Abstract
The cultivation of sweet cherry takes place in various climatic zones, where the plant may be exposed to different types of environmental stress during the growing season, which can significantly affect yield and fruit quality. The role of various physiologically active compounds is crucial for plant resistance to stressful environmental conditions. The aim of this study is to determine how the foliar application of different physiologically active substances affects the mineral composition of sweet cherry leaves. Research was performed in 2022 and 2023 at two locations (Ninski Stanovi and Murvica) in Zadar County with the Regina variety. The trials included five foliar treatments (T0—water only, T1—Ca nutritional supplement, T2—biostimulant (Ascophyllum nodosum L.), T3—proline solution, T4—salicylic acid solution). Leaf samples were collected for the analysis of the following macro-elements: total carbon (TC), total nitrogen (TN), calcium (Ca), magnesium (Mg), potassium (K), and phosphorus (P). On average, significantly higher TN content in leaves was found only in T2 (15% higher than T0). Ca, Mg, and K contents in leaf dry matter in all variants were higher by 20–29%, 13–20%, and 12–14%, respectively, compared to the control variant. The significant correlations were found between Ca and Mg, Ca and P, as well as Ca and K contents. This study shows a significant impact of the applied compounds on sweet cherry leaf mineral composition, and considering the year and locality effects, further testing of these treatments in different environments could be suggested.
1. Introduction
The increase in the world’s population is the greatest challenge in agriculture today. One obstacle to providing an adequate supply of food is the reduction in the agrochemical’s usage, leading to increasing pressure on crops and orchard productivity. By the application of certain physiologically active substances, the maximum productivity and quality of crops can be achieved, plant growth can be regulated, and the effects of the environmental stress can be reduced. In modern agriculture, biostimulators are increasingly used in addition to conventional foliar fertilizers containing macro- and microelements. Recently, according to the EU Regulation, providing plants with optimal growth conditions through fertilization, irrigation as well as phytohormones, the abiotic stress effects can be prevented to a certain extent [1]. Accordingly, the use of biostimulators containing physiologically active compounds becomes a new practice in the cultivation systems [2,3]. Biostimulants are a group of substances from natural origin that contribute to boosting plant yield and nutrient uptake, while reducing the dependency on chemical fertilizers [4]. Their functional compounds plants can use as metabolites, growth regulators, and nutrients; however, biostimulants cannot be considered biofertilizers [5].
Sweet cherry (Prunus avium L.) is a fruit species from the Rosaceae family that originates from Europe, western Asia, and the Caucasus region. Today, it is widespread and cultivated around the world, both for its delicious fruit and as an ornamental tree in gardens and parks [6]. It is an economically highly profitable crop due to its commercial, technological, and market value of the fruit [7]. Afonso et al. [8] consider sweet cherry as a highly appreciated fruit with a significant economic value, linked to production yield and quality attributes influencing consumer acceptability. In 2023, global sweet cherry production was 2,963,780.9 t on an area of 462,868 ha [9].
New regulations in agricultural fruit production including sweet cherry, restrict the use of certain chemicals that may have a long-term negative imprint on the nutritional status of plants, which is commonly evaluated using leaf mineral analysis [10]. Foliar treatment of plants is a commonly used tool in orchard management, often as a complementary measure to fertilization. Such application of fertilizers provides nutrients to the plants faster than soil application [11,12]. Moreover, this technique, if properly applied, has valuable potential for managing fruit yield and quality, offering a cost-effective approach with minimal environmental impact [13]. Accordingly, Varaldo and Giacalone [14] stated that foliar applications of macro- and micro-nutrients can mitigate cracking and improve the post-harvest performance of sweet cherries as a practical strategy to increase both yield and fruit quality in regions with adverse weather conditions.
Nutrient deficiencies in sweet cherries in Croatia exist and depend on soil type, variety, substrate, and fertilization management. Indiscriminate application is often ineffective in combating biotic and abiotic stresses and can be harmful to the environment. Macro-elements, including nitrogen (N), phosphorus (P), potassium (K), and carbon (C), are the essential nutrients for the normal growth and development of plants [15,16]. Ziogas et al. [12] consider N as an essential ingredient for plant survival, which possesses multiple principal roles in plant metabolism (aminoacids, proteins (enzymes), and storage compounds of N, etc.). N is differently accumulated plant tissues and organs [17]. Mostly, the leaves, seeds, flower buds, and fruits have the highest N content. Nitrogen plays a critical role in the vegetative growth, especially regarding leaf formation, shoot extension, and root activity [18]. It is essential for the biosynthesis of various primary metabolites (i.e., amino acids, chlorophyll, nucleic acids, lipids, and proteins) and a plethora of functional secondary metabolites, such as alkaloids, betalains, and cyanogen glucosides, etc. [19]. An important role of P is in the energy conversion cycle, the formation and function of the plant’s reproductive organs, sugar phosphates, phospholipids, DNA, RNA, and nucleotide synthesis [19,20]. Among the physiological roles of Ca and K, these nutrients are essential for reproduction involved in the metabolism of growth regulators (hormones) and sugars. The desired quantity and quality of fruit is inconceivable without an adequate supply of K and Ca [11]. Leaf area in stone fruits is necessary to meet the fruit’s C needs, which vary throughout the growing season and during the growth and development of the entire plant [21,22]. According to Gluszek et al. [20], during fruit development stages, sweet cherry requires 10 kg of magnesium (Mg) per plant. In the leaves of the sweet cherry, the optimal amount of Mg is 0.3 to 0.5% [23]. More Mg can be found in younger, developing leaves because of its essential role in plant metabolism.
Here, the study is carried out on the introduced German cultivar ‘Regina’, the late-ripening sweet cherry variety, which forms a lush tree with a well-branched and broad crown. In the Mediterranean climate, flowering takes place in the first half of April, and the fruit ripens in June. The fruits are medium-sized, heart-shaped, red in color, and of good flesh quality [24]. This variety was primarily chosen for its cold resistance and late ripening, which offer advantages in coping with increasingly frequent climate extremes, such as frost during flowering and other impacts of climate change.
The aim of this work is to investigate the effect of foliar treatments with different physiologically active substances (Ca supplement, biostimulant containing algae extract, proline solution, salicylic solution) on the mineral composition of sweet cherry leaves during two years of research at two locations.
2. Materials and Methods
2.1. Location, Soil and Climatic Features
The research was conducted in sweet cherry orchards at the locations Ninski Stanovi (44.205056, 15.235148) and Murvica (44.149544, 15.366065) in Zadar County, Republic of Croatia. The sweet cherry orchard at Ninski Stanovi is located 30 m above sea level, while at the Murvica location the position of the orchard is 90 m above sea level. The orchard in Ninski Stanovi is located on an area of slightly more than 40 hectares with over 20 varieties. The cultivation form is KGB (Kym Green Bush). The plantation was established in 2012. The planting pattern is 5 m × 2.5 m. The orchard in Murvica is located on an area of just over 6 h with 7 varieties of sweet cherry. The cultivation form is the vase. The orchard was planted in 2011. The planting pattern is 5 m × 4 m. The row arrangement in both orchards is NE-SW. The rootstock in both orchards is mahaleb cherry (Prunus mahaleb L.). The subject of research was the introduced German sweet cherry variety Regina (Prunus avium var. Regina), which is known to be cold resistant.
