Green Light Enhances the Postharvest Quality of Lettuce During Cold Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
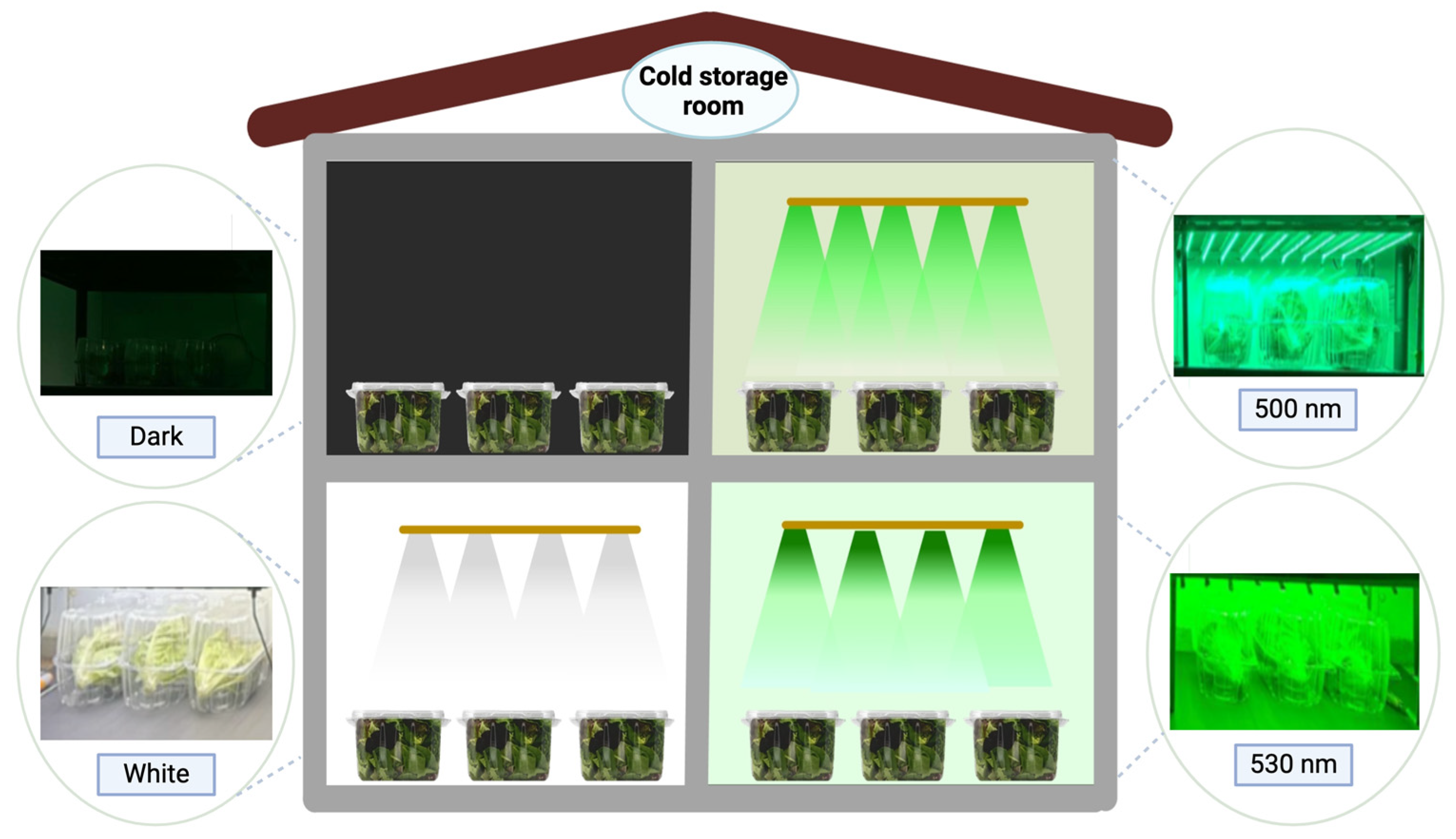
2.2. Light Sources and Experimental Conditions
2.3. Quality Assessment
2.3.1. Moisture Loss
2.3.2. Color Measurement
2.3.3. Texture Measurements
2.4. Gas Exchange Measurements
2.5. Chlorophyll Content (SPAD Index)
2.6. Relative Water Content (RWC)
2.7. Electrolyte Leakage
2.8. Assessment of Overall Visual Quality (OVQ)
2.9. Statistical Analysis
3. Results and Discussion
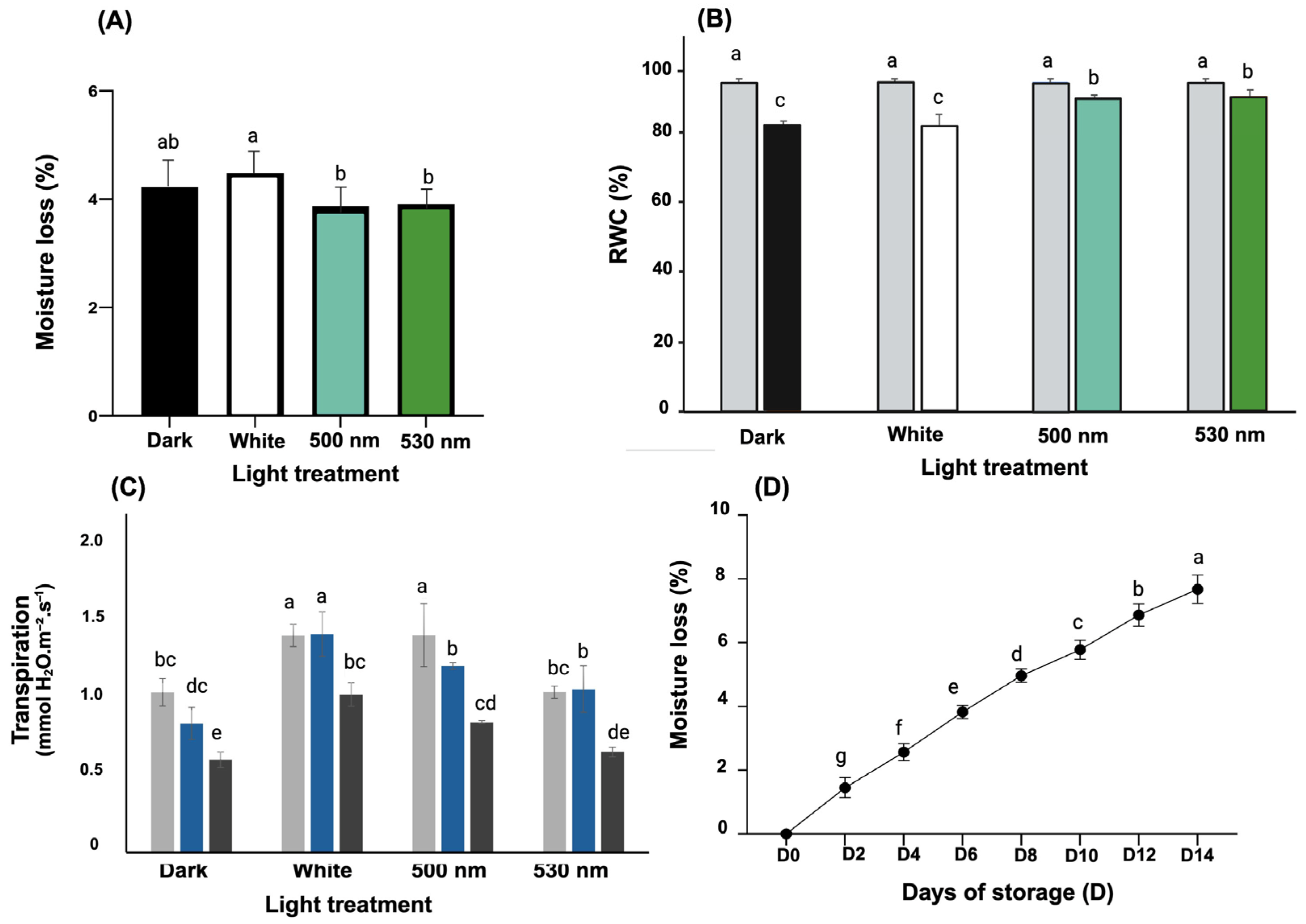
3.1. Green Light Effect on Lettuce Moisture Loss, Transpiration Rate, and RWC
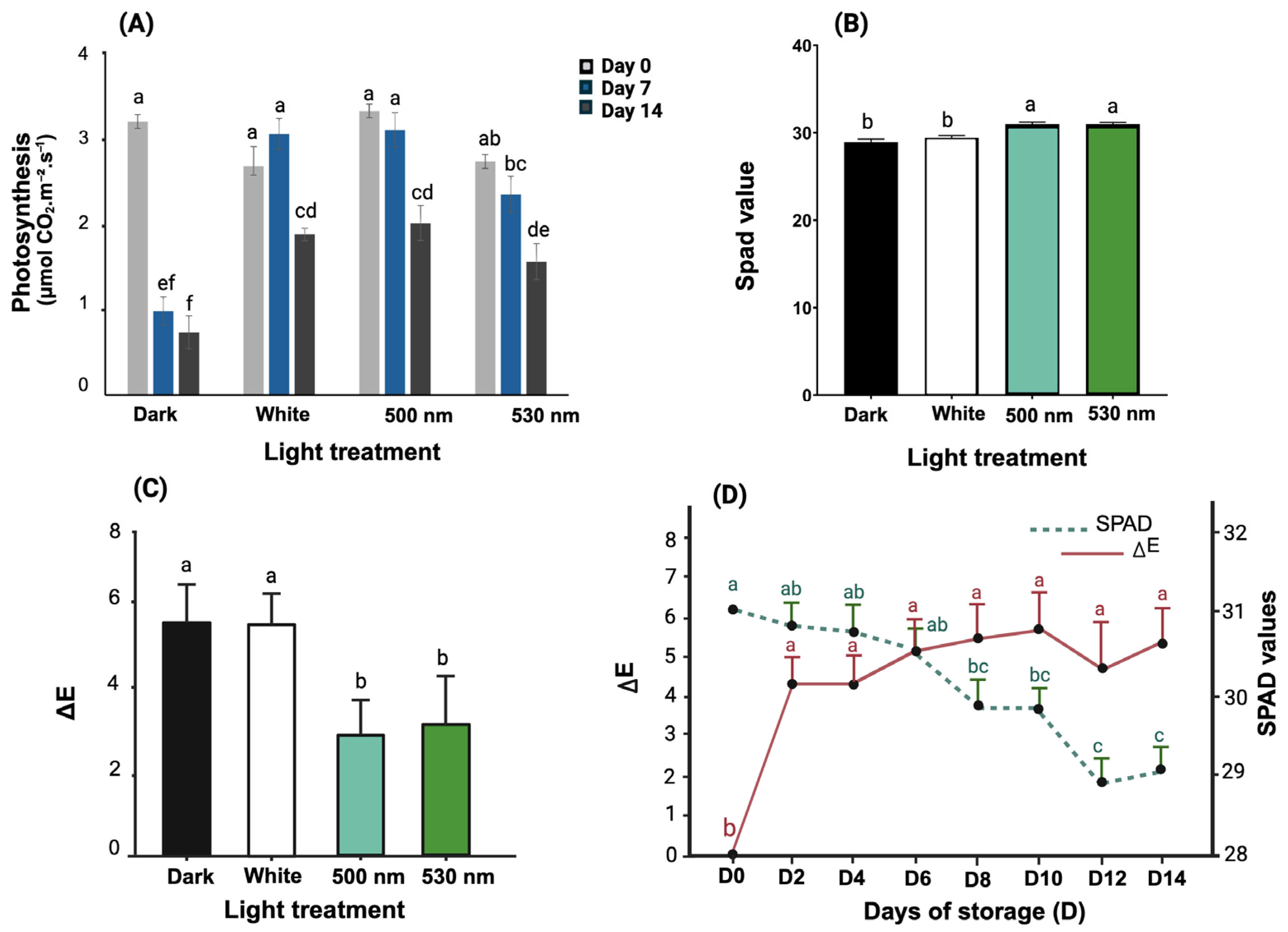
3.2. Green Light Effect on Lettuce Photosynthesis, SPAD Values, and ∆E
3.3. Green Light Effect on Lettuce Texture and EL
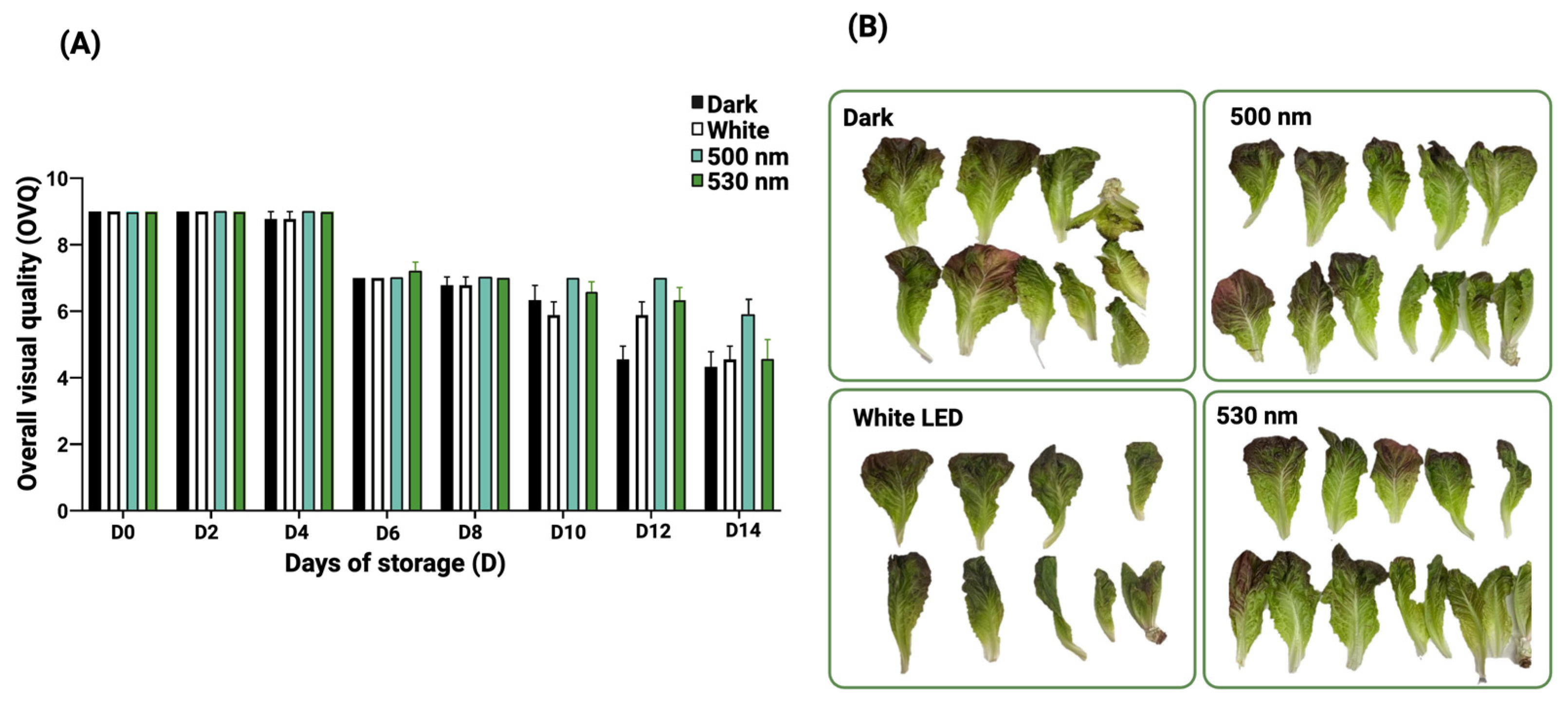
3.4. Green Light Effect on Lettuce Overall Visual Quality (OVQ) During Cold Storage
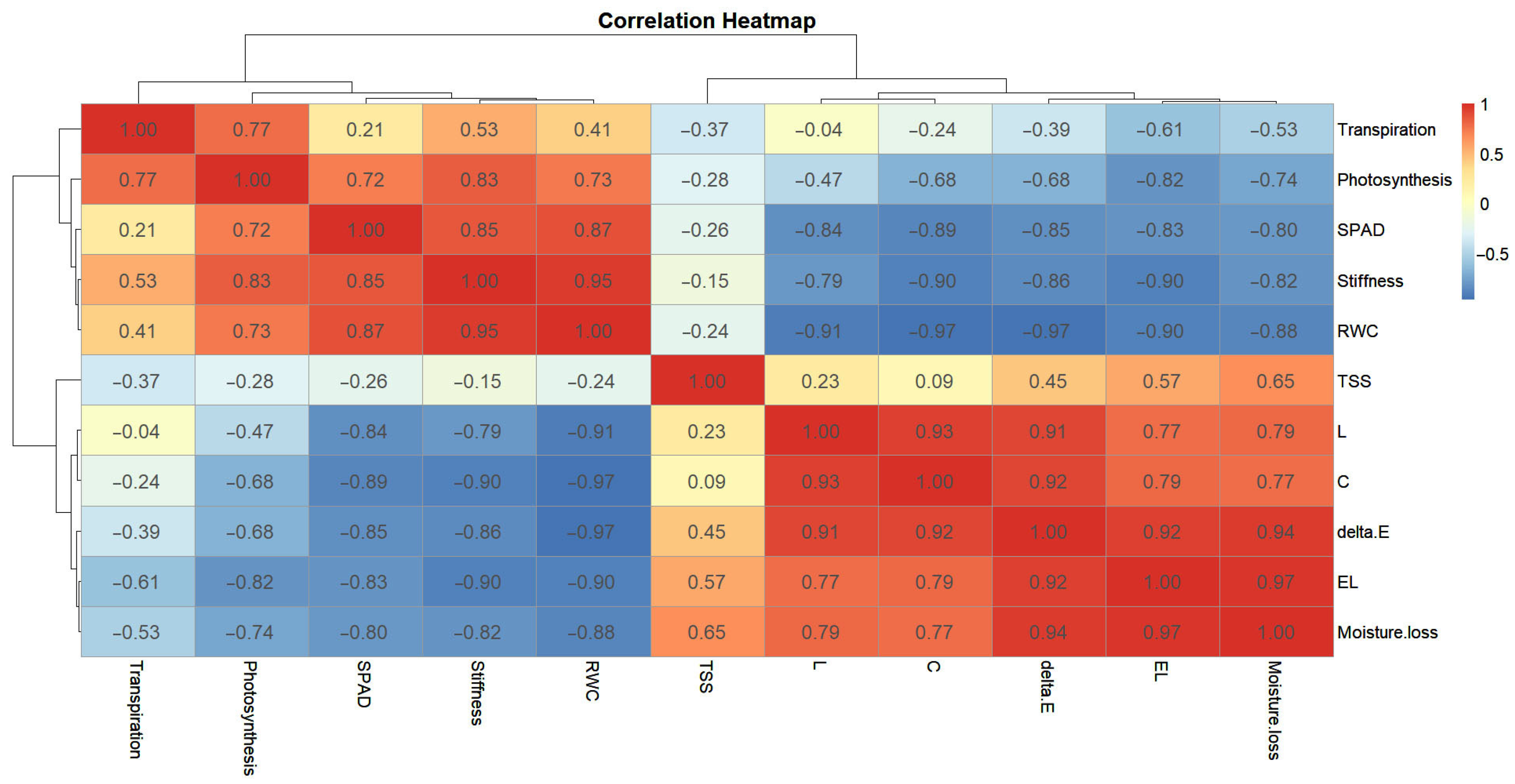
3.5. Impact of Postharvest Lighting Environment on the Correlation Matrix and PCA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shezi, S.; Magwaza, L.S.; Mditshwa, A.; Tesfay, S.Z. Changes in biochemistry of fresh produce in response to ozone postharvest treatment. Sci. Hortic. 2020, 269, 109397. [Google Scholar] [CrossRef]
- Kader, A.A. Postharvest Technology of Horticultural Crops; University of California Agriculture and Natural Resources: Davis, CA, USA, 2002. [Google Scholar]
