PVC Inhibits Radish (Raphanus sativus L.) Seedling Growth by Interfering with Plant Hormone Signal Transduction and Phenylpropanoid Biosynthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Radish Cultivation and Growth Measurement
2.3. Enzyme Activities and Oxidative Stress Marker Analysis
2.4. RNA Sequencing and Transcriptome Profiling
2.5. Metabolite Extraction and LC-MS Analysis
2.6. Statistical Analyses
3. Results
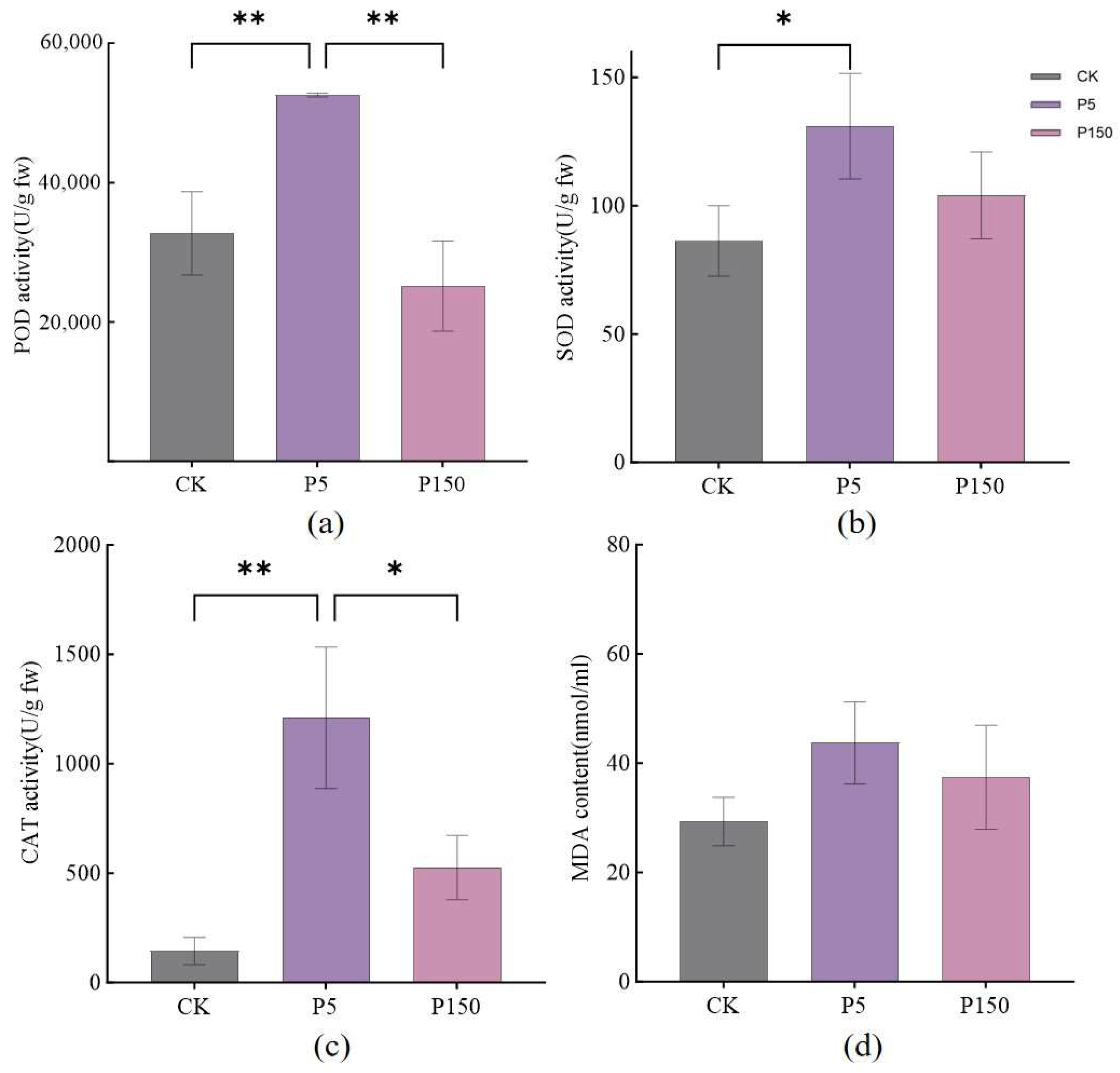