Irrigation and basic fertilization are carried out using the fertigation system or manually by the producers, according to the soil properties determined by the previous analysis. The average annual amounts were N 110 kg/ha, P 103 kg/ha, and K 130 kg/ha. Chemical analysis of the soil was carried out at both locations throughout the entire depth (0–60 cm) before setting up the experiment. At the Ninski Stanovi location, soil pH (H2O) was slightly alkaline (7.65), with medium content of organic matter, high content of N (0.17% N), very high potassium content (53.53 mg K2O/100 g), and medium P content (13.18 mg P2O5/100 g). At the Murvica location, soil pH (H2O) was slightly alkaline (7.42), with medium content of organic matter, high content of N (0.36% N), medium potassium content (23.37 mg K2O/100 g), and also medium P content (18.58 mg P2O5/100 g).
The climate of the research area at the Ninski Stanovi location belongs to the type of Mediterranean, subtype EU–Mediterranean characterized by mild, rainy, moderately windy winters and hot, dry summers, while the climate around Murvica belongs to the type of moderately warm humid climate with hot summers [25]. Climatic data for the years 2022 and 2023 were taken from the meteorological stations of the Pinova TM [26] type located within both orchards (Figure 1 and Figure 2).
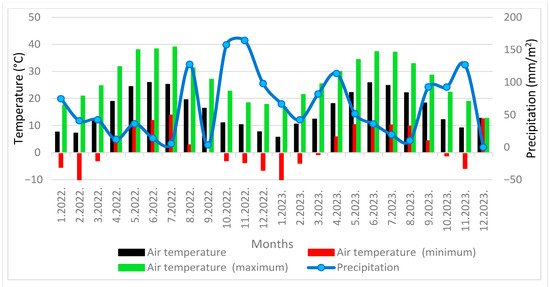
Figure 1.
Monthly temperature (minimum and maximum) and monthly precipitation at the location Ninski Stanovi (January 2022–December 2023).
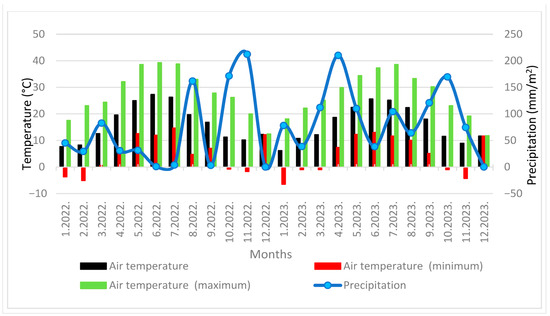
Figure 2.
Monthly temperatures (minimum and maximum) and monthly precipitation at the location Murvica (January 2022–December 2023).
The total amount of precipitation in 2022 at Ninski Stanovi locality was 734.4 mm and in 2023, 733.6 mm, while at the Murvica locality there was 772.4 mm in 2022 and 1120.4 mm in 2023. The absolute maximum temperature in Ninski Stanovi was 39 °C in 2022 and 37.3 °C in 2023, while it was recorded as 39.3 °C in 2022 and 38.6 °C in 2023 at the Murvica locality. The absolute minimum temperature at Ninski Stanovi was −10 °C in both years, while it was −5.1 °C in 2022 and −6.5 °C in 2023 at Murvica locality (Figure 1 and Figure 2).
2.2. Experimental Design
The research was conducted over two years on 75 sweet cherry trees per location, with one tree isolated among the repetitions, according to the randomized block design. Foliar applications were carried out three times during the fruit development stages, comprehending back spraying of five foliar application variants in the following three repetitions: T0—control (tap water), T1—commercial calcium (6.7 mmol/L per tree)-based nutritional supplement complexed with ammonium lignin sulfonate, and T2—commercial biostimulator based on algae (Ascophyllum nodosum L.) extract, with the trade name AlgoVital® Plus (Pro-eco d.o.o., Novi Marof, Croatia). Its composition includes carbohydrates, microelements (Zn, B, and Mg), phytohormones, amino acids, vitamins, and precursors of plant hormones. T3 is the proline solution (10 mM) and T4 is the salicylic acid solution (1 mM; Table 1). The first foliar treatment was performed at the beginning of fruit development (BBCH 73) [27], and the last was performed at the optimal harvest time for each locality, depending on the microclimate. The foliar applications were performed with an interval of 7 to 10 days.

Table 1.
Foliar applications specifications.
2.3. Leaf Sampling and Elemental Analysis
Leaf sampling was performed five days after the last foliar application. The average leaves from each repetition (5 trees) were randomly collected, equally from all sides of the crown, in total 15 to 20 leaves per repetition.
The leaf samples were dried in a dryer with hot air circulation first at 70 °C and then briefly at 105 °C to the constant mass. Dry samples were homogenized by milling (Grindomix GM 200, Retsch GmbH, Hann, Germany). Plant material was digested using the mixture of nitric acid (HNO3) and hydrogen peroxide (H2O2) in a microwave oven [28]. The samples were quantitatively transferred to plastic cuvettes and diluted to 50 mL with demineralized water. The concentrations of Ca, Mg, K and P were analyzed by ICP-MS, a coupled plasma technique on a Perkin Elmer Sciex Elan instrument [29].
The content of total C and total N was determined in dry leaf powder using C/N analyzer (Skalar Primacs SNC 100-IC, Skalar Analytical B.V., Breda, The Netherlands).
2.4. Statistical Data Processing
The statistical analysis of the data was performed with TIBCO Statistica Software Inc. v. 13.6.0 (2020) [30]. Nutrient content (N, C, Ca, Mg, K, and P) in sweet cherry leaves under the influence of foliar applications two locations in two years are groups of data statistically evaluated using three-factor analysis of variance (ANOVA) and the F test. A significant difference among treatment means for each trait was established using Fisher’s LSD test. The relationships (correlations) among the leaf contents of Ca and Mg, Ca and P, and Ca and K in the particular treatments were evaluated by linear regression analyses and t-tests.
3. Results
3.1. The Influence of Foliar Application with Physiologically Active Substances, Locations, and Year on the Mineral Composition of Sweet Cherry Leaves
The three-way ANOVA data analyses showed a significant influence of the foliar application with different physiologically active substances on mineral composition of sweet cherry leaves in four of six nutrients analyzed—N, Ca, Mg, K—while for C and P the treatment influence was not statistically significant. Also, there are the established differences between locations and years, as well as the significance of the interactions of foliar application × year, foliar application × location, location × year and foliar application × year × location (Table 2).

Table 2.
Influence of foliar application with physiologically active substances, location, and years (2022 and 2023) on the mineral content (N, C, Ca, Mg, K, and P) of sweet cherry leaves.
