Abstract
Colletotrichum capsici is an important pathogen causing anthracnose in postharvest peppers in parts of Asia, seriously compromising quality and storage life. Unveiling the pathogenic mechanism can better prevent postharvest disease in pepper. This study investigated the impacts of C. capsici infection on cell wall and phenylpropanoid metabolism in postharvest pepper. Compared to the non-inoculated peppers, C. capsici infection notably increased the disease index, damaged visual quality, and reduced the firmness. Morphological observations showed that C. capsici infection contributed to the collapse of epidermal cell structure. During the early stage, C. capsici triggered pepper’s defensive responses, including lignin deposition around the wounds, increased cellulose and hemicellulose content, and boosted disease-resistance enzymes, including phenylalanine ammonia-lyase (PAL), cinnamic acid 4-hydroxylase (C4H), 4-coumarate-CoA ligase (4CL), cinnamyl alcohol dehydrogenase (CAD), laccase (LAC), β-1,3-glucanase (β-1,3-Glu), and chitinase (CHI), alongside elevated total phenolics and flavonoids. However, as storage time progressed, the activities of carboxymethy cellulase (Cx), polygalacturonase (PG), pectin methylesterase (PME), and β-glucosidase (β-Glu) remained at a high level, leading to a reduction in cell wall components, a decline in the activities of disease-resistance enzymes, and a decrease in phenylpropanoid metabolite, resulting from disease progression in pepper. These insights highlight the need for early intervention strategies to mitigate postharvest losses by targeting pathogen-induced stress responses and cell wall integrity preservation.
1. Introduction
Bell pepper (Capsicum annuum L.) is an annual or perennial herb belonging to the Capsicum genus in the Solanaceae family [1]. It is the most widely cultivated vegetable crop and condiment in the world [2], with China being the largest producer [3]. As an important economic horticultural crop, bell peppers are rich in amino acids and biologically active substances, including carotene, vitamin C, and carotenoids [4,5]. These components can effectively remove reactive oxygen radicals in the body and reduce the risk of cardiovascular and cerebrovascular diseases and cancer [6]. In addition, the capsicine it contains can also promote digestion and enhance appetite [7].
Bell pepper is a climacteric fruit, and it is highly perishable due to its thin skin and brittle flesh, high respiration rate and moisture content, and postharvest diseases. A major threat to the pepper industry is microbial activities. The common diseases of postharvest pepper fruit are fruit rot, gray mold, and anthracnose [7]. Among these, anthracnose is considered one of the most destructive diseases of pepper globally. The typical symptoms on fruit are surfaced, yellow-brown, sunken lesions accompanied by conidia covering the surface [8]. The disease is attributed to Colletotrichum, which is also ranked as the eighth largest group of phytopathogenic fungi in the world, capable of causing a wide range of hosts, including peppers, to be susceptible to one or more species of anthracnose [9]. The common Colletotrichum species include C. gloeopsorioides, C. capsici, C. acutatum, and C. truncatum [8,10], among which C. capsici is widely prevalent in China and seriously jeopardizes the quality of pepper fruit.
Pepper fruit may become damaged in the postharvest transportation process due to vibration, compression, impact, and other factors, which lead to irreversible damage to the appearance and internal tissue structure of the fruit [11]. This facilitates the development of phytopathogenic fungi, resulting in rapid fruit deterioration.
As the primary structural barrier against pathogen infestation, the cell wall is mainly composed of polysaccharides; therefore, its strength is closely related to the disease resistance of the plants [12]. Cellulose, hemicellulose, and pectin are the main polysaccharides in the plant cell wall, which play a vital role in the structural properties of fruit [13]. Postharvest fruit softening is linked to cell wall solubilization and depolymerization, mainly resulting from combined actions of cell wall-degrading enzymes, including pectinolytic enzymes such as polygalacturonase (PG), pectin methylesterase (PME), as well as cellulolytic enzymes like cellulase, which weaken the intercellular connection and destabilize cell wall components, ultimately leading to fruit softness and rotting [13,14]. It was reported that the soluble pectin content of Zizania latifolia increased, and the fiber content decreased, decreasing hardness and textural softening during the postharvest storage process [15]. Moreover, Alternaria alternata contributes to the destruction of cantaloupe tissue structure by inducing the activity of enzymes related to cell wall metabolism [16]. Inoculation of pummelo with Diaporthe citri resulted in rind softening, browning, and tissue degradation, along with enhanced cell wall-degrading enzyme activity and gene expression [17]. The more information we know about the interaction between host and pathogen, the more effective control methods we will explore [18].
When plants experience stress, they activate their defense mechanisms. For instance, during pathogen invasion, plants trigger the phenylpropanoid metabolic pathway, producing secondary metabolites such as phenolic compounds, including flavonoids, and lignin to prevent the spread of pathogens [19]. Phenylalanine ammonia-lyase (PAL) is the key rate-limiting enzyme in the phenylpropanoid metabolic pathway, whereas compounds such as cinnamic acid 4-hydroxylase (C4H) and 4-coumarate-CoA ligase (4CL) contribute significantly to downstream metabolic flux and secondary metabolite formation [16,20]. Guo et al. [21] and Liu et al. [22] have confirmed that promoting the activities of enzymes in the phenylpropanoid metabolic pathway and accumulating the secondary metabolites can resist the attack from Alternaria alternata. The mechanism of plant disease resistance also includes the upregulation of disease-resistance proteins, such as chitinase (CHI) and β-1,3-glucanase (β-1,3-Glu). These two enzymes act directly on pathogens, breaking down their cell wall structures to mitigate the damage caused by the pathogens. Lu et al. [23] demonstrated that melatonin improves the anthracnose resistance of mango by increasing the activities of CHI and β-1,3-Glu.
Given the scarcity of research on the cell wall metabolism and phenylpropanoid metabolism of postharvest pepper fruit induced by C. capsici and its direct relation to disease development, our study assumes significant importance. We try to elucidate the infection mechanism of C. capsici and the potential disease resistance mechanism by investigating the cell wall metabolism and phenylpropanoid metabolism during C. capsici infestation. Our findings are expected to pave the way for developing effective antifungal agents and control strategies for C. capsici, thereby providing a crucial theoretical basis for future research.
2. Materials and Methods
2.1. Fungal Strains and Inoculum Preparation
Colletotrichum capsici (C. capsici) was obtained from China General Microbiological Culture Collection Center. C. capsici was cultured in potato dextrose agar (PDA) plates for 7 days at 28 ± 1 °C, with a relative humidity 95% prior to use. Spores were harvested from 7-day-old cultures, dipped in sterile water and gently shaken. Then, unnecessary mycelia were filtered out using eight layers of cheesecloth. Spore suspension was adjusted to 1 × 107 spores/mL with a hemocytometer for further use.
2.2. Plant Material and Inoculation
Fresh green bell peppers were obtained from a vegetable cultivation facility in Babu District, Hezhou City, Guangxi, China. Peppers without stains or physical injuries were selected based on their uniform shape, size, and color. Subsequently, peppers were sterilized with 0.5% (v/v) NaClO solution for 5 min and then rinsed three times with tap water. After air-drying, all fruits were criss-crossed (length of 1 cm, depth of 1 cm) with a sterilized scalpel on the equatorial surface to create four wounds. These pepper fruits were randomly separated into two groups. For the Control group, 15 μL sterile water was injected into the center of each wound, while for C. capsici treatment, equivalent prepared spore suspensions were injected instead of water [24]. After air-drying at ambient temperature, peppers were put into plastic baskets lined with polyethylene bags (70 × 110 cm, thickness 0.12 mm) and placed in an artificial climatic chamber at a temperature of 28 ± 1 °C and a humidity of 95 ± 5%. Each treatment consisted of three biological replicates, with each replicate comprising 30 fruits. The 2 mm mixture tissue (pepper skin and flesh) around the cross-wound samples were taken at 0 d, 1 d, 3 d, 6 d, and 9 d of storage periods. The samples were immediately frozen with liquid nitrogen and then stored at −80 °C for subsequent experiments.
