Revised Viticulture for Low-Alcohol Wine Production: Strategies and Limitations
Abstract
1. Introduction
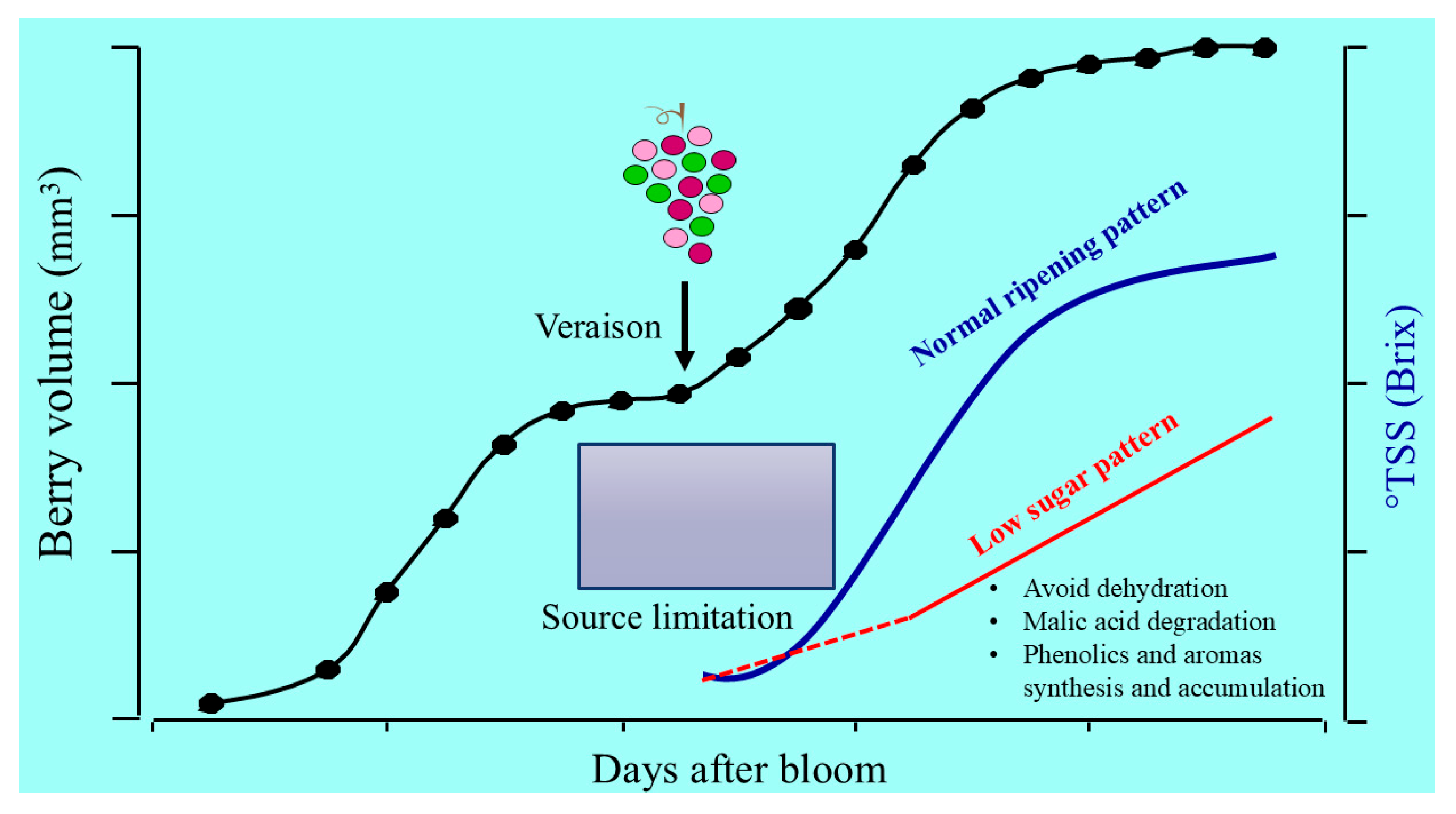
2. Ideotype of Berry Ripening for Low-Alcohol Wines
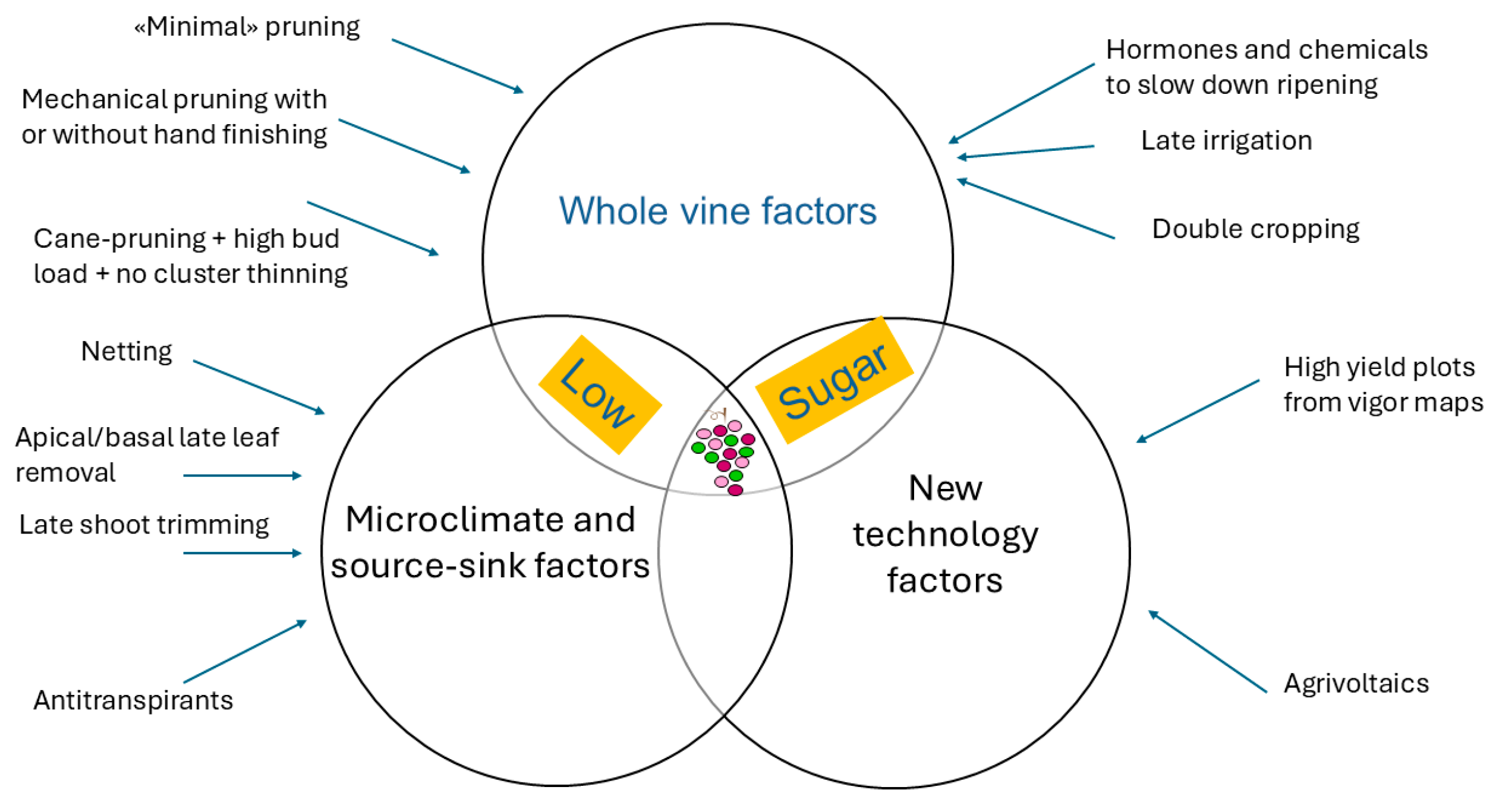
3. Aiming at Low Sugar at Harvest: Keep It Simple First
4. Aiming at Low Sugar at Harvest: A More Sophisticated Approach
5. Acting on Microclimate or Source–Sink Balance
6. The Varietal Choice for Low/No-Alcohol Wines
7. Relationship Between “Low-Sugar Viticulture“ and New Technologies
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Matese, A.; Filippo Di Gennaro, S. Technology in precision viticulture: A state of the art review. Int. J. Wine Res. 2015, 2015, 69–81. [Google Scholar] [CrossRef]
- González, P.A.; Parga-Dans, E. Natural wine: Do consumers know what it is, and how natural it really is? J. Clean. Prod. 2020, 251, 119635. [Google Scholar] [CrossRef]
- Bucher, T.; Deroover, K.; Stockley, C. Low-alcohol wine: A narrative review on consumer perception and behaviour. Beverages 2018, 4, 82. [Google Scholar] [CrossRef]
- Pickering, G.J. Low-and reduced-alcohol wine: A review. J. Wine Res. 2000, 11, 129–144. [Google Scholar] [CrossRef]
- Schmidtke, L.M.; Blackman, J.W.; Agboola, S.O. Production technologies for reduced alcoholic wines. J. Food Sci. 2012, 77, R25–R41. [Google Scholar] [CrossRef]
- Saha, B.; Torley, P.; Blackman, J.; Schmidtke, L. Review of processing technology to reduce alcohol levels in wines. In Proceedings of the 1st International Symposium Alcohol Level Reduction in Wine-Oenoviti International Network, Bordeauxq, France, 6 September 2013. [Google Scholar]
- Liguori, L.; Russo, P.; Albanese, D.; Di Matteo, M. Production of low-alcohol beverages: Current status and perspectives. In Food Processing for Increased Quality and Consumption; Elsevier: Amsterdam, The Netherlands, 2018; pp. 347–382. [Google Scholar]
- Chrysochou, P. Drink to get drunk or stay healthy? Exploring consumers’ perceptions, motives and preferences for light beer. Food Qual. Prefer. 2014, 31, 156–163. [Google Scholar] [CrossRef]
- Stubenitsky, K.; Aaron, J.; Catt, S.; Mela, D. Effect of information and extended use on the acceptance of reduced-fat products. Food Qual. Prefer. 1999, 10, 367–376. [Google Scholar] [CrossRef]
- Bucher, T.; Frey, E.; Wilczynska, M.; Deroover, K.; Dohle, S. Consumer perception and behaviour related to low-alcohol wine: Do people overcompensate? Public Health Nutr. 2020, 23, 1939–1947. [Google Scholar] [CrossRef]
- Deroover, K.; Siegrist, M.; Brain, K.; McIntyre, J.; Bucher, T. A scoping review on consumer behaviour related to wine and health. Trends Food Sci. Technol. 2021, 112, 559–580. [Google Scholar] [CrossRef]
- Coombe, B.G. Research on development and ripening of the grape berry. Am. J. Enol. Vitic. 1992, 43, 101–110. [Google Scholar] [CrossRef]
- Zhang, Y.; Keller, M. Discharge of surplus phloem water may be required for normal grape ripening. J. Exp. Bot. 2017, 68, 585–595. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coombe, B.G. Viticulture. Volume 1. Resources in Australia; Dry, P.R., Ed.; Winetitles: Adelaide, SA, Australia, 1988. [Google Scholar]
- Huang, X.M.; Huang, H.B. Early post-veraison growth in grapes: Evidence for a two-step mode of berry enlargement. Aust. J. Grape Wine Res. 2001, 7, 132–136. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Matthews, M.A.; Di Gaspero, G.; Gambetta, G.A. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta 2007, 227, 101–112. [Google Scholar] [CrossRef]