The C content in leaf dry matter was not influenced by the applied treatments, although the highest values were found in T1 and T2 treatments, while the control (T0), which was treated with water only, showed the lowest values. As for the location impact, the total C content was lower at Murvica by 3%, whereas considering the influence of the production year, C content was significantly lower in 2022, by 6%.
The total content of N in leaves had a significantly higher value only in T2, by 15% as compared to the control (T0). Considering locations, total N content was higher at the Ninski Stanovi by 12%, and looking at the production years, N content was higher in 2022 by 13%.
The content of Ca in leaves was increased significantly by all foliar applications as compared to the control (T0). The highest increase in leaf Ca content was seen in T1 foliar application (29%), as expected. The higher Ca content was determined at the Murvica location, where it was on average for all treatments and both years 17% higher compared with the location Ninski Stanovi, and considering the production year effect, Ca content was higher in 2022 by 15%.
The total content of Mg in leaves showed the highest value in biostimulator application (T2), where it was 20% higher than in control plants (T0). It is followed by T3 and T4, while T1 showed the lowest increase (13%) compared to the control. There are no statistically significant differences in Mg in sweet cherry leaves between the locations and between years, respectively.
The content of K in the leaves was insignificantly different among the foliar applications; however, in the control variant, it was significantly lower. The positive effect of the applied treatments on K accumulation in cherry leaves ranged from 12% in T3 to 14% in T4. The higher K content was obtained at the Ninski Stanovi location, where it was 9% higher than at the Murvica location, and considering the growing seasons, it was higher in 2022 by 7%.
On average for both locations and years, the P content in the leaf was not influenced by the applied foliar treatments. Considering the differences between the locations and production years, there was significantly higher P content at the Murvica locality and in 2022, respectively.
There is statistically significant influence of the interaction foliar application × year regarding Mg content, the influence of the interaction foliar application × location was significant for N and potassium content, while the interaction of location × year influenced significantly on N and Ca contents in leaves. The interaction of all three factors in this research (foliar application × year × location) influenced N, P, and potassium contents.
3.2. The Influence of Foliar Applications with Physiologically Active Substances on Particular Locations and Year on the Mineral Content of Ca, Mg, and K in Sweet Cherry Leaves
The following Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8 show the results of the two-way ANOVA statistical analysis of the content of Ca, Mg, and K in the sweet cherry leaf dry matter after foliar applications (T0, T1, T2, T3, and T4) in two consecutive years (2022 and 2023) at a specific location (Murvica and Ninski Stanovi).
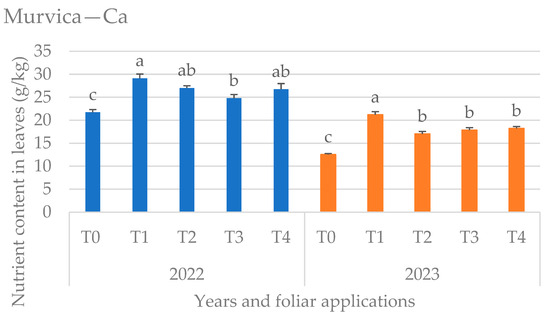
Figure 3.
Calcium (Ca) content in leaves at Murvica location by years and foliar applications (T0—water only, T1—Ca nutritional supplement, T2—biostimulant (A. nodosum L.), T3—proline solution, T4—salicylic acid solution), mean values marked with the same letters were not significantly different at p < 0.05.
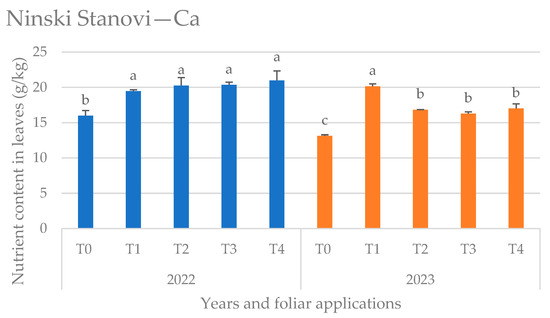
Figure 4.
Calcium (Ca) content in leaves at the Ninski Stanovi location by years and foliar applications, (T0—water only, T1—Ca nutritional supplement, T2—biostimulant (Ascophyllum nodosum L.), T3—proline solution, and T4—salicylic acid solution), mean values marked with the same letters were not significantly different at p < 0.05.
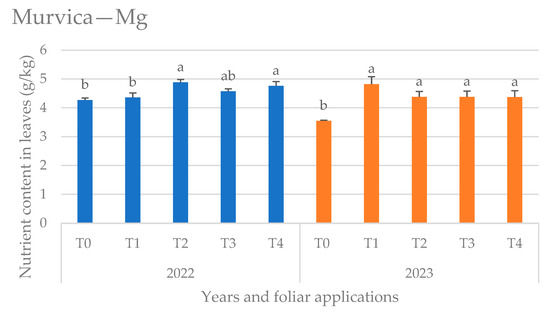
Figure 5.
Magnesium (Mg) content in leaves at the Murvica location by years and foliar applications (T0—water only, T1—Ca nutritional supplement, T2—biostimulant (A. nodosum L.), T3—proline solution, and T4—salicylic acid solution), mean values marked with the same letters were not significantly different at p < 0.05.
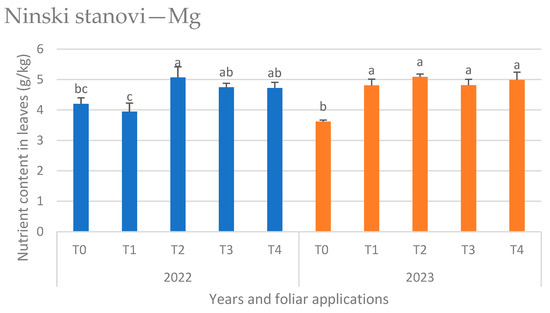
Figure 6.
Magnesium (Mg) content in leaves at the Ninski Stanovi location by years and foliar applications (T0—water only, T1—Ca nutritional supplement, T2—biostimulant (A. nodosum L.), T3—proline solution, and T4—salicylic acid solution), mean values marked with the same letters were not significantly different at p < 0.05.
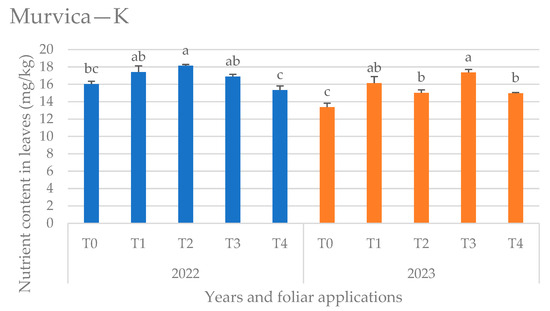
Figure 7.
Potassium (K) content in leaves at the Murvica location by years and foliar applications, (T0—water only, T1—Ca nutritional supplement, T2—biostimulant (Ascophyllum nodosum L.), T3—proline solution, and T4—salicylic acid solution), mean values marked with the same letters were not significantly different at p < 0.05.