- Jiang, A.; Zuo, J.; Zheng, Q.; Guo, L.; Gao, L.; Zhao, S.; Wang, Q.; Hu, W. Red LED irradiation maintains the postharvest quality of broccoli by elevating antioxidant enzyme activity and reducing the expression of senescence-related genes. Sci. Hortic. 2019, 251, 73–79. [Google Scholar] [CrossRef]
- Kasim, M.U.; Kasim, R. While continuous white LED lighting increases chlorophyll content (SPAD), green LED light reduces the infection rate of lettuce during storage and shelf-life conditions. J. Food Process. Preserv. 2017, 41, e13266. [Google Scholar] [CrossRef]
- Zhan, L.; Hu, J.; Pang, L.; Li, Y.; Shao, J. Light exposure reduced browning enzyme activity and accumulated total phenols in cauliflower heads during cool storage. Postharvest Biol. Technol. 2014, 88, 17–20. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Xu, B.; Mujumdar, A.S.; Guo, Z. Light-emitting diodes (below 700 nm): Improving the preservation of fresh foods during postharvest handling, storage, and transportation. Compr. Rev. Food Sci. Food Saf. 2022, 21, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Lefsrud, M.G.; Kopsell, D.A.; Sams, C.E. Irradiance from distinct wavelength light-emitting diodes affect secondary metabolites in kale. HortScience 2008, 43, 2243–2244. [Google Scholar] [CrossRef]
- Dhakal, R.; Baek, K.-H. Short period irradiation of single blue wavelength light extends the storage period of mature green tomatoes. Postharvest Biol. Technol. 2014, 90, 73–77. [Google Scholar] [CrossRef]
