Abstract
In vitro micropropagation is used to rapidly shorten the breeding process of crops, such as kale, an internationally widespread winter vegetable. The aim of this study is to develop optimised micropropagation protocols for three kale varieties. First, it was determined which seed surface disinfection method resulted in the highest germination rate and the lowest infection rate. Secondly, it was investigated which of several existing Brassica protocols and one modified protocol from the literature provided the highest regeneration efficiency of kale explant types (cotyledons, hypocotyl, root, and intact seedlings as the control) after eight weeks of cultivation. Germination was highest and fastest after disinfection with 10% NaClO for 10 min for “Frostara” and at 5% for 2.5 min for “Schatteburg”. The infection rate and speed were lowest in treatments with 10% NaClO. The regeneration efficiency and number of newly formed leaves, roots, shoots, and stems varied between media, explant type, and kale variety. Most new leaves and shoots were formed when hypocotyls were used as explant type. Roots regenerated mostly more roots than shoots, stems, and leaves. A higher ratio of auxin to cytokinin in the culture medium partially increased leaf regeneration. The addition of AgNO3 increased shoot regeneration and reduced yellowing and leaf drop. Phenotypic anomalies occurred less frequently in media with lower hormone concentrations. All tested protocols are suitable for kale micropropagation, but regeneration was highly dependent on the medium for different varieties and explant types. Therefore, this study builds a basis for future micropropagation of kale and the development of variety-specific protocols for maximum commercial success.
1. Introduction
In view of climate change and the simultaneously increasing global demand for food, strategies are needed to feed the population in a resource-efficient and future-proof manner [1]. One of these strategies is to achieve faster propagation and breeding success of climate-adapted and high-yielding vegetable varieties by circumventing the long growth period and development times. Kale (Brassica oleracea var. sabellica L.) is one of such vegetable species with a comparatively long life cycle [2], since it requires at least one year from sowing to harvesting new seeds [3]. Breeding has gained increased attention with recent recognition of the specific advantages of kale such as its fresh availability in winter [4] and its high nutritional value [5]. In general, kale has developed into one of the most important vegetables within the Brassicaceae family [6]. In addition, it has gained increasing economic importance in recent years, in which the cultivation and consumption of kale increased [5].
Newer, commercially used, high-yielding varieties that have been bred to be suitable for a wide range of growing conditions are among the most cultivated varieties. In contrast, old landraces, developed over many generations and adapted to local conditions, are less widely cultivated [7]. However, among both new and old varieties, there is currently no variety that combines the positive traits found individually in other different varieties, such as an improved composition of ingredients with positive effects on health, a high yield, and climate adaptation. Therefore, labour- and resource-intensive breeding is still required to produce new, advanced varieties in the face of ongoing global change. However, the time required to breed a new Brassica variety is eight to ten generations using conventional breeding [8]. This breeding work can be shortened and improved by using biotechnological methods such as genetic transformation but also considering in vitro micropropagation.
Micropropagation not only enables the conservation of gene pools [9] and the selective propagation of plants through cloning [10], but also the rapid and space-saving production of disease-free, uniform, and high-quality plants independent of the current season [10,11]. It is also essential for the recovery of transgenic plants [12].
In micropropagation, the organogenesis is influenced by many different parameters, such as culture medium composition, culture environment, and explant type [10,13]. In addition, the regeneration efficiency in Brassica has been shown to vary greatly between different genotypes under the same culture conditions [14,15]. Furthermore, there has been evidence of dominantly inherited traits in B. oleracea that can lead to intolerance to in vitro conditions resulting in the inability to regenerate new shoots [16]. There are also common problems such as contaminations and phenotypic anomalies [11]. Due to these problems, the development of genotype-specific protocols is necessary to achieve a time- and cost-saving and, above all, reproducible regeneration efficiency.
For each regenerable genotype, suitable sterilisation methods for aseptic culture conditions should be tested to strike a balance between the degree of contamination and the survival of the explants [17]. Various sterilisation methods have already been tested for Brassica, e.g., using sodium hypochlorite (NaClO) [18,19]. There are also various micropropagation protocols for Brassica available, with and without callus induction e.g., [8,20,21]. Currently, hypocotyl and cotyledon explants have become standard explant types for establishing Brassica in vitro cultures [6], but also regeneration from root explants has been reported, as in cauliflower (Brassica oleracea var. botrytis L.) [22]. Additives can have a large effect, e.g., addition of silver nitrate (AgNO3) had a positive effect on shoot regeneration in ornamental cabbage (Brassica oleracea L. var. acephala (D.C.) Alef.) [23]. Hormones can have various effects, for example 6-benzylaminopurine (BAP) and α-naphthaleneacetic acid (NAA) in oilseed rape (Brassica rapa L.) [13]. For kale, however, we are not aware of any published studies that have tested different sterilisation methods and applied specific protocols for micropropagation of seedling explants.
Therefore, the aim of this study is to develop and optimise an efficient in vitro regeneration protocol for kale plants to optimise the propagation and maintenance of elite plants in the future. To achieve this, we first present a comprehensive approach to investigate which NaClO disinfection method promotes seed germination and minimises infections. Secondly, we then tested a published protocol slightly modified by us as well as three other published Brassica protocols comparatively on their suitability for the micropropagation of three different kale varieties by using three different explant types.
2. Materials and Methods
2.1. Plant Material
The three kale varieties used in this study differed mainly in breeding history: Kale “Frostara” (Wolfgang Meier-Versandhandel, Fürstenwalde, Germany) is a commercially widely cultivated variety, “Schatteburg” (Dreschflegel GbR, Witzenhausen, Germany) is an old East Frisian variety that is mainly privately cultivated and has been grown in Northwestern Germany for decades, and “Oldenburger Palme” (seeds harvested at the Botanical Garden Oldenburg) is a new variety based on crossing of high-yielding commercial varieties with robust landraces that is still under development.
2.2. Seed Sterilisation Experiment
Different surface sterilisation treatments were carried out on 25 seeds of only the two varieties Frostara and Schatteburg, using different concentrations and application times of sodium hypochlorite (NaClO) (Table 1) [18,24,25]. Oldenburger Palme seeds were not tested in this experimental part due to a lack of seeds.

Table 1.
