Effect of Preharvest Aluminum-Coated Paper Bagging on Postharvest Quality, Storability, and Browning Behavior of ‘Afrata Volou’ Quince
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Trees
2.2. Treatments
2.3. Measurements
2.3.1. Field Measurements Before Harvest
2.3.2. Fruit Quality Measurements
2.3.3. Flesh Color Changes with Time After Cutting
2.3.4. Total Phenolic Content and Total Antioxidant Activity
2.3.5. Total Tannin Content
2.3.6. Bruising
2.4. Statistical Analysis
3. Results and Discussion
3.1. Fruit Temperature
3.2. Fruit Quality
3.3. Fruit Nutritional Quality
3.3.1. Fruit TPC and TTC
3.3.2. Fruit TAA
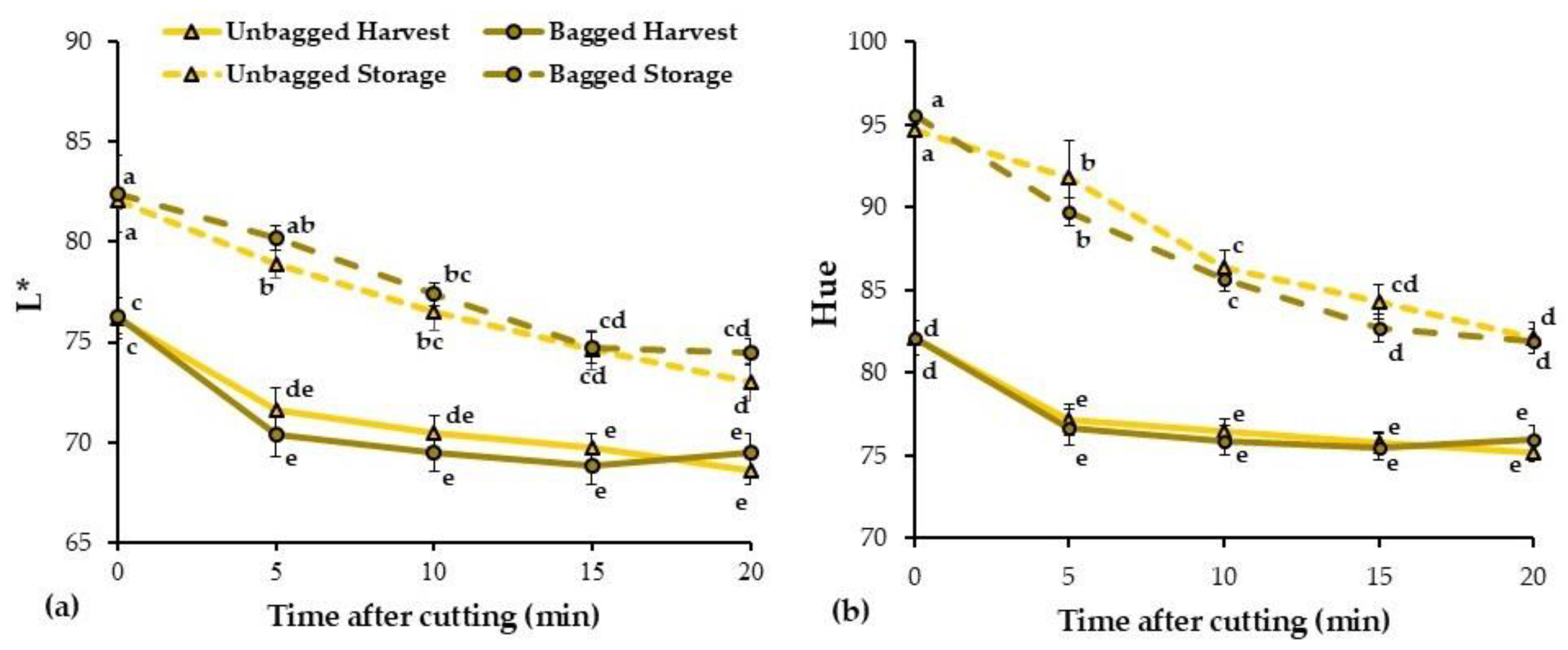
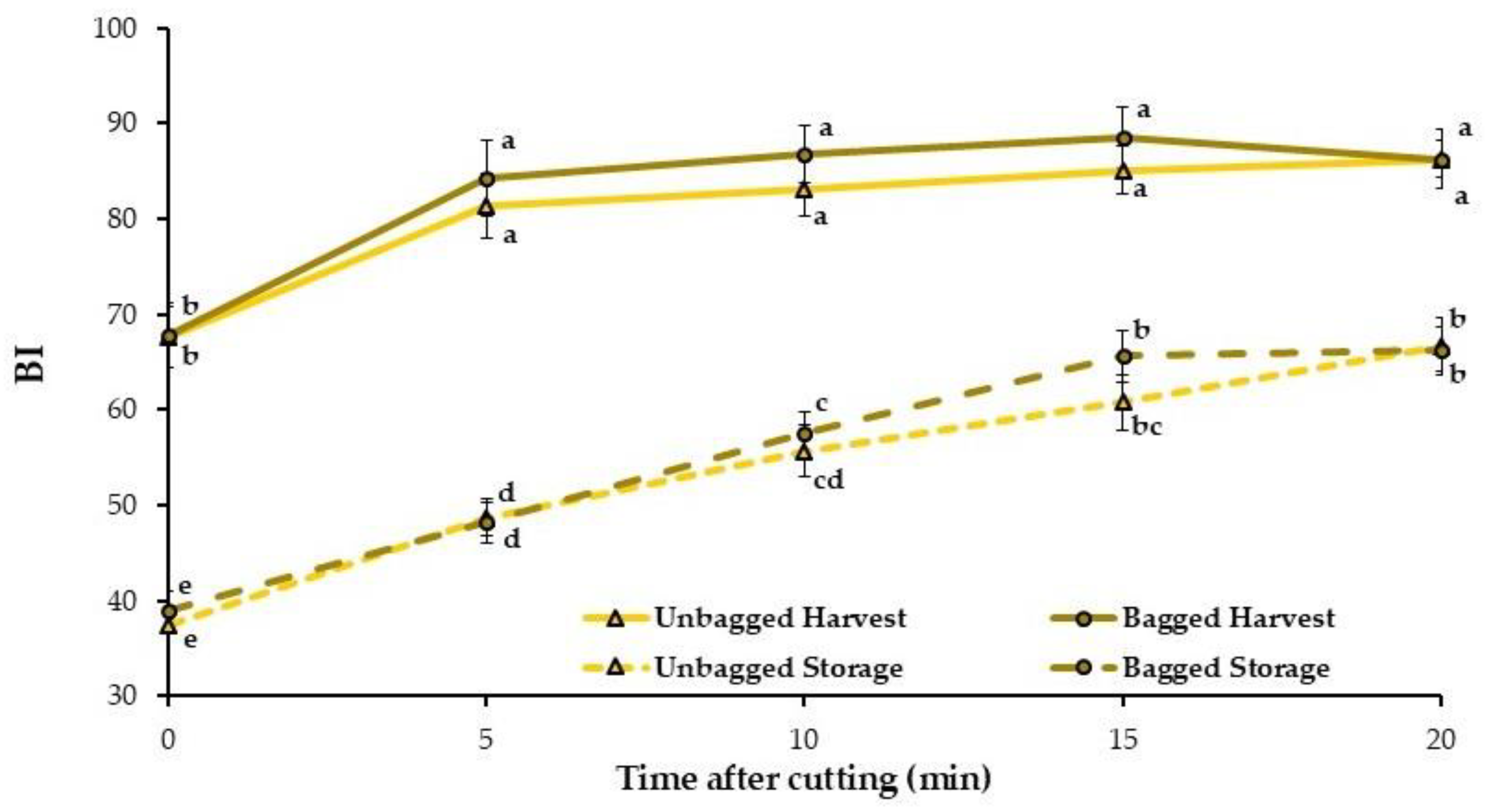
3.4. Flesh Color Changes with Time After Cutting
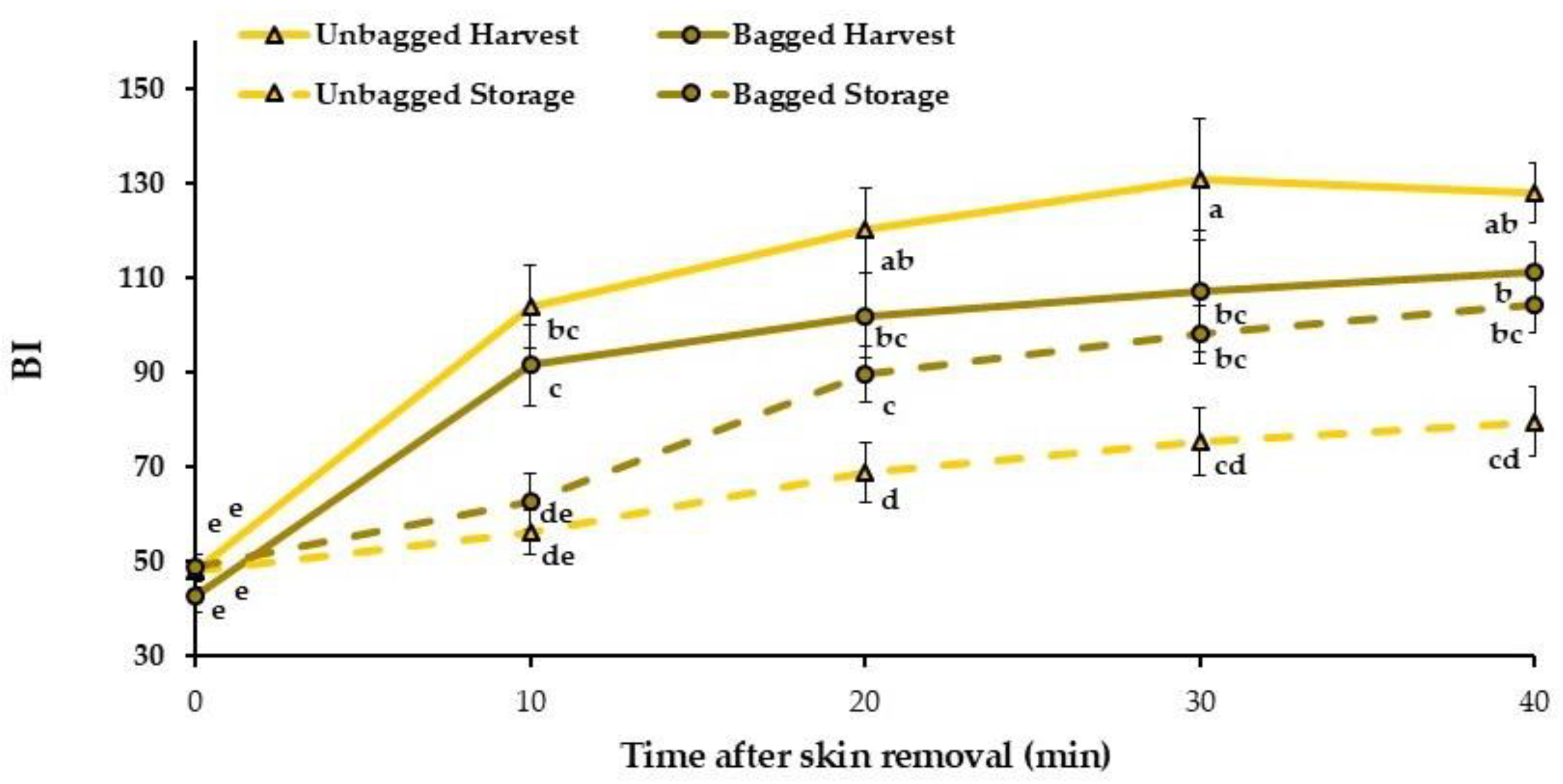
3.5. Bruised Fruit Flesh Color Changes with Time After Skin Removal
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hudina, M.; Stampar, F. Effect of fruit bagging on quality of ‘Conference’ pear (Pyrus communis L.). Eur. J. Hortic. Sci. 2011, 76, 176–181. [Google Scholar] [CrossRef]
- Feng, F.; Li, M.; Maa, F.; Cheng, L. The effects of bagging and debagging on external fruit quality, metabolites, and the expression of anthocyanin biosynthetic genes in ‘Jonagold’ apple (Malus domestica Borkh.). Sci. Hortic.-Amst. 2014, 165, 123–131. [Google Scholar] [CrossRef]
- Ali, M.M.; Anwar, R.; Yousef, A.F.; Li, B.; Luvisi, A.; De Bellis, L.; Aprile, A.; Chen, F. Influence of bagging on the development and quality of fruits. Plants 2021, 10, 358. [Google Scholar] [CrossRef]
- Sharma, R.R.; Reddy, S.V.R.; Jhalegar, M.J. Pre-harvest fruit bagging: A useful approach for plant protection and improved post-harvest fruit quality—A review. J. Hortic. Sci. Biotech. 2014, 89, 101–113. [Google Scholar] [CrossRef]
- Bastías, R.M.; Corelli-Grappadelli, L. Light quality management in fruit orchards: Physiological and technological aspects. Chil. J. Agr. Res. 2012, 72, 574–581. [Google Scholar] [CrossRef]
- Schrader, L.; Zhang, J.; Sun, J. Environmental stresses that cause sunburn of apple. Acta Hortic. 2003, 618, 397–405. [Google Scholar] [CrossRef]
- Amarante, C.; Banks, N.H.; Max, S. Effect of preharvest bagging on fruit quality and postharvest physiology of pears (Pyrus communis). N. Z. J. Crop Hort. 2002, 30, 99–107. [Google Scholar] [CrossRef]
- Buthelezi, N.M.D.; Mafeo, T.P.; Mathaba, N. Preharvest bagging as an alternative technique for enhancing fruit quality: A review. HortTechnology 2021, 31, 4–13. [Google Scholar] [CrossRef]