- Xu, F.; Cao, S.; Shi, L.; Chen, W.; Su, X.; Yang, Z. Blue light irradiation affects anthocyanin content and enzyme activities involved in postharvest strawberry fruit. J. Agric. Food Chem. 2014, 62, 4778–4783. [Google Scholar] [CrossRef]
- Song, Y.; Qiu, K.; Gao, J.; Kuai, B. Molecular and physiological analyses of the effects of red and blue LED light irradiation on postharvest senescence of pak choi. Postharvest Biol. Technol. 2020, 164, 111155. [Google Scholar] [CrossRef]
- Lester, G.E.; Makus, D.J.; Hodges, D.M. Relationship between fresh-packaged spinach leaves exposed to continuous light or dark and bioactive contents: Effects of cultivar, leaf size, and storage duration. J. Agric. Food Chem. 2010, 58, 2980–2987. [Google Scholar] [CrossRef]
- Rufyikiri, A.S. The Use of Light Emitting Diodes (LEDs) for Shelf Life Extension of Spinach and Kale; McGill University: Montreal, QC, Canada, 2018. [Google Scholar]
- Jin, P.; Yao, D.; Xu, F.; Wang, H.; Zheng, Y. Effect of light on quality and bioactive compounds in postharvest broccoli florets. Food Chem. 2015, 172, 705–709. [Google Scholar] [CrossRef]
- Jin, S.; Ding, Z.; Xie, J. Study of postharvest quality and antioxidant capacity of freshly cut amaranth after blue LED light treatment. Plants 2021, 10, 1614. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, J. The effect of red and violet light emitting diode (LED) treatments on the postharvest quality and biodiversity of fresh-cut pakchoi (Brassica rapa L. Chinensis). Food Sci. Technol. Int. 2022, 28, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Salehinia, S.; Didaran, F.; Aliniaeifard, S.; Macpherson, S.; Orsat, V.; Lefsrud, M. Effects of different light spectra and intensities on stomatal function in lettuce and basil. J. Hortic. Res. 2025, 33, 1. [Google Scholar] [CrossRef]
- Salehinia, S.; Didaran, F.; Aliniaeifard, S.; Zohrabi, S.; MacPherson, S.; Lefsrud, M. Green light enhances the phytochemical preservation of lettuce during postharvest cold storage. PLoS ONE 2024, 19, e0311100. [Google Scholar] [CrossRef]
- Firouz, M.S.; Alimardani, R.; Mobli, H.; Mohtasebi, S.S. Effect of modified atmosphere packaging on the mechanical properties of lettuce during shelf life in cold storage. Inf. Process. Agric. 2021, 8, 485–493. [Google Scholar]
- Barrs, H.; Weatherley, P. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Kiran, S. Effects of vermicompost on some morphological, physiological and biochemical parameters of lettuce (Lactuca sativa var. crispa) under drought stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 352–358. [Google Scholar] [CrossRef][Green Version]
- Kader, A.A.; Lipton, W.J.; Morris, L.L. Systems for Scoring Quality of Harvested Lettuce1. HortScience 1973, 8, 408–409. [Google Scholar] [CrossRef]