3.1. Effects of PVC Stress on Radish Growth and Antioxidant System
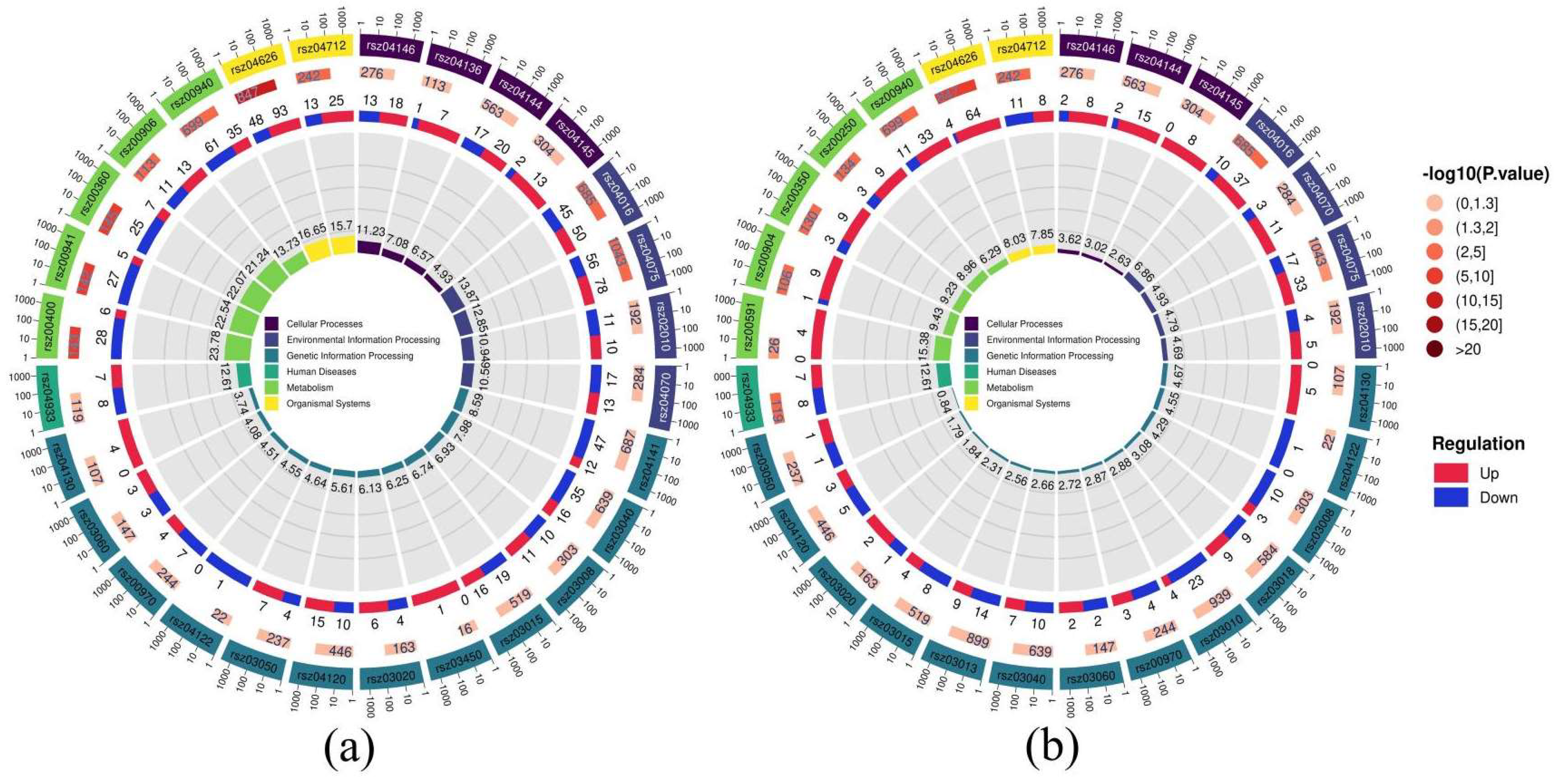
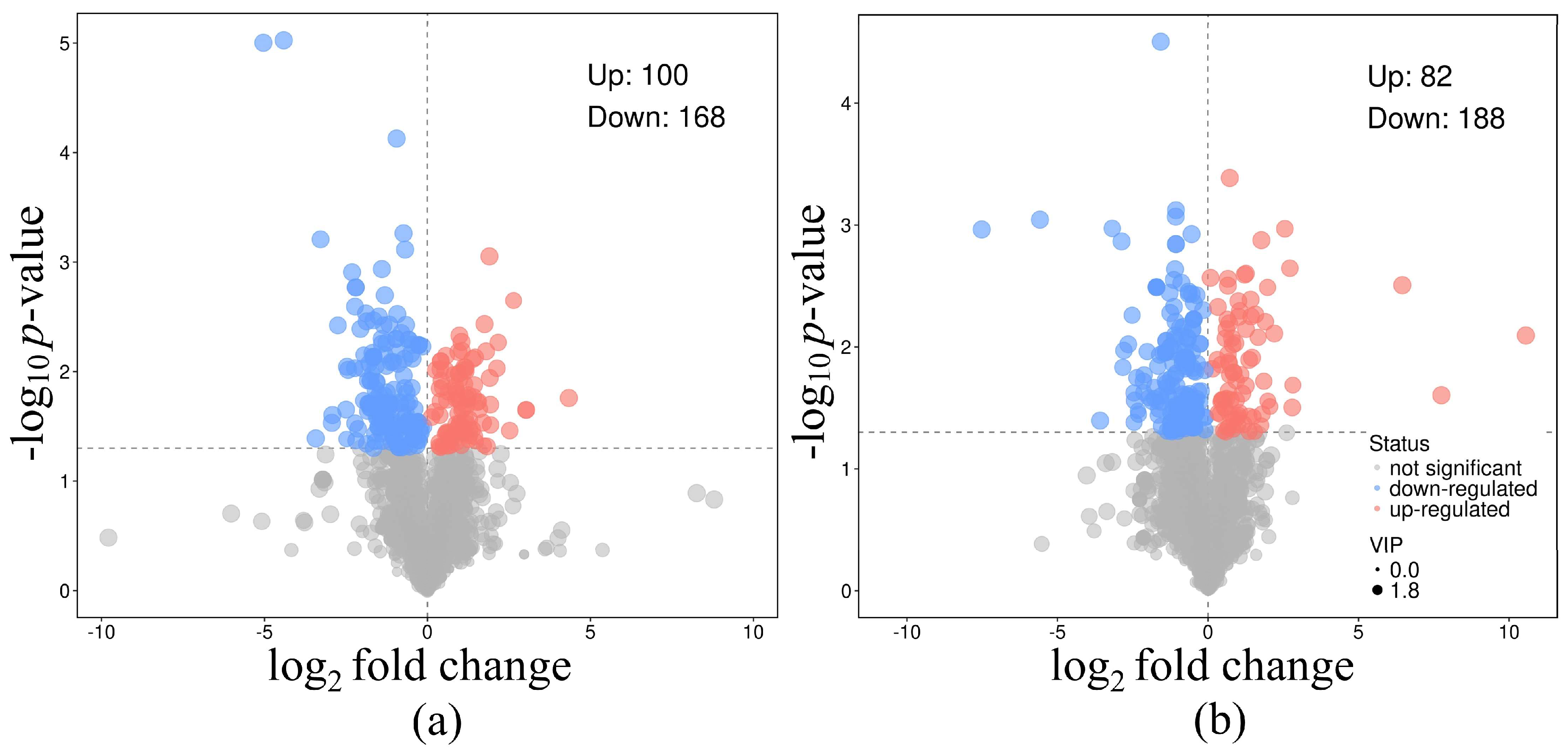
3.2. Transcriptomic Analysis of PVC Stress Responses in Radish Roots
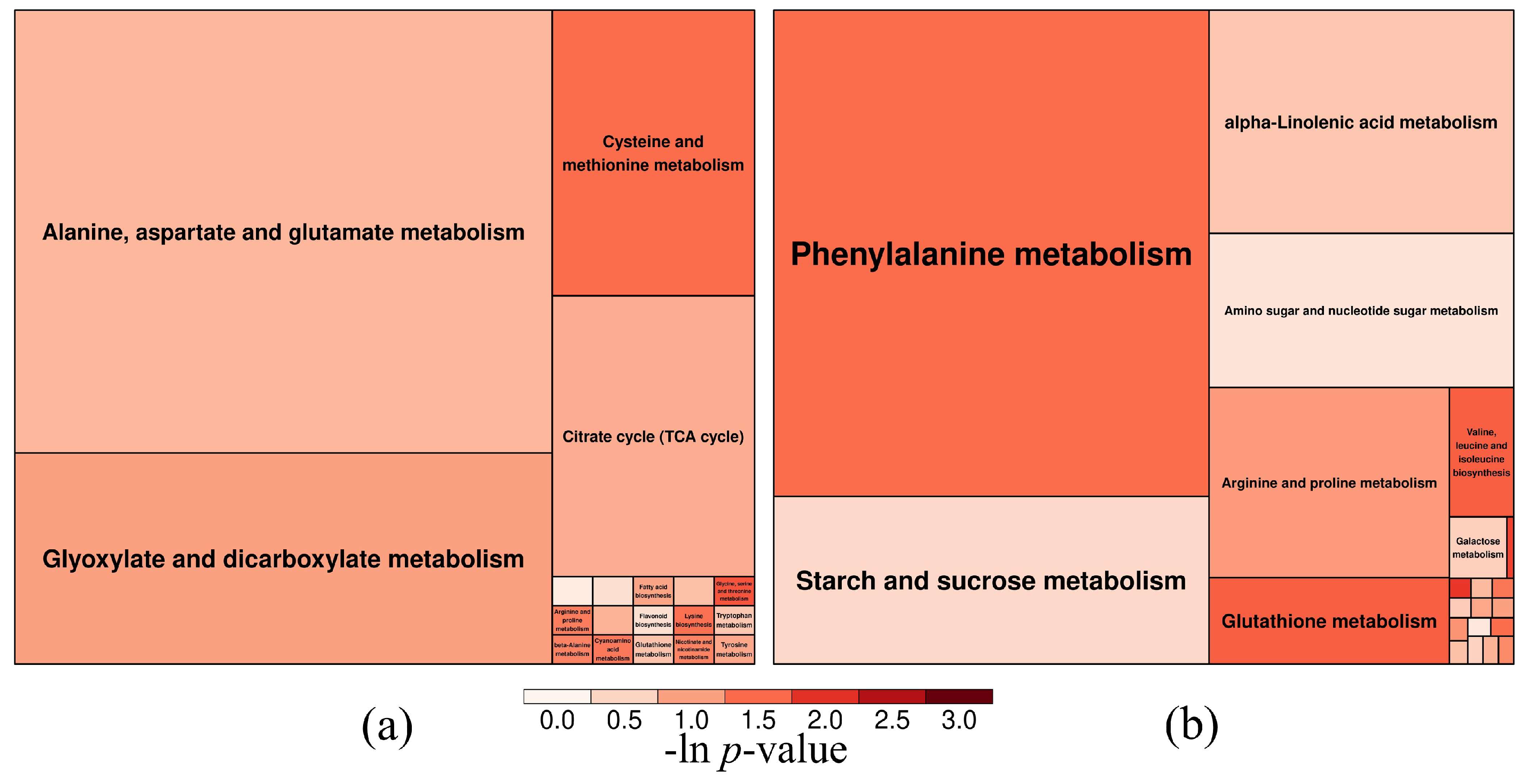
3.3. Metabolomic Response After Treatment with PVC
4. Discussion
4.1. Germination and Growth Dynamics: PVC-Induced Stress Responses and Adaptive Strategies
4.2. Antioxidant Defense Mechanisms: Enzyme System Activation Under PVC Stress