- Falginella, L.; Magris, G.; Castellarin, S.D.; Gambetta, G.A.; Matthews, M.A.; Morgante, M.; Di Gaspero, G. Grape ripening speed slowed down using natural variation. Theor. Appl. Genet. 2025, 138, 130. [Google Scholar] [CrossRef]
- García, J.; Zheng, W.; Balda, P.; Martinez De Toda, F. Varietal differences in the sugar content of red grapes at the onset of anthocyanin synthesis. Vitis 2017, 56, 15–18. [Google Scholar]
- Cameron, W.; Petrie, P.; Barlow, E.; Patrick, C.; Howell, K.; Fuentes, S. Is advancement of grapevine maturity explained by an increase in the rate of ripening or advancement of veraison? Aust. J. Grape Wine Res. 2021, 27, 334–347. [Google Scholar] [CrossRef]
- Parker, A.K.; Hofmann, R.W.; van Leeuwen, C.; McLachlan, A.R.; Trought, M.C. Leaf area to fruit mass ratio determines the time of veraison in S auvignon B lanc and P inot N oir grapevines. Aust. J. Grape Wine Res. 2014, 20, 422–431. [Google Scholar] [CrossRef]
- Kliewer, W.M.; Dokoozlian, N.K. Leaf area/crop weight ratios of grapevines: Influence on fruit composition and wine quality. Am. J. Enol. Vitic. 2005, 56, 170–181. [Google Scholar] [CrossRef]
- Howell, G.S. Sustainable grape productivity and the growth-yield relationship: A review. Am. J. Enol. Vitic. 2001, 52, 165–174. [Google Scholar] [CrossRef]
- Poni, S.; Giachino, E. Growth, photosynthesis and cropping of potted grapevines (Vitis vinifera L. cv. Cabernet Sauvignon) in relation to shoot trimming. Aust. J. Grape Wine Res. 2000, 6, 216–226. [Google Scholar] [CrossRef]
- Poni, S.; Gatti, M.; Palliotti, A.; Dai, Z.; Duchêne, E.; Truong, T.-T.; Ferrara, G.; Matarrese, A.M.S.; Gallotta, A.; Bellincontro, A. Grapevine quality: A multiple choice issue. Sci. Hortic. 2018, 234, 445–462. [Google Scholar] [CrossRef]
- Cola, G.; Mariani, L.; Salinari, F.; Civardi, S.; Bernizzoni, F.; Gatti, M.; Poni, S. Description and testing of a weather-based model for predicting phenology, canopy development and source–sink balance in Vitis vinifera L. cv. Barbera. Agric. For. Meteorol. 2014, 184, 117–136. [Google Scholar] [CrossRef]
- Longo, R.; Blackman, J.W.; Antalick, G.; Torley, P.J.; Rogiers, S.Y.; Schmidtke, L.M. Volatile and sensory profiling of Shiraz wine in response to alcohol management: Comparison of harvest timing versus technological approaches. Food Res. Int. 2018, 109, 561–571. [Google Scholar] [CrossRef]
- Sekhon, K.; Sun, Q. Opportunities and Challenges for Low-Alcohol Wine. In Global Warming and the Wine Industry-Challenges, Innovations and Future Prospects; IntechOpen: London, UK, 2024. [Google Scholar]
- King, E.S.; Heymann, H. The effect of reduced alcohol on the sensory profiles and consumer preferences of white wine. J. Sens. Stud. 2014, 29, 33–42. [Google Scholar] [CrossRef]
- Iland, P.; Dry, P.; Proffitt, T.; Tyerman, S. The Grapevine: From the Science to the Practice of Growing Vines for Wine; Patrick Iland Wine Promotions: Adelaide, Australia, 2011. [Google Scholar]
- Ferrero-del-Teso, S.; Suárez, A.; Ferreira, C.; Perenzoni, D.; Arapitsas, P.; Mattivi, F.; Ferreira, V.; Fernández-Zurbano, P.; Sáenz-Navajas, M.-P. Modeling grape taste and mouthfeel from chemical composition. Food Chem. 2022, 371, 131168. [Google Scholar] [CrossRef]
- Coombe, B.G.; McCarthy, M. Identification and naming of the inception of aroma development in ripening grape berries. Aust. J. Grape Wine Res. 1997, 3, 18–20. [Google Scholar] [CrossRef]
- Williams, P.J.; Cynkar, W.; Francis, I.L.; Gray, J.D.; Iland, P.G.; Coombe, B.G. Quantification of glycosides in grapes, juices, and wines through a determination of glycosyl glucose. J. Agric. Food Chem. 1995, 43, 121–128. [Google Scholar] [CrossRef]