2.3. Firmness Measurement
Fruit firmness was assessed using the modified version of the method outlined by Jia et al. [25]. A texture analyzer (TA. XT plus, Stable Micro Systems Ltd., Surrey, Godalming, UK) was applied to measure the firmness of the peppers. Three peppers were randomly selected from different treatments, and their hardness was determined at their sampling sites. For each pepper, measurements were taken at three points, and the average firmness was recorded in N/cm2. The test included a P2 probe, a pre-test and post-test speed of 1 mm/s, and a penetration depth of 10 mm.
2.4. Microstructure Observation of Pepper Pericarp
The microstructure of pepper pericarp was observed by scanning electron microscope [26]. The sampling site of peppers was cut into several pieces with 4 mm × 2 mm, immersed in 2% glutaraldehyde solution (0.1 mol/L pH 6.8), and fixed in a refrigerator at 4 °C for 24 h. Thereafter, the samples were taken out and rinsed with phosphate buffer three times for 15 min and then dehydrated with gradients of 50%, 70%, 80%, and 90% ethanol for 15 min, respectively, and finally dehydrated with anhydrous ethanol thrice for 15 min. After freeze-drying, the samples were observed and photographed by a scanning electron microscope (JSM-7610) after gold coating using a sputter coater.
2.5. Lignin Content Determination and Lignin Deposition Observation
Lignin content was measured following a modified version of the method by Qu et al. [27]. In brief, 1 g of pepper pericarp was well ground and mixed with 3 mL of pre-cooled 95% (v/v) ethanol, then centrifuged (TGL-16MS, Shanghai Bioridge, Shanghai, China) at 12,000 r/min for 10 min at 4 °C. The precipitate was washed thrice with 3 mL of 95% ethanol and 3 mL of ethanol–n-hexane (v:v) = 1:2 solution before being dried at 65 °C for 12 h. The final results were expressed as OD280/g.
Lignin deposition was analyzed using a modified procedure based on Huang et al. [28]. Thin slices (0.2–0.3 mm thick) were cut perpendicular to the wound surface of the peppers with a stainless-steel blade. After being soaked and rinsed several times with distilled water, the thin sections were placed on slides and stained with 1% (w/v) phloroglucinol in ethanol for 1.5 min, followed by the addition of 1–2 drops of concentrated hydrochloric acid. The red-stained lignin deposition in the pepper sections was observed using a microscope (Olympus-BX53, Tokyo, Japan, magnification 40).
2.6. Cell Wall Materials Measurement
Cell wall materials (CWMs) were extracted based on Wang et al. [29]. Briefly, 5 g of pepper tissue was homogenized with 50 mL of 95% (v/v) ethanol, then heated in boiling water for 20 min. After cooling to room temperature, the mixture was centrifuged (TGL-16MS, Shanghai Bioridge, Shanghai, China) at 4000 r/min for 10 min. The residue was washed thoroughly three times with 20 mL each of 85% (v/v) ethanol, chloroform–methanol (1:1, v/v), and acetone. The residue was subsequently dried at 40 °C for 12 h to obtain CWMs.
Cellulose and hemicellulose were measured by the anthrone method [29], with glucose as the standard. Results were expressed as mg/g.
The content of protopectin and soluble pectin in peppers was referred to by the carbazole colorimetric method of Song et al. [30]. The contents were calculated based on a standard curve made with galacturonic acid and expressed as the mass fraction of galacturonic acid (%).
2.7. Cell Wall-Degrading Enzyme Activity Determination
The extraction of crude enzyme was performed following the method of Song et al. [30] with slight modifications. Pepper pulp samples (1 g) were well mixed with 5 mL of pre-cooled 95% (v/v) ethanol at 4 °C and incubated for 10 min. The mixture was then centrifuged (TGL-16MS, Shanghai Bioridge) at 12,000 r/min for 20 min at 4 °C, and the supernatant was discarded. The residue was re-extracted with the extraction buffer. After a second centrifugation (12,000 r/min for 20 min), 2.5 mL of 50 mmol/L pre-cooled acetate buffer was added to resuspend the precipitate. The supernatant obtained from this final centrifugation was used as the crude enzyme extract.
Polygalacturonase (PG) activity and β-glucosidase (β-Glu) activity were determined following the method of Song et al. [30]. One unit of enzyme activity was defined as the amount of galacturonic acid or glucose produced per hour per gram of fresh weight (FW) and expressed as mg/h·g.
Carboxymethy cellulase (Cx) activity was evaluated using the slightly modified method from Yang et al. [31]. One unit of enzyme activity was defined as the amount of enzyme that releases 1 mg of D-glucose per hour per gram of fresh weight (FW) from carboxymethyl cellulose, expressed as mg/h·g.
Pectin methylesterase (PME) activity was assessed based on a modified procedure, as described by Chen et al. [13]. One unit of PME activity corresponded to 1 mmol/L of NaOH consumed per hour and was expressed as mg/h·g.
2.8. Total Phenolic Content and Flavonoid Content Measurement
The total phenolic and flavonoid contents were determined following the method of Li et al. [20], with slight modifications. Briefly, 6 mL of pre-cooled HCl-methanol was added to 1 g of pepper tissue. After thorough homogenization, the mixture was centrifuged (TGL-16MS, Shanghai Bioridge) at 12,000 r/m for 20 min at 4 °C, and the supernatant was collected for further analysis. The total phenolic content was measured using Folin–Ciocalteu reagent, with absorbance recorded at 765 nm. Flavonoid content was assayed using an aluminum nitrate reagent with absorbance measured at 510 nm. The results total phenolic and flavonoid content were quantified by gallic acid mg/g and rutin mg/g, respectively.
2.9. Phenylpropanoid Metabolic Pathway-Related Enzymes Determination
The activities of PAL, C4H, 4CL, and cinnamyl alcohol dehydrogenase (CAD) were determined following the method of Guo et al. [21]. The absorbance change for 0.01 at 290 nm, 340 nm, 333 nm, and 340 nm per hour was defined as one unit for PAL, C4H, 4CL, and CAD enzymatic activities, respectively. Results were recorded as U/g.
Laccase (LAC) activity was measured following the procedures of Bourbonnais and Paice [32] with minor changes. The activity was determined by the absorbance change at 420 nm after oxidation of ABTS, expressed as U/g.
2.10. Chitinase and β-1,3-Glucanase Activity Assay
Chitinase (CHI) and β-1,3-glucanase (β-1,3-Glu) activities were measured, referring to the approach of Liu et al. [33]. CHI activity was determined using colloidal chitin as the substrate. The reaction mixture contained 0.5 mL of enzyme extract and 0.5 mL of 10 g/L colloidal chitin in 0.1 mol/L sodium acetate buffer (pH 5.2), incubated at 37 °C for 1 h. Then, 0.1 mL of 30 g/L desalted snailase was added, followed by incubation at 37 °C for 1 h. For β-1,3-glucanase (β-1,3-Glu) activity, laminarin was used as the substrate. The reaction mixture contained 20 μL of crude enzyme extract and 20 μL of 4 g/L laminarin solution. The mixture was incubated at 37 °C for 40 min, followed by the addition of 0.36 mL of distilled water and 0.3 mL of DNS reagent. The reaction was terminated by heating in a boiling water bath for 3 min. After cooling, distilled water was added to bring the final volume to 5 mL. A standard curve was prepared using D-glucose. Absorbances were measured at 585 nm for CHI and 540 nm for β-1,3-Glu. One unit of CHI activity was defined as the amount of enzyme needed to produce 1 nmol of NAG per second per gram of FW. Similarly, one unit of β-1,3-Glu activity corresponded to the production of 1 nmol of glucose per second per gram of FW.