Seed surface sterilisation treatments for kale varieties Schatteburg and Frostara.
We added 25 randomly selected seeds to 30 mL of each solution for the respective application time (5% or 10% NaClO for 2.5, 5, or 10 min; Table 1). Afterwards, the disinfected seeds were rinsed three times with sterile water in a laminar flow. We then placed the rinsed seeds in Petri dishes with moistened (3 mL sterile water) filter paper and wrapped the Petri dishes with parafilm. Three Petri dishes with five seeds each were prepared for each variety and each treatment. All test samples were stored in an incubation room at 22.5 °C with a light/dark cycle of 16/8 h and a light intensity on average of 2590 lux during the day and 21 lux during the night (Table S1).
To determine the germination rate (GR), we counted the number of germinated seeds per treatment (NGS). Seeds were considered to have germinated when the radicle had broken through the testa of the seed [26]. The NGS was determined every working day until more than 75% of the seedlings had reached the seedling size required for micropropagation, and the last time at the 14th trial day (D). Using NGS and the total number of seeds (TS), the GR was calculated (Formula (1)). Mouldy seeds, if found, were excluded from the calculation by reducing the total number of seeds per Petri dish.
The germination speed (GS) was determined using the Timson index [27] modified by Khan and Ungar [28] (Formula (2)).
We also recorded the number of fungal-infected seeds (NIS) on the same days, recognised both with and without binoculars, and determined the infection rate (IR) and infection speed (IS) (Formulas (3) and (4)).
2.3. Micropropagation
2.3.1. Culture Media
Five media with different combinations and concentrations of nutrients and hormones, added to the Murashige and Skoog (MS) medium [29], were tested (Table 2):

Table 2.
Physical and chemical characteristics of the supplemented and unsupplemented Murashige and Skoog [29] media used for micropropagation (- = not included, 1 = Bhalla and Singh [8], 2 = Zhao et al. [13], 3 = Dai et al. [23]).
Medium 1: A Brassica reproduction protocol by Bhalla and Singh [8], consisting of three separate media compositions (here denominated as 1a–c; Table 2), which were applied one after the other when the media boxes were changed (Table 3) (BAP:NAA the ratios 3.75:1; 15:1; 0.001:0, respectively).

Table 3.
The tested media for each variety, explant types/control, and number of media boxes used. The three explant types used were cotyledons, hypocotyls, and roots. Intact seedlings were explanted as a control. Each media box contained one of the explant types/or the control. Each treatment (explant type/control and medium) was repeated three times.
Medium 2: A Brassica reproduction protocol by Bhalla and Singh [8], by calculating the mean value of each plant growth regulator from the three separate media 1a–c (6.25:1).
Medium 3: A medium by Zhao et al. [13], whereby the MS-medium was supplemented with BAP and NAA (4:1).
Medium 4: Medium 3 with added AgNO3.
Medium 5: MS medium following the protocol of Murashige and Skoog [29], which is free of plant growth regulators and serves as the control.
Prepared media were filled into plastic boxes and stored in the dark at 15–20 °C until use.
2.3.2. Culture Conditions of Explants
For micropropagation experiments, surface-sterilised seeds (Table 1 treatment e) of the three kale varieties were germinated in Petri dishes and, after germination, seedlings were separated into different explant types. The explants used were cotyledons (Co; approx. 1 mm long), hypocotyl (Hy; approx. 5 mm long), and roots (Ro; approx. 4 mm long). Intact seedlings (Se) were used as the control. When cutting the explants, meristems were avoided [8]. The explants were placed on the different media according to Table 3. For Oldenburger Palme, only the media 3, 4, and 5 were tested due to a lack of seeds, and only one box per medium was prepared with intact seedlings.
The explants were transferred to a fresh box of the same medium approximately every one to two weeks according to the respective protocol but they were not cut (Table 4).

Table 4.
Time of media changes. (Week 1 = start of the experiment, explants placed on first medium; x = medium was changed; - = medium was not changed).
2.3.3. Temperature and Light Conditions
The temperature in the breeding room was on average 24.2 °C (19.1–27.9 °C) during the day and 21.6 °C at night (19.8–23.3 °C). Light intensity was on average 2590 lux during the day (473–3800 lux) and 21 lux at night (0–860 lux) (raw data in Table S1, figures in File S1).
2.3.4. Regeneration and Phenotypical Anomalies
After eight weeks of culture on the media, for every explant, the number of regenerated adventitious roots, adventitious shoots, leaves, stems, and calluses were determined. We counted regenerated tissue both on the explant and on the callus. We also determined the regeneration efficiency (RE) of regenerated tissues using the number of explants showing regeneration (NREx) and the total number of explants (TNEx) (Formula (5)) [30].
Furthermore, we checked each explant for phenotypical anomalies; namely, hyperhydricity (swollen tissue), shoot tip necrosis (brown shoot tips), albinism (white tissue), yellowing (yellow tissue), leaf abscission (leaf detached from explant), and phenolic oxidation (brown tissue).
2.4. Statistical Analysis
Statistical and graphical analysis was performed using the statistical software R v.4.1.3 [31]. To detect significant differences between explants, as well as between treatments (media) of one species, we used the Kruskal–Wallis test [32], followed by the pairwise Wilcox post hoc test [33]. The graphical analysis was carried out using the packages ggplot2 [34], tidyverse [35], ggpattern [36], patternplot [37], ggh4x [38], car [39], psych [40], ggpubr [41], reshape [42], and hrbrthemes [43].
3. Results
3.1. Examination of Disinfection Methods
For Frostara, the required explant length was achieved after 11 days, for Oldenburger Palme after 7 days, and for Schatteburg after 6 days.
3.1.1. Germination Rate and Speed
The germination rate of Frostara kale seeds in Petri dishes was highest in the treatment with 10% NaClO for 10 min, being 33% higher than in the 5%/2.5 min treatment, which had the lowest germination rate (60%) (Figure 1, raw data in Table S2). In Frostara, a 100% germination rate was not achieved in any of the treatments. In contrast, the germination rate of 80% of the Schatteburg treatments reached the maximum of 100% except for the 10%/5 min treatment, whose rate was 7% lower than the others. In Frostara, seeds with infections had a reduced germination rate and speed in the 5%/2.5 min treatment (Figure 1, Figure 2, Figure 3 and Figure 4).
