellana L.) kernels and oils. J. Food Biochem. 2013, 37, 53–61. [Google Scholar] [CrossRef]
- Deutsche Gesellschaft für Fettwissenschaften (DGF). German Standard Methods for the Analysis of Fats and Other Lipids; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 1998. [Google Scholar]
- Bozkurt, E. Çakıldak Fındık Çeşidinde Rakım, yıl ve Bahçelere Göre Verimin Değişimi Üzerine Araştırmalar. Master’s Thesis, Ordu University, Ordu, Türkiye, 2010. [Google Scholar]
- Gülsoy, E.; Şimşek, M.; Çevik, C. Ordu ilinin farklı rakım ve lokasyonlarında yetiştirilen bazı fındık çeşitlerinin meyve kalite özelliklerinin belirlenmesi. Int. J. Agric. Wildl. Sci. 2019, 5, 25–30. [Google Scholar]
- Demir, T.; Beyhan, N. Samsun ilinde yetiştirilen fındıkların seleksiyonu üzerine bir araştırma. Turk. J. Agric. For. 2000, 24, 173–183. [Google Scholar]
- Yılmaz, M. Bazı Fındık Çeşit ve Genotiplerinin Pomolojik, Morfolojik ve Moleküler Karakterizasyonu. Ph.D. Thesis, Çukurova University, Adana, Türkiye, 2009. [Google Scholar]
- Bostan, S.Z.; Günay, K. Variation of important quality characteristics in hazelnut at different years and correlations between husk number and nut and kernel traits. Acta Hortic. 2009, 845, 641–646. [Google Scholar] [CrossRef]
- Balta, F.; Balta, M.F.; Karadeniz, T. The evaluations on preselection of the hazelnut ‘Tombul’ and ‘Palaz’ cultivars grown in Carsamba and Terme (Samsun) districts. Acta Hortic. 1997, 445, 109–118. [Google Scholar] [CrossRef]
- Turan, A. Giresun Ili Bulancak Ilçesi Tombul Fındık Klon Seleksiyonu. Master’s Thesis, Ondokuz Mayıs University, Samsun, Türkiye, 2007. [Google Scholar]
- Karakaya, O. Nut traits and bioactive contents of Kalınkara hazelnut cultivar grown in different region. In Proceedings of the 7th International Conference on Agriculture, Animal Sciences and Rural Development, Mus, Turkey, 18–19 September 2022; pp. 18–19. [Google Scholar]
- Serdar, Ü.; Demir, T. Yield, cluster drop and nut traits of three Turkish hazelnut cultivars. Hortic. Sci. 2005, 32, 96–99. [Google Scholar] [CrossRef]
- Külahcılar, A.; Tonkaz, T.; Bostan, S.Z. Effect of irrigation regimes by mini sprinkler on yield and pomological traits in ‘Tombul’ hazelnut. Acta Hortic. 2018, 1226, 301–308. [Google Scholar] [CrossRef]
- Beyhan, N.; Demir, T. Performance of the local and standard hazelnut cultivars grown in Samsun province, Turkey. Acta Hortic. 2001, 556, 227–234. [Google Scholar] [CrossRef]
- Karakaya, O. The intensity of the cluster drop affects the bioactive compounds and fatty acid composition in hazelnuts. Grasas Aceites 2023, 74, e487. [Google Scholar] [CrossRef]