2.11. Statistical Analysis
Each experiment was repeated independently in triplicate, and the results were represented as means ± standard error (SE). Statistical analysis was conducted by SPSS v. 19.0 (SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) with least significant difference (LSD) was performed for mean comparisons at p < 0.05. Correlation coefficients and principal component analysis (PCA) were performed in Origin, v. 2021 (OriginLab, Northampton, MA, USA).
3. Results
3.1. C. capsici Infestation on Appearance Quality and Firmness of Peppers
The visual appearance of pepper fruit is shown in Figure 1A. The control group exhibited an intact visual surface without obvious lesions during the first 6 days of storage, and a small lesion appeared on the last day. In the C. capsici-treated group, the yellow-brown lesion appeared on the wound site on the third day, and since then, the disease spot has gradually expanded. These results indicate that C. capsici is capable of causing typical anthracnose symptoms in peppers, which exhibit strong pathogenicity and rapid progression, seriously affecting the appearance and quality of peppers after harvesting.
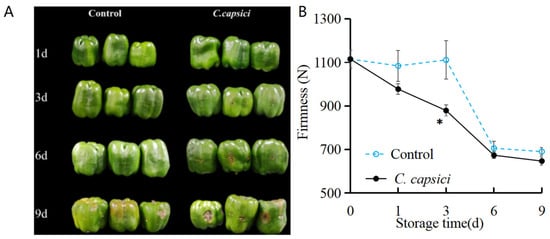
Figure 1.
Effect of C. capsici infestation on the quality of appearance (A) and firmness (B) of peppers. The asterisks represent the significant difference (* p < 0.05) between C. capsici-treated peppers and control peppers on each storage day (the same applies below).
Fruit hardness is one of the critical indicators of postharvest senescence and decay of fruits and vegetables. As shown in Figure 1B, the hardness of C. capsici-treated fruits rapidly decreased during the whole storage period. However, there was no significant change before 3 d in the control group, and after that, it decreased sharply. Among them, the hardness of the C. capsici treatment group decreased the fastest and was significantly lower than that of the control group (p < 0.05). These results indicated that mechanical injuries would reduce the hardness of C. capsici-treated fruits, and anthracnose would accelerate the decline of hardness, thus accelerating quality deterioration.
3.2. C. capsici Infestation on Microstructure of Pepper Epidermis
As the first barrier against the invasion of pathogenic bacteria, the cell wall plays an important role in protecting the cells from pathogenic bacteria. The microstructures of pepper epidermis on C. capsici treatment and control were observed. As can be seen in Figure 2, intact cell structure was observed in both the C. capsici treatment and control at 0 d. Distinct differences can be detected in the C. capsici treatment and the control during the subsequent period. In the control group, the degree of cell crumpling deepened with increased storage time. In contrast, rupture of pepper cells was observed at 3 d of storage in the C. capsici treatment group, and even a large number of anthracnose spores were enriched on the surface of the pepper epidermis at 9 d. These observations indicated that, under the tested conditions, C. capsici infestation accelerated tissue deterioration and led to substantial cellular disruption in peppers, which was consistent with the disease development shown in Figure 1.
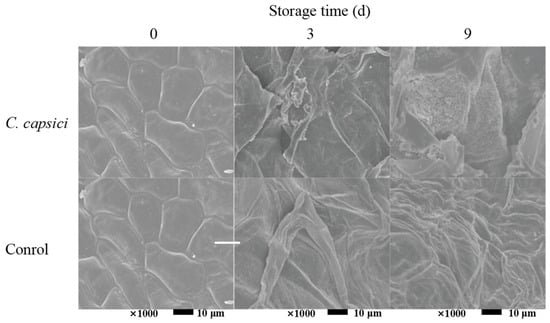
Figure 2.
Effect of C. capsici infestation on microstructure of peppers.
3.3. C. capsici Infestation on Cell-Wall Polysaccharides of Peppers
As illustrated in Figure 3A,B, the cellulose and hemicellulose contents followed a similar pattern, rising initially and peaking on the third day before declining. Notably, the cellulose content in C. capsici-treated peppers was higher than that of the control at days 3 and 6 but significantly lower at day 9 (p < 0.05). For hemicellulose, no significant difference was observed between the two groups in the early stages of storage. However, by days 6 and 9, the hemicellulose content in the C. capsici group was lower than that of the control. The protopectin contents in both the control and C. capsici-treated peppers decreased over time, with the C. capsici treatment accelerating this decline (Figure 3C). Meanwhile, the soluble pectin content in both the control and treated peppers increased as storage progressed. The C. capsici treatment notably enhanced the soluble pectin content in peppers compared to the control group (Figure 3D). These findings suggest that C. capsici infestation affects the contents of cellulose, hemicellulose, and pectin in peppers during storage. These findings align with the previously observed changes in lignin content.
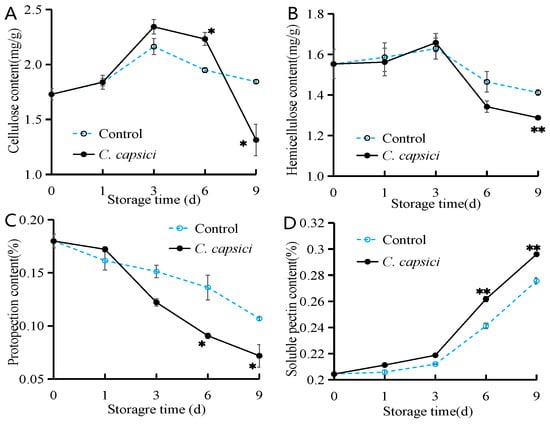
Figure 3.
Effects of C. capsici infection on cellulose content (A), hemicellulose content (B), protopectin content (C), and soluble pectin content (D) of peppers. The asterisks represent the significant difference (* p < 0.05, ** p < 0.01) between C. capsici-treated peppers and control peppers on each storage day.
3.4. C. capsici Infestation on Activities of Cell Wall Degradation Enzymes
As shown in Figure 4, C. capsici treatment significantly increased the activities of PG, β-Glu, Cx, and PME in peppers during the early and middle stages of storage, particularly Cx and PME, which surged after day 1. All enzyme activities peaked on day 6, reaching 1.3 mg/h·g for PG, 0.86 mg/h·g for β-Glu, 1.77 mg/h·g for Cx, and 111.75 mg/h·g for PME, significantly higher than those of the control group (p < 0.05). However, the activities of these enzymes declined sharply by day 9. In the control group, PG activity showed a continuous increase, while the trends for the other three enzymes mirrored those observed in the C. capsici-treated group, with only slight changes in β-Glu activity. These findings suggest that C. capsici treatment activates cell wall-degrading enzymes, which accelerates pepper softening.
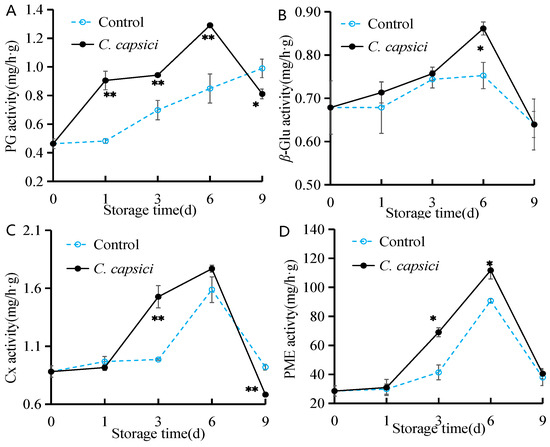
Figure 4.