- Tian, W.; Lv, Y.; Cao, J.; Jiang, W. Retention of iceberg lettuce quality by low temperature storage and postharvest application of 1-methylcyclopropene or gibberellic acid. J. Food Sci. Technol. 2014, 51, 943–949. [Google Scholar] [CrossRef]
- Alipasandi, A.; Mahmoudi, A.; Sturm, B.; Behfar, H.; Zohrabi, S. Application of meta-heuristic feature selection method in low-cost portable device for watermelon classification using signal processing techniques. Comput. Electron. Agric. 2023, 205, 107578. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Castillejo, N.; Gómez, P.A.; Crepaldi, A.; Fernández, J.A.; Egea-Gilabert, C.; Artés-Hernández, F.; Gianquinto, G. Spectral composition from led lighting during storage affects nutraceuticals and safety attributes of fresh-cut red chard (Beta vulgaris) and rocket (Diplotaxis tenuifolia) leaves. Postharvest Biol. Technol. 2021, 175, 111500. [Google Scholar] [CrossRef]
- Rufyikiri, A.-S.; Addo, P.W.; Wu, B.-S.; MacPherson, S.; Orsat, V.; Lefsrud, M. The use of LEDs for the stomatal response, light compensation points, and storage of spinach and kale. J. Photochem. Photobiol. B Biol. 2024, 257, 112959. [Google Scholar] [CrossRef]
- Lee, Y.J.; Ha, J.Y.; Oh, J.E.; Cho, M.S. The effect of LED irradiation on the quality of cabbage stored at a low temperature. Food Sci. Biotechnol. 2014, 23, 1087–1093. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red: Blue ratio provided by LED lighting. Sci. Rep. 2019, 9, 14127. [Google Scholar] [CrossRef]