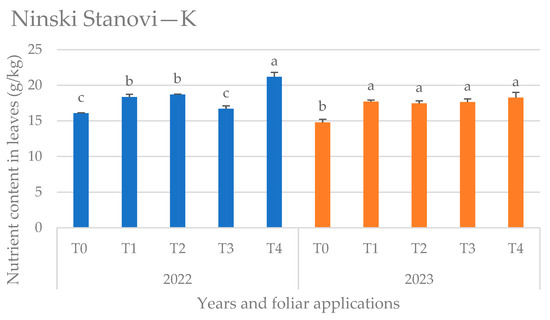
Figure 8.
Potassium (K) content in leaves at the Ninski Stanovi location by years and foliar applications, (T0—water only, T1—Ca nutritional supplement, T2—biostimulant (A. nodosum L.), T3—proline solution, and T4—salicylic acid solution), mean values followed by the same letters were not significantly different at p < 0.05.
Ca content was significantly higher in the year 2022 at the location Murvica, regardless of the foliar treatment (Figure 3). Foliar application of Ca supplement (T1) resulted in the highest leaf Ca content in both years, which was expected. The control variant (only water spraying) showed the lowest amounts of Ca in the leaf, while the effect of other foliar applications (T2, T3, and T4) was not significant in both years.
At the Ninski Stanovi location (Figure 4) in the first year (2022) of the research, the lowest Ca content in sweet cherry leaves was in the control (T0), while the other foliar applications did not differ significantly. In the second year of the research (2023), the impact of the Ca foliar application was more pronounced, so that the highest Ca content was noticed in T1 treatment.
The effect of foliar applications on the mineral content of Mg in leaves at the Murvica location (Figure 5) in 2022 resulted with statistically significant highest values in T2 and T4, while the lowest content was in T0 and T1. In 2023, the control (T0) variant showed significantly lowest content of Mg, without significant differences among the other foliar applications.
At the Ninski Stanovi location in 2022, biostimulant application (T2) resulted in the highest Mg content in the leaves, while T1 and T0 showed the lowest values. In the following year, 2023, the highest value was also detected in T2; although, it was not significantly different from other foliar applications, except for the control (T0), which showed the lowest Mg content (Figure 6).
The K content in the leaves at the Murvica location (Figure 7) in 2022 had statistically significant highest values in T2 (biostimulant application), while in 2023 it was highest in T3 (proline application) and the lowest was detected in control (T0).
At the Ninski Stanovi location (Figure 8) the highest K content was obtained in both years of the research by T4 (salicylic acid solution) treatment, differing significantly from other foliar applications in 2022, however in 2023 when K content was lower, in general, on both locations, the effect of the applied physiologically active compounds was less pronounced. However, the control variant had the lowest K content in both years on this locality.
3.3. Relationships Among Ca, Mg, P, and K Content in Sweet Cherry Leaves Under the Influence of Applied Foliar Treatments
The applied foliar treatments influenced mutual relationships of the tested macronutrients in sweet cherry leaves, whereas the relationships between Ca content and K, Mg, and P contents were the most interesting (Table 3), considering the possible influence of Ca on fruit quality.

Table 3.
Correlations between Ca and macro-elements (Mg, P, and K) in the leaves, shown with linear correlation coefficients, under influence of different foliar applications (T0—water only, T1—Ca nutritional supplement, T2—biostimulant (A. nodosum L.), T3—proline solution, and T4—salicylic acid solution).
The linear regression analysis showed lower or negative correlation coefficients between Ca and Mg contents in treated plants in comparison with the control (T0), which had a coefficient of r = 0.82 **, whereas the only positive correlation was found in T4 (r = 0.23 *). The other foliar applications (T1, T2, and T3) resulted in the following negative coefficients: r = −0.03 *, r = −0.02 *, and r = −0.12 *, respectively. The correlation coefficients between Ca and P were positive in general and ranged from 0.46 at T3 to 0.71 at T4. The correlation between Ca and potassium was very significant and positive in T0 and T2; however, negative coefficients were established between these two macronutrients in T1, T3, and T4.
4. Discussion
Optimal macro-element content in plants is crucial for healthy growth and development because these elements are essential building blocks and functional components for various plant processes. Macronutrients like N, P, and K are needed in larger quantities and play vital roles in energy production, structural support, and overall plant health [31].
Chosen foliar applications had no significant effect on TC content in sweet cherry leaves. In the studies of Roper et al. [32] on fruitful trees, the C content does not change until the second stage of fruit development. Also, the level of C in the leaf had no effect on photosynthesis in their research.
The results of total N content according to the standards (deficient, low, normal, high, and excess) for the mineral composition of sweet cherry leaf [33] belongs to the low content category (1.7–2.1%) for all foliar application values (1.82–1.90%) except T2, whose value (2.16%) was between the low and normal (2.2–2.6%) categories. The results largely coincide with the studies of Thakur et al. [34] and Sánchez-Alonso and Lachica [35], who found that the N content in the leaf begins to decrease at the end of the growing season and at fruit maturity, which may be a consequence of the onset of N remobilization before leaf fall [36]. The results obtained here are consistent with the studies of Batjer and West Wood [37], Verma and Bhandari [38] on peach, and Rehalija and Sandhu [39] on Japanese apple. Furthermore, in the study by Mikiciuk et al. [40], Ca fertilization resulted in a 12% higher N concentration in the sweet cherry leaf, while the N concentration in our T1 treatment (Ca) increased by 3% on average, compared to the control. However, the biostimulator (extract of A. nodosum L.) used in the T2 increased the total N content in the sweet cherry leaf by 15%. Similar results have been obtained in numerous studies on the application of biostimulators based on algae in some other fruit species. For example, Ismail and Ghazzi [41] mention that treatment of olives with 12% natural organic matter extracted from seaweed (A. nodosum) increased the N concentration in the leaf. Bradshaw et al. [42] found that spraying with extracts from seaweed significantly improved the vegetative growth of apple trees. Al-Hadethi [43] found that spraying with seaweed extracts increased the content of several minerals (N, K, Ca, and Fe) in apricot leaves. Eman et al. [44] also reported that the application of algae extract was very effective in stimulating the mineral composition of the leaf. Jannin et al. [45] showed that a biostimulant derived from A. nodosum L. improved N uptake by regulating the expression of genes involved in photosynthesis and N metabolism. As for the treatment with proline solution (T3), Hölzel et al. [46] reported that although the potential of foliar spray application has been demonstrated, there remains uncertainty around whether proline is taken up by cherry leaves, followed by translocation into other parts of the tree. The authors found that the percentage of N derived from proline in tree tissues was low, and considering the deciduous nature of sweet cherry, they supposed that the remobilized N from previous seasons and N from commercial fertilizer application (80 kg N ha−1 and season) likely accounted for the majority of N present in tissues. Shehata [47] stated that the positive impacts of proline extend beyond enhancing fruit quality and productivity under unfavorable conditions. Hence, further investigation is required to define the optimal dosages of proline, the ideal timing of pre-harvest application, and the frequency at which proline should be administered to fruit trees in order to attain the utmost fruit yield and quality.