Effects of C. capsici infection on PG activity (A), β-Glu activity (B), Cx activity (C), and PME activity (D) of peppers. The asterisks represent the significant difference (* p < 0.05, ** p < 0.01) between C. capsici-treated peppers and control peppers on each storage day.
3.5. C. capsici Infestation on Lignin Content and Lignin Deposition of Peppers
The lignin content, as shown in Figure 5A, exhibited an overall upward trend in the control group, with some fluctuations between days 3 and 6. Similarly, the lignin content in the C. capsici treatment group gradually increased until day 6, after which it dropped to 0.6 OD280/g. Generally, the lignin content in the control group remained higher than that in the C. capsici treatment group during both the early and later stages of storage. This suggests that C. capsici infestation may stimulate lignin production as a defense mechanism to resist pathogen invasion.
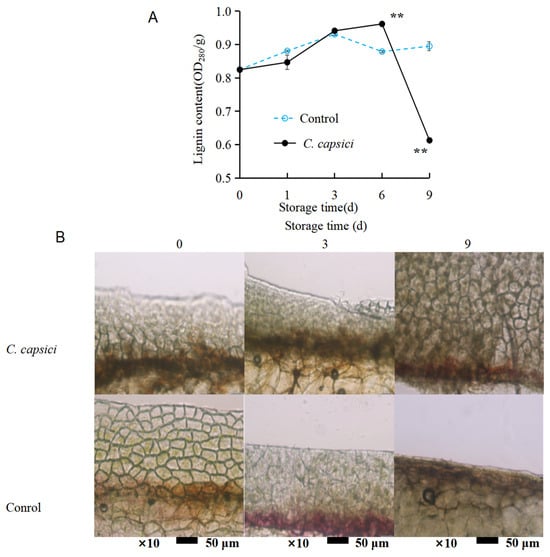
Figure 5.
Effects of C. capsici infection on lignin content (A) and lignin deposition (B) of peppers. The asterisks represent the significant difference (** p < 0.01) between C. capsici-treated peppers and control peppers on each storage day.
To better visualize lignin accumulation under different treatments, a staining method was used to detect lignin deposition in the cell wall. Phloroglucinol ethanol staining turns lignin red or purple-red. As shown in Figure 5B, this red or purple-red coloration was noticeable starting from day 3 in the control group. However, in the C. capsici treatment group, the color appeared consistently throughout the storage period, with a notably darker hue on days 3 and 6. This indicates that while mechanical wounding alone can initiate lignin accumulation as a local repair response (as seen in the control group), pathogen infection further amplifies this response, leading to more extensive lignin deposition in the infected tissue.
3.6. C. capsici Infestation on Total Phenolic Content and Flavonoid Content of Peppers
As shown in Figure 6A, the total phenolic content increased throughout the storage period. In the control group, this increase was gradual. However, after 3 d of C. capsici inoculation, the content rose significantly. It remained consistently higher than the control during the entire storage time. Figure 6B illustrates the variation trend of flavonoid content across different treatments. Both groups exhibited a similar trend, with flavonoid content increasing during the first day and then gradually declining. However, the decline in the C. capsici-treated group was notably less pronounced compared to the control (p > 0.05). These findings suggest that the pathogen may stimulate an accumulation of phenolic compounds in peppers, possibly through indirect mechanisms such as oxidative stress or localized defense responses.
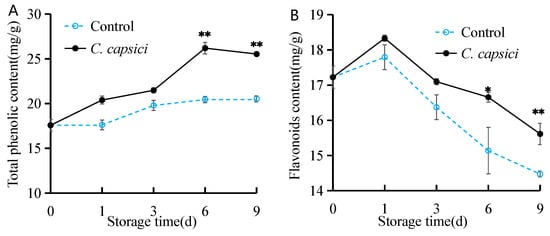
Figure 6.
Effect of C. capsici on total phenolic content (A) and flavonoid content (B). The asterisks represent the significant difference (* p < 0.05, ** p < 0.01) between C. capsici-treated peppers and control peppers on each storage day.
3.7. C. capsici Infestation on Activities of Disease Resistance-Related Enzymes of Peppers
As shown in Figure 7A, PAL activity in the control group exhibited a fluctuating increase throughout the storage period. In the C. capsici-treated group, PAL activity peaked at 131 U/g on the third day before declining sharply to its lowest point, 17.7 U/g. This decrease may reflect the collapse of host cellular structure and metabolic inactivation under advanced infection stress.
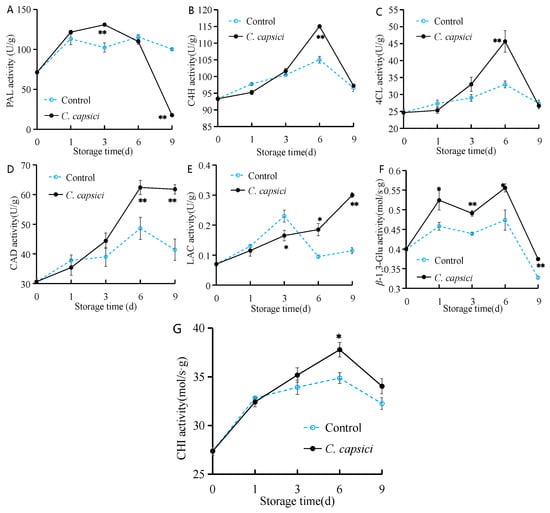
Figure 7.
Effects of C. capsici infection on PAL activity (A), C4H activity (B), 4CL activity (C), CAD activity (D), LAC activity (E), β-1,3-Glu activity (F), and CHI activity (G) of peppers. The asterisks represent the significant difference (* p < 0.05, ** p < 0.01) between C. capsici-treated peppers and control peppers on each storage day. PAL, Phenylalanine ammonia-lyase; C4H, Cinnamic acid-4-hydroxylase; 4CL, 4-coumaric acid CoA ligase; CAD, Cinnamyl alcohol dehydrogenase; LAC, Laccases; β-1,3-Glu, β-1,3-glucanase; CHI, Chitinase.
Interestingly, the activity trends of C4H and 4CL in both the control and C. capsici-treated groups were similar, showing an initial increase followed by a decrease, with peak activity observed on the sixth day (Figure 7B,C). Notably, the C. capsici-treated group displayed higher activity during the middle stage of storage.
As for CAD activity, both the control and C. capsici-treated groups showed a gradual increase, reaching their highest level on the sixth day, at 48 U/g and 62 U/g, respectively (Figure 7D). Afterwards, CAD activity declined to some extent, though overall, the C. capsici-treated group consistently exhibited higher CAD activity than the control group.
Figure 7E shows that LAC activity in the control group initially increased for the first three days, remaining higher than the C. capsici-treated group during this period. After 3 d, LAC activity in the control group dropped dramatically. Meanwhile, in the C. capsici-treated group, LAC activity steadily increased over the storage period, becoming significantly higher than in the control in the later stages, particularly on the ninth day, approximately twice that of the control group.
For β-1,3-Glu activity, both the control and C. capsici-treated groups showed two local peaks during the storage period, with the C. capsici-treated group maintaining significantly higher levels than the control at most time points (p < 0.05; Figure 7F). During the storage period, CHI activity first increased and then decreased. In the first three days of storage, there was no significant difference in CHI activity between the control and C. capsici-treated groups. However, in the mid-to-late stages, CHI activity in the C. capsici-treated group was significantly higher than that in the control group (p < 0.05; Figure 7G).