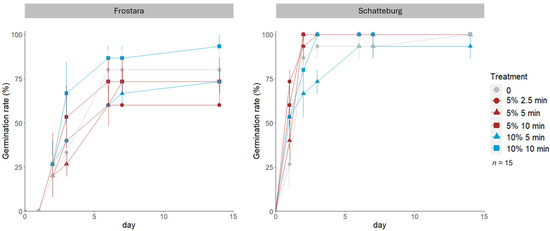
Figure 1.
Germination rate (%) of seeds of kale varieties Frostara (left) and Schatteburg (right) monitored for 15 days and treated with different concentrations of NaClO (%) in different durations (min) for disinfection; seeds were germinated on filter paper in Petri dishes in a breeding room; n = 15, means ± standard error (SE) (raw data in Table S2).
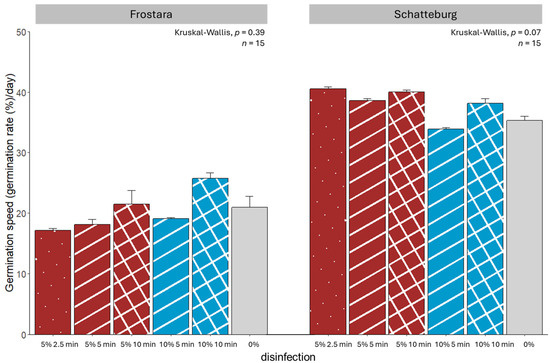
Figure 2.
Germination speed of seeds of kale varieties Frostara (left) and Schatteburg (right) treated with different concentrations of NaClO (%) in different durations (min) for disinfection; seeds were germinated on filter paper in Petri dishes in a breeding room; n = 15, means ± SE (raw data in Table S2).
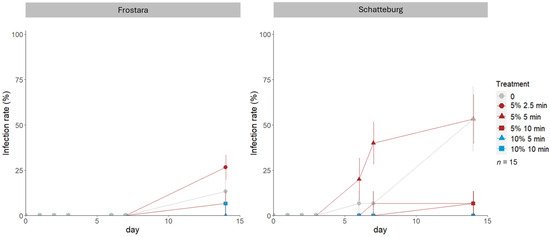
Figure 3.
Infection rate (%) of seeds of kale varieties Frostara (left) and Schatteburg (right) monitored for 15 days and treated with different concentrations of NaClO (%) in different durations (min) for disinfection; seeds were germinated on filter paper in Petri dishes in a breeding room; n = 15, mean ± SE (raw data in Table S2).
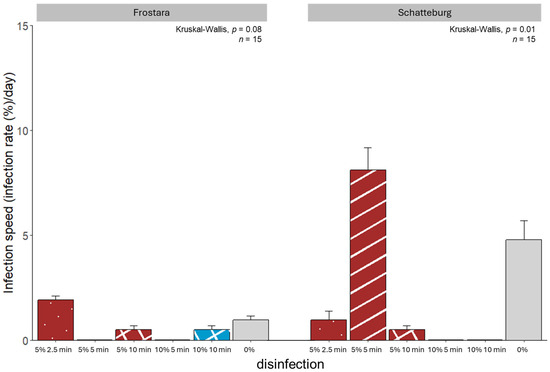
Figure 4.
Infection speed of seeds of kale varieties Frostara (left) and Schatteburg (right) treated with different concentrations of NaClO (%) in different durations (min) for disinfection; seeds were germinated on filter paper in Petri dishes in a breeding room; n = 15, mean ± SE (raw data in Table S2).
The maximum germination speed value that could be achieved in the present study was 42.9 (GS = 1800%/14 days). The germination speeds of Schatteburg seeds in Petri dishes in the breeding room were in all treatments significantly higher than those of Frostara. For Frostara, speed was highest in the 10%/10 min treatment being approximately 8% higher than in the 5%/2.5 min treatment, which had the lowest germination speed, although without significant differences (Figure 2, raw data in Table S2). In contrast, Schatteburg achieved the highest germination speed in the treatment with 5% NaClO for 2.5 min, which was 7% higher than the slowest speed in the 10%/5 min treatment, also without significant differences.
3.1.2. Infection Rate and Speed
The infection rate of Frostara seeds germinated in Petri dishes was highest when treated with 5% NaClO for 2.5 min, being 27% higher compared to the lowest infection rates when treated with either 5% or 10% NaClO for 5 min (Figure 3, raw data in Table S2). In Schatteburg, in contrast, the infection rate was highest with 5%/5 min being 53% higher compared to both 10% NaClO treatments (which had the lowest infection rates).
The maximum infection speed value that could be achieved in this study was 42.9 (IS = 1800%/14 days). Compared to Frostara, we determined higher infection speeds in Schatteburg treatments (Figure 4, raw data in Table S2). Here, the highest infection speed was achieved when treated 5 min with 5% NaClO, which was 8% higher (but not significant) than the lowest speeds in the 10%/5 min and 10%/10 min treatments. In contrast, the Frostara seeds had the highest infection speed when treated with 5%/2.5 min that was 2% higher (but not significant) than infection speed of both 10 min treatments.
3.2. Examination of Micropropagation Protocols
The required explant lengths were reached approx. 5 days after start of germination in Schatteburg, 10 days in Frostara, and 7 days in Oldenburger Palme.
3.2.1. Regeneration of Leaves, Roots, Callus, Shoots, and Stems
Formation of leaves from the explants. Between medium 1 (M1) and 2 (M2), there were on average only slight and non-significant differences in the number of leaves formed on the explant (Figure 5). However, in M2, more leaves per callus were formed in Schatteburg on all explant types compared to M1 (12–64% more), but this was not significant. For Frostara, in contrast, there was no uniform pattern.
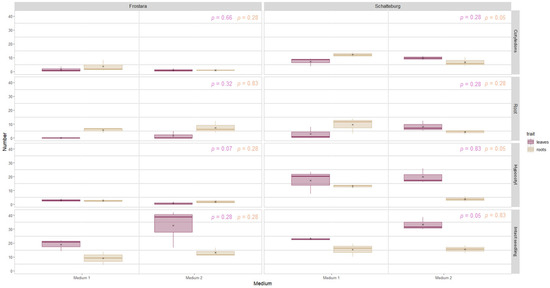
Figure 5.