The results of the mineral content of Ca in the sweet cherry leaves according to the research standards [33] belong to the category of normal content. Moreover, all the results are generally consistent with the studies of Sánchez-Alonso and Lachica [35], which show the Ca content in sweet cherry leaves throughout the growing season, with an increase in Ca content in the period up to harvest. In addition, Thakur et al. [34] found that Ca concentration increases with increasing leaf age. Mikiciuk et al. [40] showed that the leaf Ca concentration of a sweet cherry treated with Ca foliar fertilizer increased by 28% compared to the control, while in our study it increases almost equally, by 28.5%, on average, over two years and two locations. In the research of Hakimi and Lafrikhi [48] on raspberries, the foliar application of Ca at different doses caused an increase in leaf Ca content from 42 to 46%. In addition, the biostimulator used in the study by Basile et al. [49] caused a threefold increase in Ca content in petioles in two varieties of sweet cherry, while in our research the biostimulator increased Ca content by 22%. In the study by Zouari et al. [50] on olives, the treatment with the amino acid proline increased the Ca content in the leaf by 30%, while in our case the Ca content was higher by 20% compared to the control. Chen et al. [51] emphasize that exogenous application of proline to crops such as tobacco has been shown to cause a significant intracellular accumulation of calcium ions (Ca2+), which in turn triggers an oxidative burst. This elevated cytosolic Ca2+ concentration may contribute to an overall increase in calcium content in leaf tissues. When treated with salicylic acid (T4), an increase in leaf Ca content compared to the control was 24% on average for two years and two locations, which is consistent with the results of Aghaeifard et al. [52], who obtained an increase of 12% to 18% in one year when treating strawberries with salicylic acid at different doses. Salicylic acid regulates cell membrane integrity and ion transport, facilitating the uptake of beneficial macronutrients, including calcium. It also contributes to the balance of mineral metabolism, which improves the intake of Ca2+ [53].
The results of the mineral content of Mg in the sweet cherry leaves according to the research standards [33] belong to the category of normal content. Mg content in sweet cherry leaves was studied by the authors Sánchez-Alonso and Lachica [35], who observed a constant increase in the Mg content throughout the growing season, which was also confirmed by Thakur et al. [34]. Mikiciuk et al. [40] reported that the Mg concentration in the leaf of a sweet cherry treated foliar with Ca fertilizer was decreased by 17% compared to the control, which is the opposite of our study, where leaf Mg content was increased by 13%. The treatment of tomato plants with two biostimulants based on A. nodosum L. extract in the study by Dell’Aversana et al. [54] led to an increase in the Mg content in the leaf by 8 and 20% compared to the control, whereas in our research, biostimulator treatment enhanced Mg content in sweet cherry leaves by 20%. Also, the treatment with proline in the study of Zouari et al. [51] on olives increased the Mg content in the leaf by 16%; indeed, the same effect is seen in our study on sweet cherries. Treatments with different doses of salicylic acid on strawberries in the study by Aghaeifard et al. [52] showed no statistically significant differences in leaf Mg content, while in our research salicylic acid solution treatment resulted in an increase over the control of 17%.
The results of the mineral content of K in the sweet cherry leaves, according to the research standards [33], belong to the category of normal content, except for T0, which is slightly lower. The content of K in the leaves of sweet cherry and other fruit species has been studied by many authors. Some authors studied the seasonal movement of K in the leaf, while on the other hand a certain number of authors used treatments that can be compared with our experiment. The results obtained are in agreement with the study [34], where the potassium content gradually increases up to July 15, which coincides with the harvest date in our experiment. The opposite trend was found by Sánchez-Alonso and Sánchez-Alonso and Lachica [35] in their study on the accumulation of K content in sweet cherry leaves, where it decreased until harvest. The Ca-based products decreased the K content in the leaf by 8%. In our study, in T1 treatment where the Ca-based product was used, the Ca content in the leaf increases by 13%. Al-Hadethi [43] found that spraying with seaweed extracts increased the mineral K content in apricot leaves, while Ismail and Ghazzi [41] also reported that treating olives with 12% natural organic material extracted from seaweed (A. nodosum) increased the K concentration in the leaf. In addition, Zouari et al. [50] studied the impact of treatment with proline on the K content in olive leaves, where the content increased by 12%, the same 12% as in our research. The treatments with different doses of salicylic acid on strawberries in the study by Aghaeifard et al. [52] showed no statistically significant differences in the K content of the leaf, while in our study there is an increase of 13.5% in the T4 compared to the control T0.
The content of P in the sweet cherry leaves from this research showed no significant difference over the foliar applications, but it was slightly elevated according to the standards [32]. In addition, foliar Ca fertilization in the Mikiciuk et al. [40] study resulted in 8% lower P concentration in sweet cherry leaves, which is consistent with the results of this research, where P concentration in T1 (Ca) decreased by 2.5%. Ismail and Ghazzi [41] mentioned that treatment of olives with 12% natural organic matter extracted from algae (A. nodosum) increased P concentration in leaves, while Esitken et al. [55] stated in their study that different biostimulants increased the P content in sweet cherry leaves by 15–29%, which is consistent with this research where the increase of P content in the same foliar application was 0,5%. Afonso et al. [8] generally state in their review article that the P content in the leaf is increased by treatment with biostimulants. In a study by Rashedy et al. [56], proline treatment with added humic acid was found to increase the P content in pomegranate leaves by 23.8%. As stated by Hölzel et al. [46], the seasonal movement of P has a greater influence than the influence of treatment with the amino acid proline absorbed in the leaf and translocated to the fruit and other storage organs. Treatments with different doses of salicylic acid on strawberries in the study by Aghaeifard et al. [52] contributed to a 6% increase in leaf P content, while the opposite result is found in our study, where leaf P content decreased by 4% in the T4, further leading to the conclusion that the seasonal movement of P in the plant organs had a greater impact than the foliar application.
The specific relationships among plant nutrients, that is, a possible synergism or antagonism in their uptake and accumulation in plants can be defined by the correlation coefficients. Some studies have shown that there is cation antagonism between Ca, Mg, and K, so that high levels of one or more of these nutrients can lead to reduced uptake of the other nutrient, despite sufficient amounts in the soil [57,58,59,60]. Ca and Mg have similar chemical properties (both are divalent cations), so depending on the concentration, form of application, time, and soil condition, Ca can competitively inhibit Mg uptake through the same transporters in root cells. In the study by Piao et al. [61], increasing Ca availability led to a significant decrease in Mg concentrations in the leaves, and increasing Mg availability decreased Ca concentrations in the roots. Mg deficiency in the field is usually caused by ion antagonism [62], and plant Ca uptake is inhibited by Mg excess in tissues [63]. Thouraya et al. [64] reported negative relationships between K and Mg, as well as Ca and Mg, in sweet cherry leaves. These statements can be compared with the correlations obtained in our research (Table 3), in which foliar applications T1 (CaO 15%), T2 (A. nodosum L. extract) and T3 (proline 10 mM) resulted in negative relationships between Ca and Mg. A study by Sánchez-Alonso and Lachica [35] in sweet cherry leaves showed a moderate significant correlation (p < 0.05) between Ca and Mg, while in our research this relationship was very significant and positive in control plants (T0; p < 0.01) only. The authors found moderately significant negative correlation between Ca and P, as opposed to our results, where this relationship was strong (p < 0.01) and positive in all treatments. Also, the significant negative correlations (p < 0.05) of Ca and K obtained in the T1 (CaO 15%), T3 (proline 10 mM), and T4 (salicylic acid 1 mM) foliar applications are in agreement with the statements of Rhodes et al. [58] who found a significant and negative correlation between K and Ca in sugarcane leaves, and Andziak et al. [65] in apples. Wakeel et al. [66] reported that the addition of too much Ca and Mg can lead to K deficiency due to competition between Ca, Mg, and K, and excessive K supplementation can also reduce Ca and Mg intake [67].