3.8. Correlation and Principal Component Analysis (PCA)
As shown in Figure 8A, storage time exhibited a significant or highly significant positive correlation with total phenolic content (p < 0.05) and soluble pectin content (p < 0.01) while showing a negative correlation with firmness (p < 0.01) and protopectin content (p < 0.01). These correlations suggest that, over time, the degradation of structural polysaccharides such as protopectin into soluble pectin contributes to fruit softening, while the accumulation of phenolic compounds likely reflects an induced defense response. In the C. capsici group, the storage time was positively correlated with disease index (p < 0.01). Disease progression following C. capsici infestation was closely tied to changes in cell wall metabolism and phenylpropanoid metabolism. For instance, the disease index showed a significant negative correlation with firmness, hemicellulose content, protopectin content, and flavonoid content (all p < 0.05), suggesting that C. capsici infection weakens the structural components of the cell wall and suppresses the biosynthesis or stability of flavonoids, which are known to have antimicrobial properties. In contrast, it was positively correlated with soluble pectin content (p < 0.01) and LAC and CAD activity (both p < 0.05), enzymes involved in lignin biosynthesis. This indicates that as the disease advances, cell wall disassembly is accompanied by attempts to reinforce cell walls via lignification as part of the host defense. Additionally, the firmness of pepper fruit was positively associated with structural cell wall components, particularly protopectin (p < 0.01). Interestingly, firmness was negatively correlated with cell wall polysaccharide depolymerization and disease index, as evidenced by increased PG and CAD activity, and increased total phenolic content. This pattern suggests a causal chain wherein C. capsici infection induces defense responses—including the activation of phenylpropanoid metabolism and lignification—but at the cost of accelerating cell wall disassembly and fruit softening.
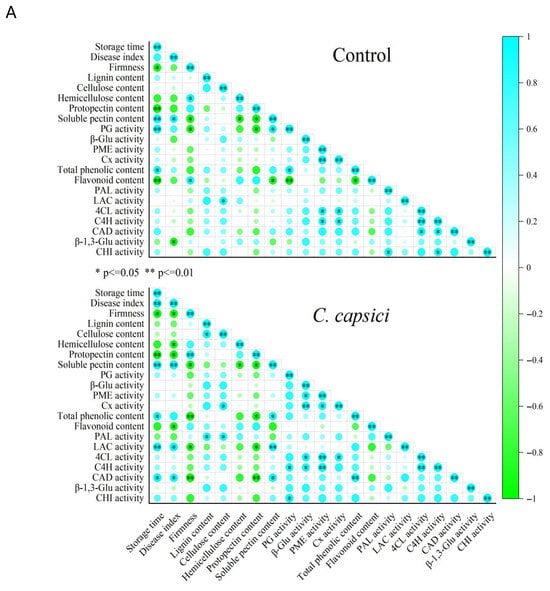
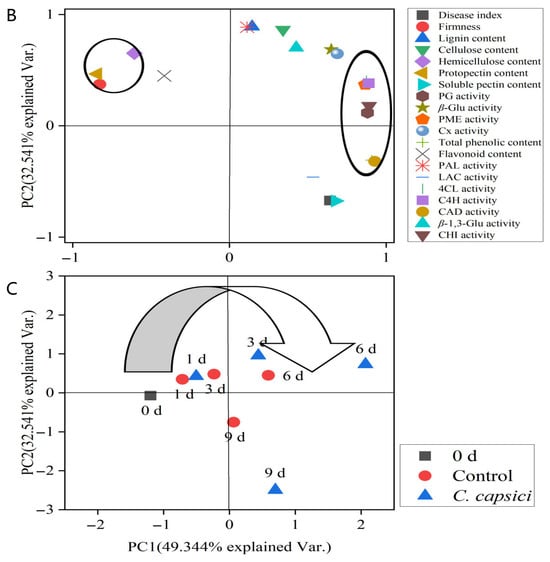
Figure 8.
Correlation matrix (A), loading plot (B), and score plot (C) of disease development and cell wall metabolism of pepper fruit treated by control and C. capsici.
Principal component analysis (PCA) was performed on the standardized data of relevant indicators during storage, and Figure 8B,C show that component 1 (PC1) and component 2 (PC2) account for 49.344% and 32.541% of the total variation, respectively. PG, PME, and Cx, soluble pectin content, and β-Glu activity were all clustered along the positive axis of PC1 (49.344%), whereas firmness, hemicellulose content, and protopectin content were positioned along the negative axis of PC1 (Figure 8B). This indicates that a higher PC1corresponds to increased soluble pectin content, PG activity, PME activity, Cx activity, and β-Glu activity, while firmness and protopectin content decrease. Therefore, PC1 is defined as the component related to cell wall metabolism. Meanwhile, disease resistance-related indicators (lignin content, flavonoid content, PAL activity, β-1,3-Glu activity, CHT activity, 4CL activity, and C4H activity) were clustered along the positive axis of PC2 (32.541%). In contrast, the disease index exhibited a negative loading on PC2, suggesting an inverse covariation with other PC2-associated variables, such as lignin content, PAL activity, β-1,3-Glu activity, CHI activity, 4CL activity, and C4H activity. Thus, PC2 may reflect a covariation pattern among variables associated with disease resistance-related biochemical responses.
The score plot (Figure 8C) showed that, over the course of storage, the trend shifted from the positive axis of PC2 to the negative axis. In combination with the loading plot, this indicated a decrease in lignin content, flavonoid content, PAL activity, β-1,3-Glu activity, CHI activity, 4CL activity, and C4H activity, while the disease index increased. In contrast to the control group, C. capsici accelerated this trend. Additionally, a shift from the negative axis to positive axis of PC1 was observed, suggesting increased PG activity, PME activity, Cx activity, and β-Glu activity, alongside a reduction in firmness. Similarly, C. capsici stimulated this change.
These results highlight that C. capsici promotes the cell wall degradation and weakens disease resistance, contributing to progression of disease.
4. Discussion
Pathogenic fungal infestation is one of the main causes of postharvest pepper spoilage and deterioration, with anthracnose caused by Colletotrichum leading to destructive loss in the pepper industry. C. capsici is one of the four pathogenic fungi responsible for pepper anthracnose in China. In this study, C. capsici infestation resulted in an increased disease index (data has not been publicly released yet), causing dark spots and even rot on the fruit surface, which severely compromised the storage quality of peppers (Figure 1). In addition, C. capsici infestation exacerbated the decline in firmness and hastened the softening of pepper fruit with the extension of storage time (Figure 1B). It also caused the breakdown of the cell wall structure and enrichment of C. capsici spores in the epidermal cells of peppers (Figure 3), leading to irreversible damage to peppers. Similarly, Alternaria alternata infestation on muskmelon increased both spot diameter and spot depth significantly, and a large number of hyphae attached were observed by SEM [16]. These indicate that fruits and vegetables, especially those with wounds, are readily susceptible to invasion and provide an excellent environment for the growth of pathogenic bacteria.