- Yılmaz, M.; Kaya, T.; Karakaya, O.; Balta, F.; Çalışkan, K. Orchard-based variations in oil content, fatty acid composition, and bioactive compounds in ‘Tombul’ and ‘Palaz’ hazelnut (Corylus avellana L.) cultivars. Appl. Fruit Sci. 2024, 66, 599–608. [Google Scholar] [CrossRef]
- Zhu, Y.; Wilkinson, K.L.; Wirthensohn, M.G. Lipophilic antioxidant content of almonds (Prunus dulcis): A regional and varietal study. J. Food Compos. Anal. 2015, 39, 120–127. [Google Scholar] [CrossRef]
- Tonkaz, T.; Şahin, S.; Bostan, S.Z.; Korkmaz, K. Effect of supplementary irrigation on total antioxidant capacity and phenolic content of hazelnut. Akad. Zir. Derg. 2019, 8, 79–84. [Google Scholar] [CrossRef]
- Cristofori, V.; Bertazza, G.; Bignami, C. Changes in kernel chemical composition during nut development of three Italian hazelnut cultivars. Fruits 2015, 70, 311–322. [Google Scholar] [CrossRef]
- Balta, M.F.; Yarılgaç, T.; Aşkın, M.A.; Kuçuk, M.; Balta, F.; Özrenk, K. Determination of fatty acid compositions, oil contents and some quality traits of hazelnut genetic resources grown in eastern Anatolia of Turkey. J. Food Compos. Anal. 2006, 19, 681–686. [Google Scholar] [CrossRef]
- Parcerisa, J.; Boatella, J.; Codony, R.; Farrà, A.; Garcia, J.; Lopez, A.; Romero, A. Influence of variety and geographical origin on the lipid fraction of hazelnuts (Corylus avellana L.) from Spain: I. Fatty acid composition. Food Chem. 1993, 48, 411–414. [Google Scholar] [CrossRef]
- Parcerisa, J.; Rafecas, M.; Castellote, A.I.; Codony, R.; Farran, A.; Garcia, J.; Boatella, J. Influence of variety and geographical origin on the lipid fraction of hazelnuts (Corylus avellana L.) from Spain: III. Oil stability, tocopherol content and some mineral contents (Mn, Fe, Cu). Food Chem. 1995, 53, 71–74. [Google Scholar] [CrossRef]
- Özdemir, M.; Açkurt, F.; Kaplan, M.; Yıldız, M.; Löker, M.; Gürcan, T.; Seyhan, F.G. Evaluation of new Turkish hybrid hazelnut (Corylus avellana L.) varieties: Fatty acid composition, α-tocopherol content, mineral composition and stability. Food Chem. 2001, 73, 411–415. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, X.P.; Yang, X.H.; Shi, X.M.; Ren, C.M.; Meng, J.; Zhang, Y. Analysis and comparison of nutritional components of Juglans regia oil and Juglans sigillata oil from different producing areas in China. China Oils Fats 2022, 47, 60–64. [Google Scholar]



| Cultivars | Geographic Origins | Nut Weight (g) | Kernel Weight (g) | Kernel Ratio (%) | Shell Thickness (mm) |
|---|---|---|---|---|---|
| Giresun | 1.68 b z | 0.97 c | 57.90 a | 0.92 a | |
| Çakıldak | Ordu | 2.13 a | 1.14 b | 53.63 b | 0.93 a |
| Samsun | 2.31 a | 1.33 a | 57.64 a | 0.87 a | |
| Significance | *** | *** | ** | ns | |
| LSD (0.05) | 0.22 | 0.11 | 2.58 | 0.14 | |
| Giresun | 2.04 ab | 1.08 a | 53.05 a | 1.12 a | |