As stated by Teklić et al. [68], the uptake of minerals, hormones, antioxidants, and other functional compounds from biostimulants may contribute to the biofortification of plants grown for food/fodder in a sustainable way and therefore should achieve more attention from plant and food scientists. Here, the applied foliar treatments with different physiologically active substances resulted in higher mineral contents in sweet cherry leaves compared to the control, which consistently had the lowest value for all nutrients analyzed.
5. Conclusions
Foliar application of physiologically active compounds in this research had a significant impact on the mineral composition of sweet cherry leaves regarding N, Ca, Mg, and K content, while the C and P contents were not affected by the applied treatments. Leaf application of Ca supplement (T1) showed the largest increase in leaf Ca content, also a negative correlation with Mg and K and a positive correlation with P. The largest increase in N and Mg content was recorded after the treatment with algae-based biostimulant (T2) which also had a strong influence on the leaf K content. Also, the applications of proline (T3) and salicylic acid solution (T4) stimulated the accumulation of Ca, Mg, and K in sweet cherry leaves. Location and year had significant influence on the nutrient content: Murvica had higher Ca and P contents, and Ninski Stanovi had higher N and K contents. The year 2022 was associated with higher C, N, Ca, and K content, while Mg and P showed inconsistent variation by year. The Ca and P relationship in sweet cherry leaves established moderate to strong positive correlations, especially in salicylic acid treatment (T4). Both positive and negative correlations between Ca and K are obtained in this study, indicating diverse and complex relationships between these two nutrients. Significant interactions between the factors (foliar application × year, foliar application × location, and location × year) further suggest that nutrient accumulation in leaves depends on the environmental factors. In 2023, lower Ca and K values were detected at both locations compared to 2022. The reason for this might be the extreme amounts of precipitation recorded at both locations in a few days during the fruit development in 2023 (Figure 1 and Figure 2). The study confirms that targeted foliar application of physiologically active substances can significantly improve the mineral composition of sweet cherry leaves, especially for N, Ca, Mg, and K, with variability influenced by environmental conditions and variants of foliar applications.
As the sweet cherry is a perennial, a woody fruit crop, it is generally necessary to continue with such research in different environments to better understand the accumulation and transport of tested nutrients in sweet cherries.
Author Contributions
Conceptualization, M.Z. (Marko Zorica), M.L. and T.T.; methodology, M.Z. (Marko Zorica) and M.Š.; validation, T.T., T.K. and M.L.; formal analysis, M.Z. (Marko Zorica); investigation, M.Z. (Marko Zorica), M.L. and M.Š.; resources, M.Z. (Marko Zorica), M.Š., M.Z. (Magdalena Zorica), J.R. and Š.K.; data curation, M.Z. (Marko Zorica), Š.K., M.Š. and M.Z. (Magdalena Zorica); writing—original draft preparation, M.Z. (Marko Zorica), T.T. and M.L.; writing—review and editing, T.T., T.K. and Š.K.; visualization, M.Z. (Marko Zorica) and J.R.; supervision, T.T. and T.K.; project administration, T.T. and M.L.; funding acquisition, M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded as a part of the institutional scientific project entitled “Treatment of seed and plantlets with wild plant-derived products” financed by the Faculty of Agrobiotechnical Sciences Osijek. URL: https://www.croris.hr/projekti/projekt/9938 (accessed on 29 July 2025).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study in the collection, analyses, or interpretation of data in the writing of the manuscript or in the decision to publish the results.
References
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003 (Text with EEA Relevance). 2019. Available online: https://eur-lex.europa.eu/homepage.html (accessed on 19 March 2024).
- Yakhin, O.; Lubyanov, A.; Yakhin, I.; Brown, P. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Xu, L.; Geelen, G. Developing biostimulants from agro-food and industrial by-products. Front. Plant Sci. 2018, 9, 1567. [Google Scholar] [CrossRef] [PubMed]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species—A review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Tian, C.; Yao, R.; Xu, X.; Ai, X.; Hu, M.; Wang, W.; Liu, X.; Li, Y.; Zhang, A. Genome-wide characterization and expression analysis of the PavC2H2 gene family to different abiotic stress in sweet cherry (Prunus avium L.). S. Afr. J. Bot. 2024, 171, 245–256. [Google Scholar] [CrossRef]
- Serrano, M.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Valero, D. Chemical constituents and antioxidant activity of sweet cherry at different ripening stages. J. Agric. Food Chem. 2005, 53, 2741–2745. [Google Scholar] [CrossRef] [PubMed]
- Afonso, S.; Oliveira, I.; Meyer, A.S.; Gonçalves, B. Biostimulants to improved tree physiology and fruit quality: A review with special focus on sweet cherry. Agronomy 2022, 12, 659. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products. 2024. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 21 May 2024).
