Abstract
Anthocyanins, a subclass of flavonoid pigments, impart vivid red, purple, and blue coloration to horticultural plants, playing essential roles in ornamental enhancement, stress resistance, and pollinator attraction. Recent studies have identified B-box (BBX) proteins as a critical class of transcription factors (TFs) involved in anthocyanin biosynthesis. Despite these advances, comprehensive reviews systematically addressing BBX proteins are urgently needed, especially given the complexity and diversity of their roles in regulating anthocyanin production. In this paper, we provide an in-depth overview of the fundamental structures, biological functions, and classification of BBX TFs, along with a detailed description of anthocyanin biosynthetic pathways and bioactivities. Furthermore, we emphasize the diverse molecular mechanisms through which BBX TFs regulate anthocyanin accumulation, including direct activation or repression of target genes, indirect modulation via interacting protein complexes, and co-regulation with other transcriptional regulators. Additionally, we summarize the known upstream regulatory signals and downstream target genes of BBX TFs, highlighting their significance in shaping anthocyanin biosynthesis pathways. Understanding these regulatory networks mediated by BBX proteins will not only advance fundamental horticultural science but also provide valuable insights for enhancing the aesthetic quality, nutritional benefits, and stress adaptability of horticultural crops.
1. Introduction
Anthocyanins are water-soluble natural pigments that are widespread in plants, contributing to red, purple, blue, and other distinct colorations in fruits and vegetables [1,2]. These pigments not only enhance the stress tolerance of plants but also serve to attract pollinators, thereby facilitating plant reproduction [3,4]. Furthermore, owing to their remarkable bioactivity and natural coloring attributes, anthocyanins find extensive applications across the food, nutraceutical, and cosmetic industries [5]. They are commonly employed as natural colorants in a variety of beverages and food products and are developed as bioactive ingredients in functional foods and cosmetics [6,7]. Moreover, anthocyanins contribute to improving food quality by extending shelf life, enhancing flavor, and protecting food components from environmental stresses, all of which have substantially contributed to the sustained increase in their market demand [8]. Moreover, anthocyanin-rich foods exhibit potent antioxidant properties, confer health benefits, and have been linked to a decreased risk of multiple chronic diseases, including cancer, obesity, and cardiovascular disorders [9,10,11,12].
Substantial advances have been achieved in elucidating the biosynthetic pathways of anthocyanins over the past several decades. However, the regulation of anthocyanin biosynthesis in plants is a complex process that involves multiple molecular components and environmental factors [13]. Anthocyanin biosynthesis is initiated from phenylalanine as a precursor and involves a cascade of enzymatic reactions catalyzed by enzymes such as phenylalanine ammonia-lyase (PAL), chalcone synthase (CHS), chalcone isomerase (CHI), flavanone-3-hydroxylase, dihydroflavonol 4-reductase (DFR), anthocyanidin synthase (ANS), and UDP-glucose: flavonoid 3-glucosyltransferase (UFGT) [14,15]. The genes mediating anthocyanin biosynthesis are mainly regulated by MYB, bHLH, and WD40 transcription factors, which may act individually or synergistically by forming the MYB/bHLH/WD40 (MBW) transcriptional complex to modulate gene expression [9,16].
However, beyond the well-established MBW complex, recent research has highlighted the significant role of other transcription factors in regulating anthocyanin biosynthesis. Notably, B-box (BBX) proteins—a subclass of zinc finger transcription factors characterized by the presence of one or two B-box domains—have emerged as important regulators of secondary metabolite biosynthesis, especially in the context of light signal transduction [17]. For instance, PpBBX16 and CsBBX22 have been shown to indirectly modulate light-induced anthocyanin accumulation through interaction with key light signaling components, such as HY5 [18,19].
Although previous studies have preliminarily revealed the important role of BBX proteins in the regulation of secondary metabolism, especially in anthocyanin biosynthesis, there are still several obvious deficiencies in current research. First, most studies have focused on model plants (such as Arabidopsis thaliana), while systematic functional analyses of BBX transcription factors in horticultural plants remain relatively scarce, limiting the applicability and extension of these research findings. Second, existing research mainly emphasizes single-gene overexpression or interference, neglecting the potential synergistic or antagonistic interactions among BBX family members, and a comprehensive interaction network has yet to be established. Third, although BBX proteins are often thought to participate in light signaling pathways, there has been limited research on their functions in anthocyanin accumulation as regulated by other environmental factors, such as temperature or salt stress. In addition, most current studies are descriptive, lacking the use of high-throughput approaches such as chromatin immunoprecipitation sequencing (ChIP-seq) or spatio-temporal expression profiling to systematically identify their direct target genes and downstream networks. These issues collectively restrict a deeper understanding of the regulatory mechanisms by which BBX transcription factors control anthocyanin biosynthesis.
This review provides a comprehensive overview of the fundamental structures, classification, and diverse biological functions of the plant B-box (BBX) transcription factor family, with particular emphasis on elucidating the molecular mechanisms through which BBX proteins mediate the biosynthesis of anthocyanins in horticultural plants. In addition, the review discusses the potential roles of BBX proteins in enhancing ornamental traits, economic value, and commercial viability of horticultural plants and explores their promising applications in molecular breeding and genetic improvement. By synthesizing recent research advances and highlighting prospective research directions, this review aims to offer a theoretical framework for deepening our understanding of BBX transcription factor functions, thereby providing novel insights and strategies for the molecular improvement of horticultural crops.
2. Overview of the BBX TF Family
2.1. Structural Features of BBX TFs
B-box proteins (BBXs) represent a plant-specific class of zinc finger transcription factors. Initial investigations into this protein family primarily focused on Arabidopsis thaliana, and the increasing number of related reports has gradually brought these transcription factors to the forefront of plant molecular biology research. In Arabidopsis thaliana, the BBX family consists of 32 members, which are classified into five subclasses according to their distinct domain architectures [20] (Figure 1). All BBX proteins contain at least one B-box domain, whereas certain members additionally harbor a C-terminal CCT (CONSTANS, CONSTANS-like, TOC1) domain. The first identified and characterized member of the BBX family is CONSTANS (CO, also referred to as BBX1), which belongs to subclass I and possesses two N-terminal B-box domains in addition to a single C-terminal CCT domain [21].
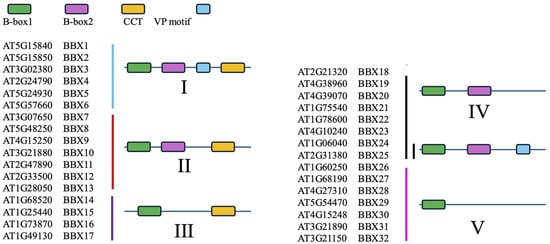
Figure 1.
Structure and classification of the 32 BBX proteins in Arabidopsis thaliana.
The B-box domain is a conserved zinc finger motif comprising approximately 40 amino acids and is characterized by the consensus sequence C-X2-C-X7–9-C-X2-D-X4-C-X2-C-X3–4-H-X4–8-H. Within this motif, cysteine (C), aspartic acid (D), and histidine (H) residues coordinate two zinc ions, thereby stabilizing the structural integrity and function of the domain [22]. Based on the differences in the spacing of zinc-coordinating residues within the conserved sequence, B-boxes are classified into two types: B-box1 and B-box2. Of the 32 BBX proteins identified in Arabidopsis thaliana, 21 members (BBX1–13 and BBX18–25) possess two tandemly arranged B-box domains, whereas the remaining 11 members (BBX14–17 and BBX26–32) contain only a single B-box domain. The C-termini of certain BBX proteins also harbor a VP (Valine–Proline) motif positioned adjacent to the CCT domain, which is defined by the conserved sequence G-I/V-V-P-S/T-F. Notably, this motif is typically situated only 16–20 amino acid residues from the CCT domain [23].
The CCT domain is a highly conserved basic sequence comprising 42–43 amino acids and is known to play a critical role in transcriptional regulation and nucleocytoplasmic transport. In Arabidopsis thaliana, 17 BBX proteins (BBX1–17) harbor the CCT domain. Specifically, class I (BBX1–6) and class II (BBX7–13) members possess two B-box domains and a single CCT domain, whereas class III members (BBX14–17) contain only one B-box and one CCT domain [23].
2.2. Functions of BBX TFs
From early studies to the present, the functions of the BBX transcription factor family have been extensively and progressively elucidated. Accumulating evidence indicates that BBX family members fulfill diverse and indispensable regulatory functions in plant growth, development, secondary metabolism, and stress responses (Figure 2).
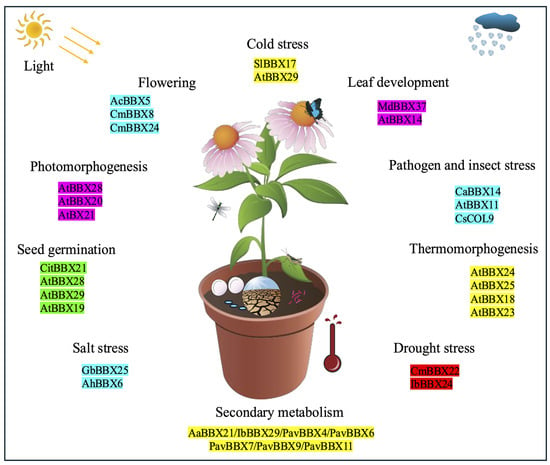
Figure 2.
Biological functions of BBX transcription factors in plants.
2.2.1. Seed Germination
Seed germination marks the initiation of the plant life cycle and is tightly regulated to ensure that plants commence growth under favorable environmental conditions. Abscisic acid (ABA) and gibberellins (GA) are two key endogenous hormones that play antagonistic roles in regulating seed germination; the balance between these hormones determines whether seeds remain dormant or initiate germination. In general, ABA acts as a negative regulator of seed germination, while GA promotes this process [24]. Light, as a crucial environmental cue, also exerts significant influence on seed germination [25].
ABI5 is a central transcription factor in the ABA signaling pathway and has been extensively studied for its role and regulatory mechanisms. ABI5 inhibits seed germination by activating the expression of downstream target genes through binding to ABA-responsive elements (ABREs) [26,27]. Recent studies have revealed that B-box (BBX) transcription factors form a complex regulatory network with ABA and GA signaling to finely modulate seed germination. For example, AtBBX19 directly binds to the GT1 cis-element in the promoter region of ABI5, activating its transcription and thereby enhancing the inhibitory effect of ABA on seed germination. This regulatory pathway functions independently of GA signaling and DELLA proteins and does not require the involvement of the key light-responsive transcription factor HY5. Conversely, HY5 can cooperate with BBX21 to suppress ABI5 expression, thereby promoting seed germination. In parallel, GA facilitates germination by promoting the degradation of DELLA proteins, which alleviates ABA-mediated inhibition [28].
In addition to ABA and GA, BBX transcription factors have also been shown to interact with components of other hormonal signaling pathways. For instance, they can crosstalk with key transcription factors in the brassinosteroid (BR) signaling pathway, collectively contributing to the regulation of seed germination. For instance, in Arabidopsis thaliana, AtBBX28 and AtBBX29 integrate light and brassinosteroid (BR) signaling, thereby positively regulating the BR pathway to suppress excessive seedling elongation. These two BBX proteins interact with pivotal BR pathway transcription factors, including BEE1, BEE2, and BEE3, augmenting their DNA-binding affinity at target promoters and facilitating the transcription of BR-responsive genes [29]. Furthermore, AtBBX28 and AtBBX29 promote the accumulation of BZR1 protein, thereby further enhancing BR signal transduction. BR treatment stabilizes the protein abundance of both AtBBX28 and AtBBX29, a process that is at least partially dependent on BRI1 and BIN2.
2.2.2. Leaf Development
BBX participates in leaf development through multiple mechanisms. MdBBX37 has been identified as a key regulator of leaf senescence. MdBBX37 interacts with MdbHLH93, enhancing the latter’s transcriptional activation of MdSAG18 and thus promoting leaf senescence. Meanwhile, the jasmonate signaling repressor MdJAZ2 disrupts the interaction between MdBBX37 and MdbHLH93, thereby attenuating their pro-senescence activity. The E3 ubiquitin ligase MdSINA3 facilitates the ubiquitination and subsequent degradation of MdBBX37, thereby functioning as a negative regulator of leaf senescence at the post-translational level [30]. Additionally, MdBBX37 physically interacts with MdABI5 and MdEIL1, participating in abscisic acid (ABA)- and ethylene (ET)-mediated pathways regulating leaf senescence. Under adverse environmental conditions such as nitrogen starvation and darkness, AtBBX14 can effectively delay leaf senescence by directly repressing the transcriptional activity of EIN3, a key transcription factor in the ethylene signaling pathway. Moreover, the transcription factor AtMYB44 further enhances this senescence-delaying effect by promoting AtBBX14 expression. In contrast, AtPIF4 plays an antagonistic regulatory role by accelerating leaf senescence; it suppresses the expression of AtBBX14, thereby releasing EIN3 from AtBBX14-mediated repression [31].