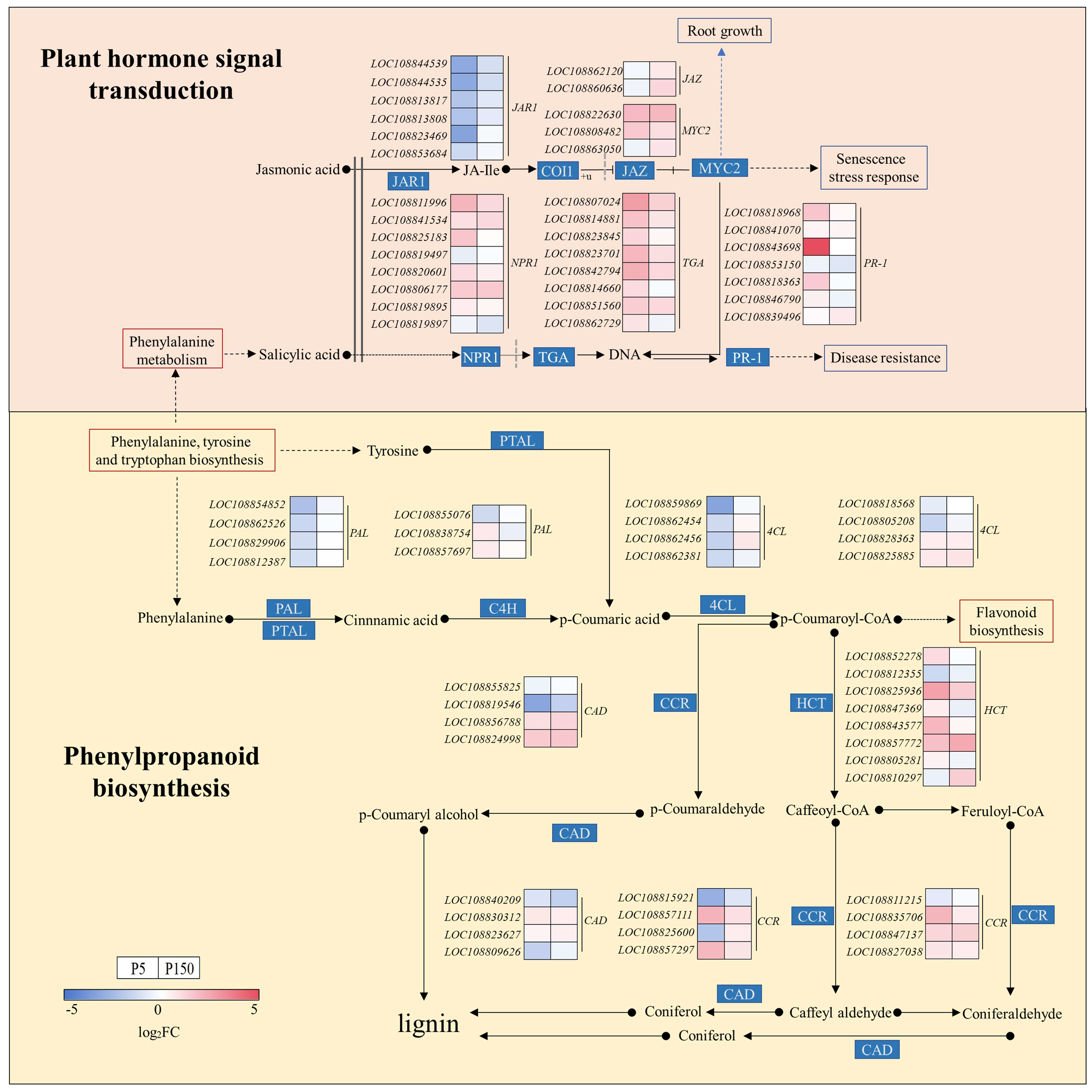
4.3. Phytohormone Signaling Networks: Modulation and Crosstalk Under PVC Stress
4.4. Phenylpropane Biosynthesis: Metabolic Reprogramming in Response to PVC Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xiao, M.; Ding, J.; Luo, Y.; Zhang, H.; Yu, Y.; Yao, H.; Zhu, Z.; Chadwick, D.R.; Jones, D.; Chen, J.; et al. Microplastics shape microbial communities affecting soil organic matter decomposition in paddy soil. J. Hazard. Mater. 2022, 431, 128589. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Marine pollution. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, K.; Guo, Y.; Xu, J.; Szafran, M. (Ba, Sr) TiO3/polymer dielectric composites-progress and perspective. Prog. Mater. Sci. 2021, 121, 100813. [Google Scholar] [CrossRef]
- Plastics–the Facts. An Analysis of European Plastics Production, Demand and Waste Data for 2011. Available online: https://plasticseurope.org (accessed on 22 January 2025).