- Fan, X.; Mattheis, J.P. Bagging ‘Fuji’ apples during fruit development affects color development and storage quality. HortScience 1998, 33, 1235–1238. [Google Scholar] [CrossRef]
- Hudina, M.; Stampar, F.; Orazem, P.; Petkovsek, M.M.; Veberic, R. Phenolic compounds profile, carbohydrates and external fruit quality of the ‘Concorde’ pear (Pyrus communis L.) after bagging. Can. J. Plant Sci. 2012, 92, 67–75. [Google Scholar] [CrossRef]
- Zhi, C.; Ali, M.M.; Zhang, J.; Shi, M.; Ma, S.; Chen, F. Effect of Paper and Aluminum Bagging on Fruit Quality of Loquat (Eriobotrya japonica Lindl.). Plants 2021, 10, 2704. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.A.; Rahman, M.H.; Islam, M.T.; Hossain, M.S. Effect of pre-harvest fruit bagging on biotic stresses and postharvest quality of banana. J. Appl. Hortic. 2023, 25, 194–198. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, Y.; Wang, H.; Liu, X.; Zhao, Z. Effects of different pre-harvest bagging times on fruit quality of apple. Foods 2024, 13, 1243. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, D.; Wang, Y.; Li, P.; Ma, F. Effects of fruit bagging on the contents of phenolic compounds in the peel and flesh of ‘Golden Delicious’, ‘Red Delicious’, and ‘Royal Gala’ apples. Sci. Hortic. 2012, 142, 68–73. [Google Scholar] [CrossRef]
- Elmizadeh, A.; Shahedi, M.; Hamdami, N. Quality assessment of electrohydrodynamic and hot-air drying of quince slice. Ind. Crop Prod. 2018, 116, 35–40. [Google Scholar] [CrossRef]
- Peršić, M.; Veberic, R.; Mikulic-Petkovsek, M. Phenolic profiles of quince (Cydonia oblonga Mill.) leaf extracts obtained by different extraction methods. Acta Bot. Croat. 2019, 78, 175–180. [Google Scholar] [CrossRef]
- Najman, K.; Adrian, S.; Hallmann, E.; Sadowska, A.; Buczak, K.; Waszkiewicz-Robak, B.; Szterk, A. Effect of various drying methods on physicochemical and bioactive properties of quince fruit (Cydonia oblonga Mill.). Agriculture 2023, 13, 446. [Google Scholar] [CrossRef]
- Ňorbová, M.; Vollmannová, A.; Fedorková, S.; Musilová, J.; Lidiková, J. The forgotten fruit (Cydonia oblonga Mill.) and its chemical composition: A review. Eur. Food Res. Technol. 2024, 250, 2093–2102. [Google Scholar] [CrossRef]
- Sabir, S.; Khan, M.Q.; Mehmood, A.; Hussain, K.; Raza, A.; Shakil, M.; Safeer, S. Quince (Cydonia oblonga) fruits: A rich source of nutrition, phenolics, flavonoids, antioxidant and antibacterial activity. Int. J. Agric. Biol. 2024, 31, 263–268. [Google Scholar] [CrossRef]