2.2.3. Flowering
Accumulating evidence indicates that in Arabidopsis thaliana, flowering time is orchestrated by the concerted action of multiple genetic pathways, including the photoperiod, autonomous, vernalization, aging, and gibberellin (GA) signaling pathways [32]. Over the past several decades, comprehensive genetic analyses have elucidated the molecular mechanisms underlying key regulatory genes involved in these flowering pathways. Among these, the FLOWERING LOCUS T (FT) gene serves as a central integrator of diverse flowering pathways; its encoded protein functions as a pivotal florigen [33]. The FT protein interacts with the bZIP transcription factor FD to form a transcriptional activation complex, which induces the expression of downstream floral meristem identity genes, including APETALA1 (AP1), LEAFY (LFY), FRUITFULL (FUL), and CAULIFLOWER (CAL), ultimately promoting floral transition [34]. By contrast, the homologous protein TERMINAL FLOWER 1 (TFL1) acts antagonistically to FT by repressing the initiation of flowering in Arabidopsis thaliana. Within the photoperiod pathway, the pivotal regulatory factor CONSTANS (CO) integrates endogenous circadian rhythm–mediated signals with external photoperiodic cues, thereby allowing plants to initiate flowering under optimal day-length conditions [35,36]. Simultaneously, FLOWERING LOCUS C (FLC), a MADS-box transcription factor, is broadly recognized as a potent floral repressor in Arabidopsis thaliana as well as in various crop species [37,38]. Recent research has demonstrated that light-responsive BBX family transcription factors also play significant roles in the regulation of flowering. Specific BBX proteins have been shown to directly modulate the expression of floral integrator genes, thereby affecting flowering time. For instance, in pineapple, AcBBX5 acts as a negative regulator of flowering time by binding to and repressing the AcFT promoter while concurrently promoting the enlargement of floral organs [39]. Likewise, BBX24 promotes flowering in Arabidopsis thaliana by repressing FLC expression [40].
BBX transcription factors also participate in flowering regulation via the gibberellin (GA) signaling pathway. For example, under long-day conditions, CmBBX24 serves as a transcriptional repressor by downregulating the expression of GA biosynthetic enzyme genes GA20ox and GA3ox, thereby reducing the accumulation of bioactive gibberellins GA1 and GA4. Consequently, this represses the expression of downstream flowering integrators Cm-FTL3 and Cm-SOC1, thereby impeding the transduction of photoperiod and GA signals to the floral meristem and ultimately delaying flowering in chrysanthemum [41]. Furthermore, BBXs are implicated in the regulation of flowering mediated by other phytohormones, such as ethylene (ET) and abscisic acid (ABA). In chrysanthemum, the ethylene response factor CmERF3 interacts with the B-box protein CmBBX8 to form a transcriptional repression complex. CmERF3 is highly expressed during the vegetative phase, and by binding to CmBBX8, it attenuates CmBBX8-mediated activation of the flowering integrator CmFTL1, thereby repressing CmFTL1 expression and delaying flowering. Upon the transition to the reproductive stage, CmERF3 expression decreases, thereby lifting the inhibition on CmBBX8 and permitting full activation of CmFTL1 expression to initiate floral development. Thus, the CmERF3–CmBBX8–CmFTL1 module enables finely tuned regulation of the vegetative-to-reproductive transition in response to ethylene signaling [42]. In addition, drought-induced ABA accumulation also contributes to the regulation of flowering. ABA enhances GIGANTEA (GI) signaling and promotes CO protein activity, thereby upregulating FT expression in leaves and, through a CO-independent mechanism, inducing TSF expression. Moreover, ABA delays flowering by repressing SOC1 expression at the shoot apex. Collectively, these mechanisms enable ABA to exert precise control over flowering timing under stress conditions [43].
2.2.4. Secondary Metabolism
BBX transcription factors have also been implicated in the regulation of plant secondary metabolite biosynthesis. During light-regulated artemisinin biosynthesis, AaBBX21 interacts with the crucial photomorphogenic transcription factor AaHY5 to constitute a regulatory module. Under illumination, AaHY5 directly binds to the promoters of essential artemisinin biosynthetic genes (e.g., AaGSW1, AaMYB108, and AaORA), with AaBBX21 potentiating its transcriptional activation efficacy. Collectively, these factors synergistically promote the expression of downstream genes, thereby enhancing artemisinin biosynthesis and accumulation. AaHY5 and AaBBX21 are capable of physically interacting with the E3 ubiquitin ligase AaCOP1. In darkness, AaCOP1 mediates the proteasomal degradation of both proteins, thereby attenuating their regulatory influence on downstream targets and ultimately inhibiting artemisinin biosynthesis. Conversely, under light conditions, AaCOP1 activity is suppressed, permitting the accumulation of AaHY5 and AaBBX21 and enabling their positive regulatory effects on target gene expression [44]. In sweet potato, IbBBX29 has been shown to form a complex with IbMYB308L, synergistically activating flavonoid biosynthetic genes (e.g., IbCHS, IbCHI1, IbF3′H) and thereby enhancing flavonoid accumulation [45]. Additionally, a study investigating BBX genes in sweet cherry (Prunus avium) fruit revealed that, during fruit development, the expression levels of PavBBX4, PavBBX6, PavBBX7, PavBBX9, and PavBBX11 were significantly upregulated, and their expression profiles closely paralleled those of anthocyanin biosynthesis-related genes, including PavPAL, PavCHS, PavCHI, PavF3H, PavF3′H, PavDFR, PavANS, PavUFGT, and PavMYB10. Notably, the transcript levels of PavBBX6 and PavBBX9 were markedly induced by light exposure and by hormonal treatments, including abscisic acid (ABA), gibberellin (GA), and brassinosteroid (BR). Moreover, their expression showed a strong correlation with that of genes involved in anthocyanin biosynthesis [46]. Collectively, these findings suggest that PavBBX genes may participate in the regulation of anthocyanin biosynthesis by integrating light and hormonal signaling pathways.
2.2.5. Cold Stress
BBX transcription factors participate not only in the regulation of plant development but also play critical roles in mediating plant responses to cold stress. Under low-temperature conditions, the kinases SlMPK1 and SlMPK2 in tomato (Solanum lycopersicum) can activate and phosphorylate SlBBX17 at the threonine 122 (Thr-122) residue, thereby facilitating its interaction with the light signaling transcription factor SlHY5. The phosphorylated SlBBX17 enhances the transcriptional activation of key cold-responsive genes, such as SlCBF, by stabilizing the SlHY5 protein, thereby markedly improving the cold tolerance of tomato plants [47]. In contrast, AtBBX29 in Arabidopsis thaliana exhibits a negative regulatory effect on the plant’s cold stress response. It directly represses the expression of a suite of cold-induced transcription factors, including ZAT12, PRR9, RVE1, and MYB96, thereby diminishing the plant’s tolerance to low-temperature stress [48]. Collectively, these findings suggest that BBX transcription factors exhibit dual regulatory roles within plant cold stress signaling pathways, with distinct family members functioning as either positive or negative regulators of cold tolerance, thus highlighting their broad potential in plant stress adaptation mechanisms.
2.2.6. Salt Stress
BBX transcription factors likewise play pivotal roles in modulating plant responses to salt stress. For instance, in transgenic poplar, GbBBX25 enhances salt tolerance by upregulating the expression of several salt-associated genes—such as RabE1b, ERF76, bZIP60, WRKY106, and NAC130—thereby activating salt-responsive molecular signaling pathways [49]. In peanut (Arachis hypogaea), silencing the AhBBX6 gene has been shown to significantly reduce the activities of antioxidant enzymes—such as CAT, SOD, and POD—yet, paradoxically, enhance the plant’s salt tolerance [50]. This phenomenon suggests that members of the BBX family may exert diverse, or even opposing, regulatory roles across different plant species.
2.2.7. Drought Stress
Drought stress induces the expression of BBX transcription factors, which subsequently regulate the transcription of drought-responsive genes, thereby enhancing plant drought tolerance. For instance, functional analyses have revealed that CmBBX22 operates as a transcriptional activator in chrysanthemum, negatively modulating drought tolerance through the regulation of abscisic acid (ABA) responses, stomatal conductance, and antioxidant activity [51]. Similarly, in sweet potato, IbBBX24 binds to the GT-1 cis-regulatory element within the IbPRX17 promoter, thereby activating its transcription. As a member of the peroxidase gene family, IbPRX17 encodes a product capable of scavenging reactive oxygen species, consequently enhancing drought tolerance in sweet potato [52].
2.2.8. Photomorphogenesis
As pivotal regulators in light signaling, BBX proteins function cooperatively with the canonical transcription factor HY5 during plant photomorphogenesis to exert essential regulatory roles. Studies in Arabidopsis thaliana have demonstrated that AtBBX proteins can directly interact with HY5 to inhibit its transcriptional activation capability, thereby precisely modulating the expression of light-responsive genes. Furthermore, the stability of BBX proteins is regulated by COP1-mediated ubiquitination and subsequent proteasomal degradation. Under light conditions, negative regulators such as BBX28 accumulate and competitively bind to the promoter regions of target genes, thereby attenuating the regulatory function of HY5 and negatively influencing photomorphogenic development [53]. Another study has further demonstrated that BBX proteins can also act synergistically with the karrikin (KAR) signaling pathway to regulate photomorphogenesis in plant seedlings. Under normal growth conditions, SMAX1 and SMXL2 proteins restrict the activation of light signaling by suppressing the functions of BBX20 and BBX21. Upon perception of KAR signals, the SCF-type E3 ubiquitin ligase complex, comprising KAI2 and MAX2, targets SMAX1 and SMXL2 for proteasomal degradation, thereby alleviating their repression of BBX proteins. This process subsequently activates the HY5–BBX regulatory module, promotes the expression of downstream genes involved in photomorphogenesis, and ultimately suppresses excessive hypocotyl elongation [54]. Collectively, these findings suggest that BBX transcription factors cross-regulate with light signaling pathways through multiple mechanisms, playing indispensable roles in plant photomorphogenesis and highlighting their complex regulatory potential in environmental sensing and developmental coordination.
2.2.9. Pathogen and Insect Defense
BBX transcription factors are additionally implicated as pivotal regulators in plant defense mechanisms against both pathogenic microorganisms and insect herbivores. Recent studies have demonstrated that the expression of CaBBX14 in pepper (Capsicum annuum) is significantly upregulated following Phytophthora capsici infection or exogenous salicylic acid (SA) application. Functional analyses have revealed that silencing CaBBX14 markedly impairs resistance to Phytophthora capsici, which is concomitant with reduced endogenous SA levels and the downregulation of SA-responsive and disease resistance genes, including those encoding pathogenesis-related (PR) proteins. Collectively, these findings indicate that CaBBX14 positively modulates plant immune responses to pathogenic challenge, likely by activating the SA signaling cascade [55]. In the context of stomatal immunity, the transcription factor NAC053 directly activates AtBBX11 expression, conferring upon it essential regulatory roles in response to pathogen-associated molecular pattern (PAMP) elicitation. AtBBX11 suppresses COR (cold-responsive protein)-mediated stomatal reopening and participates in flg22-induced PAMP-triggered immunity (PTI) by promoting reactive oxygen species (ROS) accumulation, activating the mitogen-activated protein kinase (MAPK) signaling cascade, and facilitating callose deposition, thereby strengthening defense against bacterial infection [56]. BBX factors have also been shown to play significant roles in conferring resistance to insect herbivores. Silencing the CsCOL9 gene in citrus markedly increases the survival and host preference of whitefly (Bemisia tabaci), concomitant with suppressed expression of key antioxidant defense genes, including CsSOD, CsRBOH, and CsPOD. Correspondingly, both the enzymatic activities of SOD and POD, as well as hydrogen peroxide (H2O2) levels, are substantially diminished. By contrast, CsCOL9 overexpression reduces whitefly adaptability and feeding preference, which is accompanied by elevated expression and enzymatic activity of associated defense genes, as well as increased accumulation of reactive oxygen species (ROS) [57]. Collectively, these findings underscore that BBX transcription factors orchestrate plant immunity via multifaceted signaling pathways and defense mechanisms, thereby fulfilling central and multifarious roles in conferring resistance to both pathogens and insect herbivores.
2.2.10. Thermomorphogenesis
BBX transcription factors are also implicated in mediating plant morphological responses to elevated temperatures, a phenomenon known as thermomorphogenesis. Studies have shown that at warm temperatures, both the expression levels and protein accumulation of AtBBX24 and AtBBX25 are markedly increased. This induction antagonizes the function of the thermosensor ELF3, thereby alleviating its repression of PIF4 transcription and protein expression. Moreover, AtBBX24 and AtBBX25 can directly interact with the PIF4 protein, enhancing its stability and transcriptional activation capability, thus promoting heat-induced hypocotyl elongation and leaf expansion—hallmark responses of thermomorphogenesis [58]. Similarly, under high temperature, AtBBX18 and AtBBX23 modulate the protein stability of ELF3 through interactions with both ELF3 and COP1, thereby indirectly influencing PIF4 activity. This regulatory mechanism further amplifies the PIF4-mediated heat response, thus positively regulating typical thermomorphogenic processes such as hypocotyl elongation in Arabidopsis thaliana [59].