| Palaz | Ordu | 1.87 b | 0.96 b | 51.24 a | 1.08 a |
| Samsun | 2.20 a | 1.16 a | 52.68 a | 1.17 a | |
| Significance | * | ** | ns | ns | |
| LSD (0.05) | 0.21 | 0.12 | 2.25 | 0.22 | |
| Giresun | 1.84 b | 1.00 b | 54.51 ab | 0.97 a | |
| Tombul | Ordu | 1.83 b | 0.98 b | 53.50 b | 0.99 a |
| Samsun | 2.12 a | 1.19 a | 56.22 a | 0.96 a | |
| Significance | * | ** | * | ns | |
| LSD (0.05) | 0.27 | 0.11 | 2.64 | 0.16 | |
| Cultivars | Geographic Origins | Nut Length (mm) | Nut Width (mm) | Nut Thickness (mm) | Nut Size (mm) | Kernel Length (mm) | Kernel Width (mm) | Kernel Thickness (mm) | Kernel Size (mm) |
|---|---|---|---|---|---|---|---|---|---|
| Giresun | 18.65 b z | 15.93 b | 14.74 b | 16.36 b | 14.99 b | 11.91 b | 11.37 c | 12.66 c | |
| Çakıldak | Ordu | 20.32 a | 17.55 a | 16.35 a | 18.00 a | 16.02 a | 13.16 a | 12.15 b | 13.68 b |
| Samsun | 20.10 a | 17.37 a | 16.08 a | 17.77 a | 16.65 a | 13.48 a | 12.84 a | 14.23 a | |
| Significance | *** | ** | *** | *** | ** | *** | *** | *** | |
| LSD (0.05) | 0.60 | 0.94 | 0.46 | 0.50 | 0.88 | 0.62 | 0.21 | 0.49 | |
| Giresun | 16.45 b | 18.81 ab | 15.86 b | 16.99 b | 12.91 a | 14.91 a | 12.59 ab | 13.42 ab | |
| Palaz | Ordu | 16.57 b | 18.72 b | 15.98 b | 17.05 b | 12.22 a | 14.40 a | 12.30 b | 12.93 b |
| Samsun | 17.65 a | 19.42 a | 16.92 a | 17.97 a | 13.16 a | 14.82 a | 13.03 a | 13.64 a | |
| Significance | *** | * | *** | *** | ns | ns | * | * | |
| LSD (0.05) | 0.49 | 0.62 | 0.29 | 0.29 | 1.41 | 0.92 | 0.54 | 0.50 | |
| Giresun | 18.02 a | 16.57 a | 15.06 a | 16.50 b | 14.00 b | 12.70 a | 11.94 b | 12.85 b | |
| Tombul | Ordu | 18.75 a | 16.79 a | 15.32 a | 16.90 ab | 14.76 ab | 12.46 a | 11.63 b | 12.88 b |
| Samsun | 18.68 a | 17.42 a | 15.68 a | 17.21 a | 14.95 a | 13.14 a | 12.95 a | 13.65 a | |
| Significance | ns | ns | ns | * | * | ns | ** | ** | |
| LSD (0.05) | 1.08 | 1.09 | 0.67 | 0.55 | 0.79 | 0.69 | 0.62 | 0.43 | |
| Cultivars | Geographic Origins | Total Phenolics (mg g−1) | Total Flavonoids (mg kg−1) | DPPH (mmol kg−1) | FRAP (mmol kg−1) |
|---|---|---|---|---|---|
| Giresun | 23.05 b z | 83.2 a | 37.08 a | 46.35 a | |
| Çakıldak | Ordu | 23.62 b | 75.3 b | 34.41 b | 15.95 c |
| Samsun | 27.92 a | 80.9 ab | 35.31 b | 23.06 b | |
| Significance | *** | * | ** | *** | |
| LSD (0.05) | 1.81 | 7.8 | 1.14 | 2.78 | |
| Giresun | 13.24 a | 51.7 b | 8.26 a | 9.72 b | |
| Palaz | Ordu | 12.34 a | 56.2 a | 8.96 a | 12.79 a |
| Samsun | 7.68 b | 57.2 a | 2.93 b | 4.27 c | |
| Significance | *** | ** | *** | *** | |
| LSD (0.05) | 0.91 | 3.5 | 2.47 | 1.40 | |
| Giresun | 13.67 b | 49.8 b | 24.32 a | 10.43 b | |
| Tombul | Ordu | 5.50 c | 57.2 b | 3.46 b | 5.86 c |
| Samsun | 20.83 a | 85.7 a | 26.62 a | 18.22 a | |