- Bindon, K.; Varela, C.; Kennedy, J.; Holt, H.; Herderich, M. Relationships between harvest time and wine composition in Vitis vinifera L. cv. Cabernet Sauvignon 1. Grape and wine chemistry. Food Chem. 2013, 138, 1696–1705. [Google Scholar] [CrossRef]
- Bindon, K.; Holt, H.; Williamson, P.O.; Varela, C.; Herderich, M.; Francis, I.L. Relationships between harvest time and wine composition in Vitis vinifera L. cv. Cabernet Sauvignon 2. Wine sensory properties and consumer preference. Food Chem. 2014, 154, 90–101. [Google Scholar] [CrossRef]
- Coombe, B.G.; McCarthy, M. Dynamics of grape berry growth and physiology of ripening. Aust. J. Grape Wine Res. 2000, 6, 131–135. [Google Scholar] [CrossRef]
- Ford, C. The biochemistry of organic acids in the grape. In The Biochemistry of the Grape Berry; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012; pp. 67–88. [Google Scholar]
- Longo, R.; Ristic, R.; Schimdtke, L. Reducing alcohol: Low alcohol wines: Blending with an early harvest or dealcoholisation of a later harvest? Wine Vitic. J. 2019, 34, 32–35. [Google Scholar]
- Sam, F.E.; Ma, T.-Z.; Salifu, R.; Wang, J.; Jiang, Y.-M.; Zhang, B.; Han, S.-Y. Techniques for dealcoholization of wines: Their impact on wine phenolic composition, volatile composition, and sensory characteristics. Foods 2021, 10, 2498. [Google Scholar] [CrossRef]
- Schelezki, O.J.; Šuklje, K.; Boss, P.K.; Jeffery, D.W. Comparison of consecutive harvests versus blending treatments to produce lower alcohol wines from Cabernet Sauvignon grapes: Impact on wine volatile composition and sensory properties. Food Chem. 2018, 259, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Kontoudakis, N.; Esteruelas, M.; Fort, F.; Canals, J.; Zamora, F. Use of unripe grapes harvested during cluster thinning as a method for reducing alcohol content and pH of wine. Aust. J. Grape Wine Res. 2011, 17, 230–238. [Google Scholar] [CrossRef]
- Nuzzo, V.; Matthews, M. Berry size and yield paradigms on grapes and wines quality. In Proceedings of the International Workshop on Advances in Grapevine and Wine Research 754, Venosa, Italy, 15–17 September 2005; pp. 423–436. [Google Scholar]
- Keller, M.; Mills, L.J.; Wample, R.L.; Spayd, S.E. Cluster thinning effects on three deficit-irrigated Vitis vinifera cultivars. Am. J. Enol. Vitic. 2005, 56, 91–103. [Google Scholar] [CrossRef]
- Poni, S.; Bernizzoni, F.; Presutto, P.; Rebucci, B. Performance of Croatina under short-cane mechanical hedging: A successful case of adaptation. Am. J. Enol. Vitic. 2004, 55, 379–388. [Google Scholar] [CrossRef]
- Jackson, D.; Steans, G.; Hemmings, P. Vine response to increased node numbers. Am. J. Enol. Vitic. 1984, 35, 161–163. [Google Scholar] [CrossRef]
- Clingeleffer, P. Effect of varying node number per bearer on yield and juice composition of Cabernet Sauvignon grapevines. Aust. J. Exp. Agric. 1989, 29, 701–705. [Google Scholar] [CrossRef]
- McLoughlin, S.; Petrie, P.R.; Dry, P.R. Impact of node position and bearer length on the yield components in mechanically pruned Cabernet Sauvignon (Vitis vinifera L.). Aust. J. Grape Wine Res. 2011, 17, 129–135. [Google Scholar] [CrossRef]
- Poni, S.; Tombesi, S.; Palliotti, A.; Ughini, V.; Gatti, M. Mechanical winter pruning of grapevine: Physiological bases and applications. Sci. Hortic. 2016, 204, 88–98. [Google Scholar] [CrossRef]