Although earlier studies have demonstrated that BBX proteins fulfill multiple vital roles in plants, recent years have witnessed a surge in research focusing on BBX family members in a wide array of plant species. However, the functions of the majority of BBX genes have yet to be fully elucidated, and their downstream target genes remain largely unidentified (Table 1).

Table 1.
The numbers of the BBX family, key BBX, and their functions in various plant species.
3. Classification, Bioactivities, and Biosynthetic Pathways of Plant Anthocyanins
Anthocyanins are the products of anthocyanidins after glycosylation modification. Glycosylation can significantly improve the stability of anthocyanidins, enabling their effective accumulation in plant cells. The most common anthocyanins in plants are the derivatives of delphinidin, pelargonidin, cyanidin, petunidin, malvidin, and peonidin [1] (Figure 3B).
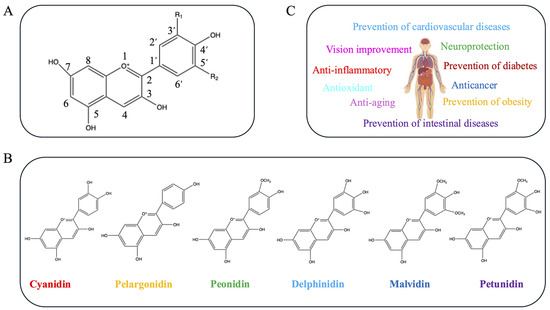
Figure 3.
Basic structure, classification, and biological activities of anthocyanins. (A). The basic structure of anthocyanins. Cyanidin: R1 = OH, R2 = H; pelargonidin: R1 = H, R2 = H; delphinidin: R1 = OH, R2 = OH; peonidin: R1 = OCH3, R2 = H; malvidin: R1 = OCH3, R2 = OCH3; and petunidin: R1 = OCH3, R2 = OH. (B). Classification of anthocyanins. (C). Bioactive functions of anthocyanins in human health.
3.1. Classification
3.1.1. Delphinidins
Delphinidins are distinguished by hydroxyl substitutions at the 3′ and 5′ positions of the B-ring. These compounds are abundant in blue or purple plant tissues, including those of blueberries and eggplants. Moreover, delphinidin-3-O-sambubioside has been identified as a potent xanthine oxidase (XO) inhibitor, with potential application in the prevention and treatment of hyperuricemia [82].
3.1.2. Pelargonidins
Pelargonidins possess a relatively simple structure, defined by the absence of substitutions at the 3′ and 5′ positions of the B-ring. These anthocyanidins are primarily responsible for the red pigmentation in fruits such as strawberries. Recent studies have demonstrated that pelargonidins also possess notable anti-inflammatory properties. For instance, pelargonidins have been shown to ameliorate inflammation and cartilage degeneration in osteoarthritis by inhibiting the NF-κB signaling pathway, suppressing the secretion of inflammatory cytokines, and preventing extracellular matrix degradation in chondrocytes [83].
3.1.3. Cyanidins
Cyanidins are ubiquitous throughout the plant kingdom. Distinct from other anthocyanidins, cyanidins possess a hydroxyl substitution at the 3′ position of the B-ring. Notably, cyanidin-3-glucoside accounts for the vast majority of the total cyanidins. These compounds are major contributors to dark pigmentation observed in various plant tissues. In addition to their pigmentary roles, cyanidins exhibit significant health-promoting properties. For instance, cyanidin-3-O-glucoside and its phenolic metabolites can ameliorate intestinal disorders by modulating immune responses within the intestinal mucosa [84]. Furthermore, cyanidins have been shown to suppress adipogenesis in 3T3-L1 preadipocytes by activating the PLC-IP3 signaling pathway, thereby highlighting their potential utility in obesity management [85].
3.1.4. Petunidins
Petunidins are characterized by the presence of a methoxy group at the 3′ position and a hydroxyl group at the 5′ position of the B-ring. Among petunidins, petunidin-3-O-glucoside is the predominant derivative and represents one of the major anthocyanins identified in Chinese wild berries [86]. Petunidins have also been reported to exhibit notable antioxidant activity. For example, petunidin targets NOX4 to suppress reactive oxygen species (ROS) production, attenuates oxidative stress, and modulates the Bcl-2 signaling pathway to prevent cardiomyocyte apoptosis [87].
3.1.5. Peonidins
Peonidins are methylated derivatives of cyanidin, distinguished by the presence of a methoxy group at the 3′ position and a hydroxyl group at the 5′ position of the B-ring. Among the various forms, peonidin-3-O-glucoside is the most prevalent and is particularly abundant in blueberries. It is also present in litchi pericarp, Prunus armeniaca, and Myrciaria vexator [86]. Recent studies indicate that peonidins possess therapeutic potential for the management of nonalcoholic fatty liver disease (NAFLD) [88], as well as psoriasis [89].
3.1.6. Malvidins
Malvidin, an O-methylated anthocyanidin and the 3′,5′-dimethoxy derivative of delphinidin, is one of the most structurally modified members of the anthocyanidin family. It is renowned for imparting the most intense red coloration among all monomeric anthocyanins. Its glucoside form, malvidin-3-O-glucoside, serves as the principal pigment contributing to the coloration of grapes and is also widely distributed across a variety of other fruits [86].
3.2. Bioactivities
Anthocyanins serve as essential pigments that impart color to plant fruits and flowers while also playing a role in improving plant resilience to environmental stresses. Moreover, these compounds confer multiple health benefits in humans, such as antioxidant effects, cardiovascular protection, anti-carcinogenic activity, anti-aging potential, glycemic control, and enhancement of visual function (Figure 3C).
3.2.1. Roles of Anthocyanins in Plants
Anthocyanins are responsible for the intense pigmentation observed in plant fruits and flowers, resulting in distinct coloration such as purple, pink, red, and blue. These color traits play a key role in attracting pollinators and seed dispersers [90]. Regarding light regulation, anthocyanins confer photoprotection to apple leaves by absorbing excess visible light; however, this protective effect may concomitantly reduce photosynthetic efficiency to some degree [91]. Under conditions of high-intensity light or ultraviolet (UV) stress, anthocyanins operate via a dual mechanism of light shielding and antioxidation. Specifically, they absorb excess light energy and scavenge reactive oxygen species (ROS), thereby preserving chloroplast structure, protecting the photosynthetic machinery, and mitigating photooxidative damage [92]. Additionally, exposure to abiotic stresses—including high salinity, extreme temperatures (heat or cold), and heavy metal contamination—often results in the accumulation of large quantities of free radicals in plants, thereby inducing oxidative stress. These reactive species can inflict severe damage on biological macromolecules, such as cellular membranes, proteins, and DNA [93]. Owing to their distinctive chemical structure, anthocyanins exhibit robust ROS-scavenging activity and mitigate oxidative stress through multiple mechanisms, including direct radical scavenging, inhibition of lipid peroxidation, and metal ion chelation [92,94]. Furthermore, anthocyanins contribute to plant defense against biotic stress by enhancing pathogen resistance and modulating the structure and stability of phyllosphere microbial communities through ROS-mediated signaling [95,96]. Notably, under nutrient-deficient conditions—such as nitrogen or phosphorus starvation—plants frequently accumulate elevated levels of anthocyanins, thereby bolstering their antioxidative capacity [97,98]. In summary, these studies offer compelling evidence that anthocyanins are integral to the maintenance of normal physiological processes in plants and substantially enhance their adaptability to various environmental stresses.
3.2.2. Antioxidant Activity
As natural antioxidants, anthocyanins rank among the most effective, safe, and water-soluble free radical scavengers discovered to date. Their potent antioxidant activity is intrinsically linked to their distinctive chemical structure. In general, simpler anthocyanin structures are associated with stronger antioxidant capacity [99]. Mechanistically, anthocyanins scavenge free radicals predominantly via two mechanisms: hydrogen atom transfer (HAT) and single electron transfer (SET). During these reactions, hydroxyl groups serve as hydrogen or electron donors, participating in redox processes to generate more stable radical intermediates, thereby interrupting oxidative cascades and exerting pronounced antioxidant effects [100]. In addition, anthocyanins bolster endogenous antioxidant defenses by upregulating the transcription of genes encoding antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx). Recent studies have systematically investigated the antioxidant properties of both anthocyanins and proanthocyanidins. In vitro studies have demonstrated that anthocyanins markedly reduce intracellular reactive oxygen species (ROS) levels; in vivo experiments further revealed that anthocyanin administration prolongs lifespan, enhances antioxidant enzyme activity, and decreases oxidative stress-associated biomarkers [101]. Notably, glycosylation generally diminishes the antioxidant activity of anthocyanins, although in specific contexts it may paradoxically enhance their efficacy. This variability is influenced by the anthocyanin subtype, the chemical nature of the sugar moiety, and the oxidative environment or properties of the target oxidant. In contrast, acylation is widely acknowledged to significantly potentiate the antioxidant activity of anthocyanins.
3.2.3. Prevention of Cardiovascular Diseases
Cardiovascular disease (CVD) has emerged as the foremost cause of mortality globally. With its incidence on the rise, the development of alternative preventive and therapeutic strategies has become a central focus of contemporary research, aiming to mitigate the escalating burden that CVD imposes on global healthcare systems. In recent years, anthocyanins have garnered increasing attention for their promising role in the prevention of CVD. For instance, a 2015 study demonstrated that dietary supplementation with blueberry-derived anthocyanins significantly attenuated cyclophosphamide (CTX)-induced cardiac injury in rats by exerting anti-inflammatory, antioxidant, and anti-apoptotic effects. This intervention improved cardiac function and suppressed cardiomyocyte apoptosis and inflammatory signaling pathways [102]. Subsequently, a comprehensive meta-analysis further confirmed that increased dietary intake of anthocyanins is significantly associated with a reduced risk of cardiovascular disease [103]. Therefore, promoting the regular consumption of anthocyanin-rich fruits and vegetables in daily diets holds considerable practical significance for the primary prevention of CVD.
3.2.4. Anti-Cancer Activity
Cancer continues to represent one of the foremost threats to global human health. According to the Global Cancer Statistics 2022, nearly 20 million new cancer cases were diagnosed worldwide, and cancer-related deaths approached 9.7 million [104]. These alarming figures underscore the persistently high mortality associated with cancer and emphasize the urgent need for more effective therapeutic interventions. In recent years, accumulating evidence has demonstrated that plant-derived anthocyanins possess potent anticancer properties, contributing to both cancer prevention and therapy. For example, a study on lung cancer revealed that anthocyanins exert anticancer effects via multiple molecular mechanisms, including inhibition of critical signaling pathways (PI3K/Akt/mTOR, p38 MAPK, JNK); downregulation of VEGF expression to impede angiogenesis; modulation of apoptotic regulators (e.g., upregulation of Bax and Caspase-3/9, downregulation of Bcl-2); and suppression of EMT-related markers such as N-cadherin and vimentin [105]. Moreover, a comprehensive review on colorectal cancer highlighted that anthocyanins exert robust anticancer effects through a multifaceted mode of action, encompassing anti-inflammatory, antioxidant, anti-proliferative, pro-apoptotic, and epigenetic regulatory pathways [106]. Taken together, these findings underscore the therapeutic promise of anthocyanins as potential agents in cancer prevention and treatment.
3.2.5. Anti-Aging Activity
Aging is a natural and inevitable physiological process and represents a major risk factor for numerous age-related diseases. It not only poses a serious threat to individual health but also imposes a substantial burden on public healthcare systems. A growing body of evidence suggests that anthocyanins possess the potential to delay aging and alleviate aging-associated disorders [107]. For example, cyanidin 3-O-[6-O-(E-p-coumaroyl-2-O-(β-D-xylopyranosyl)-β-D-glucopyranoside)]-5-O-β-D glucopyranoside, isolated from Sambucus canadensis, has been shown to significantly reduce cellular senescence and lens aging by inhibiting the PI3K/AKT/mTOR signaling pathway. This compound promotes the apoptosis of senescent cells, enhances autophagy and mitophagy flux, and improves mitochondrial and cellular renewal capacity, thereby maintaining cellular homeostasis and slowing the aging process [108]. In aging models, treatment with anthocyanins isolated from black chokeberry significantly reduced the transcription of inflammatory cytokines (COX2, TGF-β1, and IL-1) and the levels of DNA damage in the mouse brain, indicating that anthocyanins may mitigate aging by suppressing DNA damage and reducing the accumulation of inflammatory factors [109]. Moreover, anthocyanins exert anti-aging effects through multiple coordinated mechanisms, including the modulation of inflammation-related pathways, age-associated signaling proteins, transcription factors, gene expression, and microRNAs [110]. In addition, another study revealed that anthocyanins can also exert health-promoting effects through interactions with the gut microbiota. Specifically, anthocyanins and their microbial metabolites promote healthy aging by exhibiting bioactivities such as anti-inflammatory, antioxidant, and neuroprotective effects [111]. Collectively, these findings highlight the important role of anthocyanins in combating aging.