- Wojdyło, A.; Teleszko, M.; Oszmiański, J. Antioxidant property and storage stability of quince juice phenolic compounds. Food Chem. 2014, 152, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Majeed, T.; Wani, I.A.; Muzzaffar, S. Postharvest Biology and Technology of Quince. In Postharvest Biology and Technology of Temperate Fruits; Mir, S.A., Shah, M.A., Mir, M.M., Eds.; Springer: Cham, Switzerland, 2018; pp. 273–284. [Google Scholar] [CrossRef]
- Gunes, N.T. Ripening regulation during storage in quince (Cydonia oblonga Mill.) fruit. Acta Hortic. 2008, 796, 191–196. [Google Scholar] [CrossRef]
- Nanos, G.D.; Mpezou, A.; Georgoudaki, T. Effects of 1-MCP and storage temperature on quince fruit quality. Acta Hortic. 2015, 1079, 453–458. [Google Scholar] [CrossRef]
- Pastopoulos, S.; Nanos, G.D. Kaolin sprays and individual fruit bagging effects on quince fruit quality. Acta Hortic. 2012, 940, 381–386. [Google Scholar] [CrossRef]
- Gunes, N.T.; Poyrazoğlu, E.S. Influence of hot water and 1-methylcyclopropene treatments on air-stored quince fruit. Agronomy 2022, 12, 458. [Google Scholar] [CrossRef]
- Tatari, M.; Mahlouji, M.; Ghorbani, E. Determination of the appropriate harvest time and storability of some cultivars and promising genotypes of quince (Cydonia oblonga Mill.) in cold storage conditions. J. Hortic. Sci. 2019, 33, Fa639–Fa653. [Google Scholar] [CrossRef]
- Fan, X. Chemical inhibition of polyphenol oxidase and cut surface browning of fresh-cut apples. Crit. Rev. Food. Sci. 2022, 63, 8737–8751. [Google Scholar] [CrossRef]
- Yildiz, G.; Izli, G.; Aadil, R.M. Comparison of chemical, physical, and ultrasound treatments on the shelf life of fresh-cut quince fruit (Cydonia oblonga Mill.). J. Food Process. Preserv. 2020, 44, e14366. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Barroca, M.J. Quantification of browning kinetics and colour change for quince (Cydonia oblonga Mill.) exposed to atmospheric conditions. Agric. Eng. Int. CIGR J. 2014, 16, 285–298. [Google Scholar]
- Queiroz, C.; Jorge Ribeiro da Silva, A.J.; Mendes Lopes, M.L.; Fialho, E.; Valente-Mesquita, V.L. Polyphenol oxidase activity, phenolic acid composition and browning in cashew apple (Anacardium occidentale, L.) after processing. Food Chem. 2011, 125, 128–132. [Google Scholar] [CrossRef]