- Allegro, G.; Martelli, R.; Valentini, G.; Pastore, C.; Mazzoleni, R.; Pezzi, F.; Filippetti, I. Effects of mechanical winter pruning on vine performances and management costs in a trebbiano romagnolo vineyard: A five-year study. Horticulturae 2022, 9, 21. [Google Scholar] [CrossRef]
- Morris, J.R. Development and commercialization of a complete vineyard mechanization system. HortTechnology 2007, 17, 411–420. [Google Scholar] [CrossRef]
- Kurtural, S.K.; Fidelibus, M.W. Mechanization of pruning, canopy management, and harvest in winegrape vineyards. Am. J. Enol. Vitic. 2021, 5, 29–44. [Google Scholar] [CrossRef]
- Intrieri, C.; Poni, S. Integrated evolution of trellis training systems and machines to improve grape quality and vintage quality of mechanized Italian vineyards. Am. J. Enol. Vitic. 1995, 46, 116–127. [Google Scholar] [CrossRef]
- Intrigliolo, D.S.; Castel, J.R. Effects of irrigation on the performance of grapevine cv. Tempranillo in Requena, Spain. Am. J. Enol. Vitic. 2008, 59, 30–38. [Google Scholar] [CrossRef]
- McCarthy, M. The effect of transient water deficit on berry development of cv. Shiraz (Vitis vinifera L.). Aust. J. Grape Wine Res. 1997, 3, 2–8. [Google Scholar] [CrossRef]
- Sadras, V.O.; Moran, M.A. Elevated temperature decouples anthocyanins and sugars in berries of Shiraz and Cabernet Franc. Aust. J. Grape Wine Res. 2012, 18, 115–122. [Google Scholar] [CrossRef]
- Martinez de Toda, F.; Balda, P. Quantifying the effect of temperature on decoupling anthocyanins and sugars of the grape (Vitis vinifera L.’Maturana Tinta de Navarrete’). Vitis-Geilweilerhof- 2015, 54, 117–120. [Google Scholar]
- Downton, W.; Grant, W. Photosynthetic physiology of spur pruned and minimal pruned grapevines. Funct. Plant Biol. 1992, 19, 309–316. [Google Scholar] [CrossRef]
- Schultz, H.; Kraml, S.; Werwitzke, U.; Zimmer, T.; Schmid, J. Adaptation and utilization of minimal pruning systems for quality production in cool climates. Am. J. Enol. Vitic 2000, 51, 185–190. [Google Scholar]
- Zheng, W.; del Galdo, V.; García, J.; Balda, P.; de Toda, F.M. Use of minimal pruning to delay fruit maturity and improve berry composition under climate change. Am. J. Enol. Vitic. 2017, 68, 136–140. [Google Scholar] [CrossRef]
- Sommer, K.; Clingeleffer, P.; Shulman, Y. Comparative study of vine morphology, growth, and canopy development in cane-pruned and minimal-pruned Sultana. Aust. J. Exp. Agric. 1995, 35, 265–273. [Google Scholar] [CrossRef]
- Hed, B.; Ngugi, H.K.; Travis, J.W. Relationship between cluster compactness and bunch rot in Vignoles grapes. Plant Dis. 2009, 93, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Del Zozzo, F.; Poni, S. Climate change affects choice and management of training systems in the grapevine. Aust. J. Grape Wine Res. 2024, 2024, 7834357. [Google Scholar] [CrossRef]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of anthocyanins in red-wine grape under high temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef]
- Rustioni, L.; Fracassetti, D.; Prinsi, B.; Geuna, F.; Ancelotti, A.; Fauda, V.; Tirelli, A.; Espen, L.; Failla, O. Oxidations in white grape (Vitis vinifera L.) skins: Comparison between ripening process and photooxidative sunburn symptoms. Plant Physiol. Biochem. 2020, 150, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, C.; Burbidge, C.A.; Boss, P.K.; Davies, C. Interactions between ethylene and auxin are crucial to the control of grape (Vitis vinifera L.) berry ripening. BMC Plant Biol. 2013, 13, 222. [Google Scholar] [CrossRef]