| Significance | *** | *** | *** | *** | |
| LSD (0.05) | 2.17 | 13.2 | 2.35 | 1.99 | |
| Cultivars | Geographic Origins | Palmitic Acid (C16:0) | Palmitoleic Acid (C16:1) | Stearic Acid (C18:0) | Oleic Acid (C18:1) | Linoleic Acid (C18:2) | Linolenic Acid (C18:3) | Arachidic Acid (C20:0) | 11-Eicosenoic Acid (C20:1) |
|---|---|---|---|---|---|---|---|---|---|
| Giresun | 8.03 a z | 0.00 c | 1.75 b | 84.28 a | 5.95 b | 0.00 | 0.00 | 0.00 | |
| Çakıldak | Ordu | 6.14 c | 0.17 b | 2.24 a | 83.74 a | 7.70 a | 0.00 | 0.00 | 0.00 |
| Samsun | 7.24 b | 0.54 a | 1.96 b | 82.62 a | 7.64 a | 0.00 | 0.00 | 0.00 | |
| Significance | ** | *** | ** | ns | *** | - | - | - | |
| LSD (0.05) | 0.72 | 0.06 | 0.24 | 2.84 | 0.29 | - | - | - | |
| Giresun | 7.91 a | 0.00 c | 2.24 b | 83.28 a | 6.57 b | 0.00 b | 0.00 b | 0.00 c | |
| Palaz | Ordu | 4.86 c | 0.17 b | 1.77 c | 78.90 b | 14.06 a | 0.09 a | 0.00 b | 0.14 a |
| Samsun | 6.52 b | 0.24 a | 2.61 a | 84.56 a | 5.83 c | 0.00 b | 0.11 a | 0.13 b | |
| Significance | *** | *** | *** | ** | *** | * | * | * | |
| LSD (0.05) | 0.65 | 0.03 | 0.27 | 3.29 | 0.38 | 0.001 | 0.001 | 0.004 | |
| Giresun | 5.51 b | 0.00 c | 2.48 a | 84.73 a | 7.28 c | 0.00 | 0.00 | 0.00 | |
| Tombul | Ordu | 5.73 b | 0.15 b | 2.27 a | 81.25 b | 10.60 a | 0.00 | 0.00 | 0.00 |
| Samsun | 8.05 a | 0.25 a | 2.43 a | 80.40 b | 8.87 b | 0.00 | 0.00 | 0.00 | |
| Significance | *** | *** | ns | * | *** | - | - | - | |
| LSD (0.05) | 0.65 | 0.03 | 0.24 | 3.28 | 0.36 | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurt, H.; Karakaya, O. Geographical Origin Affects the Nut Traits, Bioactive Compounds, and Fatty Acid Composition of Turkish Hazelnut Cultivars (Corylus avellana L. cvs. Çakıldak, Palaz, and Tombul). Horticulturae 2025, 11, 987. https://doi.org/10.3390/horticulturae11080987
Kurt H, Karakaya O. Geographical Origin Affects the Nut Traits, Bioactive Compounds, and Fatty Acid Composition of Turkish Hazelnut Cultivars (Corylus avellana L. cvs. Çakıldak, Palaz, and Tombul). Horticulturae. 2025; 11(8):987. https://doi.org/10.3390/horticulturae11080987
Chicago/Turabian StyleKurt, Haydar, and Orhan Karakaya. 2025. "Geographical Origin Affects the Nut Traits, Bioactive Compounds, and Fatty Acid Composition of Turkish Hazelnut Cultivars (Corylus avellana L. cvs. Çakıldak, Palaz, and Tombul)" Horticulturae 11, no. 8: 987. https://doi.org/10.3390/horticulturae11080987
APA StyleKurt, H., & Karakaya, O. (2025). Geographical Origin Affects the Nut Traits, Bioactive Compounds, and Fatty Acid Composition of Turkish Hazelnut Cultivars (Corylus avellana L. cvs. Çakıldak, Palaz, and Tombul). Horticulturae, 11(8), 987. https://doi.org/10.3390/horticulturae11080987