- Roversi, A.; Malvicini, G.L.; Porro, D.; Plessi, C. Sweet cherry leaf composition as influenced by genotype, rootstock and orchard management. Acta Hortic. 2010, 868, 243–246. [Google Scholar] [CrossRef]
- Nagy, P.T.; Thurzó, S.; Kincses, I.; Szabó, Z.; Nyéki, J. Effect of foliar fertilization on leaf mineral composition, sugar and organic acid contents of sweet cherry. Int. J. Hortic. Sci. 2008, 14, 45–48. [Google Scholar] [CrossRef]
- Ziogas, V.; Michailidis, M.; Karagiannis, E.; Tanou, G.; Molassiotis, A. Manipulating Fruit Quality Through Foliar Nutrition; Elsevier: Amsterdam, The Netherlands, 2020; pp. 401–417. [Google Scholar] [CrossRef]
- Tagliavini, M.; Drahorad, W.; Dalla Via, J. Preface. Acta Hortic. 2002, 594, 9. [Google Scholar] [CrossRef]
- Varaldo, A.; Giacalone, G. Enhancing cracking resistance and post-harvest quality of sweet cherries (Prunus avium L.) through Ca and potassium-based foliar treatments. Horticulturae 2025, 11, 30. [Google Scholar] [CrossRef]
- Barczak, B.; Nowak, K. Effect of sulphur fertilisation on the content of microelements’ and their ionic ratios in potato tubers. J. Elem. 2015, 20, 37–47. [Google Scholar] [CrossRef]
- Heyburn, J.; McKenzie, P.; Crawley, M.J.; Fornara, D.A. Effects of grassland management on plant C:N:P stoichiometry: Implications for soil element cycling and storage. Ecosphere 2017, 8, e01963. [Google Scholar] [CrossRef]
- San Martino, L.; Sozzi, G.O.; San Martino, S.; Lavado, R.S. Isotopically labelled N uptake and partitioning in sweet cherry as influenced by timing of fertilizer application. Sci. Hortic. 2010, 126, 42–49. [Google Scholar] [CrossRef]
- Shah, I.H.; Jinhui, W.; Li, X.; Hameed, M.K.; Manzoor, M.A.; Li, P.; Zhang, Y.; Niu, O.; Chang, L. Exploring the role of nitrogen and potassium in photosynthesis implications for sugar: Accumulation and translocation in horticultural crops. Sci. Hortic. 2024, 27, 112832. [Google Scholar] [CrossRef]
- Tariq, A.; Zeng, F.; Graciano, C.; Ullah, A.; Sadia, S.; Ahmed, Z.; Murtaza, G.; Ismoilov, K.; Zhang, Z. Regulation of metabolites by nutrients in plants. In Plant Ionomics: Sensing, Signaling, and Regulation; Singh, V.P., Siddiqui, M.H., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2023; pp. 1–18. [Google Scholar] [CrossRef]
- Głuszek, S.; Derkowska, E.; Sas-Paszt, L.; Sitarek, M.; Sumorok, B. Influence of bioproducts and mycorrhizal fungi on the growth and yielding of sweet cherry trees. Hort. Sci. 2020, 47, 122–129. [Google Scholar] [CrossRef]
- Dejong, T.M.; Walton, E.F. Carbohydrate requirements of peach fruit growth and respiration. Tree Physiol. 1989, 5, 329–335. [Google Scholar] [CrossRef]
- Penzel, M.; Möhler, M.; Weltzien, C.; Herppich, W.; Zude-Sasse, M. Estimation of daily carbon demand in sweet cherry (Prunus avium L.) production. J. Appl. Bot. Food Qual. 2020, 93, 149–158. [Google Scholar] [CrossRef]
- Bergmann, W. Farbatlas—Ernährungsstörungen bei kulturpflanzen. Jena VEB Gustav Fischer Verlag. 1986, 1, 306. [Google Scholar]
- Sagredo, K.X.; Cassasa, V.; Vera, R.; Carroza, I. Pollination and fruit set for ‘Kordia’ and ‘Regina’ sweet cherry trees in the south of Chile. Acta Hortic. 2017, 1161, 353–360. [Google Scholar] [CrossRef]
- Šegota, T.; Filipčić, A. Köppenova podjela klima i hrvatsko nazivlje. Geoadria 2003, 8, 17–37. [Google Scholar] [CrossRef]
- PinovaMeteo Agriculture Weather Station. Available online: https://pinova-meteo.com (accessed on 12 March 2024).
- Fadón, E.; Herrero, M.; Rodrigo, J. Flower development in sweet cherry framed in the BBCH scale. Sci. Hortic. 2015, 192, 141–147. [Google Scholar] [CrossRef]
- Wu, S.L.; Feng, X.B.; Wittmeier, A. Microwave digestion of plant and grain reference materials in nitric acid or a mixture of nitric acid and hydrogen peroxide for the determination of multi-elements by inductively coupled plasma mass spectrometry. J. Anal. Atomic Spectr. 1997, 12, 797–806. [Google Scholar] [CrossRef]
- Matusiewicz, H.; Sturgeon, R.E.; Berman, S.S. Trace element analysis of biological material following pressure digestion with nitric acid—Hydrogen peroxide and microwave heating. J. Anal. At. Spectrom. 1989, 4, 323–327. [Google Scholar] [CrossRef]
- TIBCO Statistica, v. 13.6.0; TIBCO Software Inc.: Palo Alto, CA, USA, 2020. Available online: https://docs.tibco.com/products/spotfire-statistica/archive (accessed on 15 April 2024).
- Monib, A.W.; Alimyar, O.; Mohammad, M.U.; Akhundzada, M.S.; Niazi, P. Macronutrients for plants growth and humans health. J. Res. Appl. Sci. Biotechnol. 2023, 2, 268–279. [Google Scholar] [CrossRef]
- Roper, T.R.; Keller, J.D.; Loescher, W.H.; Rom, C.R. Photosynthesis and carbohydrate partitioning in sweet cherry: Fruiting effeets. Physiol. Plant. 1988, 72, 42–47. [Google Scholar] [CrossRef]
- Leece, D.R. Diagnostic leaf analysis for stone fruit. 5. Sweet cherry. Aust. J. Exp. Agric. Anim. Husb. 1975, 15, 118–122. [Google Scholar] [CrossRef]
- Thakur, D.; Rehalia, A.S.; Kumar, J. Standardization of foliar sampling techniques for macronutrients in sweet cherry (Prunus avium L.) Cv. Stella. Agric. Sci. Digest. 2013, 33, 274–278. [Google Scholar] [CrossRef]
- Sánchez-Alonso, F.; Lachica, M. Seasonal trends in the elemental content of sweet cherry leaves. Commun. Soil Sci. Plant Anal. 1987, 18, 17–29. [Google Scholar] [CrossRef]
- Clark, J.; Smith, G.S. Seasonal changes in mineral nutrient content of persimmon leaves. Sci. Horti. 1990, 42, 85–97. [Google Scholar] [CrossRef]
- Batjer, I.P.; Westwood, M.N. Seasonal trend of several mineral elements in leaves and fruit of Elberta Peach. Proc. Amer. Soc. Hort. Sci. 1958, 71, 116–126. [Google Scholar]
- Verma, K.S.; Bhandari, A.R. Standardization of leaf sampling techniques for macronutrient element in temperate peaches. Indian, J. Hort. 1990, 47, 140–153. [Google Scholar]
- Rehalia, A.S.; Sandhu, R.D. Standardization of foliar sampling technique for micronutrients in persimmon (Diospyrous Kaki L.). Acta Hortic. 2005, 696, 265–268. [Google Scholar] [CrossRef]
- Mikiciuk, G.; Mikiciuk, M.; Możdżer, E.; Statkiewicz, M.; Chylewska, U. The effects of foliar nutrition with inca fertilizer on the chemical composition of leaves and fruits of sweet cherry. J. Ecol. Eng. 2015, 16, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.A.; Ghazzi, A.K. Response of olive transplants to seaweed extract as soil application and foliar application of Mg. Iraqi J. Agric. Sci. 2012, 34, 119–131. [Google Scholar]