Pathogens can pass through the mechanical barriers of fruits and vegetables and colonize and grow in the epidermis [34], which is consistent with our results (Figure 2). The cell wall is a vital structure for maintaining the integrity of plant cells and also functions as a physical barrier against pathogen attack. It is a dynamic and rigid structure composed mainly of polysaccharides such as cellulose, hemicellulose, and pectin [35]. Cellulose was encapsulated in a matrix of hemicellulose and pectin, forming microfibrils to maintain cellular rigidity and preserve cellular structural integrity [36]. Hemicellulose, a branched-chain heteropolysaccharide, is primarily found in the primary cell wall, forming a structural framework in combination with microfibrils [36]. Pectin is a complex structure of polysaccharides, mainly in the middle layer of the cell wall, responsible for cell adhesion, mainly including soluble pectin and protopectin [14,37]. Thus, rearranging the cell wall components makes fruit flaccid and soft [13,35]. Previous studies demonstrated that pathogen infection could affect cell wall reorganization. For example, Phomopsis longanae Chi infection decreased the cellulose and hemicellulose contents but promoted the water-soluble pectin content [38]. Similarly, pummelo fruit presented low contents of protopectin, cellulose, and hemicellulose after Diaporthe citri invasion [17]. The results of this study showed that C. capsici infestation increased the soluble pectin content (Figure 3D). Interestingly, cellulose, hemicellulose, and protopectin levels initially rose before declining in both C. capsici-infected and control fruit (Figure 3A–C). This transient increase may reflect an early stress response, during which structural components are reinforced at damage sites to delay cell wall disassembly under mechanical or pathogenic stress [39,40,41,42]. The cell wall can be reinforced at the specific site during the process. Nonetheless, with the extension of the storage time, particularly on the third and sixth day, obvious symptom onset occurred on the pepper surface, in accordance with low cellulose, hemicellulose, and protopectin levels, while soluble pectin was high (Figure 3). Due to the weakening capacity of wound healing and the intensification of pathogen infection, this ultimately leads to progressive disassembly of the cell wall structure, gradually compromising its integrity and weakening its protective function. As seen in Figure 1A, the control group without C. capsici infection also exhibited slight surface deterioration on the ninth day, which may be attributed to natural senescence under prolonged high-humidity storage conditions.
The variations in cell wall composites are correlated with the interaction of cell wall metabolism-related enzymes. Cell wall-degrading enzymes, such as polygalacturonase (PG), pectin methylesterase (PME), β-glucosidase (β-Glu), and cellulase, contribute to the decomposition of cell wall polysaccharides, leading to the loss of integrity of the middle layer of the cell wall and of cellular toughness, which induces softening of the fruits and the loss of resistance to disease, which, in turn, facilitates the infestation of pathogenic bacteria [36,43]. In this study, we found that C. capsici infestation effectively increased the activities of PG, PME, β-Glu, and Cx but dramatically dropped after the sixth day (Figure 4). These results are similar to the responses observed in grape [43], longan [38], and Valencia orange [42] following pathogen infestation. Our results, although indicating an increase in cell wall-degrading enzyme activities and a decrease in fruit firmness, notably show that the content of cell wall components remains relatively high. This could be explained by the fact that, under stress responses (biotic or abiotic stress), the activity of cell wall-degrading enzymes increases, but the cell wall components are reinforced through various pathways to maintain the defense mechanism of the cell wall [44,45].
Cellulose, hemicellulose, and pectins form the primary cell wall, while the monolignols, precursors of lignin, are deposited into the cell wall matrix and polymerized in situ through oxidative reactions, resulting in lignin formation and the development of the secondary cell wall [44]. Cell wall-degrading enzymes such as cellulase hydrolyze cellulose when plants are subjected to pathogen or mechanical stress. Simultaneously, lignin is deposited at the wound site, reinforcing the barrier and filling in the defects in the cell wall. This study found that C. capsici infestation accelerated lignin deposition at the wound and also increased lignin content in the early stage, but the lignin content decreased sharply in the late storage period due to the severe disease (Figure 5). Similarly, inoculation of jujube with A. alternata showed a trend of increasing and then decreasing lignin content [22]. The monomer phenolic substrates are produced by the phenylpropanoid metabolism pathway and catalyzed by CAD and LAC [22]. Guo et al. [21] reported that caffeic acid enhanced CAD activity in pear under A. alternata inoculation, while Liu et al. [22] showed that methionine enhanced LAC activity in jujube to boost lignin accumulation against A. alternata. In this study, when the disease was not obvious, the activities of CAD and LAC were weak but both showed an increasing trend, which may be due to mechanical injury beginning to stimulate the resistance of pepper. After the appearance of disease spots, the activities of CAD and LAC significantly increased, possibly because the growth of pathogenic bacteria further stimulated the resistance of pepper fruit. The results correspond to the enrichment of lignin.
Above all, these findings highlight that the dynamic interplay between lignin deposition, pectin solubilization, and fruit firmness plays a crucial role in maintaining tissue integrity during C. capsici infestation. In the early stages, the fruit responds to wounding and pathogen attack by activating phenylpropanoid metabolism, leading to localized lignin accumulation. This temporary reinforcement stabilizes the cell wall and helps preserve firmness despite the increased activity of cell wall-degrading enzymes. As infection advances, lignin levels decline while soluble pectin increases and structural polysaccharides (protopectin, cellulose, hemicellulose) degrade. This shift from defense to disintegration corresponds with rapid softening. The inverse pattern of lignin and soluble pectin suggests that failure to maintain lignification accelerates pectin depolymerization, weakening cell adhesion and promoting tissue collapse. Thus, fruit softening results not only from enzymatic degradation but also from the inability to sustain cell wall reinforcement. These coordinated changes mark the breakdown of structural integrity and the loss of resistance, underscoring the central role of cell wall remodeling in disease progression and postharvest deterioration.
Total phenolics and flavonoids are synthesized mainly by the phenylpropanoid metabolism pathway, which is regulated by several key enzymes, such as PAL, C4H, and 4CL, to strengthen disease resistance in fruits and vegetables [20,21]. In this study, C. capsici infestation led to an accumulation of total phenolic content and flavonoids (Figure 6), in line with the changes in activities of PAL, C4H, and 4CL (Figure 7A–C). Moreover, both phenols and flavonoids can be oxidized into quinones, which have toxic effects on pathogens. This can also explain the decrease in total phenolic and flavonoid content in the later stages of storage in our study. The changes in β-1,3-Glu and CHI activity related to disease resistance further confirm this. The pepper fruit exhibited higher activities of β-1,3-Glu and CHI during the early stage of the disease; however, as the disease progresses, enzyme activity decreases (Figure 7F,G). Similarly, Yan et al. [16] demonstrated that NO could induce high β-1,3-Glu and CHI activities during the process of inducing disease resistance in muskmelons. However, it should be noted that the upregulation of these enzymes may also be mediated by endogenous signaling molecules such as H2O2, salicylic acid (SA), jasmonic acid (JA), or ethylene (ET), independent of the physical presence of the pathogen. Further studies are needed to dissect these regulatory mechanisms.
5. Conclusions
Post-inoculation with C. capsici accelerates disease development over time, leading to firmness loss and quality decline in harvested pepper fruit. This study uncovered that the decline in firmness is closely associated with cell wall disassembly, as indicated by increased activities of PG, PME, and Cx, which degrade pectin and cellulose—key components responsible for maintaining cell wall integrity. This degradation weakens the physical barrier, causing it to soften and facilitating fungal invasion. Simultaneously, disease progression is linked to disruptions of the phenylpropanoid metabolic pathway, as evidenced by reduced activities of PAL, C4H, 4CL, CAD, LAC, CHI, and β-1,3-Glu, along with a decline in phenolic compounds and lignin, undermine the fruit’s natural pathogenic resistance. Interestingly, this study also found that C. capsici infection triggers a transient early-stage stress response characterized by the reinforcement of the cell wall structure and the strengthening of disease resistance. From a practical perspective, lignin and soluble pectin may serve as biomarkers for the early detection and tracking of disease progression in postharvest pepper. Moreover, treatments aimed at enhancing lignin biosynthesis or delaying pectin solubilization could help strengthen fruit resistance and extend shelf life during storage. In summary, the probable mechanism diagram is depicted below (Figure 9).