- Tian, W.; Song, P.; Zhang, H.; Duan, X.; Wei, Y.; Wang, H.; Wang, S. Microplastic materials in the environment: Problem and strategical solutions. Prog. Mater. Sci. 2023, 132, 101035. [Google Scholar] [CrossRef]
- Chigwada, A.D.; Tekere, M.; Ogola, H.J.O. Ecological and genomic perspectives on fungal plastic biodegradation: Research progress, opportunities and challenges. Ecol. Genet. Genom. 2025, 36, 100378. [Google Scholar] [CrossRef]
- Bradney, L.; Wijesekara, H.; Palansooriya, K.N.; Obadamudalige, N.; Bolan, N.S.; Ok, Y.S.; Rinklebe, J.; Kim, K.H.; Kirkham, M.B. Particulate plastics as a vector for toxic trace-element uptake by aquatic and terrestrial organisms and human health risk. Environ. Int. 2019, 131, 104937. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Q.; Xie, Z.; Zhou, Y.; Tu, C.; Fu, C.; Mi, W.; Ebinghaus, R.; Christie, P.; Luo, Y. Occurrences of organophosphorus esters and phthalates in the microplastics from the coastal beaches in north China. Sci. Total Environ. 2018, 616–617, 1505–1512. [Google Scholar] [CrossRef]
- Zhao, P.; Yang, S.; Zheng, Y.; Zhang, L.; Li, Y.; Li, J.; Wang, W.; Wang, Z. Polylactic acid microplastics have stronger positive effects on the qualitative traits of rice (Oryza sativa L.) than polyethylene microplastics: Evidence from a simulated field experiment. Sci. Total Environ. 2024, 917, 170334. [Google Scholar] [CrossRef] [PubMed]
- Colzi, I.; Renna, L.; Bianchi, E.; Castellani, M.B.; Coppi, A.; Pignattelli, S.; Loppi, S.; Gonnelli, C. Impact of microplastics on growth, photosynthesis and essential elements in Cucurbita pepo L. J. Hazard. Mater. 2022, 423 Pt B, 127238. [Google Scholar] [CrossRef]
- Ng, E.L.; Huerta Lwanga, E.; Eldridge, S.M.; Johnston, P.; Hu, H.W.; Geissen, V.; Chen, D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef]
- Lu, S.; Huo, Z.; Niu, T.; Zhu, W.; Wang, J.; Wu, D.; He, C.; Wang, Y.; Zou, L.; Sheng, L. Molecular mechanisms of toxicity and detoxification in rice (Oryza sativa L.) exposed to polystyrene nanoplastics. Plant Physiol. Biochem. 2023, 199, 107605. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, L.; Spanò, C.; Muccifora, S.; Bottega, S.; Barbieri, F.; Bellani, L.; Ruffini Castiglione, M. Exploring the interaction between polystyrene nanoplastics and Allium cepa during germination: Internalization in root cells, induction of toxicity and oxidative stress. Plant Physiol. Biochem. 2020, 149, 170–177. [Google Scholar] [CrossRef]
- Li, Z.; Li, R.; Li, Q.; Zhou, J.; Wang, G. Physiological response of cucumber (Cucumis sativus L.) leaves to polystyrene nanoplastics pollution. Chemosphere 2020, 255, 127041. [Google Scholar] [CrossRef] [PubMed]
- Zainab, R.; Hasnain, M.; Ali, F.; Abideen, Z.; Siddiqui, Z.S.; Jamil, F.; Hussain, M.; Park, Y.K. Prospects and challenges of nanopesticides in advancing pest management for sustainable agricultural and environmental service. Environ. Res. 2024, 261, 119722. [Google Scholar] [CrossRef]
- Abideen, Z.; Hanif, M.; Munir, N.; Nielsen, B.L. Impact of nanomaterials on the regulation of gene expression and metabolomics of plants under salt stress. Plant 2022, 11, 691. [Google Scholar] [CrossRef]
- Wagner, S.; Reemtsma, T. Things we know and don’t know about nanoplastic in the environment. Nat. Nanotechnol. 2019, 14, 300–301. [Google Scholar] [CrossRef]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of nanoplastic in the environment and possible Impact on Human Health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef] [PubMed]
- Doerge, D.R.; Divi, R.L.; Churchwell, M.I. Identification of the colored guaiacol oxidation product produced by peroxidases. Anal. Biochem. 1997, 250, 10–17. [Google Scholar] [CrossRef]
- Minami, M.; Yoshikawa, H. A simplified assay method of superoxide dismutase activity for clinical use. Clin. Chim. Acta Int. J. Clin. Chem. 1979, 92, 337–342. [Google Scholar] [CrossRef]
- Johansson, L.H.; Borg, L.A. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal. Biochem. 1988, 174, 331–336. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Reprint of: Photoperoxidation in isolated chloroplasts I. kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 2022, 726, 109248. [Google Scholar] [CrossRef]