- Iqbal, A.; Murtaza, A.; Marszałek, K.; Iqbal, M.A.; Chughtai, M.J.F.; Hu, W.; Barba, F.J.; Bi, J.; Liu, X.; Xu, X. Inactivation and structural changes of polyphenol oxidase in quince (Cydonia oblonga Mill.) juice subjected to ultrasonic treatment. J. Sci. Food Agr. 2020, 100, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Wei, W.; Hu, X.; Bai, Q.; Guo, Y.; Zhang, M.; Li, S.; Pan, Q. Inhibitory effect and molecular mechanism on tyrosinase and browning of fresh-cut apple by longan shell tannins. Int. J. Biol. Macromol. 2024, 274, 133326. [Google Scholar] [CrossRef]
- Sharma, R.; Joshi, V.K.; Rana, J.C. Nutritional composition and processed products of quince (Cydonia oblonga Mill.). Indian J. Nat. Prod. Resour. 2011, 2, 354–357. [Google Scholar]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Maskan, M. Kinetics of colour change of kiwifruits during hot air and microwave drying. J. Food Eng. 2001, 48, 169–175. [Google Scholar] [CrossRef]
- Chen, C.R.; Ramaswamy, H.S. Color and texture change kinetics in ripening bananas. LWT-Food Sci. Technol. 2002, 35, 415–419. [Google Scholar] [CrossRef]
- Zambrano-Zaragoz, M.L.; Mercado-Silva, E.; Del Real, L.A.; Gutiérrez-Cortez, E.; Cornejo-Villegas, M.A.; Quintanar-Guerrero, D. The effect of nano-coatings with α-tocopherol and xanthan gum on shelf-life and browning index of fresh-cut “Red Delicious” apples. Innov. Food Sci. Emerg. 2014, 22, 188–196. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. J. Sci. Food Agr. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Brand-Willians, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Nair, R.; Ghakker, N.; Sharma, A. Spectrophotometric estimation of tannins in raw and processed form (Paan Masala) of Areca nut. Int. J. Educ. Sci. Res. Rev. 2015, 2, 51–56. [Google Scholar]
- Opara, L.U. Bruise susceptibilities of ‘Gala’ apples as affected by orchard management practices and harvest date. Postharvest Biol. Technol. 2007, 43, 47–54. [Google Scholar] [CrossRef]
- Wang, S.; Lin, T.; Man, G.; Li, H.; Zhao, L.; Wu, J.; Liao, X. Effects of anti-browning combinations of ascorbic acid, citric acid, nitrogen and carbon dioxide on the quality of banana smoothies. Food Bioprocess. Tech. 2014, 7, 161–173. [Google Scholar] [CrossRef]
- Sharma, R.R.; Sethi, S.; Asrey, R.; Sagar, V.R.; Bhan, C. On-the-tree fruit bagging: A safe approach for production of quality fruits. Indian J. Agr. Sci. 2021, 91, 3–7. [Google Scholar] [CrossRef]