- Böttcher, C.; Boss, P.K.; Davies, C. Delaying Riesling grape berry ripening with a synthetic auxin affects malic acid metabolism and sugar accumulation, and alters wine sensory characters. Funct. Plant Biol. 2012, 39, 745–753. [Google Scholar] [CrossRef]
- Poni, S.; Del Zozzo, F.; Santelli, S.; Gatti, M.; Magnanini, E.; Sabbatini, P.; Frioni, T. Double cropping in Vitis vinifera L. cv. Pinot Noir: Agronomical and physiological validation. Aust. J. Grape Wine Res. 2021, 27, 508–518. [Google Scholar] [CrossRef]
- Lavee, S.; May, P. Dormancy of grapevine buds-facts and speculation. Aust. J. Grape Wine Res. 1997, 3, 31–46. [Google Scholar] [CrossRef]
- Velappan, Y.; Chabikwa, T.G.; Considine, J.A.; Agudelo-Romero, P.; Foyer, C.H.; Signorelli, S.; Considine, M.J. The bud dormancy disconnect: Latent buds of grapevine are dormant during summer despite a high metabolic rate. J. Exp. Bot. 2022, 73, 2061–2076. [Google Scholar] [CrossRef]
- Martinez, F.; Toda, J.G.; Balda, P. Preliminary results on forcing vine regrowth to delay ripening to a cooler period. Vitis 2019, 58, 17–22. [Google Scholar]
- Martinez De Toda, F. Global warming allows two grape crops a year, with about two months apart in ripening dates and with very different grape composition-the forcing vine regrowth to obtain two crops a year. Vitis 2021, 60, 119–124. [Google Scholar]
- Castellano, S.; Mugnozza, G.S.; Russo, G.; Briassoulis, D.; Mistriotis, A.; Hemming, S.; Waaijenberg, D. Plastic nets in agriculture: A general review of types and applications. Appl. Eng. Agric. 2008, 24, 799–808. [Google Scholar] [CrossRef]
- Pallotti, L.; Silvestroni, O.; Dottori, E.; Lattanzi, T.; Lanari, V. Effects of shading nets as a form of adaptation to climate change on grapes production: A review. OENO One 2023, 57, 467–476. [Google Scholar] [CrossRef]
- Pallotti, L.; Partida, G.; Laroche-Pinel, E.; Lanari, V.; Pedroza, M.; Brillante, L. Late-season source limitation practices to cope with climate change: Delaying ripening and improving colour of Cabernet-Sauvignon grapes and wine in a hot and arid climate. OENO One 2025, 59, 8323. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Chen, C.C.L.; Brillante, L.; Kurtural, S.K. Mitigating heat wave and exposure damage to “Cabernet Sauvignon” wine grape with partial shading under two irrigation amounts. Front. Plant Sci. 2020, 11, 579192. [Google Scholar] [CrossRef] [PubMed]
- Poni, S.; Gatti, M.; Bernizzoni, F.; Civardi, S.; Bobeica, N.; Magnanini, E.; Palliotti, A. Late leaf removal aimed at delaying ripening in cv. S angiovese: Physiological assessment and vine performance. Aust. J. Grape Wine Res. 2013, 19, 378–387. [Google Scholar]
- Poni, S.; Frioni, T.; Gatti, M. Summer pruning in Mediterranean vineyards: Is climate change affecting its perception, modalities, and effects? Front. Plant Sci. 2023, 14, 1227628. [Google Scholar] [CrossRef]
- Candolfi-Vasconcelos, M.d.C. Compensation and stress recovering related to leaf removal in Vitis vinifera. Ph.D. Thesis, Technical University of Lisbon, Lisbon, Portugal, 1990. [Google Scholar]
- Petrie, P.; Trought, M.T.; Howell, G. Influence of leaf ageing, leaf area and crop load on photosynthesis, stomatal conductance and senescence of grapevine (Vitis vinifera L. cv. Pinot noir) leaves. Vitis-Geilweilerhof- 2000, 39, 31–36. [Google Scholar]
- Ferreira, V.; Lopez, R. The actual and potential aroma of winemaking grapes. Biomolecules 2019, 9, 818. [Google Scholar] [CrossRef]
- Ryona, I.; Pan, B.S.; Intrigliolo, D.S.; Lakso, A.N.; Sacks, G.L. Effects of cluster light exposure on 3-isobutyl-2-methoxypyrazine accumulation and degradation patterns in red wine grapes (Vitis vinifera L. cv. Cabernet Franc). J. Agric. Food Chem. 2008, 56, 10838–10846. [Google Scholar] [CrossRef]