- Salehinia, S. Effects of Different Light Emitting Diode Spectra and Intensities on the Stomatal Functioning of Lettuce and Basil; McGill University: Montreal, QC, Canada, 2023. [Google Scholar]
- Roura, S.I.; Davidovich, L.; Valle, C.D. Quality loss in minimally processed Swiss chard related to amount of damaged area. LWT-Food Sci. Technol. 2000, 33, 53–59. [Google Scholar] [CrossRef]
- Woltering, E.; Witkowska, I. Effects of pre-and postharvest lighting on quality and shelf life of fresh-cut lettuce. In Proceedings of the VIII International Symposium on Light in Horticulture 1134, East Lansing, MI, USA, 22–26 May 2016. [Google Scholar]
- Rahman, M.M.; Field, D.L.; Ahmed, S.M.; Hasan, M.T.; Basher, M.K.; Alameh, K. LED illumination for high-quality high-yield crop growth in protected cropping environments. Plants 2021, 10, 2470. [Google Scholar] [CrossRef]
- Zhan, L.; Hu, J.; Li, Y.; Pang, L. Combination of light exposure and low temperature in preserving quality and extending shelf-life of fresh-cut broccoli (Brassica oleracea L.). Postharvest Biol. Technol. 2012, 72, 76–81. [Google Scholar] [CrossRef]
- Hasperué, J.H.; Rodoni, L.M.; Guardianelli, L.M.; Chaves, A.R.; Martínez, G.A. Use of LED light for Brussels sprouts postharvest conservation. Sci. Hortic. 2016, 213, 281–286. [Google Scholar] [CrossRef]
- Loi, M.; Liuzzi, V.C.; Fanelli, F.; De Leonardis, S.; Creanza, T.M.; Ancona, N.; Paciolla, C.; Mulè, G. Effect of different light-emitting diode (LED) irradiation on the shelf life and phytonutrient content of broccoli (Brassica oleracea L. var. italica). Food Chem. 2019, 283, 206–214. [Google Scholar] [CrossRef]