- Konarska, A. Morphological, histological and ultrastructural changes in fruit epidermis of apple Malus domestica cv. ligol (Rosaceae) at fruit set, maturity and storage. Acta Biol. Cracov. Bot. 2015, 56, 35–48. [Google Scholar] [CrossRef]
- Karabourniotis, G.; Liakopoulos, G.; Nikolopoulos, D.; Bresta, P. Protective and defensive roles of non-glandular trichomes against multiple stresses: Structure–function coordination. J. For. Res. 2020, 31, 1–12. [Google Scholar] [CrossRef]
- Huang, C.; Yu, B.; Teng, Y.; Su, J.; Shu, Q.; Cheng, Z.; Zeng, L. Effects of fruit bagging on coloring and related physiology, and qualities of red Chinese sand pears during fruit maturation. Sci. Hortic.-Amst. 2009, 121, 149–158. [Google Scholar] [CrossRef]
- Guan, Y.; Qin, X.; Wei, C.; Feng, Y.; Cheng, Y.; Zhang, Y.; Guan, J. Influence of bagging on fruit quality, incidence of peel browning spots, and lignin content of ‘Huangguan’ pears. Plants 2024, 13, 516. [Google Scholar] [CrossRef]
- Abdollahi, H. Quince. In Temperate Fruits, Production, Processing, and Marketing, 1st ed.; Mandal, D., Wermund, U., Phavaphutanon, L., Cronje, R., Eds.; CRC: New York, NY, USA, 2021; pp. 183–246. [Google Scholar]
- Conesa, A.; Manera, F.C.; Brotons, J.M.; Fernandez-Zapata, J.C.; Simon, I.; Simon-Grao, S.; Alfosea-Simon, M.; Martinez Nicolas, J.J.; Valverde, J.M.; Garcia-Sanchez, F. Changes in the content of chlorophylls and carotenoids in the rind of Fino 49 lemons during maturation and their relationship with parameters from CIELAB color space. Sci. Hortic.-Amst. 2019, 243, 252–260. [Google Scholar] [CrossRef]
- Sharma, R.R.; Asrey, R.; Sagar, V.R.; Sethi, S. Influence of pre-harvest fruit bagging on Golden Delicious apple (Malus × domestica). Indian J. Agr. Sci. 2017, 87, 1170–1173. [Google Scholar] [CrossRef]
- Brizzolara, S.; Manganaris, G.A.; Fotopoulos, V.; Watkins, C.B.; Tonutti, P. Primary metabolism in fresh fruits during storage. Front. Plant Sci. 2020, 11, 80. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, V.P.; Jat, R.; Ahamad, S.; Kumar, V. Pre-harvest fruit bagging for quality improvement in fruit crops: A review. Pharma Innov. J. 2021, 10, 530–541. [Google Scholar]
- Moradi, S.; Saba, M.K.; Mozafari, A.A.; Abdollahi, H. Physical and biochemical changes of some Iranian quince (Cydonia oblonga Mill) genotypes during cold storage. J. Agr. Sci. Tech.-Iran. 2017, 19, 377–388. [Google Scholar]
- Göksel, Z. Comparison of physicochemical and bioactive contents of 36 different quince cultivars and genotypes. Genet. Resour. Crop Evol. 2024, 71, 4499–4518. [Google Scholar] [CrossRef]