3.2.6. Other Bioactivities
A growing body of evidence indicates that, beyond their well-documented bioactivities, anthocyanins possess considerable therapeutic potential in diverse areas, including diabetes [112], visual function enhancement [113], obesity management [114], neuroprotection [115], and the prevention of gastrointestinal disorders [84]. These diverse bioactivities not only lay a robust foundation for the development of functional foods, nutraceuticals, and pharmaceuticals, but also open promising avenues for leveraging plant-derived natural compounds in the prevention and treatment of chronic diseases.
3.3. Biosynthetic Pathway
The biosynthetic pathway of plant anthocyanins can be delineated into three distinct stages (Figure 4). The first stage involves the initiation of flavonoid biosynthesis, beginning with phenylalanine as the precursor, which is stepwise converted to 4-coumaroyl-CoA through the catalytic actions of phenylalanine ammonia-lyase (PAL), cinnamate-4-hydroxylase (C4H), and 4-coumaroyl-CoA ligase (4CL). The second stage represents a critical phase in anthocyanin biosynthesis, during which 4-coumaroyl-CoA is sequentially transformed into various intermediate compounds by the coordinated activities of key enzymes, including chalcone synthase (CHS), chalcone isomerase (CHI), flavanone-3-hydroxylase (F3H), flavonoid-3′-hydroxylase (F3′H), and flavonoid-3′,5′-hydroxylase (F3′5′H). In the third stage, the intermediate compounds are converted into three principal types of anthocyanidins through the actions of dihydroflavonol 4-reductase (DFR) and anthocyanidin synthase (ANS) and subsequently stabilized via glycosylation by flavonoid-3-O-glucosyltransferase (3GT/UFGT), resulting in the formation of structurally stable anthocyanins [116]. Finally, anthocyanins may undergo further modifications, such as glycosylation, methylation, and acetylation, to generate diverse derivatives, and are subsequently transported from the cytoplasm to the vacuole for storage via specific transport proteins.
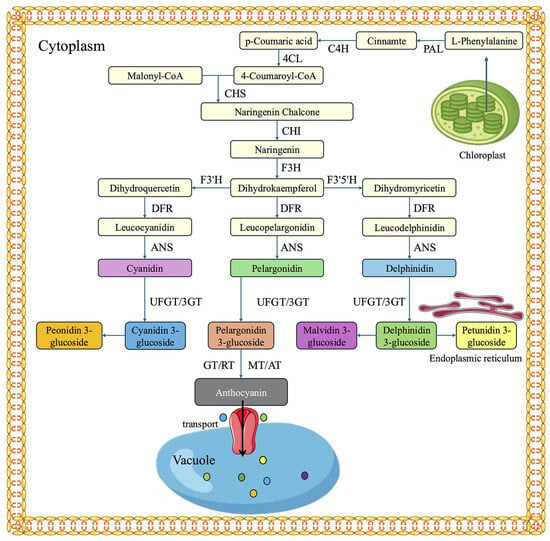
Figure 4.
Biosynthetic pathway of anthocyanins in plants.
4. Molecular Mechanisms of BBX TFs Regulating Plant Anthocyanin Biosynthesis
BBX transcription factors exhibit complex and diverse functions in the regulation of anthocyanin biosynthesis in plants. Recent studies indicate that BBX proteins finely tune anthocyanin accumulation in plant cells via multiple mechanisms, including direct and indirect regulation and co-regulation (Figure 5).
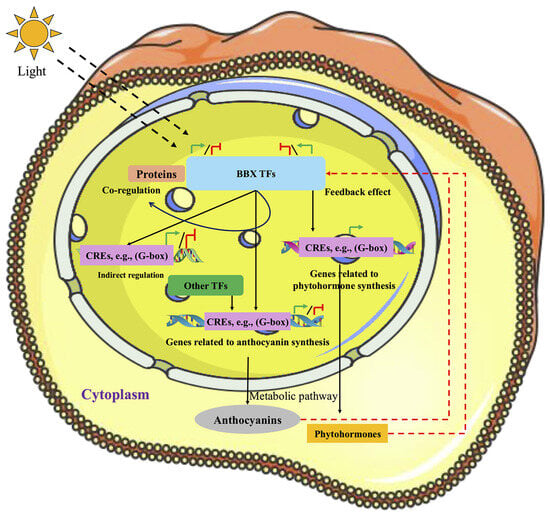
Figure 5.
Molecular regulatory mechanism model of BBX-mediated anthocyanin biosynthesis in plants.
4.1. Direct Regulation
BBX proteins are capable of directly binding to the promoters of pivotal structural genes within the anthocyanin biosynthetic pathway, thereby exerting either positive or negative regulatory effects on their transcriptional activity and ultimately modulating anthocyanin accumulation. For instance, transient overexpression studies have demonstrated that ectopic expression of ChBBX6 and ChBBX18 in Cerasus humilis fruit significantly enhances anthocyanin accumulation and markedly upregulates the transcription of anthocyanin biosynthesis-related genes, including chalcone synthase (ChCHS) and flavanone 3-hydroxylase (ChF3H). Subsequent yeast one-hybrid and dual-luciferase reporter assays further confirmed that ChBBX6 and ChBBX18 directly bind to and activate the promoters of ChCHS and ChF3H, with the G-box motif (CACGTG) identified as their specific binding site [117]. Similarly, SlBBX20 has been reported to directly associate with the promoter of the anthocyanin biosynthetic gene SlDFR in Solanum lycopersicum, activating its expression and thus promoting anthocyanin accumulation [118].
4.2. Indirect Regulation
In certain instances, BBX transcription factors do not directly target the structural genes of the anthocyanin biosynthetic pathway but instead modulate anthocyanin biosynthesis through the indirect regulation of other critical regulatory elements. For example, blue light induction has been shown to elevate the expression of MiBBX24 and MiBBX27, both of which can directly interact with G-box elements located within the promoter region of MiMYB1—a key MYB-type transcription factor gene regulating anthocyanin biosynthesis—thereby activating its transcription. Subsequently, MiMYB1 protein directly regulates the expression of downstream structural genes, including MiANS, MiUFGT1, and MiUFGT3, thereby facilitating anthocyanin accumulation in the peel of mango (Mangifera indica L.) [119]. In addition, under light signaling, MdBBX22 is capable of binding to the promoter region of the mdm-miR858 gene and activating its transcription. The resulting mdm-miR858 subsequently targets and represses the expression of transcription factor genes such as MdMYB9, MdMYB11, and MdMYB12, thereby reducing proanthocyanidin accumulation and promoting anthocyanin biosynthesis in apple (Malus domestica) [120]. These findings imply the existence of a competitive relationship between proanthocyanidin and anthocyanin metabolic pathways.
4.3. Co-Regulation
BBX transcription factors can also participate in the precise regulation of anthocyanin biosynthesis by interacting with other proteins or light signaling components, such as HY5. For instance, during light-induced flower coloration in grape hyacinth (Muscari armeniacum), MaBBX20 forms a complex with MaHY5 to synergistically activate the transcription of MaMybA and MaDFR, thereby enhancing anthocyanin synthesis. In contrast, MaBBX51 interacts with both MaHY5 and MaBBX20, hindering complex formation and interfering with the binding of MaMybA to the promoters of its downstream targets, thus repressing anthocyanin biosynthesis [121]. The allelic variant PyBBX24DN14 of PyBBX24 interacts with PyHY5 to form a transcriptional complex that binds to the G-box elements within the promoters of anthocyanin biosynthesis-related genes, such as PyUFGT and PyMYB10, thereby activating their transcription and promoting anthocyanin accumulation [122]. Another study showed that the Ppbbx24-del mutant, which lacks the VP domain, evades PpPUB59-mediated ubiquitination and subsequent degradation, thereby stably activating PpCHS expression and promoting anthocyanin accumulation. Meanwhile, PpBBX24 can negatively modulate anthocyanin biosynthesis by suppressing PpHY5-mediated activation of the PpCHS promoter. PpPUB59 further fine-tunes anthocyanin accumulation by mediating the ubiquitination and degradation of both PpBBX24 and PpHY5, ultimately resulting in the red pigmentation phenotype characteristic of the “Red Zaosu” pear [123].
Notably, beyond the regulatory mechanisms described above, the expression of BBX transcription factors may be subject to feedback induction by excessive anthocyanin accumulation or hormonal cues, thereby sustaining the homeostasis of anthocyanin biosynthesis via positive or negative regulatory pathways. For instance, in grape (Vitis vinifera), VvBBX44 and VvMYBA1 constitute a transcriptional feedback regulatory loop that precisely modulates anthocyanin biosynthesis. Specifically, under light conditions, VvHY5 is initially activated and subsequently induces the expression of VvMYBA1. Acting as a transcriptional activator, VvMYBA1 directly upregulates the key structural gene VvUFGT, thereby promoting anthocyanin accumulation. Concurrently, VvMYBA1 also induces the expression of VvBBX44. As anthocyanin levels rise, exogenous anthocyanin can further enhance VvBBX44 expression. VvBBX44 binds to the T/G-box element within the VvMYBA1 promoter, represses its expression, and interferes with VvMYBA1-mediated activation of both VvUFGT and VvBBX44 promoters, ultimately downregulating the expression of VvUFGT and VvMYBA1 [124]. This negative feedback circuitry results in attenuated anthocyanin biosynthesis and maintains cellular anthocyanin homeostasis. Plant hormones play pivotal roles in the feedback regulation of anthocyanin biosynthesis. For instance, in bicolored sweet cherry (Prunus avium cv. Rainier) fruit, light induces the expression of transcription factors PavBBX6 and PavBBX9, both of which can directly bind to the promoter of the key anthocyanin biosynthetic gene PavUFGT, thereby enhancing its transcriptional activity and promoting anthocyanin accumulation. Simultaneously, light upregulates the expression of the abscisic acid (ABA) biosynthetic gene PavNCED1, promoting ABA synthesis. Accumulated ABA further upregulates PavBBX6 and PavBBX9 expression, forming a positive feedback loop that sustains the transcription of anthocyanin biosynthesis-associated genes [125].
In summary, recent studies have identified several recurring patterns in the regulation of anthocyanin biosynthesis by BBX transcription factors. On the one hand, whether BBX proteins participate in anthocyanin biosynthesis directly, indirectly, or synergistically, their functions invariably depend on light signaling, highlighting light as a critical environmental determinant of BBX activity. On the other hand, in the context of BBX-mediated responses to light signaling, the transcription factor HY5 is almost universally engaged and exerts a positive regulatory influence on anthocyanin biosynthesis in multiple species, such as apple, Artemisia annua, pear, and grape, thereby indicating that the BBX-HY5 regulatory module is highly conserved across the plant kingdom. Furthermore, distinct members of the BBX family can function as either positive or negative regulators of anthocyanin biosynthesis, thereby underscoring the functional diversity within this gene family. Notably, earlier research has suggested that BBX activity may be modulated by feedback from anthocyanin metabolites or plant hormones; however, the precise molecular mechanisms underlying this regulation remain largely elusive. Therefore, a substantial knowledge gap remains regarding the regulatory mechanisms through which BBX modulates anthocyanin biosynthesis. Additional research is warranted to thoroughly elucidate the functional roles of BBX, which will offer a strong foundation for the strategic utilization of BBX and its interactions with target genes in the molecular breeding of horticultural crops (Table 2).

Table 2.
BBX proteins, target genes, and types of regulation in anthocyanin biosynthesis in plants.
5. Characterized BBX TFs Involved in the Regulation of Horticultural Plant Anthocyanin Biosynthesis
Although current research on the regulation of anthocyanin biosynthesis by BBX transcription factors remains relatively limited, available evidence has demonstrated their pivotal roles in various horticultural plant species (Figure 6). For instance, in lily (Lilium spp.), light induces the expression of LvBBX24, whose encoded protein can bind to the G-box element within the LvMYB5 promoter and activate LvMYB5 transcription. As an R2R3-MYB transcription factor, LvMYB5 subsequently upregulates the expression of structural genes associated with anthocyanin biosynthesis (such as LvANS), thereby enhancing anthocyanin accumulation. Additionally, LvbZIP44 and LvBBX24 form a heterodimer that cooperatively activates LvMYB5 expression and jointly regulates anthocyanin biosynthesis [126]. In woody plants, investigations in poplar (Populus trichocarpa) have further elucidated the complexity of BBX- and light-mediated co-regulation. PtrBBX23 can assemble with PtrHY5 into a transcriptional activation complex, which targets both upstream transcription factors (such as PtrMYB115 and PtrMYB119) and downstream structural genes (including PtrCHS4, PtrF3H3, PtrDFR1, and PtrANS1), thereby markedly promoting the biosynthesis of anthocyanins and proanthocyanidins. High light treatment not only induces PtrBBX23 expression but also elevates the transcription of downstream structural genes. Notably, the expression peak of PtrHY5 occurs prior to that of PtrBBX23, implying potential temporal and functional partitioning within their regulatory network. Furthermore, PtrBBX23 can suppress putative negative regulators (such as PtrMYB57 and PtrMYB182), further enhancing anthocyanin accumulation [127]. Herbaceous plants also exhibit diverse regulatory mechanisms involving BBX factors. For example, in chrysanthemum (Chrysanthemum morifolium), CmBBX28 expression is upregulated in darkness, and it interacts with the key anthocyanin biosynthetic transcription factor CmMYB9a, impeding its binding to target gene promoters and reducing its protein stability, thereby repressing the expression of structural genes such as CmCHS, CmDFR, and CmUFGT. In contrast, light significantly upregulates CmMYB9a and represses CmBBX28 expression, thus promoting anthocyanin biosynthesis. This light-dependent regulatory mechanism highlights the bidirectional regulatory role of BBX factors in pigment accumulation in response to environmental signals [128]. Moreover, BBX proteins have been shown to participate in more intricate regulatory networks through interactions with core photomorphogenic factors. In Lagerstroemia indica, LiBBX4 not only responds to light signals to regulate its own expression but also interacts with key photomorphogenesis components such as LiHY5, LiHYH, and LiCOP1. By downregulating a suite of anthocyanin biosynthetic structural genes (e.g., LiCHS, LiCHI, LiF3H, LiDFR, LiANS, and LiUFGT), LiBBX4 acts as an overall negative regulator, thereby suppressing anthocyanin accumulation [129].