Number of regenerated leaves (purple) and roots (beige) of micropropagated kale (Frostara and Schatteburg) from different explant types (displayed in rows) grown on media 1 and 2 after eight weeks. Data represent mean ± SE of three independent boxes each containing five explants (in the case of cotyledons, there were 10 each) (deviations for Frostara M1 Ro: n = 4, Frostara M2 Co: n = 9). Kruskal–Wallis test, α = 0.05, NA = no stat. test possible, p-value colours correspond to the colours indicated in the legend (raw data in Table S3).
In Frostara-M1, the explant type cotyledons (hereafter abbreviated as Co) produced on average 42% more leaves compared to M2, and explant type hypocotyls (Hy) produced 75% more leaves (not significant) (Figure 5). In contrast, for both explant types, roots (Ro) and intact seedling (Se), more leaves were formed in M2 than in M1 (Ro 100% more, Se 68% more, not significant).
For medium 3 (M3) and 4 (M4), no positive effect was apparent for any of the two media with regard to the formation of a more significant number of leaves on any of the explant types (Figure 6). In medium 5 (M5), no leaves at all were formed on the callus, and no leaves were formed on the explant for the explant type Ro.
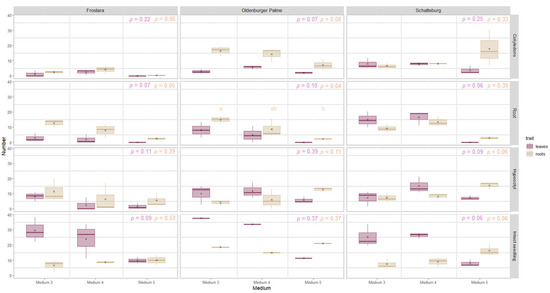
Figure 6.
Number of regenerated leaves (purple) and roots (beige) of micropropagated kale (Frostara, Oldenburger Palme, and Schatteburg) from different explant types (displayed in rows) grown on media 3, 4, and 5 after eight weeks. Data represent mean ± SE of three independent boxes each containing five (for Schatteburg and Frostara) or three explants (for Oldenburger Palme), respectively (in the case of cotyledons, there were 10 or 6 each, respectively) (deviation for Frostara M3 Ro: n = 4). Kruskal–Wallis test, α = 0.05, NA = no stat. test possible, p-value colours correspond to the colours indicated in the legend (raw data in Table S3).
From the explant type Se, compared to the other explant types, most leaves were formed on the explant and callus in Oldenburger Palme and Frostara, but not in Schatteburg. From Hy, often more leaves were formed on the callus in all varieties within the respective medium than from Ro and Co (8 to 56% more, in some cases significantly more).
The regeneration efficiency of leaves on both the explant and callus was not significantly different and without any clear trend between medium 1 and 2 and also not between medium 3 and 4 (File S2: Figures S5–S8). In medium 5, the regeneration efficiency of leaves at both the explant and callus was again zero in most cases and thus lower than in the other media (up to 100% lower, not significant) (Figures S5 and S6).
Concerning the explant types, the regeneration efficiency of leaves at both the explant and at the callus was almost always the highest for Se in all media (41 to 50% higher). The regeneration efficiency of leaves at the callus was lower in the explant type roots in medium 1 and 2 compared to the other explant types (up to 85% lower).
Formation of roots. The number of roots formed at the callus was not significantly higher in medium 1 than in medium 2 (Figure 5, raw data in Table S3, photos in Figure S13). There were also no significant differences between medium 3 and 4 except for Schatteburg Hy and no clear trend regarding more or less new roots in any of the media, both between and within the varieties (Figure 6).
Comparing the explant types, on average most roots on medium 1 and 2 were formed on the callus of the Se explant (between 15% and 94% more than with the other explants, not significant) (Figures S5 and S6). On medium 3 and 4, in contrast, this was mostly the case for the Ro explant (10 to 81% more, only significant for Schatteburg Hy). On the explant itself, however, roots almost never formed on medium 1 to 4. In medium 5, in contrast, they formed much more frequently, in particular when using the explant types Hy and Se (not significant).
The regeneration efficiency of roots at the callus is higher with M1 than with M2 for all explant types (7–20% higher, not significant), and on average higher in M3 than in M4 (up to 7% higher, not significant) (Figures S5 and S6). In M5, the regeneration efficiency was in almost all cases zero for callus-roots and thus significantly lower compared to the other media. The regeneration efficiency of roots at the explant instead was in media 1–4 in all cases zero except for the cotyledons in Oldenburger Palme (not significant). With medium 5, in contrast to all other media, maximum regeneration (100%) of explant-roots was achieved in almost all varieties and with all explant types.
The regeneration efficiency of Ro at the callus was up to 45% higher compared to Co in Frostara and often even reached its maximum with Ro and Se (significant in media 1 and 5). In Oldenburger Palme, on the other hand, the regeneration efficiency of Co was on average similar to that of Ro and Se, reaching a maximum of 100%, while for Hy, the regeneration efficiency was 18–45% lower, but not significant.
Formation of calluses. In the micropropagation experiment, callus formation occurred in media 1, 2, 3, and 4 in all explant types and all tested kale varieties, whereas in medium 5 it only occurred in hypocotyls and cotyledons (Figure 7 and Figure 8, raw data in Table S3, photos in Figure S13). Significantly more calluses formed when using cotyledons as explant type in media 1–4 for all varieties analysed. In contrast, the other explant types (i.e., root, hypocotyl) and the intact seedling usually produced 50% less calluses in media 1–4 (p < 0.05). In medium 5, on the other hand, calluses almost never formed, and if they did, then only in hypocotyls and cotyledons, with on average between 47% and 84% less calluses than in the other media.
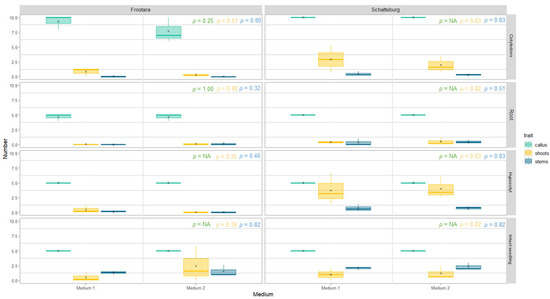
Figure 7.