- Bradshaw, T.L.; Berkett, L.P.; Griffith, M.C.; Kingsley-Richards, S.L.; Darby, H.M.; Parsons, R.L.; Moran, R.E.; Garcia, M.E. Assessment of kelp extract biostimulants on arthropod incidence and damage in a certified organic apple orchard. Acta Hortic. 2013, 1001, 139–145. [Google Scholar] [CrossRef]
- Al-Hadethi, M.E.; Al-Qatan, Y.F. Effect of algae extract and ascorbic acid spray with different levels on yield and growth of apricot trees. Egypt. J. Appl. Sci. 2013, 28, 93–101. [Google Scholar]
- Eman, A.; El Moniem, A.; AbdAllah, A.S.E. Effect of algae extract as foliar spray on vegetative growth, yield and berries quality of Superior grapevines. Am. Eurasian. J. Agric. Environ. Sci. 2008, 4, 427–433. [Google Scholar]
- Jannin, L.; Arkoun, M.; Etienne, P.; Laîné, P.; Goux, D.; Garnica, M.; Fuentes, M.; Francisco, S.; Baigorri, R.; Cruz, F.; et al. Brassica napus growth is promoted by Ascophyllum nodosum (L.) Le Jol. seaweed extract: Microarray analysis and physiological characterization of N, C, and S metabolisms. J. Plant Growth Regul. 2013, 32, 31–52. [Google Scholar] [CrossRef]
- Hölzel, N.; Close, D.C.; Bound, S.A.; Quin, P.R.; Visentin, D.C.; Swarts, N.D. Uptake and translocation of foliar-applied L-proline in sweet cherry (Prunus avium L.). Agronomy 2023, 13, 958. [Google Scholar] [CrossRef]
- Shehata, R.S. Proline in action: Enhancing fruit quality. DYSONA—Appl. Sci. 2025, 6, 8–15. [Google Scholar] [CrossRef]
- Hakimi, F.; Lafrikhi, H. Effects of foliar Ca applications on raspberry fruit quality and shelf life. Intern. J. Proc. Sci. Technol. 2021, 29, 30–36. [Google Scholar]
- Basile, B.; Brown, N.; Valdes, J.M.; Cardarelli, M.; Scognamiglio, P.; Mataffo, A.; Rouphael, Y.; Bonini, P.; Colla, G. Plant-based biostimulant as sustainable alternative to synthetic growth regulators in two sweet cherry cultivars. Plants 2021, 10, 619. [Google Scholar] [CrossRef]
- Zouari, M.; Ben Ahmed, C.; Elloumi, N.; Bellassoued, K.; Delmail, D.; Labrousse, P.; Ben Abdallah, F.; Ben Rouina, B. Impact of proline application on cadmium accumulation, mineral nutrition and enzymatic antioxidant defense system of Olea europaea L. cv Chemlali exposed to cadmium stress. Ecotoxicol. Environ. Saf. 2016, 128, 195–205. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Wang, C.; Lü, W.; Jin, J.B.; Hua, X. Proline induces calcium-mediated oxidative burst and salicylic acid signaling. J. Amino Acids 2011, 40, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Aghaeifard, F.; Babalar, M.; Fallahi, E.; Ahmadi, A. Influence of humic acid and salicylic acid on yield, fruit quality, and leaf mineral elements of strawberry (Fragaria ananassa Duch.) Cv. Camarosa. J. Plant. Nutr. 2015, 39, 1821–1829. [Google Scholar] [CrossRef]
- Khan, M.I.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front Plant Sci. 2015, 30, 462. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aversana, E.; Cirillo, V.; Van Oosten, M.J.; Di Stasio, E.; Saiano, K.; Woodrow, P.; Ciarmiello, L.F.; Maggio, A.; Carillo, P. Ascophyllum nodosum based extracts counteract salinity stress in tomato by remodeling leaf N metabolism. Plants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Esitken, A.; Pirlak, L.; Turan, M.; Sahin, F. Effects of floral and foliar application of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrition of sweet cherry. Sci. Hort. 2006, 110, 324–327. [Google Scholar] [CrossRef]
- Rashedy, A.A.; Abd-Einafea, M.H.; Khedr, E.H. Co-application of proline or Ca and humic acid enhances productivity of salt stressed pomegranate by improving nutritional status and osmoregulation mechanisms. Sci. Rep. 2022, 12, 14285. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Wu, R.Y.; Chuang, K.C.; Hsieh, T.F.; Chung, R.S. Effects of chemical and organic fertilizers on the growth, flower quality andnutrient uptake of Anthurium andreanum, cultivated for cut flower production. Sci. Hortic. 2010, 125, 434–441. [Google Scholar] [CrossRef]
- Gransee, A.; Fuhrs, H. Mg mobility in soils as a challenge for soil and plant analysis Mg fertilization and root uptake under adverse growth conditions. Plant Soil. 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Ertiftik, H.; Zengin, M. Response of maize for grain to potassium and Mg fertilizers in soils with high lime contents. J. Plant Nutr. 2017, 40, 93–103. [Google Scholar] [CrossRef]
- Rhodes, R.; Miles, N.; Hughes, J.C. Interactions between potassium, Ca and Mg in sugarcane grown on two contrasting soils in South Africa. Field Crop. Res. 2018, 223, 1–11. [Google Scholar] [CrossRef]
- Piao, H.-C.; Li, S.-L.; Yan, Z.; Li, C. Understanding nutrient allocation based on leaf N isotopes and elemental ratios in the karst region of Southwest China. Agric. Ecosyst. Environ. 2020, 294, 106864. [Google Scholar] [CrossRef]
- Rose, T.J.; Raymond, C.A.; Bloomfield, C.; King, G.J. Perturbation of nutrient source—Sink relationships by post anthesis stresses results in differential accumulation of nutrients in wheat grain. J. Plant Nutr. Soil Sci. 2015, 178, 89–98. [Google Scholar] [CrossRef]
- Kobayashi, H.; Masaoka, Y.; Sato, S. Effects of excess Mg on the growth and mineral content of rice and Echinochloa. Plant Prod. Sci. 2005, 8, 38–43. [Google Scholar] [CrossRef]
- Thouraya, A.; Albouchi, A.; Campoy, J.; Mezni, M.; Ben Ahmed, H.; Youssef, A. Effect of soil mineralogical composition on fruit quality of sweet cherry cultivars. Int. J. Agron. Agric. Res. 2016, 9, 45–56. [Google Scholar]
- Andziak, J.; Tomala, K.; Sadowski, A.; Dziuban, R. Nutritional status and quality of ‘šampion’ apples depending on rootstock. Acta Sci. Pol. Hortum Cultus 2004, 3, 179–187. [Google Scholar]
- Wakeel, A.; Gul, M.; Zörb, C. Potassium for sustainable agriculture. In Soil Science: Agricultural and Environmental Prospectives; Hakeem, K.R., Akhtar, J., Sabir, M., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Schonewille, J.T. Mg in dairy cow nutrition: An overview. Plant Soil. 2013, 368, 167–178. [Google Scholar] [CrossRef]
- Teklić, T.; Parađiković, N.; Špoljarević, M.; Zeljković, S.; Lončarić, Z.; Lisjak, M. Linking abiotic stress, plant metabolites, biostimulants and functional food. Ann. Appl. Biol. 2021, 178, 169–191. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).