- Pignattelli, S.; Broccoli, A.; Renzi, M. Physiological responses of garden cress (L. sativum) to different types of microplastics. Sci. Total Environ. 2020, 727, 138609. [Google Scholar] [CrossRef]
- Bosker, T.; Bouwman, L.J.; Brun, N.R.; Behrens, P.; Vijver, M.G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 2019, 226, 774–781. [Google Scholar] [CrossRef]
- Lian, J.; Wu, J.; Xiong, H.; Zeb, A.; Yang, T.; Su, X.; Su, L.; Liu, W. Impact of polystyrene nanoplastics (PSNPs) on seed germination and seedling growth of wheat (Triticum aestivum L.). J. Hazard. Mater. 2020, 385, 121620. [Google Scholar] [CrossRef]
- Barbale, M.; Chinaglia, S.; Gazzilli, A.; Pischedda, A.; Degli-Innocenti, F. Hazard profiling of compostable shopping bags. towards an ecological risk assessment of littering. Polym. Degrad. Stab. 2021, 188, 109592. [Google Scholar] [CrossRef]
- Shi, R.; Liu, W.; Lian, Y.; Wang, Q.; Zeb, A.; Tang, J. Phytotoxicity of polystyrene, polyethylene and polypropylene microplastics on tomato (Lycopersicon esculentum L.). J. Environ. Manag. 2022, 317, 115441. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, Y.; Zheng, Q.; Yang, Q.; Wang, J.; Zhu, J.; Zhan, X. Toxicity of photoaged polyvinyl chloride microplastics to wheat seedling roots. J. Hazard. Mater. 2023, 463, 132816. [Google Scholar] [CrossRef] [PubMed]
- Qaiser, Z.; Khalid, N.; Mahmood, A.; Rizvi, Z.F.; Lee, S.Y.; Aqeel, M. Spatial distribution and impacts of microplastics on potato growth and yield in agroecosystems in Sialkot, Pakistan. J Hazard Mater. 2024, 480, 136262. [Google Scholar] [CrossRef]
- Jalal, A.; de Oliveira Junior, J.C.; Ribeiro, J.S.; Fernandes, G.C.; Mariano, G.G.; Trindade, V.D.R.; dos Reis, A.R. Hormesis in plants: Physiological and biochemical responses. Ecotoxicol. Environ. Saf. 2021, 207, 11125. [Google Scholar] [CrossRef]
- Ren, D.; Yang, H.; Zhang, S. Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. J. Biol. Chem. 2002, 277, 559–565. [Google Scholar] [CrossRef]
- Yuan, H.M.; Huang, X. Inhibition of root meristem growth by cadmium involves nitric oxide-mediated repression of auxin accumulation and signalling in Arabidopsis. Plant Cell Environ. 2016, 39, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Duijts, K.; Pasini, C.; van Santen, J.E.; Lamers, J.; de Zeeuw, T.; Verstappen, F.; Wang, N.; Zeeman, S.C.; Santelia, D.; et al. Effective root responses to salinity stress include maintained cell expansion and carbon allocation. New Phytol. 2023, 238, 1942–1956. [Google Scholar] [CrossRef]
- Yan, F.; Zhao, H.; Wu, L.; Huang, Z.; Niu, Y.; Qi, B.; Zhang, L.; Fan, S.; Ding, Y.; Li, G.; et al. Basic gognition of melatonin regulation of plant growth under salt stress: A Meta-Analysis. Antioxidants 2022, 11, 1610. [Google Scholar] [CrossRef]
- Mészáros, E.; Bodor, A.; Kovács, K.; Papp, S.; Kovács, E.; Perei, K.; Frei, K.; Feigl, G. Plastic contamination from latex and nitrile disposable gloves has the potential to influence plant productivity and soil health. J. Hazard. Mater. Adv. 2025, 17, 100605. [Google Scholar] [CrossRef]
- Dong, Y.; Song, Z.; Liu, Y.; Gao, M. Polystyrene particles combined with di-butyl phthalate cause significant decrease in photosynthesis and red lettuce quality. Environ. Pollut. 2021, 278, 116871. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yun, Z.; Zhang, D.; Yang, C.; Zhu, H.; Jiang, Y.; Duan, X. Proteomic analysis of differentially expressed proteins involved in ethylene-induced chilling tolerance in harvested banana fruit. Front. Plant Sci. 2015, 6, 845. [Google Scholar] [CrossRef]
- Yuan, B.; Yao, M.; Wang, X.; Sato, A.; Okazaki, A.; Komuro, H.; Hayashi, H.; Toyoda, H.; Pei, X.; Hu, X.; et al. Antitumor activity of arsenite in combination with tetrandrine against human breast cancer cell line MDA-MB-231 in vitro and in vivo. Cancer Cell Int. 2018, 18, 113. [Google Scholar] [CrossRef]