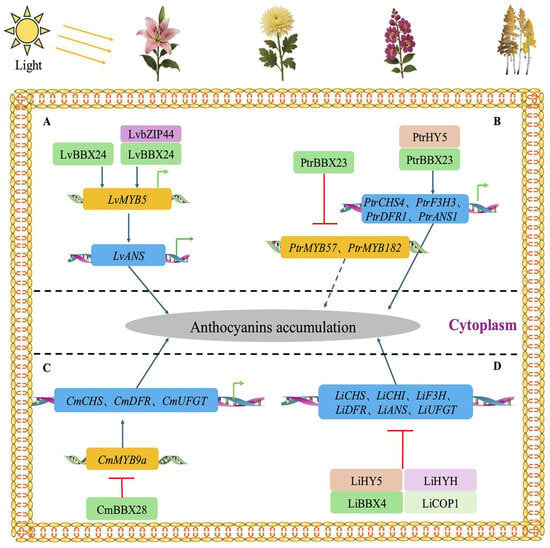
Figure 6.
The regulatory functions of BBXs in the anthocyanin biosynthetic pathway in Lilium spp. (A), Populus trichocarpa (B), Chrysanthemum morifolium (C), and Lagerstroemia indica (D).
Notably, BBX transcription factors seldom directly bind to the promoters of structural genes during the regulation of anthocyanin biosynthesis. Rather, they predominantly activate the transcription of key structural genes involved in anthocyanin biosynthesis through indirect mechanisms, such as interacting with other proteins or transcription factors, or by forming multi-protein complexes. Importantly, the transcription factor HY5 exerts a critical synergistic effect in modulating the regulatory functions of BBX proteins. Furthermore, these regulatory mechanisms are predominantly orchestrated under the influence of light signaling pathways. Collectively, these findings offer valuable insights and a solid foundation for future investigations into the regulatory mechanisms of BBX transcription factors and their roles in plant secondary metabolism.
6. Prospects
Anthocyanins constitute a major class of plant secondary metabolites, playing pivotal roles in augmenting ornamental value, enhancing nutritional quality, and fortifying plant responses to abiotic stress. BBX transcription factors, as newly identified regulators of anthocyanin biosynthesis, have garnered considerable attention in recent years and represent a focal point of research interest. To fully exploit the functional potential of BBX transcription factors, future investigations should be directed along the following strategic avenues.
6.1. Application of Genome Editing Technologies in Anthocyanin Biosynthesis in Horticultural Plants
Recent studies have demonstrated that BBX transcription factors can act as either positive or negative regulators of anthocyanin biosynthesis in plants. Notably, BBX proteins that function as negative regulators typically suppress anthocyanin accumulation, thereby detrimentally affecting the coloration and ornamental qualities of horticultural plants and ultimately diminishing their aesthetic and commercial value. With the rapid advancement of genome editing technologies, the targeted knockout of negatively regulating BBX genes using precise tools such as CRISPR/Cas has emerged as an effective strategy to enhance anthocyanin content in horticultural species. This approach can markedly increase anthocyanin accumulation in plant tissues, improve flower coloration and visual appeal, and ultimately promote the economic value of ornamental and horticultural crops. Moreover, the design of artificially engineered BBX transcription factors offers a novel avenue for the targeted regulation of anthocyanin biosynthesis. By constructing BBX transcription factors with strong activation capabilities and directing their expression in plants, it is possible to further upregulate the transcription of key genes in the anthocyanin biosynthetic pathway, thereby achieving precise control over flower coloration and overall plant quality.
6.2. Optimizing Light Conditions Promotes Anthocyanin Biosynthesis
As prototypical light-responsive transcription factors, BBX proteins are profoundly modulated by light signaling pathways in the regulation of anthocyanin biosynthesis. Accumulating evidence demonstrates that distinct light wavelengths and intensities differentially modulate anthocyanin biosynthesis. For instance, exposure to red light markedly enhances the activities of key enzymes involved in anthocyanin synthesis—such as phenylalanine ammonia-lyase (PAL) and 4-coumarate-CoA ligase (4CL)—in grape berries, thereby significantly promoting anthocyanin accumulation and imparting deeper, more visually appealing skin coloration [130]. By contrast, blue light exerts a comparatively weaker stimulatory effect, whereas green light exhibits only a minimal influence. Furthermore, related studies in tea plants have shown that at a light intensity of 8000 lx, the expression of CsAN1 and multiple anthocyanin biosynthetic genes (ABGs) is significantly induced, and anthocyanin accumulation increases markedly; conversely, 50% shading nearly halves the anthocyanin content [131]. Notably, analogous phenomena have also been documented in the present study. For instance, MiBBX24 and MiBBX27 function as positive regulators of anthocyanin biosynthesis in mango (Mangifera indica) fruit. Under specific blue light conditions, the expression of these genes is upregulated, and through binding to G-box elements in the MiMYB1 promoter, they upregulate MiMYB1 expression, thereby facilitating blue light-induced anthocyanin accumulation in mango peel [119]. Conversely, MdBBX37 has been identified as a negative regulator, repressing the expression of MdMYB1, MdMYB9, and MdHY5, which consequently leads to diminished anthocyanin accumulation. However, exposure to light suppresses MdBBX37 expression, thereby alleviating its inhibitory influence and promoting anthocyanin accumulation, at least in part [132]. These findings collectively suggest that the manipulation of light parameters—such as wavelength and intensity—can effectively induce the expression of BBX transcription factors that enhance anthocyanin biosynthesis or suppress negative regulators, thereby enabling the precise regulation of anthocyanin accumulation. This strategy holds substantial promise for practical applications.
Additionally, this study observed that BBX proteins are involved in the regulation of anthocyanin biosynthesis in response to hormonal cues; however, investigations in this area remain limited. Notably, light and hormonal signals may exert synergistic effects in mediating the regulatory roles of BBX proteins. Thus, future studies should further investigate the complex interactions among BBX proteins, light signaling, and hormonal pathways and aim to construct systematic regulatory network models to provide a robust theoretical framework for comprehensively elucidating their mechanisms of action.
6.3. The Research Potential and Agricultural Applications of BBX as a "Regulatory Hub"
BBX transcription factors are central regulators in plant development and secondary metabolism, distinguished by their multifunctionality and integrative roles. In the context of anthocyanin biosynthesis, BBX proteins rarely act in isolation; rather, they serve as integrative nodes, coordinating light and hormone signaling pathways to form complex, multidimensional regulatory networks that enable precise modulation of anthocyanin synthesis. Consequently, future research should emphasize the “hub” function of BBX proteins in orchestrating light and hormone signal transduction as well as metabolic regulation, with particular attention to elucidating the mechanisms underlying fruit and flower coloration and other key quality traits. Notably, BBX genes represent promising targets for molecular breeding programs.
It is important to highlight that functional characterizations of BBX genes have thus far been predominantly confined to model species such as Arabidopsis and only a limited number of fruit and ornamental plants. In contrast, investigations of BBX gene function in vegetables and staple crops are comparatively sparse, thus constraining the potential application of BBX in enhancing production scale, yield, and quality in horticultural crops. Therefore, future studies should broaden their scope to encompass a diverse array of fruit, vegetable, and cereal crops and leverage advanced genome-editing techniques such as CRISPR/Cas9 and transient expression systems to comprehensively dissect the regulatory functions of BBX genes.
Furthermore, the molecular mechanisms governing interactions between BBX proteins and other key transcription factors remain insufficiently understood, particularly with respect to their interactions with HY5, COP1, and components of the MYB-bHLH complex. Systematic approaches—such as ChIP-seq and yeast two-hybrid assays—are recommended to delineate their regulatory targets and clarify the molecular basis of protein complex assembly. In addition, the identification of superior BBX allelic variants, in tandem with marker-assisted selection, holds significant promise for improving breeding materials, thereby facilitating the enhancement of pigment accumulation or stress resistance in horticultural crops.
Collectively, although the involvement of BBX genes in flower and fruit coloration has been characterized in several ornamental and horticultural plants, their roles in other types of ornamental species or horticultural crops remain largely unexplored. Consequently, future research should be expanded to encompass a broader diversity of fruit-bearing and flowering horticultural plants to clarify the universal mechanisms by which BBX genes regulate anthocyanin biosynthesis. Moreover, integrating large-scale functional analyses such as high-throughput genetic screening, along with advanced multi-omics approaches—including genomics, transcriptomics, proteomics, and metabolomics—will be instrumental in unraveling the complex regulatory networks underlying BBX-mediated pigment biosynthesis. These strategies will not only deepen our mechanistic understanding but also provide a robust theoretical foundation and practical framework for targeted trait improvement and precision breeding in horticultural crops.
Author Contributions
D.X. conceived the study and reviewed the manuscript writing. H.L., K.D., Y.Z. and D.X. wrote the initial draft and conducted the writing review. D.X. and Y.Z. supervised, funded, and administered the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research received financial support from various sources, including the National Natural Science Foundation of China (32260089), the Science and Technology Department Foundation of Guizhou Province (QKHJC-MS [2025]371), the Guizhou Provincial Special Fund for Innovation Capacity Building of the Scientific Research Institutions of China (Qian Kehe service enterprise [2024]008-1, [2024]008-2), the Horizontal Project of the Zunyi Academy of Agricultural Sciences in 2025 (G-257), the Industry–University Collaborative Education Project of Ministry of Education of China (230901414190927), the Employment-oriented Education Project for Supply–Demand Docking of the Ministry of Education of China (2025030310008), the Future Outstanding Teacher Training Program of Zunyi Medical University (XJ2023-JX-01-06).
Data Availability Statement
Not applicable.
Acknowledgments
We thank Servier Medical Art a lot for the assistance in figure drawing, licensed under a Creative Commons Attribution 3.0 unported license, and media library (https://ian.umces.edu/media-library/symbols/, (accessed on 1 July 2025)). We also used PowerPoint drawing in Microsoft Office 2021; we are equally grateful to them for their assistance.
Conflicts of Interest
All authors approve the final version and declare that there are no conflicts of interest that could be perceived as prejudicial to the impartiality of the reported research.