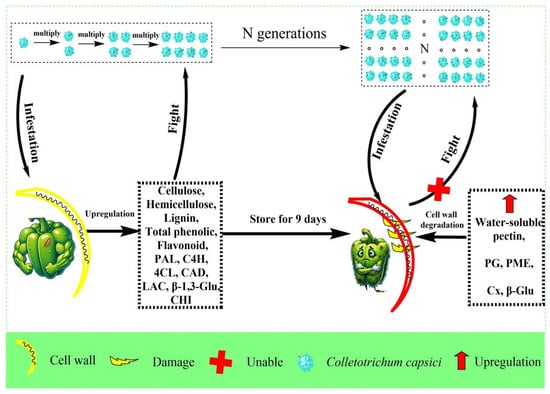
Figure 9.
The probable mechanism schematic presenting the effect of Colletorichum capsici infection on cell wall and disease resistance in pepper.
Author Contributions
Y.L. (Yunfen Liu): designed the research program, formal analysis, writing—review, and editing. Q.S.: investigation, formal analysis, writing—original draft. F.Y.: investigation, funding acquisition. Y.L. (Yuanli Liang): investigation. M.S.: investigation. M.H.: investigation, funding acquisition, writing—review, and editing. L.S.: conceptualization, resources, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Guangxi Province (2024GXNSFBA010323; 2023GXNSFBA026112), and the Scientific Research Fund for Doctor of Hezhou University (2024BSQD06).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Queiroz, A.G.D.; Ramos, E.G.; Armond, C.; Felipini, R.B.; Di Piero, R.M. Coating based on gelatin and propolis for the control of anthracnose in postharvest of bell pepper. Food Control 2024, 166, 110689. [Google Scholar] [CrossRef]
- Liu, C.; Wan, H.; Yang, Y.; Ye, Q.; Zhou, G.; Wang, X.; Ahammed, G.J.; Cheng, Y. Post-Harvest LED light irradiation affects firmness, bioactive substances, and amino acid compositions in chili pepper (Capsicum annum L.). Foods 2022, 11, 2712. [Google Scholar] [CrossRef] [PubMed]
- Tiamiyu, Q.O.; Adebayo, S.E.; Ibrahim, N. Recent advances on postharvest technologies of bell pepper: A review. Heliyon 2023, 9, e15302. [Google Scholar] [CrossRef]
- Barik, S.; Ponnam, N.; Reddy, A.C.; Readdy, D.C.L.; Saha, K.; Acharya, G.C.; Reddy, K.M. Breeding peppers for industrial uses: Progress and prospects. Ind. Crops Prod. 2022, 178, 114626. [Google Scholar] [CrossRef]
- González-Saucedo, A.; Barrera-Necha, L.L.; Ventura-Aguilar, R.I.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Hernández-López, M. Extension of the postharvest quality of bell pepper by applying nanostructured coatings of chitosan with Byrsonima crassifolia extract (L.) Kunth. Postharvest Biol. Technol. 2019, 149, 74–82. [Google Scholar] [CrossRef]
- de SÁ Mendes, N.; Branco De Andrade Gonçalves, É.C. The role of bioactive components found in peppers. Trends Food Sci. Technol. 2020, 99, 229–243. [Google Scholar] [CrossRef]
- Feng, P.; Zhang, X.; Godana, E.A.; Ngolong Ngea, G.L.; Dhanasekaran, S.; Gao, L.; Li, J.; Zhao, L.; Zhang, H. Control of postharvest soft rot of green peppers by Bacillus subtilis through regulating ROS metabolism. Physiol. Mol. Plant Pathol. 2024, 131, 102280. [Google Scholar] [CrossRef]
- Diao, Y.; Zhang, C.; Liu, F.; Wang, W.; Liu, L.; Cai, L.; Liu, X. Colletotrichum species causing anthracnose disease of chili in China. Persoonia 2017, 38, 20–37. [Google Scholar] [CrossRef]
- Ali, A.; Bordoh, P.K.; Singh, A.; Siddiqui, Y.; Droby, S. Post-harvest development of anthracnose in pepper (Capsicum spp): Etiology and management strategies. Crop Prot. 2016, 90, 132–141. [Google Scholar] [CrossRef]
- Boukaew, S.; Chumkaew, K.; Petlamul, W.; Srinuanpan, S.; Nooprom, K.; Zhang, Z. Biocontrol effectiveness of Trichoderma asperelloides SKRU-01 and Trichoderma asperellum NST-009 on postharvest anthracnose in chili pepper. Food Control 2024, 163, 110490. [Google Scholar] [CrossRef]
- Ma, L.; Zheng, Y.; Sang, Z.; Ge, Y.; Bai, C.; Fu, A.; Wang, Q.; Watkins, C.B.; Zuo, J. Multi-omics analysis reveals the mechanism of calcium-reduced quality deterioration in mechanically injured green pepper fruit. Postharvest Biol. Technol. 2023, 204, 112437. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, X.; Liang, J.; Fu, Y.; Wang, J.; Jiang, M.; Pan, L. Cell wall and reactive oxygen metabolism responses of strawberry fruit during storage to low voltage electrostatic field treatment. Postharvest Biol. Technol. 2022, 192, 112017. [Google Scholar] [CrossRef]
- Chen, C.; Nie, Z.; Wan, C.; Gan, Z.; Chen, J. Suppression on postharvest juice sac granulation and cell wall modification by chitosan treatment in harvested pummelo (Citrus grandis L. Osbeck) stored at room temperature. Food Chem. 2021, 336, 127636. [Google Scholar] [CrossRef]
- Li, T.; Shi, D.; Wu, Q.; Yin, C.; Li, F.; Shan, Y.; Duan, X.; Jiang, Y. Mechanism of cell wall polysaccharides modification in harvested ‘Shatangju’ mandarin (Citrus reticulate Blanco) fruit caused by Penicillium italicum. Biomolecules 2019, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, W.; Fang, X.; Chen, H.; Han, Y.; Niu, B.; Gao, H. Zizania latifolia cell wall polysaccharide metabolism and changes of related enzyme activities during postharvest storage. Foods 2022, 11, 392. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, Z.; Zhang, P.; Zhu, X.; Jing, Y.; Wei, J.; Wu, B. Nitric oxide enhances resistance against black spot disease in muskmelon and the possible mechanisms involved. Sci. Hortic. 2019, 256, 108650. [Google Scholar] [CrossRef]
- Chen, C.; Cai, N.; Wan, C.; Huang, Q.; Chen, J. Cell wall modification and lignin biosynthesis involved in disease resistance against Diaporthe citri in harvested pummelo fruit elicited by carvacrol. J. Sci. Food Agric. 2022, 102, 3140–3149. [Google Scholar] [CrossRef]
- Palou, L.; Smilanick, J.L.; Droby, S. Alternatives to conventional fungicides for the control of citrus postharvest green and blue moulds. Stewart Postharvest Rev. 2008, 2, 2. [Google Scholar] [CrossRef]
- Li, N.; Chen, W.; Wang, B.; Zhang, C.; Wang, Y.; Li, R.; Yan, Y.; He, J. Arbuscular mycorrhizal fungi improve the disease resistance of Lycium barbarum to root rot by activating phenylpropane metabolism. Front. Plant Sci. 2024, 15, 1459651. [Google Scholar] [CrossRef]
- Li, C.; Wang, M.; Guo, Y.; Zhang, S.; Xu, H.; Ge, Y. Activation of the calcium signaling, mitogen-activated protein kinase cascade and phenylpropane metabolism contributes to the induction of disease resistance in pear fruit upon phenylalanine treatment. Postharvest Biol. Technol. 2024, 210, 112782. [Google Scholar] [CrossRef]
- Guo, Y.; Li, C.; Wang, M.; Xu, H.; Zhang, S.; Liu, J.; Jin, Y.; Ge, Y. Postharvest caffeic acid dipping enhances disease resistance and storage capacity of ‘Zaosu’ pear fruit via regulating phenylpropane metabolism. Postharvest Biol. Technol. 2024, 209, 112716. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, X.; Deng, B.; Chen, O.; Deng, L.; Zeng, K. Methionine enhances disease resistance of jujube fruit against postharvest black spot rot by activating lignin biosynthesis. Postharvest Biol. Technol. 2022, 190, 111935. [Google Scholar] [CrossRef]