Number of regenerated calluses, shoots, and stems of micropropagated kale from different explant types grown on different media after eight weeks. Data represent mean ± SE of three independent boxes each containing five (for Schatteburg and Frostara) or three explants (for Oldenburger Palme), respectively (in the case of cotyledons, there were 10 or 6 replicates each, respectively) (deviations for Frostara M1 Ro: n = 4, Frostara M2 Co: n = 9). Kruskal–Wallis test, α = 0.05, NA = no stat. test possible, p-value colours correspond to the colours indicated in the legend (raw data in Table S3).
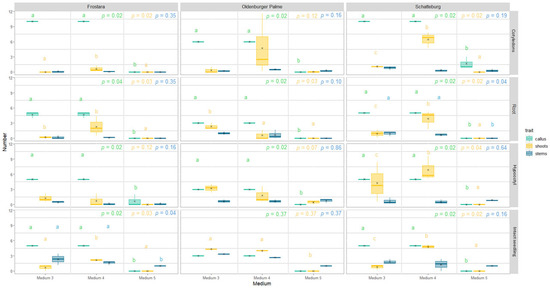
Figure 8.
Number of regenerated calluses, shoots, and stems of micropropagated kale from different explant types grown on different media after eight weeks. Data represent mean ± SE of three independent boxes each containing five (for Schatteburg and Frostara) or three explants (for Oldenburger Palme), respectively (in the case of cotyledons, there were 10 or 6 replicates each, respectively) (deviation for Frostara M3 Ro: n = 4). Kruskal–Wallis test, α = 0.05, NA = no stat. test possible, p-value colours correspond to the colours indicated in the legend (raw data in Table S3).
The regeneration efficiencies of calluses in media 1–4 reached in most cases 100% and differed only slightly between the media, being on average 84 to 87% higher than those of medium 5, which were zero for most explant types (no formation of calluses) (File S2: Figures S7 and S8).
Formation of shoots. The average number of shoots formed on the explant was slightly higher on medium 4 for all varieties compared to all other media (significant differences between media; however, only in Schatteburg for cotyledons) (Figure 7 and Figure 8, raw data in Table S3, photos in Figure S13). The formation of shoots in Frostara was low and in most cases close to zero. In addition, there was no clear trend towards higher regeneration of shoots in any of the media.
In medium 5, shoots at the callus were formed only in Oldenburger Palme using hypocotyls as the explant, although these newly formed shoots were significantly less than in the other media. In all other treatments, no new shoots appeared on medium 5. In general, most new shoots were formed at the callus in Schatteburg. Within Schatteburg, 22% to 89% more shoots at the callus were formed with the explant type hypocotyls compared to the others (not significant). The other explant types produced less newly formed shoots here.
The regeneration efficiency of shoots on the explant showed no significant differences and no trend between medium 1 and 2 (Figure S7). In contrast, the mean regeneration efficiency for cotyledons was higher on medium 4 than on medium 3 (for Frostara 100%, for Schatteburg 52%, for Oldenburger Palme 91% higher, not significant) (Figure S8). For the other explant types on these media, however, there was again no clear trend. For medium 5, the regeneration efficiency was in almost all cases zero (no new shoots), despite two exceptions in which some shoots at the Oldenburger Palme explants had formed.
In a comparison of the explants, the regeneration efficiency of Se was between 48 and 94% higher than that of the other explant types in Frostara and Oldenburger Palme (but only significant for Oldenburger Palme on M3) (Figure S7 and S8). In Schatteburg, on the other hand, the regeneration efficiency of Hy and Co was on average higher than that of Se (between 30 and 70%, not significant).
Formation of stems. The number of newly formed stems did not differ significantly between medium 1 and 2 for all three varieties (Figure 7, raw data in Table S3, photos in Figure S13). In medium 3, the number of new stems was in most cases slightly higher, but not significantly higher compared to medium 4 (Figure 8). The number of stems in medium 5 differed on average only slightly from the other media, even though, in contrast to the other media, M5 never produced more than one stem.
The number of newly formed stems was in all media up to 100% higher in the intact seedling compared to the other explant types (often significant).
On average, the regeneration efficiency of stems showed no clear trend towards higher regeneration between medium 1 and 2, nor between medium 3 and 4 (Figures S7 and S8).
The highest regeneration efficiency of stems has clearly been achieved with Se (up to 100% higher, often significant differences) reaching in almost all treatments the maximum value, in contrast to the other explants. The second highest regeneration efficiency of shoots has been achieved in Frostara with the explant type Hy, while in Schatteburg and Oldenburger Palme, there was no clear trend.
3.2.2. Abnormal Morphological Development
Albinism. In the micropropagation experiment, albinism as abnormal morphological development was almost absent in both M1 and M2, and if it did occur here then only in Se (Figure 9, Figure S9, raw data in Table S4). There was no trend towards more or less albinism between medium 3 and 4 (Figure S10). Albinism occurred up to 100% more frequently in Se than in all other explant types. Albinism rarely occurred in Frostara and Oldenburger Palme, in contrast to Schatteburg.

Figure 9.
Phenotypic anomalies: (a) yellowing (sample Frostara M2 Se), (b) hyperhydricity (sample Schatteburg M2 Co), (c) albinism (sample Frostara M5 Se), (d) shoot tip necrosis (sample Schatteburg M1 Se) (e) leaf abscission (sample Schatteburg M2 Hy), (f) phenolic oxidation (sample Frostara M1 Se). The red arrows mark the respective anomalies.
Leaf abscission. Leaf abscission occurred slightly more frequently on M2 compared to M1 (Figure S9). In a comparison of all media, it occurred most frequently on M3 (being significant in some cases) (Figures S9 and S10). Leaf abscission most frequently occurred again within Se and never within Ro and Hy (often significant). In Frostara, leaf abscission occurred significantly less frequently on average than in the other two varieties, with Schatteburg having the highest rate.
Hyperhydricity. The occurrence of hyperhydricity showed no clear trend towards increased occurrence in either M1 or M2 (Figure S9). Hyperhydricity occurred up to 13% more frequently in M4 than in M3 (Figure S10). In medium 5, on the other hand, hyperhydricity was only marginal and occurred significantly less. The intact seedling was again most affected and Ro the least (with often significant differences).