- Moradi, S.; Saba, M.K.; Mozafari, A.A.; Abdollahi, H. Antioxidant bioactive compounds changes in fruit of quince genotypes over cold storage. J. Food Sci. 2016, 81, H1833–H1839. [Google Scholar] [CrossRef]
- Campbell, J.R.; Thomas, W.; Waterhouse, A.L.; Kennedy, J.A. Effect of applied shading during fruit ripening on tannin structure-activity relationships of grape skin extracts. J. Sci. Food Agr. 2024, 104, 352–361. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Tian, H.; Luo, X.; Qi, X.; Chen, X. Molecular Progress in Research on Fruit Astringency. Molecules 2015, 20, 1434–1451. [Google Scholar] [CrossRef]
- Montes-Ávila, J.; López-Angulo, G.; Delgado-Vargas, F. Tannins in Fruits and Vegetables: Chemistry and Biological Functions. In Fruit and Vegetable Phytochemicals; Yahia, E.M., Ed.; Wiley: Hoboken, NJ, USA, 2017; pp. 221–268. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, Q.; Wang, W.; Grierson, D.; Yin, X. Molecular basis of the formation and removal of fruit astringency. Food Chem. 2022, 372, 131234. [Google Scholar] [CrossRef]
- Papp, N.; Szabó, T.; Szabó, Z.; Nyéki, J.; Stefanovits-Bányai, É.; Hegedûs, A. Antioxidant capacity and total polyphenolic content in quince (Cydonia oblonga Mill.) fruit. Int. J. Hortic. Sci. 2013, 19, 33–35. [Google Scholar] [CrossRef]
- Singh, S.; Alam, M.S. Preservation of fresh-cut fruits and vegetables: Current status and emerging technologies. Stewart Postharvest Rev. 2012, 2, 5. [Google Scholar] [CrossRef]
- Serra, S.; Anthony, B.; Boscolo Sesillo, F.; Masia, A.; Musacchi, S. Determination of Post-Harvest Biochemical Composition, Enzymatic Activities, and Oxidative Browning in 14 Apple Cultivars. Foods 2021, 10, 186. [Google Scholar] [CrossRef]
- Murata, M. Food chemistry and biochemistry of enzymatic browning. Food Sci. Technol. Res. 2022, 28, 1–12. [Google Scholar] [CrossRef]
- Al-Zughbi, I.; Krayem, M. Quince fruit Cydonia oblonga Mill nutritional composition, antioxidative properties, health benefits and consumers preferences towards some industrial quince products: A review. Food Chem. 2022, 393, 133362. [Google Scholar] [CrossRef] [PubMed]
- Croguennec, T. Enzymatic Browning. In Handbook of Food Science and Technology 1: Food Alteration and Food Quality, 1st ed.; Jeantet, R., Croguennec, T., Schuck, P., Brulé, G., Eds.; Wiley-ISTE: London, UK; Hoboken, NJ, USA, 2016; pp. 159–181. [Google Scholar] [CrossRef]