- Friedel, M.; Frotscher, J.; Nitsch, M.; Hofmann, M.; Bogs, J.; Stoll, M.; Dietrich, H. Light promotes expression of monoterpene and flavonol metabolic genes and enhances flavour of winegrape berries (Vitis vinifera L. cv. Riesling). Aust. J. Grape Wine Res. 2016, 22, 409–421. [Google Scholar] [CrossRef]
- Bureau, S.M.; Razungles, A.J.; Baumes, R.L. The aroma of Muscat of Frontignan grapes: Effect of the light environment of vine or bunch on volatiles and glycoconjugates. J. Sci. Food Agric. 2000, 80, 2012–2020. [Google Scholar] [CrossRef]
- Joubert, C.; Young, P.R.; Eyéghé-Bickong, H.A.; Vivier, M.A. Field-grown grapevine berries use carotenoids and the associated xanthophyll cycles to acclimate to UV exposure differentially in high and low light (shade) conditions. Front. Plant Sci. 2016, 7, 786. [Google Scholar] [CrossRef] [PubMed]
- Sivilotti, P.; Falchi, R.; Herrera, J.C.; Škvarč, B.; Butinar, L.; Sternad Lemut, M.; Bubola, M.; Sabbatini, P.; Lisjak, K.; Vanzo, A. Combined effects of early season leaf removal and climatic conditions on aroma precursors in Sauvignon Blanc grapes. J. Agric. Food Chem. 2017, 65, 8426–8434. [Google Scholar] [CrossRef]
- Voce, S.; Pizzamiglio, G.; Mosetti, D.; Bigot, G.; Lonardi, A.; Comuzzo, P.; Sivilotti, P. Effects of leaf removal on aromatic precursor dynamics during maturation of Ribolla Gialla grapes (Vitis vinifera L.). In Proceedings of the BIO Web of Conferences, Logroño, La Rioja, Spain, 7–9 November 2018; p. 03008. [Google Scholar]
- Valentini, G.; Allegro, G.; Pastore, C.; Colucci, E.; Filippetti, I. Post-veraison trimming slow down sugar accumulation without modifying phenolic ripening in Sangiovese vines. J. Sci. Food Agric. 2019, 99, 1358–1365. [Google Scholar] [CrossRef]
- Caccavello, G.; Giaccone, M.; Scognamiglio, P.; Forlani, M.; Basile, B. Influence of intensity of post-veraison defoliation or shoot trimming on vine physiology, yield components, berry and wine composition in Aglianico grapevines. Aust. J. Grape Wine Res. 2017, 23, 226–239. [Google Scholar] [CrossRef]
- Zheng, W.; García, J.; Balda, P.; Martínez de Toda, F. Effects of severe trimming after fruit set on the ripening process and the quality of grapes. Vitis 2017, 56, 27–33. [Google Scholar]
- Mphande, W.; Farrell, A.D.; Kettlewell, P.S. Commercial uses of antitranspirants in crop production: A review. Outlook Agric. 2023, 52, 3–10. [Google Scholar] [CrossRef]
- Di Vaio, C.; Villano, C.; Lisanti, M.T.; Marallo, N.; Cirillo, A.; Di Lorenzo, R.; Pisciotta, A. Application of anti-transpirant to control sugar accumulation in grape berries and alcohol degree in wines obtained from thinned and unthinned vines of cv. Falanghina (Vitis vinifera L.). Agronomy 2020, 10, 345. [Google Scholar] [CrossRef]
- Morales, F.; Irigoyen, J.J.; Antolín, M.C.; Goicoechea, N.; Santesteban, H.; Oyarzun, M.; Luquin, J.; Barbarin, M.; Urdiain, A.; Pascual, I. Novel, technical advance: A new grapevine transpiration prototype for grape berries and whole bunch based on relative humidity sensors. Comput. Electron. Agric. 2022, 196, 106890. [Google Scholar] [CrossRef]
- Gatti, M.; Galbignani, M.; Garavani, A.; Bernizzoni, F.; Tombesi, S.; Palliotti, A.; Poni, S. Manipulation of ripening via antitranspirants in cv. B arbera (Vitis vinifera L.). Aust. J. Grape Wine Res. 2016, 22, 245–255. [Google Scholar] [CrossRef]