Shoot tip necrosis. Shoot tip necrosis occurred on average more frequently in M2 than in M1 (up to 100%, partly significant differences between the media) (Figure S9). There was no clear trend towards more frequent occurrence in either medium 3 or 4 (Figure S10). In contrast, shoot tip necrosis occurred only little in medium 5 and significantly less frequently than in the other media. The necrosis was most frequent in Se and least frequent in Co (partly significant).
Yellowing. Yellowing occurred in medium 1 and 2 about equally frequently in all varieties (Figure S9). In medium 3, it occurred on average up to 40% more frequently than in medium 4 (significant only in Schatteburg Ro and Schatteburg Hy) (Figure S10). Yellowing also occurred most frequently when using Se in all varieties and media (often with significant differences). It was more pronounced in Schatteburg than in Frostara and Oldenburger Palme. Of all the anomalies analysed, yellowing occurred most frequently.
Phenolic oxidation. Phenolic oxidation usually occurred more with M2 than with M1 (up to 20%) (Figure S11). In the Frostara variety, it occurred more in M3 than in M4, but not in the other varieties (Figure S12). In M5, phenolic oxidation occurred the least across varieties and was often absent (0%). Cotyledons were affected up to 90% more frequently than roots and hypocotyls (in some cases significantly).
4. Discussion
4.1. Disinfection Experiment
Prior to our micropropagation experiment, we tested various methods for seed disinfection. The assumed general improvement of the germination rate and germination speed as well as a reduction in the infection rate and infection speed by the different experiments could not be proven (Figure 1, Figure 2, Figure 3 and Figure 4). The main factors influencing the disinfection and germination seem to be age, structure, and quality of the seed and genotypic differences.
In terms of seed age, the Frostara seeds were considerably older than the ones of Schatteburg (Table 1). This may have led to the lower germination success observed in Frostara. In addition, this may have resulted in a different response of the seeds to NaClO, as a loss of membrane integrity can occur with age [44]. We conclude that the age of the seeds seems to be a more important factor for germination than the sterilisation but does not correlate with the infection rate.
In terms of seed structure, the reduction in the germination rate and germination speed in Frostara NaClO treatments compared to the control could be due to an altered permeability of the seed coat caused by NaClO [45]. A loss of cell membrane integrity by NaClO and consequently an increased leakage of important substances from the seed is known to be able to affect germination [46]. An increase in such leakage aggregates has already been observed in cauliflower (B. oleracea var. botrytis L.) and broccoli (B. oleracea var. italica Plenck.) [45]. In contrast to Frostara, an increase in the germination rate and speed has been observed in most disinfection treatments of Schatteburg, which can also be explained by changes in the seed coat caused by NaClO. NaClO has been shown to break dormancy by Mahajan et al. [47] in B. tournefortii Gouan. However, precise information on the seed dormancy and available nutrient supply of the kale seeds used here is not available.
The generally lower infection rates observed in NaClO applications in Schatteburg compared to the control is assumed to be caused by chlorine binding to cytoplasmic components and forming highly toxic N-chlorine compounds [48]. These compounds are known to be toxic for microorganisms [48]. Such a reduction in infections by NaClO was also observed in B. napus by Shu et al. [49].
The above mentioned factors are probably also reasons for the discrepancies between our results and those of Shu et al. [49]. These authors performed a series of experiments with different NaClO disinfection methods (0.1–30.0% for 10–30 min) and found a concentration of 2–3% NaClO to be the best method for disinfection of B. napus. However, since in our study disinfection with 10% NaClO was the best for kale, closely related species seem to have noticeable differences in the reaction of NaClO with cytoplasmic components.
The use of NaClO for seed disinfection has many advantages such as ease of use, availability, cost effectiveness, and low potential for seed damage. Therefore, further NaClO disinfection methods with variations in duration and concentration should be tested. Additionally, testing alternatives to NaClO sterilisation such as ethanol, hydrogen peroxide, acetic acid, and hypochlorous acid disinfection could improve seed disinfection [50].
4.2. Micropropagation Experiment
4.2.1. Auxin to Cytokinin Ratio and Media Compositions
In the micropropagation experiment, in all media, more cytokinin was present than auxin, but differences in the ratio between auxin and cytokinin led to different effects on the regeneration of leaves, roots, shoots, and stems (Figure 5, Figure 6, Figure 7 and Figure 8. The more frequent formation of leaves on the callus in M2 (6.25:1 BAP:NAA) compared to M1 (a 3.75:1 BAP:NAA; b 15:1; c 0.001:0) could be attributed to a higher ratio of auxin in M2, because appropriate combinations of auxin and cytokinin are known to control the formation of cell phenotype and maintenance of cell division [10]. The higher regeneration of leaves at higher BAP:NAA ratios observed here was also found in other Brassica studies such as for B. oleracea L. var. acephala [23], B. oleracea var. italica [51], and B. rapa [52]. High concentrations of cytokinin relative to auxin lead to shoot regeneration [9] and may even be sufficient on its own in some cases [10]. This could be confirmed with our results (highest shoot formation was observed in a medium with a higher BAP to NAA ratio). However, for B. rapa, Zhao et al. [53] reported that a lower cytokinin to auxin ratio (2 mg L−1 BAP and 0.5 mg L−1 NAA) led to the highest shoot formations.
For the other explant types, the small differences in shoot, root, and stem regeneration between M1 and M2 suggests that the different hormone ratios were not critical for greater regeneration. This would also explain the strong callus formation in many treatments, which occurs as a natural wound-healing process when moderate to high concentrations of both hormone classes are present [10].
Cotyledons can synthesise auxins, and auxin levels in plants decrease from the tip to the base of the stem [16]. This may also have led to differential regeneration between explant types, since different levels of such endogenous hormone concentrations in the explant tissue interact with exogenous hormones present in the media [10].
A higher ratio of auxin to cytokinin is required for root initiation, as known for other plant species [54]. This is also reflected in the stronger root formation in M1, in which the concentrations of auxin and cytokinin are more similar to each other at the end of the cultivation period. Also, medium 3 (Table 3) seems to have initiated and/or enhanced root formation.