Abbreviations
The following abbreviations are used in this manuscript:
| PAL | Phenylalanine ammonia-lyase |
| B-box | BBX |
| TFs | Transcription factors |
| MBW | MYB/bHLH/WD40 |
| ChIP-seq | Chromatin immunoprecipitation sequencing |
| CHS | Chalcone synthase |
| VP | Valine–proline |
| ABA | Abscisic acid |
| GA | Gibberellins |
| ABREs | ABA-responsive elements |
| BR | Brassinosteroid |
| ET | Ethylene |
| FT | FLOWERING LOCUS T |
| AP1 | APETALA1 |
| LFY | LEAFY |
| FUL | FRUITFULL |
| TFL1 | TERMINAL FLOWER 1 |
| CAL | CAULIFLOWER |
| FLC | FLOWERING LOCUS C |
| GI | GIGANTEA |
| KAR | Karrikin |
| SA | Salicylic acid |
| PR | Pathogenesis-related |
| PAMP | Pathogen-associated molecular pattern |
| COR | Cold-responsive protein |
| PTI | PAMP-triggered immunity |
| MAPK | Mitogen-activated protein kinase |
| ROS | Reactive oxygen species |
| H2O2 | Hydrogen peroxide |
| XO | Xanthine oxidase |
| NAFLD | Nonalcoholic fatty liver disease |
| HAT | Hydrogen atom transfer |
| SET | Single electron transfer |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GPx | Glutathione peroxidase |
| CAD | Cardiovascular disease |
| ABGs | Anthocyanin biosynthetic genes |
| CHI | Chalcone isomerase |
| DFR | Flavanone-3-hydroxylase, dihydroflavonol 4-reductase |
| ANS | Anthocyanidin synthase |
| UFGT | UDP-glucose: flavonoid 3-glucosyltransferase |
References
- Lu, Z.; Wang, X.; Lin, X.; Mostafa, S.; Zou, H.; Wang, L.; Jin, B. Plant anthocyanins: Classification, biosynthesis, regulation, bioactivity, and health benefits. Plant Physiol. Biochem. 2024, 217, 109268. [Google Scholar] [CrossRef]
- Dangles, D. Anthocyanins as natural food colorings: The chemistry behind and challenges still ahead. J. Agric. Food Chem. 2024, 72, 12356–12372. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Tang, Q.; Li, Z.; Chen, N.; Luo, X.; Zhao, Q. Natural pigments derived from plants and microorganisms: Classification, biosynthesis, and applications. Plant Biotechnol. J. 2025, 23, 592–614. [Google Scholar] [CrossRef]
- Tang, R.; He, Y.; Fan, K. Recent advances in stability improvement of anthocyanins by efficient methods and its application in food intelligent packaging: A review. Food Biosci. 2023, 56, 103164. [Google Scholar] [CrossRef]
- Xie, J.; Xu, Y.; Chen, W. Discovery and characterization of novel anthocyanin-metal complex for blue food coloring. Food Chem. 2025, 485, 144485. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, C.; Li, Y.; Yuan, K.; Zhang, W.; Cai, D.; Peng, Z.; Hu, Y.; Sun, J.; Bai, W. Bioactivity and application of anthocyanins in skin protection and cosmetics: An extension as a functional pigment. Phytochem. Rev. 2023, 22, 1441–1467. [Google Scholar] [CrossRef]
- Belwal, T.; Singh, G.; Jeandet, P.; Pandey, A.; Giri, L.; Ramola, S.; Bhatt, D.I.; Venskutonis, P.R.; Georgiev, M.I.; Clément, C.; et al. Anthocyanins, multi-functional natural products of industrial relevance: Recent biotechnological advances. Biotechnol. Adv. 2020, 43, 107600. [Google Scholar] [CrossRef]
- Bian, R.; Yao, J.; Nie, Y.; Zhang, Y.; Wu, Z.; Zhang, J.; Zhang, Z. A novel function for the transcription factor sensitive to proton rhizotoxicity1 in promoting anthocyanin accumulation in strawberry. Plant Biotechnol. J. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Kowalczyk, T.; Muskała, M.; Merecz-Sadowska, A.; Sikora, J.; Picot, L.; Sitarek, P. Anti-inflammatory and anticancer effects of anthocyanins in in vitro and in vivo studies. Antioxidants 2024, 13, 1143. [Google Scholar] [CrossRef]
- Randeni, N.; Luo, J.; Xu, B. Critical review on anti-obesity effects of anthocyanins through PI3K/Akt signaling pathways. Nutrients 2025, 17, 1126. [Google Scholar] [CrossRef] [PubMed]
- Maaz, M.; Muhammad Sultan, T.; Noman, A.; Zafar, S.; Tariq, N.; Hussain, M.; Imran, M.; Mujtaba, A.; Yehuala, T.F.; Mostafa, E.M.; et al. Anthocyanins: From natural colorants to potent anticancer agents. Food Sci. Nutr. 2025, 13, e70232. [Google Scholar] [CrossRef]
- Chachar, Z.; Lai, R.; Ahmed, N.; Ma, L.; Chachar, S.; Paker, N.P.; Qi, Y. Cloned genes and genetic regulation of anthocyanin biosynthesis in maize: A comparative review. Front. Plant Sci. 2024, 15, 1310634. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Shi, Y.; Lv, S.; Zhu, C.; Xu, C.; Zhang, B.; Allan, A.; Grierson, D.; Chen, K.; et al. The R2R3 MYB Ruby1 is activated by two cold-responsive ethylene response factors, via the retrotransposon in its promoter, to positively regulate anthocyanin biosynthesis in citrus. Plant J. 2024, 119, 1433–1448. [Google Scholar] [CrossRef]
- Zhen, Z.; Cui, C.; Hong, L.; Jiang, C.; Yuhui, Z.; Guo, Y. The VvHY5-VvMYB24-VvMYBA1 transcription factor cascade regulates the biosynthesis of anthocyanin in grape. Hortic. Plant J. 2025, 11, 1066–1077. [Google Scholar] [CrossRef]
- Leng, X.; Li, C.; Wang, P.; Ren, Y.; Chen, J.; Liu, G.; Hakeem, A.; Liu, Y.; Shi, X.; Hou, T.; et al. The transcription factor VvMYB44-1 plays a role in reducing grapevine anthocyanin biosynthesis at high temperature. Plant Physiol. 2025, 197, kiae657. [Google Scholar] [CrossRef]
- He, W.; Liu, H.; Li, Y.; Wu, Z.; Xie, Y.; Yan, X.; Wang, X.; Miao, Q.; Chen, T.; Rahman, S.; et al. Genome-wide characterization of B-box gene family in Artemisia annua L. and its potential role in the regulation of artemisinin biosynthesis. Ind. Crops Prod. 2023, 199, 116736. [Google Scholar] [CrossRef]
- Fu, J.; Liao, L.; Jin, J.; Lu, Z.; Sun, J.; Song, L.; Huang, Y.; Liu, S.; Huang, D.; Xu, Y.; et al. A transcriptional cascade involving BBX22 and HY5 finely regulates both plant height and fruit pigmentation in citrus. J. Integr. Plant Biol. 2024, 66, 1752–1768. [Google Scholar] [CrossRef]
- Bai, S.; Tao, R.; Tang, Y.; Yin, L.; Ma, Y.; Ni, J.; Yan, X.; Yang, Q.; Wu, Z.; Zeng, Y.; et al. BBX16, a B-box protein, positively regulates light-induced anthocyanin accumulation by activating MYB10 in red pear. Plant Biotechnol. J. 2019, 17, 1985–1997. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lyu, S.; Yang, Z.; Xu, G.; Zhang, Y.; Liu, Y.; Jin, J.; Deng, S. Genome-wide characterization of tobacco B-BOX gene family identified two close members play contrast roles under cold stress. Environ. Exp. Bot. 2023, 216, 105533. [Google Scholar] [CrossRef]
- Khanna, R.; Kronmiller, B.; Maszle, D.; Coupland, G.; Holm, M.; Mizuno, T.; Shu-Wu, H. The Arabidopsis B-box zinc finger family. Plant Cell 2009, 21, 3416–3420. [Google Scholar] [CrossRef]
- Song, Z.; Bian, Y.; Liu, J.; Sun, Y.; Xu, D. B-box proteins: Pivotal players in light-mediated development in plants. J. Integr. Plant Biol. 2020, 62, 1293–1309. [Google Scholar] [CrossRef]
- Gangappa, S.N.; Botto, J. The BBX family of plant transcription factors. Trends Plant Sci. 2014, 19, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yoshida, H.; Chu, C.; Matsuoka, M.; Sun, J. Seed dormancy and germination in rice: Molecular regulatory mechanisms and breeding. Mol. Plant 2025, 18, 960–977. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, S.; Lin, R. The role of light in regulating seed dormancy and germination. J. Integr. Plant Biol. 2020, 62, 1310–1326. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Zheng, Y. Integration of ABA, GA, and light signaling in seed germination through the regulation of ABI5. Front. Plant Sci. 2022, 13, 1000803. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Datta, S. BBX30/miP1b and BBX31/miP1a form a positive feedback loop with ABI5 to regulate ABA-mediated postgermination seedling growth arrest. New Phytol. 2023, 238, 1908–1923. [Google Scholar] [CrossRef]
- Bai, M.; Sun, J.; Liu, J.; Ren, H.; Wang, K.; Wang, Y.; Wang, C.; Dehesh, K. The B-box protein BBX19 suppresses seed germination via induction of ABI5. Plant J. 2019, 99, 1192–1202. [Google Scholar] [CrossRef]
- Cao, J.; Liang, Y.; Yan, T.; Wang, X.; Zhou, H.; Chen, C.; Zhang, Y.; Zhang, B.; Zhang, S.; Liao, J.; et al. The photomorphogenic repressors BBX28 and BBX29 integrate light and brassinosteroid signaling to inhibit seedling development in Arabidopsis. Plant Cell 2022, 34, 2266–2285. [Google Scholar] [CrossRef]
- An, J.-P.; Zhang, C.-L.; Li, H.; Wang, G.-L.; You, C. Apple SINA E3 ligase MdSINA3 negatively mediates JA-triggered leaf senescence by ubiquitinating and degrading the MdBBX37 protein. Plant J. 2022, 111, 457–472. [Google Scholar] [CrossRef]
- Buelbuel, S.; Sakuraba, Y.; Sedaghatmehr, M.; Watanabe, M.; Hoefgen, R.; Balazadeh, S.; Mueller-Roeber, B. Arabidopsis BBX14 negatively regulates nitrogen starvation- and dark-induced leaf senescence. Plant J. 2023, 116, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, A.; Richter, R. Genetic and molecular basis of floral induction in Arabidopsis thaliana. J. Exp. Bot. 2020, 71, 2490–2504. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xuan, L.; Jiang, Y.; Yu, H. Regulation by FLOWERING LOCUS T and TERMINAL FLOWER 1 in flowering time and plant architecture. Small Struct. 2021, 2, 2000125. [Google Scholar] [CrossRef]
- Niu, F.; Rehmani, M.S.; Yan, J. Multilayered regulation and implication of flowering time in plants. Plant Physiol. Biochem. 2024, 213, 108842. [Google Scholar] [CrossRef]
- Yu, B.; Hu, Y.; Hou, X. More than flowering: CONSTANS plays multifaceted roles in plant development and stress responses. J. Integr. Plant Biol. 2025, 67, 425–439. [Google Scholar] [CrossRef]
- Romero, J.M.; Serrano-Bueno, G.; Camacho-Fernández, C.; Vicente, M.H.; Ruiz, M.T.; Perez-Castiñeira, J.R.; Pérez-Hormaeche, J.; Nogueira, F.T.S.; Valverde, F. CONSTANS, a hub for all seasons: How photoperiod pervades plant physiology regulatory circuits. Plant Cell 2024, 36, 2086–2102. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.-D.; Lin, Y.; Ren, Q.-P.; Wang, Y.-Y.; Xiong, F.; Wang, X.-L. RNA splicing of FLC modulates the transition to flowering. Front. Plant Sci. 2019, 10, 1625. [Google Scholar] [CrossRef]
- Michaels, S.; Amasino, R. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 1999, 11, 949–956. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhang, X.; Wei, Y.; He, Y.; Zhang, X.; Li, Z.; Wang, C.; Zhang, H. AcBBX5, a B-box transcription factor from pineapple, regulates flowering time and floral organ development in plants. Front. Plant Sci. 2022, 13, 1060276. [Google Scholar] [CrossRef]
- Xu, X.; Xu, J.; Yuan, C.; Chen, Q.; Liu, Q.; Wang, X.; Qin, C. BBX17 interacts with CO and negatively regulates flowering time in Arabidopsis thaliana. Plant Cell Physiol. 2022, 63, 401–409. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, C.; Xu, Y.; Wei, Q.; Imtiaz, M.; Lan, H.; Gao, S.; Cheng, L.; Wang, M.; Fei, Z.; et al. A zinc finger protein regulates flowering time and abiotic stress tolerance in chrysanthemum by modulating gibberellin biosynthesis. Plant Cell 2014, 26, 2038–2054. [Google Scholar] [CrossRef]
- Cheng, H.; Yu, Y.; Zhai, Y.; Wang, L.; Wang, L.; Chen, S.; Chen, F.; Jiang, J. An ethylene-responsive transcription factor and a B-box protein coordinate vegetative growth and photoperiodic flowering in chrysanthemum. Plant Cell Environ. 2023, 46, 440–450. [Google Scholar] [CrossRef]
- Riboni, M.; Test, A.R.; Galbiati, M.; Tonelli, C.; Conti, L. ABA-dependent control of GIGANTEA signalling enables drought escape via up-regulation of FLOWERING LOCUS T in Arabidopsis thaliana. J. Exp. Bot. 2016, 67, 6309–6322. [Google Scholar] [CrossRef]