- Brillante, L.; Belfiore, N.; Gaiotti, F.; Lovat, L.; Sansone, L.; Poni, S.; Tomasi, D. Comparing kaolin and pinolene to improve sustainable grapevine production during drought. PLoS ONE 2016, 11, e0156631. [Google Scholar] [CrossRef] [PubMed]
- Palliotti, A.; Panara, F.; Famiani, F.; Sabbatini, P.; Howell, G.S.; Silvestroni, O.; Poni, S. Postveraison application of antitranspirant di-1-p-menthene to control sugar accumulation in Sangiovese grapevines. Am. J. Enol. Vitic. 2013, 64, 378–385. [Google Scholar] [CrossRef]
- Jones, J.E.; Kerslake, F.L.; Close, D.C.; Dambergs, R.G. Viticulture for sparkling wine production: A review. Am. J. Enol. Vitic. 2014, 65, 407–416. [Google Scholar] [CrossRef]
- Frioni, T.; Collivasone, R.; Canavera, G.; Gatti, M.; Gabrielli, M.; Poni, S. Identifying the best parameters to determine genotype capability to retain adequate malic acid at harvest and in final wines: This article is published in cooperation with the 22nd GiESCO International Meeting, hosted by Cornell University in Ithaca, NY, July 17–21, 2023. OENO One 2023, 57, 247–256. [Google Scholar]
- Italiano, L.; Kumar, Y.; Schmitt, M.; Christmnann, M. Comparison of the principal production methods for dealcoholised wine based on analytical parameters: This is an original research article submitted in cooperation with Macrowine 2025. OENO One 2025, 59, 8488. [Google Scholar] [CrossRef]
- Kunter, B.; Unal, O.B.; Keskin, S.; Hatterman-Valenti, H.; Kaya, O. Comparison of the sugar and organic acid components of seventeen table grape varieties produced in Ankara (Türkiye): A study over two consecutive seasons. Front. Plant Sci. 2024, 15, 1321210. [Google Scholar] [CrossRef]
- Segade, S.R.; Giacosa, S.; Torchio, F.; de Palma, L.; Novello, V.; Gerbi, V.; Rolle, L. Impact of different advanced ripening stages on berry texture properties of ‘Red Globe’and ‘Crimson Seedless’ table grape cultivars (Vitis vinifera L.). Sci. Hortic. 2013, 160, 313–319. [Google Scholar] [CrossRef]
- Guelfat-Reich, S.; Safran, B. Indices of maturity for table grapes as determined by variety. Am. J. Enol. Vitic. 1971, 22, 13–18. [Google Scholar] [CrossRef]
- Saltveit, M.E. Synthesis and metabolism of phenolic compounds. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 115–124. [Google Scholar]
- Ma, W.; Guo, A.; Zhang, Y.; Wang, H.; Liu, Y.; Li, H. A review on astringency and bitterness perception of tannins in wine. Trends Food Sci. Technol. 2014, 40, 6–19. [Google Scholar] [CrossRef]
- Johnson, S.; Rogers, R. Progress in mechanization of wine grape economic factors. Calif. Agric. 1974, 28, 4–6. [Google Scholar]
- Van Leeuwen, C.; Seguin, G. The concept of terroir in viticulture. J. Wine Res. 2006, 17, 1–10. [Google Scholar] [CrossRef]
- Tardaguila, J.; Stoll, M.; Gutiérrez, S.; Proffitt, T.; Diago, M.P. Smart applications and digital technologies in viticulture: A review. Smart Agric. Technol. 2021, 1, 100005. [Google Scholar] [CrossRef]
- Ammoniaci, M.; Kartsiotis, S.-P.; Perria, R.; Storchi, P. State of the art of monitoring technologies and data processing for precision viticulture. Agriculture 2021, 11, 201. [Google Scholar] [CrossRef]
- Bramley, R. Precision Viticulture: Managing vineyard variability for improved quality outcomes. In Managing Wine Quality; Elsevier: Amsterdam, The Netherlands, 2022; pp. 541–586. [Google Scholar]
- Ozdemir, G.; Sessiz, A.; Pekitkan, F.G. Precision Viticulture tools to production of high quality grapes. Sci. Pap. Ser. B Hortic. 2017, 61, 209–218. [Google Scholar]
- Whelan, B.; McBratney, A. The “null hypothesis” of precision agriculture management. Precis. Agric. 2000, 2, 265–279. [Google Scholar] [CrossRef]