It should also be noted that M1 and M2 did not only differ in the hormones auxin (NAA) and cytokinin (BAP). For example, M1 contained the additives gibberellic acid (GA3) and AgNO3, which were not present in M2 (Table 3). GA3 is known to promote stem elongation [55] and AgNO3 stimulates cell division and growth [56] and promotes shoot regeneration in oilseed rape [57]. It is, therefore, difficult to disentangle their effect from a lower auxin–cytokinine ratio. Medium 1 was also supplied with vitamins for a shorter period but at a higher concentration than M2, which means that more nutrients were potentially available in M1 at the appropriate regeneration stage. Finally, polyvinylpyrrolidone (PVP 40.000) and adenine hemisulfate were applied in M1 over a shorter period but at higher concentrations. These substances are added to reduce phenotypic anomalies [58], and thus lead to a stronger and more effective regeneration.
In the control (M5), observed callus formation was probably caused by injury of the explants during transfer to the media and not by the composition of the medium. In addition, no regeneration was observed in any of the explants, only changes in the explant due to the natural growth of the intact seedling on the medium.
The next steps to optimise regeneration in kale could be a continuation of the experiment to clarify the minimum and maximum limits of the auxin–cytokinin ratio in the media, as investigated by Doğru et al. [59] for Brassica oleracea convar. capitata.
4.2.2. Impact of AgNO3
The observation that tissue regeneration in a medium with AgNO3 addition (M4) was only partially higher than in the unsupplemented medium suggests that the different types of explants react differently to this addition in terms of higher regeneration. The positive effect of AgNO3 on shoot regeneration in kale shown here is consistent with previous results from cotyledon and hypocotyl explants in ornamental kale [23], oilseed rape [57], and anthers in B. oleracea [60]. Those in vitro cultured explants, stems, leaves, and roots on the callus, and roots and shoots on the explant, in which almost no improvement or even a worse regeneration occurred after AgNO3 addition, probably produced more ethylene than the other explants, which reduced the regeneration efficiency. Apparently, silver ions acted as a competitive inhibitor of the ethylene effect, or, they were able to inhibit ethylene synthesis per se [61]. Although the actual mechanism of ethylene on regeneration has not been elucidated [23], the results of previous studies support the hypothesis that silver nitrate is a suppressor of an ethylene signalling pathway and thus promotes the ability of explants to regenerate [60].
In addition to the putative effect of AgNO3 on ethylene, AgNO3 is known to increase polyamine production [62] and stimulate cell division and growth [63,64]. This could be an alternative explanation for the higher number of shoots and (partially) roots formed on the callus in the AgNO3 treatment, as well as the higher regeneration efficiency in leaves of Oldenburger Palme and Schatteburg.
In general, AgNO3 only had a positive effect on regeneration at the callus and not directly at the explant. To investigate which concentration of AgNO3 is best suited to achieve the highest regeneration rate in kale, we recommend to also test higher concentrations.
4.2.3. Performance of Different Explant Types
Genetic differences between the varieties could be responsible for the strong differences between the most suitable explants for the regeneration of roots in M1 (Frostara: roots; Schatteburg: hypocotyls) and stems in M3 (Frostara: hypocotyls; Schatteburg, Oldenburger Palme: roots). One likely genetic difference may be differences in production of hormones that may have different effects on regeneration.
The unconfirmed expectation that the cotyledon explant type leads to the highest regeneration and the root explant type to the lowest regeneration for all traits and in all media can be mainly attributed to the portion of endogenous hormones, the media composition, and genetic differences between varieties. For example, the auxin production of cotyledons is likely to have influenced the cytokinin/auxin ratio in the medium, resulting in lower regeneration from cotyledons. Consequently, if the propagation of plantlets by using cotyledons as primary explants is the aim, the cytokinin concentration should be increased. Our observations are in agreement with the results of Dai et al. [23], who found for ornamental kale that 4-day-old hypocotyl explants regenerated better during in vitro micropropagation than cotyledons on MS medium with 3 mg dm−3 6-BAP and 0.1 mg dm−3 NAA. On the other hand, there are also discrepancies between our results and another study in which 5-day-old cotyledons of Brassica rapa L. var. parachinensis were found to be the most suitable explant for shoot regeneration compared to hypocotyl on MS medium with 1 mg L−1 BAP, 0.5 mg L−1 NAA, and 2.5 mg L−1 AgNO3 [13].
In addition, endogenous hormone levels [65] present in the explants influence the results and potentially explain greater callus formation but less leaf formation in cotyledons compared to hypocotyls and the intact seedling [10]. It has been reported by Ċosiċ et al. [66], that levels of endogenous phytohormones vary depending on plant genotype and type of explant and Mostafa et al. [65] reported that higher levels stimulate callus formation and propagation.
The observation that the root explants regenerated more roots in M3 and M4 for Frostara and Oldenburger Palme, compared to other regeneration traits, is potentially important. These media do not contain GA3, potassium iodide, adenine hemisulfate, and PVP, which all may—despite their beneficial impacts—also exert a negative effect on regeneration efficiency (e.g., George et al. [10]). For example GA3 is present in M1 and M2 and is known to often reduce the formation of adventitious roots and shoots [10]. Furthermore, our results regarding the regenerative capacity of roots are consistent with the fact that root explants show the weakest and slowest shoot regeneration compared to cotyledons and hypocotyls in B. napus [30]. The fact that Farooq et al. [30] and our study used the same medium (Brassica regeneration protocol-III in Farooq’s study corresponds to medium 1 in ours) may indicate that this medium may not be suitable for shoot regeneration from root explants in all Brassica. It is also known that applied plant growth regulators induce specific mRNA molecules [67]. In response to these regulators, specific genes are expressed [10], which may have been up-regulated in specific explant types and cultivars, resulting in different regeneration in terms of explant type and regeneration traits.
Our additional expectation that the intact seedling shows the best regeneration in almost all traits compared to the other explants was confirmed, which we attribute to the fact that phytohormonal signalling is not disturbed here, but it is in the other explants [66].
It should be noted that the seeds of the three varieties germinated and grew at different rates, which means that the used explants for micropropagation were of different ages between varieties and, in the case of Frostara, even within the variety. However, as the ability to regenerate decreases with increasing age of Brassica explants [8,57], this could also have led to differences in the frequency of regeneration between varieties.