- Ashrostaghi, T.; Aliniaeifard, S.; Shomali, A.; Azizinia, S.; Abbasi Koohpalekani, J.; Moosavi-Nezhad, M.; Gruda, N.S. Light intensity: The role player in cucumber response to cold stress. Agronomy 2022, 12, 201. [Google Scholar] [CrossRef]
- Serrano, M.; Martinez-Romero, D.; Guillen, F.; Castillo, S.; Valero, D. Maintenance of broccoli quality and functional properties during cold storage as affected by modified atmosphere packaging. Postharvest Biol. Technol. 2006, 39, 61–68. [Google Scholar] [CrossRef]
- Olarte, C.; Sanz, S.; Echávarri, J.F.; Ayala, F. Effect of plastic permeability and exposure to light during storage on the quality of minimally processed broccoli and cauliflower. LWT-Food Sci. Technol. 2009, 42, 402–411. [Google Scholar] [CrossRef]
- Ayala, F.; Echávarri, J.F.; Olarte, C.; Sanz, S. Quality characteristics of minimally processed leek packaged using different films and stored in lighting conditions. Int. J. Food Sci. Technol. 2009, 44, 1333–1343. [Google Scholar] [CrossRef]
- Huyskens-Keil, S.; Eichholz-Dündar, I.; Hassenberg, K.; Herppich, W. Impact of light quality (white, red, blue light and UV-C irradiation) on changes in anthocyanin content and dynamics of PAL and POD activities in apical and basal spear sections of white asparagus after harvest. Postharvest Biol. Technol. 2020, 161, 111069. [Google Scholar] [CrossRef]