- Gao, M.; Liu, Y.; Song, Z. Effects of polyethylene microplastic on the phytotoxicity of di-n-butyl phthalate in lettuce (Lactuca sativa L. var. ramosa Hort). Chemosphere 2019, 237, 124482. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Y.; Yin, S.; Xiao, K.; Xiong, Q.; Bian, S.; Liang, S.; Hou, H.; Hu, J.; Yang, J. Metabolomics revealing the response of rice (Oryza sativa L.) exposed to polystyrene microplastics. Environ. Pollut. 2020, 266 Pt 1, 115159. [Google Scholar] [CrossRef]
- Wu, Z.; Yuan, L.; Sun, C.; Xu, X.; Shi, W.; Han, L.; Wu, C. Effects of selenite on the responses of lettuce (Lactuca sativa L.) to polystyrene nano-plastic stress. Ecotoxicol. Environ. Saf. 2023, 262, 115138. [Google Scholar] [CrossRef]
- Weckx, J.E.J.; Clijsters, H.M.M. Oxidative damage and defense mechanisms in primary leaves of phaseolus vulgaris as a result of root assimilation of toxic amounts of copper. Physiol. Plant. 2010, 96, 506–512. [Google Scholar] [CrossRef]
- Jamil, A.; Ahmad, A.; Moeen-Ud-Din, M.; Zhang, Y.; Zhao, Y.; Chen, X.; Cui, X.; Tong, Y.; Liu, X. Unveiling the mechanism of micro-and-nano plastic phytotoxicity on terrestrial plants: A comprehensive review of omics approaches. Environ. Int. 2025, 195, 109257. [Google Scholar] [CrossRef]
- Zhou, C.Q.; Lu, C.H.; Mai, L.; Bao, L.J.; Liu, L.Y.; Zeng, E.Y. Response of rice (Oryza sativa L.) roots to nanoplastic treatment at seedling stage. J. Hazard. Mater. 2021, 401, 123412. [Google Scholar] [CrossRef]
- Fàbregas, N.; Yoshida, T.; Fernie, A.R. Role of Raf-like kinases in SnRK2 activation and osmotic stress response in plants. Nat. Commun. 2020, 11, 6184. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.S.; Ahmed, S.; Ikram, A.U.; Hannan, F.; Yasin, M.U.; Wang, J.; Zhao, B.; Islam, F.; Chen, J. Phytomelatonin: A key regulator of redox and phytohormones signaling against biotic/abiotic stresses. Redox Biol. 2023, 64, 102805. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; I Sergeeva, L.; Vreugdenhil, D. Natural variation of hormone levels in Arabidopsis roots and correlations with complex root architecture. J. Integr. Plant Biol. 2018, 60, 292–309. [Google Scholar] [CrossRef]
- Sharma, M.; Singh, D.; Saksena, H.B.; Sharma, M.; Tiwari, A.; Awasthi, P.; Botta, H.K.; Shukla, B.N.; Laxmi, A. Understanding the intricate web of phytohormone signalling in modulating root system architecture. Int. J. Mol. Sci. 2021, 22, 5508. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Møller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed; Sinauer Associates: Sunderland, MA, USA, 2015. [Google Scholar]
- Griffiths, G. Jasmonates: Biosynthesis, perception and signal transduction. Essays Biochem. 2020, 64, 501–512. [Google Scholar] [CrossRef]
- Rahman, M.M.; Mostofa, M.G.; Keya, S.S.; Ghosh, P.K.; Abdelrahman, M.; Anik, T.R.; Gupta, A.; Tran, L.P. Jasmonic acid priming augments antioxidant defense and photosynthesis in soybean to alleviate combined heat and drought stress effects. Plant Physiol. Biochem. PPB 2024, 206, 108193. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Kui, M.; He, K.; Yang, M.; Du, J.; Jiang, Y.; Hu, Y. Jasmonate-regulated root growth inhibition and root hair elongation. J. Exp. Bot. 2023, 74, 1176–1185. [Google Scholar] [CrossRef]
- Pandey, P.; Tripathi, A.; Dwivedi, S.; Lal, K.; Jhang, T. Deciphering the mechanisms, hormonal signaling, and potential applications of endophytic microbes to mediate stress tolerance in medicinal plants. Front. Plant Sci. 2023, 14, 1250020. [Google Scholar] [CrossRef] [PubMed]
- Pokotylo, I.; Hodges, M.; Kravets, V.; Ruelland, E. A ménage à trois: Salicylic acid, growth inhibition, and immunity. Trends Plant Sci. 2022, 27, 460–471. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, J.; Zhai, Q.; Zhou, W.; Qi, L.; Xu, L.; Wang, B.; Chen, R.; Jiang, H.; Qi, J.; et al. The basic helix-loop-helix transcription factor MYC2 directly represses PLETHORA expression during jasmonate-mediated modulation of the root stem cell niche in Arabidopsis. Plant Cell 2011, 23, 3335–3352. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, A.; Xu, B.; Wang, H.; Yu, J.; Liu, J.; Jian, L.; Quan, C.; Du, J. Exogenous melatonin enhances salt tolerance by regulating the phenylpropanoid biosynthesis pathway in common bean at sprout stage. Plant Stress 2024, 14, 100589. [Google Scholar] [CrossRef]