- Houborg, R.; McCabe, M.F. High-Resolution NDVI from planet’s constellation of earth observing nano-satellites: A new data source for precision agriculture. Remote Sens. 2016, 8, 768. [Google Scholar] [CrossRef]
- Gatti, M.; Garavani, A.; Vercesi, A.; Poni, S. Ground-truthing of remotely sensed within-field variability in a cv. Barbera plot for improving vineyard management. Aust. J. Grape Wine Res. 2017, 23, 399–408. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Lee, D.B.; Lee, H.I.; Myint, Z.L.; Min, S.Y.; Kim, B.M.; Oh, W.; Jung, J.H.; Yun, H.K. Grapevine growth and berry development under the agrivoltaic solar panels in the vineyards. J. Bio-Environ. Control 2022, 31, 356–365. [Google Scholar] [CrossRef]
- Strub, L.; Wittke, M.; Trommsdorff, M.; Stoll, M.; Kammann, C.; Loose, S. Assessing the economic performance of agrivoltaic systems in vineyards–framework development, simulated scenarios and directions for future research. Front. Hortic. 2024, 3, 1473072. [Google Scholar] [CrossRef]
- Weselek, A.; Ehmann, A.; Zikeli, S.; Lewandowski, I.; Schindele, S.; Högy, P. Agrophotovoltaic systems: Applications, challenges, and opportunities. A review. Agron. Sustain. Dev. 2019, 39, 35. [Google Scholar] [CrossRef]
- Ferrara, G.; Boselli, M.; Palasciano, M.; Mazzeo, A. Effect of shading determined by photovoltaic panels installed above the vines on the performance of cv. Corvina (Vitis vinifera L.). Sci. Hortic. 2023, 308, 111595. [Google Scholar] [CrossRef]




| Treatments | Leaf N (Veraison) % | Main PW (g) | Lateral PW (g) | Total PW (g) | Single Cane Weight (g) | Main LA (m2) | Lateral LA (m2) | Total LA (m2) |
|---|---|---|---|---|---|---|---|---|
| High | 1.59a | 706a | 189a | 895a | 77.3a | 3.214a | 1.321a | 4.535a |
| Medium | 1.50a | 607b | 147b | 754b | 59.2b | 3.247a | 1.098b | 4.345a |
| Low | 1.36b | 420c | 65c | 485c | 44.9c | 2.846b | 0.605c | 3.451b |
| Sig. | ** | ** | ** | ** | ** | ** | ** | ** |
| Yield (kg) | Cluster Number | Cluster Weight (g) | Berry Weight (g) | Cluster Compactness (g/cm) | Rachis Length (cm) | |||
| High | 5.9a | 19.9a | 291a | 3.0a | 23.5a | 12.5a | ||
| Medium | 5.3a | 19.8a | 265b | 2.6b | 21.5b | 11.8b | ||
| Low | 3.2b | 17.0b | 181c | 2.3c | 17.5c | 11.4b | ||
| Sig. | ** | ** | ** | ** | ** | ** | ||
| TSS (°Brix) | TA (g L−1) | pH | K+ (ppm) | Tartrate (g L−1) | Malate (g L−1) | Total Anthocyanins (g kg−1) | Total Phenolics (g kg−1) | |
| High | 22.0b | 11.3a | 3.11a | 1805a | 6.4b | 4.7a | 0.893c | 1.674c |
| Medium | 22.5b | 11.2a | 3.07a | 1622b | 5.9c | 4.5a | 1.060b | 1.888b |
| Low | 24.9a | 10.1b | 3.01b | 1470c | 7.2a | 3.3b | 1.560a | 2.656a |
| Sig. | ** | ** | ** | ** | ** | ** | ** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poni, S.; Frioni, T. Revised Viticulture for Low-Alcohol Wine Production: Strategies and Limitations. Horticulturae 2025, 11, 932. https://doi.org/10.3390/horticulturae11080932
Poni S, Frioni T. Revised Viticulture for Low-Alcohol Wine Production: Strategies and Limitations. Horticulturae. 2025; 11(8):932. https://doi.org/10.3390/horticulturae11080932
Chicago/Turabian StylePoni, Stefano, and Tommaso Frioni. 2025. "Revised Viticulture for Low-Alcohol Wine Production: Strategies and Limitations" Horticulturae 11, no. 8: 932. https://doi.org/10.3390/horticulturae11080932
APA StylePoni, S., & Frioni, T. (2025). Revised Viticulture for Low-Alcohol Wine Production: Strategies and Limitations. Horticulturae, 11(8), 932. https://doi.org/10.3390/horticulturae11080932