- He, W.; Liu, H.; Wu, Z.; Miao, Q.; Hu, X.; Yan, X.; Wen, H.; Zhang, Y.; Fu, X.; Ren, L.; et al. The AaBBX21-AaHY5 module mediates light-regulated artemisinin biosynthesis in Artemisia annua L. J. Integr. Plant Biol. 2024, 66, 1735–1751. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, H.; Li, X.; Bai, Y.; Peng, K.; Wang, Z.; Dai, Z.; Bian, X.; Zhang, Q.; Jia, L.; et al. The B-box transcription factor IbBBX29 regulates leaf development and flavonoid biosynthesis in sweet potato. Plant Physiol. 2023, 191, 496–514. [Google Scholar] [CrossRef]
- Wang, Y.; Zhai, Z.; Sun, Y.; Feng, C.; Peng, X.; Zhang, X.; Xiao, Y.; Zhou, X.; Wang, W.; Jiao, J.; et al. Genome-wide identification of the B-BOX genes that respond to multiple ripening related signals in sweet cherry fruit. Int. J. Mol. Sci. 2021, 22, 1622. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Lin, R.; Tang, M.; Wang, L.; Fan, P.; Xia, X.; Yu, J.; Zhou, Y. SlMPK1- and SlMPK2-mediated SlBBX17 phosphorylation positively regulates CBF-dependent cold tolerance in tomato. New Phytol. 2023, 239, 1887–1902. [Google Scholar] [CrossRef]
- Wang, S.; Shen, Y.; Deng, D.; Guo, L.; Zhang, Y.; Nie, Y.; Du, Y.; Zhao, X.; Ye, X.; Huang, J.; et al. Orthogroup and phylotranscriptomic analyses identify transcription factors involved in the plant cold response: A case study of Arabidopsis BBX29. Plant Commun. 2023, 4, 100684. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, C.; Xu, M.; Wang, G.; Xu, L.; Wu, Y. Overexpression of Ginkgo BBX25 enhances salt tolerance in transgenic Populus. Plant Physiol. Biochem. 2021, 167, 946–954. [Google Scholar] [CrossRef]
- Tang, H.; Yuan, C.; Shi, H.; Liu, F.; Shan, S.; Wang, Z.; Sun, Q.; Sun, J. Genome-wide identification of peanut B-boxes and functional characterization of AhBBX6 in salt and drought stresses. Plants 2024, 13, 955. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, H.; Cheng, P.; Wang, C.; Li, J.; Liu, Y.; Song, A.; Chen, S.; Chen, F.; Wang, L.; et al. The BBX gene CmBBX22 negatively regulates drought stress tolerance in chrysanthemum. Hortic. Res. 2022, 9, uhac181. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Li, X.; Gao, X.; Dai, Z.; Cui, Y.; Zhi, Y.; Liu, Q.; Zhai, H.; Gao, S.; et al. The IbBBX24-IbTOE3-IbPRX17 module enhances abiotic stress tolerance by scavenging reactive oxygen species in sweet potato. New Phytol. 2022, 233, 1133–1152. [Google Scholar] [CrossRef]
- Lin, F.; Lin, F.; Jiang, Y.; Li, J.; Yan, T.; Fan, L.; Liang, J.; Chen, Z.; Xu, D.; Deng, X.W.; et al. B-BOX DOMAIN PROTEIN28 negatively regulates photomorphogenesis by repressing the activity of transcription factor HY5 and undergoes COP1-mediated degradation. Plant Cell 2018, 30, 2006–2019. [Google Scholar] [CrossRef]
- Bursch, K.; Niemann, E.T.; David Nelson, C.; Johansson, H. Karrikins control seedling photomorphogenesis and anthocyanin biosynthesis through a HY5-BBX transcriptional module. Plant J. 2021, 107, 1346–1362. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Y.; Yu, T.; Li, J.; Qiu, X.; Zhu, C.; Liu, J.; Dang, F.; Yang, Y. Characterization of the B-BOX gene family in pepper and the role of CaBBX14 in defense response against Phytophthora capsici infection. Int. J. Biol. Macromol. 2023, 237, 124071. [Google Scholar] [CrossRef]
- Luo, S.; Tetteh, C.; Song, Z.; Zhang, C.; Jin, P.; Hao, X.; Liu, Y.; Ge, S.; Chen, J.; Ye, K.; et al. Positive regulation of BBX11 by NAC053 confers stomatal and apoplastic immunity against bacterial infection in Arabidopsis. New Phytol. 2025, 246, 1816–1833. [Google Scholar] [CrossRef]
- Xie, S.; Shi, B.; Miao, M.; Zhao, C.; Bai, R.; Yan, F.; Lei, C. A B-Box (BBX) transcription factor from cucumber, CsCOL9, positively regulates resistance of host plant to Bemisia tabaci. Int. J. Mol. Sci. 2025, 26, 324. [Google Scholar] [CrossRef]
- Malakar, B.C.; Singh, S.; Garhwal, V.; Chandramohan, R.; Upadhyaya, G.; Sethi, V.; Sreeramaiah Gangappa, N. BBX24 and BBX25 antagonize the function of thermosensor ELF3 to promote PIF4-mediated thermomorphogenesis in Arabidopsis. Plant Commun. 2025, 6, 101391. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, S.; Song, S.; Jiang, Y.; Han, J.; Lu, S.; Li, L.; Liu, J. Two B-box domain proteins, BBX18 and BBX23, interact with ELF3 and regulate thermomorphogenesis in Arabidopsis. Cell Rep. 2018, 25, 1718–1728.e4. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, H.; Jin, Z.; Ding, Y.; Guo, M. Comprehensive characterization of B-box zinc finger genes in Citrullus lanatus and their response to hormone and abiotic stresses. Plants 2023, 12, 2634. [Google Scholar] [CrossRef]
- Shi, J.; Tang, Y.; Li, H.; Xing, H. The BBX family and their response to abiotic stress in ginger (Zingiber officinale Roscoe). BMC Genom. 2025, 26, 548. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, H.; Huang, J.; Tao-Shi, X.; Meng, Z.; Chen, Q.; Deng, J. Genome-wide analysis of BBX gene family in Tartary buckwheat (Fagopyrum tataricum). PeerJ 2021, 9, e11939. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Sun, H.; Liu, S.; He, Y.; Zhao, S.; Wang, J.; Wang, T.; Zhang, J.; Gao, J.; Yang, Q.; et al. Identification of BBX gene family and its function in the regulation of microtuber formation in yam. BMC Genom. 2023, 24, 354. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Li, B.; Wu, H.; Sha, Y.; Qin, L.; Chen, X.; Liu, Y.; Tang, H.; Yang, L. The function of BBX gene family under multiple stresses in Nicotiana tabacum. Genes 2022, 13, 1841. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Chen, J.; Huang, B.; Huang, Z.; Zhang, Z.-J. The BBX gene family in Moso bamboo (Phyllostachys edulis): Identification, characterization and expression profiles. BMC Genom. 2021, 22, 533. [Google Scholar] [CrossRef]
- Yin, L.; Wu, R.; An, R.; Feng, Y.; Qiu, Y.; Zhang, M. Genome-wide identification, molecular evolution and expression analysis of the B-box gene family in mung bean (Vigna radiata L.). BMC Plant Biol. 2024, 24, 532. [Google Scholar] [CrossRef]
- Li, S.; Guo, S.; Gao, X.; Wang, X.; Liu, Y.; Wang, J.; Li, X.; Zhang, J.; Fu, B. Genome-wide identification of B-box zinc finger (BBX) gene family in Medicago sativa and their roles in abiotic stress responses. BMC Genom. 2024, 25, 110. [Google Scholar] [CrossRef]
- Cao, Y.; Meng, D.; Han, Y.; Chen, T.; Jiao, C.; Chen, Y.; Jin, Q.; Cai, Y. Comparative analysis of B-BOX genes and their expression pattern analysis under various treatments in Dendrobium officinale. BMC Plant Biol. 2019, 19, 245. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, Y.; Li, X.; Wang, G.; Zhou, Q.; Chen, Q.; Li, J.; Wang, X.; Tang, H. An evolutionary analysis of B-box transcription factors in strawberry reveals the role of FaBBx28c1 in the regulation of flowering time. Int. J. Mol. Sci. 2021, 22, 11766. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Bao, G.; Shi, T.; Zhai, L.; Bian, S.; Li, X. Genome-wide identification of BBX gene family and their expression patterns under salt stress in soybean. BMC Genom. 2022, 23, 820. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, J.; Hao, J.; Bao, X.; Kuang, L.; Zhang, D.; Zong, C. Identification of the BBX gene family in blueberry at different chromosome ploidy levels and fruit development and response under stress. BMC Genom. 2025, 26, 100. [Google Scholar] [CrossRef]
- Chen, X.; Niu, M.; Wu, X.; Peng, Y.; Zheng, R.; Cheng, M.; Zhao, K.; Zhou, Y.; Peng, D. BBX genes of Cymbidium ensifolium exhibited intense response to blue light in meristem induction through artificial control. Plants 2024, 13, 2375. [Google Scholar] [CrossRef] [PubMed]
- Obel, H.O.; Cheng, C.; Li, Y.; Tian, Z.; Njogu, M.; Li, J.; Lou, Q.; Yu, X.; Yang, Z.; Ogweno, J.; et al. Genome-wide identification of the B-box gene family and expression analysis suggests their potential role in photoperiod-mediated β-carotene accumulation in the endocarp of cucumber (Cucumis sativus L.) fruit. Genes 2022, 13, 658. [Google Scholar] [CrossRef]
- Nian, L.; Zhang, X.; Liu, X.; Li, X.; Liu, X.; Yang, Y.; Haider, F.; Zhu, X.; Ma, B.; Mao, Z.; et al. Characterization of B-box family genes and their expression profiles under abiotic stresses in Melilotus albus. Front. Plant Sci. 2022, 13, 990929. [Google Scholar] [CrossRef]
- Song, H.; Ding, G.; Zhao, C.; Li, Y. Genome-wide identification of B-box gene family and expression analysis suggest its roles in responses to Cercospora leaf spot in sugar beet (Beta vulgaris L.). Genes 2023, 14, 1248. [Google Scholar] [CrossRef]
- Feng, Z.; Li, M.; Li, Y.; Yang, X.; Wei, H.; Fu, X.; Ma, L.; Lu, J.; Wang, H.; Yu, S. Comprehensive identification and expression analysis of B-box genes in cotton. BMC Genom. 2021, 22, 439. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Lei, S.; Li, J.; Tian, B.; Li, C.; Cao, J.; Lu, J.; Ma, C.; Chang, C.; Zhang, H. In silico analysis of the wheat BBX gene family and identification of candidate genes for seed dormancy and germination. BMC Plant Biol. 2024, 24, 334. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tong, Y.; Ye, J.; Zhang, C.; Li, B.; Hu, S.; Xue, X.; Tian, Q.; Wang, Y.; Li, L.; et al. Genome-wide characterization of B-box gene family in Salvia miltiorrhiza. Int. J. Mol. Sci. 2023, 24, 2146. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Liu, Q.; Zhang, T.; Chong, X.; Yuan, H. Genome-wide identification and expression analysis of BBX transcription factors in Iris germanica L. Int. J. Mol. Sci. 2021, 22, 8793. [Google Scholar] [CrossRef]
- Shi, G.; Ai, K.; Yan, X.; Zhou, Z.; Cai, F.; Bao, M.; Zhang, J. Genome-wide analysis of the BBX genes in Platanus × acerifolia and their relationship with flowering and/or dormancy. Int. J. Mol. Sci. 2023, 24, 8576. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, G.; Chen, J.; Ying, Y.; Yao, L.; Li, X.; da Silva, J.A.T.; Yu, Z. Role of Rubus chingii BBX gene family in anthocyanin accumulation during fruit ripening. Front. Plant Sci. 2024, 15, 1427359. [Google Scholar] [CrossRef]
- Xie, J.; Cui, H.; Xu, Y.; Xie, L.; Chen, W. Delphinidin-3-O-sambubioside: A novel xanthine oxidase inhibitor identified from natural anthocyanins. Food Qual. Saf. 2021, 5, fyaa038. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, H.; Luo, C.; Hu, W.; Weng, T.; Shuang, F. Pelargonidin ameliorates inflammatory response and cartilage degeneration in osteoarthritis via suppressing the NF-κB pathway. Arch. Biochem. Biophys. 2023, 743, 109668. [Google Scholar] [CrossRef]
- Cheng, Z.; Si, X.; Tan, H.; Zang, Z.; Tian, J.; Shu, C.; Sun, X.; Li, Z.; Jiang, Q.; Meng, X.; et al. Cyanidin-3-O-glucoside and its phenolic metabolites ameliorate intestinal diseases via modulating intestinal mucosal immune system: Potential mechanisms and therapeutic strategies. Crit. Rev. Food Sci. Nutr. 2023, 63, 1629–1647. [Google Scholar] [CrossRef] [PubMed]
- Kongthitilerd, P.; Barras, E.D.; Rong, W.; Thibodeaux, A.; Rigdon, M.; Yao, S.; Adisakwattana, S.; Suantawee, T.; Cheng, H. Cyanidin inhibits adipogenesis in 3T3-L1 preadipocytes by activating the PLC-IP3 pathway. Biomed. Pharmacother. 2023, 162, 114677. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Huo, J.; Liu, J.; Yu, J.; Zhou, J.; Sun, C.; Wang, Y.; Leng, F. Anthocyanins distribution, transcriptional regulation, epigenetic and post-translational modification in fruits. Food Chem. 2023, 411, 135540. [Google Scholar] [CrossRef]