- Yu, A.; Zhao, J.; Wang, Z.; Cheng, K.; Zhang, P.; Tian, G.; Liu, X.; Guo, E.; Du, Y.; Wang, Y. Transcriptome and metabolite analysis reveal the drought tolerance of foxtail millet significantly correlated with phenylpropanoids-related pathways during germination process under PEG stress. BMC Plant Biol. 2020, 20, 274. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Y.; Mu, K.; Cai, W.; Zhao, Y.; Shen, H.; Wang, X.; Ma, H. Phenylalanine ammonia lyase GmPAL1.1 promotes seed vigor under high-temperature and -humidity stress and enhances seed germination under salt and drought stress in transgenic arabidopsis. Plants 2022, 11, 3239. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, J.L.; Austin, M.B.; Stewart, C., Jr.; Noel, J.P. Structure and function of enzymes involved in the biosynthesis of phenylpropanoids. Plant Physiol. Biochem. PPB 2008, 46, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Su, W.; Zhang, H.; Zhan, Y.; Zeng, F. Fraxinus mandshurica 4-coumarate-CoA ligase 2 enhances drought and osmotic stress tolerance of tobacco by increasing coniferyl alcohol content. Plant Physiol. Biochem. PPB 2020, 155, 697–708. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wang, J.P.; Li, Q.; Chen, H.C.; Liu, J.; Loziuk, P.; Song, J.; Williams, C.; Muddiman, D.C.; Sederoff, R.R.; et al. 4-Coumaroyl and caffeoyl shikimic acids inhibit 4-coumaric acid: Coenzyme A ligases and modulate metabolic flux for 3-hydroxylation in monolignol biosynthesis of Populus trichocarpa. Mol. Plant 2015, 8, 176–187. [Google Scholar] [CrossRef]
- Allina, S.M.; Pri-Hadash, A.; Theilmann, D.A.; Ellis, B.E.; Douglas, C.J. 4-Coumarate: Coenzyme A ligase in hybrid poplar. Properties of native enzymes, cDNA cloning, and analysis of recombinant enzymes. Plant Physiol. 1998, 116, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Shinde, S.; Puri, H.; Palmer, N.; Sarath, G.; Sattler, S.E.; Louis, J. Dynamic regulation of phenylpropanoid pathway metabolites in modulating sorghum defense against fall armyworm. Front. Plant Sci. 2022, 13, 1019266. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Saito, K. Integrated metabolomics for abiotic stress responses in plants. Curr. Opin. Plant Biol. 2015, 24, 10–16. [Google Scholar] [CrossRef]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Editor’s choice: Growth defense tradeoffs in plants: A balancing act to optimize fitness. Mol. Plant 2014, 7, 1267. [Google Scholar] [CrossRef]
- Wegner, A.; Besseling, E.; Foekema, E.M.; Kamermans, P.; Koelmans, A.A. Effects of nanopolystyrene on the feeding behavior of the blue mussel (Mytilus edulis L.). Environ. Toxicol. Chem. 2012, 31, 2490–2497. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, R.; Zhang, S.; Sun, Y.; Wang, F. Uptake and translocation of nano/microplastics by rice seedlings: Evidence from a hydroponic experiment. J. Hazard. Mater. 2022, 421, 126700. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Liu, Z.; Li, W.; Yang, Y.; Yu, Z.; Fan, J.; Guo, L.; Guo, C.; Fu, W. PVC Inhibits Radish (Raphanus sativus L.) Seedling Growth by Interfering with Plant Hormone Signal Transduction and Phenylpropanoid Biosynthesis. Horticulturae 2025, 11, 896. https://doi.org/10.3390/horticulturae11080896
Jiang L, Liu Z, Li W, Yang Y, Yu Z, Fan J, Guo L, Guo C, Fu W. PVC Inhibits Radish (Raphanus sativus L.) Seedling Growth by Interfering with Plant Hormone Signal Transduction and Phenylpropanoid Biosynthesis. Horticulturae. 2025; 11(8):896. https://doi.org/10.3390/horticulturae11080896
Chicago/Turabian StyleJiang, Lisi, Zirui Liu, Wenyuan Li, Yangwendi Yang, Zirui Yu, Jiajun Fan, Lixin Guo, Chang Guo, and Wei Fu. 2025. "PVC Inhibits Radish (Raphanus sativus L.) Seedling Growth by Interfering with Plant Hormone Signal Transduction and Phenylpropanoid Biosynthesis" Horticulturae 11, no. 8: 896. https://doi.org/10.3390/horticulturae11080896
APA StyleJiang, L., Liu, Z., Li, W., Yang, Y., Yu, Z., Fan, J., Guo, L., Guo, C., & Fu, W. (2025). PVC Inhibits Radish (Raphanus sativus L.) Seedling Growth by Interfering with Plant Hormone Signal Transduction and Phenylpropanoid Biosynthesis. Horticulturae, 11(8), 896. https://doi.org/10.3390/horticulturae11080896