- Lu, D.; Ren, Y.; Yan, T.; Jia, X.; Xu, H.; Yang, B.; Zhang, X.; He, J. Melatonin improves the postharvest anthracnose resistance of mango fruit by regulating antioxidant activity, the phenylpropane pathway and cell wall metabolism. Eur. J. Plant Pathol. 2025, 171, 17–36. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, W.; Li, X.; Chen, H.; Takac, T.; Samajova, O.; Fabrice, M.R.; Xie, L.; Ma, J.; Samaj, J.; et al. Expression and distribution of extensins and AGPs in susceptible and resistant banana cultivars in response to wounding and Fusarium oxysporum. Sci. Rep. 2017, 7, 42400. [Google Scholar] [CrossRef]
- Jia, S.; Zhang, N.; Dong, C.; Zheng, P.; Ji, H.; Yu, J.; Yan, S.; Chen, C.; Liang, L. Effect of cold plasma treatment on the softening of winter jujubes (Ziziphus jujuba Mill. cv. Dongzao). Horticulturae 2023, 9, 986. [Google Scholar] [CrossRef]
- Lu, M.; Wen, T.; Guo, M.; Li, Q.; Peng, X.; Zhang, Y.; Lu, Z.; Wang, J.; Xu, Y.; Zhang, C. Regulation of intracellular reactive oxygen species levels after the development of Phallus rubrovolvatus rot disease due to Trichoderma koningii Mycoparasitism. J. Fungi 2023, 9, 525. [Google Scholar] [CrossRef]
- Qu, L.; Xu, Z.; Huang, W.; Han, D.; Dang, B.; Ma, X.; Liu, Y.; Xu, J.; Jia, W. Selenium-molybdenum interactions reduce chromium toxicity in Nicotiana tabacum L. by promoting chromium chelation on the cell wall. J. Hazard. Mater. 2024, 461, 132641. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhu, N.; Zhu, C.; Wu, D.; Chen, K. Morphology and cell wall composition changes in lignified cells from loquat fruit during postharvest storage. Postharvest Biol. Technol. 2019, 157, 110975. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, X.; Wu, C.; Fan, G.; Li, T.; Dong, C. Retardation of postharvest softening of blueberry fruit by methyl jasmonate is correlated with altered cell wall modification and energy metabolism. Sci. Hortic. 2021, 276, 109752. [Google Scholar] [CrossRef]
- Song, X.; Dai, H.; Wang, S.; Ji, S.; Zhou, X.; Li, J.; Zhou, Q. Putrescine treatment delayed the softening of postharvest blueberry fruit by inhibiting the expression of cell wall metabolism key gene VcPG1. Plants 2022, 11, 1356. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Sang, Y.; Ma, Y.; Guo, M.; Bai, G.; Cheng, S.; Chen, G. Influences of ice-temperature storage on cell wall metabolism and reactive oxygen metabolism in Xinjiang (Diaogan) apricot. Postharvest Biol. Technol. 2021, 180, 111614. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Paice, M.G. Oxidation of non-phenolic substrates. FEBS Lett. 1990, 267, 99–102. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Bi, Y.; Jiang, Q.; Mao, R.; Liu, Z.; Huang, Y.; Zhang, M.; Prusky, D.B. Induction of defense response against Alternaria rot in Zaosu pear fruit by exogenous L-lysine through regulating ROS metabolism and activating defense-related proteins. Postharvest Biol. Technol. 2021, 179, 111567. [Google Scholar] [CrossRef]
- Li, Y.; He, H.; Hou, Y.; Kelimu, A.; Wu, F.; Zhao, Y.; Shi, L.; Zhu, X. Salicylic acid treatment delays apricot (Prunus armeniaca L.) fruit softening by inhibiting ethylene biosynthesis and cell wall degradation. Sci. Hortic. 2022, 300, 111061. [Google Scholar] [CrossRef]
- Chen, Y.; Hung, Y.; Chen, M.; Lin, H. Effects of acidic electrolyzed oxidizing water on retarding cell wall degradation and delaying softening of blueberries during postharvest storage. LWT 2017, 84, 650–657. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Lin, H.; Lu, W.; Wang, H.; Chen, Y.; Lin, Y.; Fan, Z. The role of cell wall polysaccharides disassembly in Lasiodiplodia theobromae-induced disease occurrence and softening of fresh longan fruit. Food Chem. 2021, 351, 129294. [Google Scholar] [CrossRef] [PubMed]
- Gwanpua, S.G.; Van Buggenhot, S.; Verlinden, B.E.; Christiaens, S.; Shpigelman, A.; Vicent, V.; Kermani, Z.J.; Nicolai, B.M.; Hendrickx, M.; Geeraerd, A. Pectin modifications and the role of pectin-degrading enzymes during postharvest softening of Jonagold apples. Food Chem. 2014, 1582, 83–91. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Lin, H.; Sun, J.; Lin, Y.; Wang, H.; Lin, M.; Shi, J. Phomopsis longanae Chi-induced changes in activities of cell wall-degrading enzymes and contents of cell call components in pericarp of harvested longan fruit and its relation to disease development. Front. Microbiol. 2018, 9, 1051. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, X.; Jing, G.; Ouyang, Q.; Tao, N. Cinnamaldehyde inhibits the mycelial growth of Geotrichum citri-aurantii and induces defense responses against sour rot in citrus fruit. Postharvest Biol. Technol. 2017, 129, 23–28. [Google Scholar] [CrossRef]
- Savatin, D.V.; Gramegna, G.; ModestiI, V.; Cervone, F. Wounding in the plant tissue: The defense of a dangerous passage. Front. Plant Sci. 2014, 5, 470. [Google Scholar] [CrossRef]
- Narváez-Barragán, D.A.; Tovar-Herrera, O.E.; Guevara-García, A.; Serrano, M.; Martinez-Anaya, C. Mechanisms of plant cell wall surveillance in response to pathogens, cell wall-derived ligands and the effect of expansins to infection resistance or susceptibility. Front. Plant Sci. 2022, 13, 969343. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Deng, L.; Zhou, Y.; Ming, J.; Yao, S.; Zeng, K. Wound healing in citrus fruit is promoted by chitosan and Pichia membranaefaciens as a resistance mechanism against Colletotrichum gloeosporioides. Postharvest Biol. Technol. 2018, 145, 134–143. [Google Scholar] [CrossRef]
- Li, J.; Wu, Z.; Zhu, Z.; Xu, L.; Wu, B.; Li, J. Botrytis cinerea mediated cell wall degradation accelerates spike stalk browning in Munage grape. J. Food Biochem. 2022, 46, e14271. [Google Scholar] [CrossRef] [PubMed]
- Le Gall, H.; Philippe, F.; Domon, J.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Gigli-Bisceglia, N.; Engelsdorf, T.; Hamann, T. Plant cell wall integrity maintenance in model plants and crop species-relevant cell wall components and underlying guiding principles. Cell. Mol. Life Sci. 2020, 77, 2049–2077. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).