- Cai, X.Y.; Yang, C.; Shao, L.; Zhu, H.M.; Wang, Y.X.; Huang, X.; Wang, S.; Hong, L. Targeting NOX4 by petunidin improves anoxia/reoxygenation-induced myocardium injury. Eur. J. Pharmacol. 2020, 888, 173414. [Google Scholar] [CrossRef]
- Lee, G.-H.; Hoang, T.-H.; Jung, E.-S.; Jung, S.-J.; Han, S.-K.; Chung, M.-J.; Chae, S.-W.; Chae, H.-J. Anthocyanins attenuate endothelial dysfunction through regulation of uncoupling of nitric oxide synthase in aged rats. Aging Cell 2020, 19, e13279. [Google Scholar] [CrossRef]
- Chung, I.-C.; Yuan, S.-N.; OuYang, C.-N.; Hu, S.-I.; Lin, H.-C.; Huang, K.-Y.; Lin, W.-N.; Chuang, Y.-T.; Chen, Y.-J.; Ojcius, D.M.; et al. EFLA 945 restricts AIM2 inflammasome activation by preventing DNA entry for psoriasis treatment. Cytokine 2020, 127, 154951. [Google Scholar] [CrossRef]
- Shi, L.; Li, X.; Fu, Y.; Li, C. Environmental stimuli and phytohormones in anthocyanin biosynthesis: A comprehensive review. Int. J. Mol. Sci. 2023, 24, 16415. [Google Scholar] [CrossRef]
- Zhao, S.; Blum, J.A.; Ma, F.; Wang, Y.; Borejsza-Wysocka, E.; Ma, F.; Cheng, L.; Li, P. Anthocyanin accumulation provides protection against high light stress while reducing photosynthesis in apple leaves. Int. J. Mol. Sci. 2022, 23, 12616. [Google Scholar] [CrossRef]
- Li, Z.; Ahammed, G. Plant stress response and adaptation via anthocyanins: A review. Plant Stress 2023, 10, 100230. [Google Scholar] [CrossRef]
- Fallah, S.; Yusefi-Tanha, E.; Peralta-Videa, J. Interaction of nanoparticles and reactive oxygen species and their impact on macromolecules and plant production. Plant Nano Biol. 2024, 10, 100105. [Google Scholar] [CrossRef]
- Naing, A.H.; Kim, C. Abiotic stress-induced anthocyanins in plants: Their role in tolerance to abiotic stresses. Physiol. Plant. 2021, 172, 1711–1723. [Google Scholar] [CrossRef]
- Yu, D.; Wei, W.; Fan, Z.; Chen, J.; You, Y.; Huang, W.; Zhan, J. VabHLH137 promotes proanthocyanidin and anthocyanin biosynthesis and enhances resistance to Colletotrichum gloeosporioides in grapevine. Hortic. Res. 2022, 10, uhac261. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, Y.; Du, H.; Tian, J.; Zhu, F.; Zhang, J.; Zhang, Q.; Wang, X.; Ge, L. Anthocyanins promote the abundance of endophytic lactic acid bacteria by reducing ROS in Medicago truncatula. Plant J. 2025, 122, e70127. [Google Scholar] [CrossRef]
- Zeng, Z.; Liao, Y.; Wang, J.; Liang, X.; Duan, L.; Huang, Y.; Han, Z.; Lin, K.; Hu, H.; Ye, K.; et al. Combined transcriptomic, metabolomic and physiological analysis reveals the key role of nitrogen, but not phosphate and potassium in regulating anthocyanin biosynthesis induced by nutrient deficiency in Eucalyptus. Int. J. Biol. Macromol. 2024, 283 Pt 1, 137564. [Google Scholar] [CrossRef]
- Li, H.; He, K.; Zhang, Z.; Hu, Y. Molecular mechanism of phosphorous signaling inducing anthocyanin accumulation in Arabidopsis. Plant Physiol. Biochem. 2023, 196, 121–129. [Google Scholar] [CrossRef]
- Qi, Q.; Chu, M.; Yu, X.; Xie, Y.; Li, Y.; Du, Y.; Liu, X.; Zhang, Z.; Shi, J.; Yan, N. Anthocyanins and proanthocyanidins: Chemical structures, food sources, bioactivities, and product development. Food Rev. Int. 2022, 39, 4581–4609. [Google Scholar] [CrossRef]
- Garcia, C.; Blesso, C. Antioxidant properties of anthocyanins and their mechanism of action in atherosclerosis. Free Radic. Biol. Med. 2021, 172, 152–166. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Antioxidant activity of anthocyanins and anthocyanidins: A critical review. Int. J. Mol. Sci. 2024, 25, 12001. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, D.; Shi, L.; Liu, X.; Zhang, Y.; Tong, C.; Song, D.; Hou, M. Blueberry anthocyanins-enriched extracts attenuate cyclophosphamide-induced cardiac injury. PLoS ONE 2015, 10, e0127813. [Google Scholar] [CrossRef] [PubMed]
- Neyestani, T.; Yari, Z.; Rasekhi, H.; Nikooyeh, B. How effective are anthocyanins on healthy modification of cardiometabolic risk factors: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2023, 15, 106. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Alsharairi, N.A. Insights into the mechanisms of action of proanthocyanidins and anthocyanins in the treatment of nicotine-induced non-small cell lung cancer. Int. J. Mol. Sci. 2022, 23, 7905. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, R.D.P.; da Machado, F.A.P. The preventive and therapeutic effects of anthocyanins on colorectal cancer: A comprehensive review based on up-to-date experimental studies. Food Res. Int. 2023, 170, 113028. [Google Scholar] [CrossRef]
- Wang, B.; Tang, X.; Mao, B.; Zhang, Q.; Tian, F.; Zhao, J.; Cui, S.; Chen, W. Anti-aging effects and mechanisms of anthocyanins and their intestinal microflora metabolites. Crit. Rev. Food Sci. Nutr. 2024, 64, 2358–2374. [Google Scholar] [CrossRef]
- Hu, X.; Yang, Y.; Tang, S.; Chen, Q.; Zhang, M.; Ma, J.; Qin, J.; Yu, H. Anti-aging effects of anthocyanin extracts of Sambucus canadensis caused by targeting mitochondrial-induced oxidative stress. Int. J. Mol. Sci. 2023, 24, 1528. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, G.; Zhang, X.; Xu, D.; Gao, J.; Fan, J.; Zhou, Z. Anthocyanins from black chokeberry (Aronia melanocarpa Elliot) delayed aging-related degenerative changes of brain. J. Agric. Food Chem. 2017, 65, 5973–5984. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, X.; Han, L.; Bian, J.; He, C.; El-Omar, E.; Gong, L.; Wang, M. The potential roles of dietary anthocyanins in inhibiting vascular endothelial cell senescence and preventing cardiovascular diseases. Nutrients 2022, 14, 2836. [Google Scholar] [CrossRef]
- Chen, Y.; Song, G.; Zhao, C.; Qi, W.; Wang, Y. Interactions between anthocyanins and gut microbiota in promoting healthy aging. J. Future Foods 2025, 5, 229–238. [Google Scholar] [CrossRef]
- Kozłowska, A.; Nitsch-Osuch, A. Anthocyanins and type 2 diabetes: An update of human study and clinical trial. Nutrients 2024, 16, 1674. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Guo, Y.; Liu, M.; Chen, X.; Xiao, X.; Wang, S.; Gong, P.; Ma, Y.; Chen, F. Structure and function of blueberry anthocyanins: A review of recent advances. J. Funct. Foods 2022, 88, 104864. [Google Scholar] [CrossRef]
- Franco-Sebastián, D.; Alaniz-Monreal, S.; Rabadán-Chávez, G.; Vázquez-Manjarrez, N.; Hernández-Ortega, M.; Gutiérrez-Salmeán, G. Anthocyanins: Potential therapeutic approaches towards obesity and diabetes mellitus type 2. Molecules 2023, 28, 1237. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.N.; Bickford, P. Anthocyanins and their metabolites as therapeutic agents for neurodegenerative disease. Antioxidants 2019, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Han, T.; Lyu, L.; Li, W.; Wu, W. Research progress in understanding the biosynthesis and regulation of plant anthocyanins. Sci. Hortic. 2023, 321, 112374. [Google Scholar] [CrossRef]
- Hu, Y.; Gong, Z.; Yan, Y.; Zhang, J.; Shao, A.; Li, H.; Wang, P.; Zhang, S.; Cheng, C.; Zhang, J. ChBBX6 and ChBBX18 are positive regulators of anthocyanins biosynthesis and carotenoids degradation in Cerasus humilis. Int. J. Biol. Macromol. 2024, 282 Pt 4, 137195. [Google Scholar] [CrossRef]
- Luo, D.; Xiong, C.; Lin, A.; Zhang, C.; Sun, W.; Zhang, J.; Yang, C.; Lu, Y.; Li, H.; Ye, Z.; et al. SlBBX20 interacts with the COP9 signalosome subunit SlCSN5-2 to regulate anthocyanin biosynthesis by activating SlDFR expression in tomato. Hortic. Res. 2021, 8, 163. [Google Scholar] [CrossRef]
- Pan, C.; Liao, Y.; Shi, B.; Zhang, M.; Zhou, Y.; Wu, J.; Wu, H.; Qian, M.; Bai, S.; Teng, Y.; et al. Blue light-induced MiBBX24 and MiBBX27 simultaneously promote peel anthocyanin and flesh carotenoid biosynthesis in mango. Plant Physiol. Biochem. 2025, 219, 109315. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, H.; Qu, D.; Zhen-Zhu, Z.; Ya-Yang, Z.; Zhao, Z. The MdBBX22-miR858-MdMYB9/11/12 module regulates proanthocyanidin biosynthesis in apple peel. Plant Biotechnol. J. 2022, 20, 1683–1700. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Tian, S.; Hao, W.; Du, L. Two B-Box proteins, MaBBX20 and MaBBX51, coordinate light-induced anthocyanin biosynthesis in grape hyacinth. Int. J. Mol. Sci. 2022, 23, 5678. [Google Scholar] [CrossRef]
- Yang, G.; Sun, M.; Brewer, L.; Tang, Z.; Nieuwenhuizen, N.; Cooney, J.; Xu, S.; Sheng, J.; Andre, C.M.; Xue, C.; et al. Allelic variation of BBX24 is a dominant determinant controlling red coloration and dwarfism in pear. Plant Biotechnol. J. 2024, 22, 1468–1490. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ou, C.; Liu, X.; Wang, F.; Zhang, Y.; Qi, L.; Jiang, S.; Li, H. Plant U-box E3 ligase PpPUB59 regulates anthocyanin accumulation by ubiquitinating PpBBX24 in ‘Zaosu’ pear and its red bud mutation. Plant Physiol. Biochem. 2025, 219, 109354. [Google Scholar] [CrossRef]
- Liu, W.; Mu, H.; Yuan, L.; Li, Y.; Li, Y.; Li, S.; Ren, C.; Duan, W.; Fan, P.; Dai, Z.; et al. VvBBX44 and VvMYBA1 form a regulatory feedback loop to balance anthocyanin biosynthesis in grape. Hortic. Res. 2023, 10, uhad176. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Y.; Sun, Y.; Zhang, X.; Du, B.; Turupu, M.; Qi-Yao, X.; Gai, S.; Tong, S.; Huang, J.; et al. Two B-box proteins, PavBBX6/9, positively regulate light-induced anthocyanin accumulation in sweet cherry. Plant Physiol. 2023, 192, 2030–2048. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Sun, Y.; Zhu, Z.; Ni, N.; Sun, S.; Nie, M.; Du, W.; Irfan, M.; Chen, L.; Zhang, L. Transcription factors LvBBX24 and LvbZIP44 coordinated anthocyanin accumulation in response to light in lily petals. Hortic. Res. 2024, 11, uhae211. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Pei, J.; Yan, X.; Cui, X.; Tsuruta, M.; Liu, Y.; Lian, C. A poplar B-box protein PtrBBX23 modulates the accumulation of anthocyanins and proanthocyanidins in response to high light. Plant Cell Environ. 2021, 44, 3015–3033. [Google Scholar] [CrossRef]
- Zhou, L.; Peng, J.; Chen, C.; Wang, Y.; Wang, Y.; Li, Y.; Song, A.; Jiang, J.; Chen, S.; Chen, F. CmBBX28-CmMYB9a module regulates petal anthocyanin accumulation in response to light in chrysanthemum. Plant Cell Environ. 2025, 48, 3750–3765. [Google Scholar] [CrossRef]
- Deng, F.; Zhang, Y.; Chen, Y.; Li, Y.; Li, L.; Lei, Y.; Li, Z.; Pi, B.; Chen, J.; Qiao, Z. Genome-wide identification and expression analysis of the BBX gene family in Lagerstroemia indica grown under light stress. Int. J. Biol. Macromol. 2025, 297, 139899. [Google Scholar] [CrossRef]
- Deng, F.; Zhang, Y.; Chen, Y.; Li, Y.; Li, L.; Lei, Y.; Li, Z.; Pi, B.; Chen, J.; Qiao, Z. Evaluation of light irradiation on anthocyanins and energy metabolism of grape (Vitis vinifera L.) during storage. Food Chem. 2024, 431, 137141. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhao, H.; Xu, J.; Zheng, P.; Liu, S.; Sun, B. CsHY5 regulates light-induced anthocyanin accumulation in Camellia sinensis. Int. J. Mol. Sci. 2025, 26, 3253. [Google Scholar] [CrossRef] [PubMed]
- An, J.-P.; Wang, X.-F.; Espley, R.V.; Wang, K.-L.; Bi, S.-Q.; You, C.-X.; Hao, Y.-J. An apple B-Box protein MdBBX37 modulates anthocyanin biosynthesis and hypocotyl elongation synergistically with MdMYBs and MdHY5. Plant Cell Physiol. 2020, 61, 130–143. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).