- Yuan, X.; Zhan, Z.; Lin, W.; Zhang, C.; Wang, B. The membrane may be a key factor influencing browning: A mini review on browning mechanisms of fresh-cut fruit and vegetables from a multi-omics perspective. Front. Nutr. 2025, 12, 1534594. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Bielicki, P. Polyphenolic composition, antioxidant activity, and polyphenol oxidase (PPO) activity of quince (Cydonia oblonga Miller) varieties. J. Agric. Food Chem. 2013, 61, 2762–2772. [Google Scholar] [CrossRef]
- Arnold, M.; Gramza-Michałowska, A. Enzymatic browning in apple products and its inhibition treatments: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5038–5076. [Google Scholar] [CrossRef]
- He, Q.; Luo, Y. Enzymatic browning and its control in fresh-cut produce. Stewart Postharvest Rev. 2007, 6, 3. [Google Scholar] [CrossRef]
- Fan, J.; Du, W.; Chen, Q.-L.; Zhang, J.-G.; Yang, X.P.; Hussain, S.B.; Hu, H.J. Comparative Transcriptomic Analyses Provide Insights into the Enzymatic Browning Mechanism of Fresh-Cut Sand Pear Fruit. Horticulturae 2021, 7, 502. [Google Scholar] [CrossRef]
- Sultan, M.Z.; Farouk, K.A.; Elbagoury, M.M.; Yahia, E.M. Trends in biochemical, anatomical mechanisms and molecular aspects in enzymatic browning of apples: A review. Eur. Food Res. Technol. 2025. [Google Scholar] [CrossRef]
- Navina, B.; Huthaash, K.K.; Velmurugan, N.K.; Korumilli, T. Insights into recent innovations in anti browning strategies for fruit and vegetable preservation. Trends Food Sci. Tech. 2023, 139, 104128. [Google Scholar] [CrossRef]
- Liu, H.; Song, L.; You, Y.; Li, Y.; Duan, X.; Jiang, Y.; Joyce, D.C.; Ashraf, M.; Lu, W. Cold storage duration affects litchi fruit quality, membrane permeability, enzyme activities and energy charge during shelf time at ambient temperature. Postharvest Biol. Technol. 2011, 60, 24–30. [Google Scholar] [CrossRef]
- Opara, L.U.; Pathare, P.B. Bruise damage measurement and analysis of fresh horticultural produce-A review. Postharvest Biol. Technol. 2014, 91, 9–24. [Google Scholar] [CrossRef]
- Li, D.; Cheng, Y.; Dong, Y.; Shang, Z.; Gua, J. Effects of low temperature conditioning on fruit quality and peel browning spot in ‘Huangguan’ pears during cold storage. Postharvest Biol. Technol. 2017, 131, 68–73. [Google Scholar] [CrossRef]




| Time | Treatment | Fruit Mass (g) | Fuzz Mass (g) |
|---|---|---|---|
| Harvest | Unbagged | 326 ± 13 | 0.21 ± 0.02 b |
| Bagged | 321 ± 26 | 0.38 ± 0.03 a | |
| 3 months storage + 2 d SL | Unbagged | 316 ± 17 | - |
| Bagged | 315 ± 16 | - | |