| Day 0 | Day 14 | ||||
|---|---|---|---|---|---|
| - | Dark | White | 500 nm | 530 nm | |
| Crispness (mN.mm−1) | 2.83 a | 1.717 c | 2.17 b | 2.5 a | 2.52 a |
| Standard error (+/−) | 0.21 | 0.132 | 0.232 | 0.367 | 0.315 |
| Electrolyte leakage | 17.483 b | 38.148 a | 33.42 a | 29.873 a | 31.90 a |
| Standard error (+/−) | 1.85 | 2.04 | 1.776 | 1.684 | 1.716 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehinia, S.; Didaran, F.; Gariepy, Y.; Aliniaeifard, S.; MacPherson, S.; Lefsrud, M. Green Light Enhances the Postharvest Quality of Lettuce During Cold Storage. Horticulturae 2025, 11, 792. https://doi.org/10.3390/horticulturae11070792
Salehinia S, Didaran F, Gariepy Y, Aliniaeifard S, MacPherson S, Lefsrud M. Green Light Enhances the Postharvest Quality of Lettuce During Cold Storage. Horticulturae. 2025; 11(7):792. https://doi.org/10.3390/horticulturae11070792
Chicago/Turabian StyleSalehinia, Shafieh, Fardad Didaran, Yvan Gariepy, Sasan Aliniaeifard, Sarah MacPherson, and Mark Lefsrud. 2025. "Green Light Enhances the Postharvest Quality of Lettuce During Cold Storage" Horticulturae 11, no. 7: 792. https://doi.org/10.3390/horticulturae11070792
APA StyleSalehinia, S., Didaran, F., Gariepy, Y., Aliniaeifard, S., MacPherson, S., & Lefsrud, M. (2025). Green Light Enhances the Postharvest Quality of Lettuce During Cold Storage. Horticulturae, 11(7), 792. https://doi.org/10.3390/horticulturae11070792