| Significance | Time | NS | - |
| Treatment | NS | *** | |
| LSD0.05 | 53 | - | |
| Time | Treatment | FF (N) | SSC (%) | TA (%) | SSC/TA |
|---|---|---|---|---|---|
| Harvest | Unbagged | 67.8 ± 2.8 | 17.3 ± 0.1 b | 0.69 ± 0.01 c | 25.1 ± 0.5 a |
| Bagged | 69.2 ± 2.4 | 17.1 ± 0.2 b | 0.76 ± 0.01 b | 22.5 ± 0.9 b | |
| 3 months storage + 2 d SL | Unbagged | 70.3 ± 5.8 | 16.4 ± 0.2 c | 0.68 ± 0.02 c | 24.1 ± 0.7 ab |
| Bagged | 62.5 ± 2.2 | 17.8 ± 0.2 a | 0.90 ± 0.03 a | 19.8 ± 0.6 c | |
| Significance | Time | NS | NS | ** | * |
| Treatment | NS | *** | *** | NS | |
| LSD0.05 | 10.2 | 0.5 | 0.06 | 1.8 | |
| Time | Treatment | ΔE0–5 | ΔE0–20 |
|---|---|---|---|
| Harvest | Unbagged | 6.32 ± 0.54 | 9.25 ± 0.97 b |
| Bagged | 7.27 ± 0.72 | 8.42 ± 0.57 b | |
| 3 months storage + 2 d SL | Unbagged | 7.94 ± 1.71 | 13.41 ± 1.25 a |
| Bagged | 7.07 ± 1.49 | 14.65 ± 0.98 a | |
| Significance | Time | NS | *** |
| Treatment | NS | NS | |
| LSD0.05 | 2.45 | 2.76 | |
| Time | Treatment | ΔE0–10 | ΔE0–40 |
|---|---|---|---|
| Harvest | Unbagged | 19.0 ± 1.6 a | 27.7 ± 1.3 a |
| Bagged | 19.7 ± 1.0 a | 27.4 ± 1.1 a | |
| 3 months storage + 2 d SL | Unbagged | 5.4 ± 1.8 b | 16.2 ± 2.7 c |
| Bagged | 9.3 ± 2.2 b | 22.3 ± 1.9 b | |
| Significance | Time | *** | *** |
| Treatment | NS | NS | |
| LSD0.05 | 3.9 | 4.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgoudaki, T.; Maletsika, P.; Nanos, G.D. Effect of Preharvest Aluminum-Coated Paper Bagging on Postharvest Quality, Storability, and Browning Behavior of ‘Afrata Volou’ Quince. Horticulturae 2025, 11, 881. https://doi.org/10.3390/horticulturae11080881
Georgoudaki T, Maletsika P, Nanos GD. Effect of Preharvest Aluminum-Coated Paper Bagging on Postharvest Quality, Storability, and Browning Behavior of ‘Afrata Volou’ Quince. Horticulturae. 2025; 11(8):881. https://doi.org/10.3390/horticulturae11080881
Chicago/Turabian StyleGeorgoudaki, Triantafyllia, Persefoni Maletsika, and George D. Nanos. 2025. "Effect of Preharvest Aluminum-Coated Paper Bagging on Postharvest Quality, Storability, and Browning Behavior of ‘Afrata Volou’ Quince" Horticulturae 11, no. 8: 881. https://doi.org/10.3390/horticulturae11080881
APA StyleGeorgoudaki, T., Maletsika, P., & Nanos, G. D. (2025). Effect of Preharvest Aluminum-Coated Paper Bagging on Postharvest Quality, Storability, and Browning Behavior of ‘Afrata Volou’ Quince. Horticulturae, 11(8), 881. https://doi.org/10.3390/horticulturae11080881