A follow-up study could address the question of which signalling pathways of growth regulators within the cell lead to effects on genes and thus to the growth of new tissue. Regarding the subsequent acclimatisation of kale plants, it remains to be clarified whether it can be positively supported by further minerals and bacteria added to the medium, as has been demonstrated for other species [11,68]. We also suggest addressing the question of how micropropagation affects somaclonal variation, genetic stability, and varietal identity of clonally propagated kale plants.
4.2.4. Phenotypic Anomalies
Phenotypic anomalies can increase due to explant size and the literature suggests that size is a more critical parameter for anomalies than age [69]. In our study, for example, some cotyledon explants were too small to be held in the medium by the petiole and, therefore, had a larger contact surface with the medium in relation to the size of the explant. This could have led to increased hyperhydricity, especially in Frostara.
Our observation that in medium 5 with a lower hormone concentration fewer anomalies occurred suggests that the lack of hormone supplementation resulted in a generally reduced regeneration and led to a lower incidence of anomalies. This is also supported by the fact that Se as an undamaged seedling grew faster and stronger and, at the same time, showed more anomalies compared to all other explant types. A reduction in phenotypic anomalies was also observed for the genus Lippia in MS medium without added growth regulators [70,71].
Compared to the unsupplemented medium 5, the high concentration of auxin in medium 1–4 may have caused an accumulation of ethylene gas [10], which is known to lead to an increased incidence of yellowing and leaf drop [11]. Hormones may also have been responsible for albinism by triggering changes in the transcription during chloroplast synthesis [72].
It should also be noted that it is not only the addition of different hormones that has an effect, but also the other micro- and macronutrients and vitamins present, which were higher in M1 and M2 than in M3–M5. For example, it is known that a high calcium content (higher in M1 and M2) can reduce hyperhydricity and shoot tip necrosis [11,73], but this reduction did not occur here, suggesting that the impact of hormones was stronger than that of calcium. Furthermore, nutrient imbalance can increase shoot tip necrosis, suggesting that nutrient imbalance was present in the treatment in which shoot tip necrosis was highest (at Schatteburg M4 Se). The result that the addition of AgNO3 to the media led to a reduction in leaf abscission in this experiment can be explained by the fact that silver nanoparticles limit ethylene synthesis, as discussed above, and inhibit the enzymatic hydrolysis of pectin and cellulose. These hydrolytic enzymes lead to decomposition of the cell walls, making the leaves more susceptible to abscission [74]. The hypothesis that AgNO3 reduces this phenotypic anomaly is also supported by the observation that there was less abscission than yellowing when AgNO3 was applied, since abscission is only the subsequent stage of yellowing. A reduction in leaf abscission by silver thiosulphate has previously been shown in Citrus australasica F. Muell. [75].
However, the fact that the medium with added AgNO3 did not lead to a reduction in the other anomalies investigated in this study suggests that other pathways triggered these anomalies, which have not yet been adequately studied [11]. Generally, in vitro micropropagation would benefit from investigating the molecular base of anomalies, since the causes are not yet fully understood [10,76].
4.2.5. Implications of Our Findings
The results of the disinfection experiment indicate that NaClO is an effective disinfectant, but that its concentration and application should be carefully matched to the seeds in question. Many factors relating to the seed and the experimental design cause the effect of NaClO to vary considerably, even within a single variety. For example, in Frostara, 10% NaClO/10 min already appeared to have a damaging effect to the seeds, whereas in Schatteburg, no damaging effect had been reached, yet. To ensure optimal germination and infection prevention, the concentration and application time of NaClO should be optimised for variety and seed age. Methods recommending 5%/5 min as standard in Brassica micropropagation protocols [25] should therefore not be adopted without prior testing to save costs, resources, and time, especially when seeds are limited.
The results from the micropropagation experiment indicate that all tested kale varieties are capable of in vitro regeneration and that all tested MS media with additives are suitable for the regeneration of kale. For best regeneration of leaves and shoots, we recommend hypocotyls as the explant type and the use of medium 1, 2, or 3. For the regeneration of roots and shoots, we recommend using roots as the explant type in medium 4. For high shoot and leaf regeneration, we also recommend medium 2, i.e., with a permanently higher auxin–cytokinin concentration. Additionally, one could use a root initiation medium as in Bhalla and Singh [8] afterwards, to stimulate root formation, which is important for subsequent acclimatisation.
Concerning AgNO3, even if its use did not result here in improved regeneration and did not reduce anomalies in all traits, the use of AgNO3 still seems recommendable, but likely at a higher concentration than tested in this study, since a positive effect is known for other Brassicas (e.g., Tang et al. [57]).
Since the explant types and varieties tested reacted differently—and this is probably the case with other varieties as well—adapted protocols need to be developed for each variety separately to ensure optimum regeneration. If the aim is to produce as many new plants as possible from a seedling in a short time, cotyledons are the preferred explant type, since two cotyledons per seedling are likely to regenerate more adventitious shoots than a single hypocotyl.
The phenotypic anomalies observed require further optimisation of the culture media to maximise regeneration and minimise such anomalies. This is particularly important with regard to one of the main problems of tissue culture, hyperhydricity [73], which was shown here to be one of the main factors along with yellowing and shoot tip necrosis. Our results on albinism also indicate that micropropagation in MS media with additives led to genetic changes in the kale varieties.
5. Conclusions
This study presents the first comprehensive and reproducible protocol for in vitro micropropagation of kale using seedling explants and intact seedlings. The results clearly demonstrate that regeneration efficiency is strongly dependent on disinfection method, explant type, kale genotype, and the combination of growth regulators. By optimising these factors, our results lay a solid foundation for micropropagation strategies in B. oleracea var. acephala. Micropropagation could be an essential method and opportunity to support and shape future agriculture, especially concerning the transfer of traits that cannot be crossed through conventional breeding. Further research of the physiological and biochemical mechanisms in micropropagation will be essential to realise its potential for crop improvement and to support resilient food systems in the face of global agricultural challenges.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae11070767/s1, File S1: Climatic conditions—figures (Figure S1+S2: Breeding room temperature; Figure S3+S4: Breeding room light intensity); File S2: Results—supplementary figures (Figure S5: Regeneration efficiency of regenerated leaves and roots on medium 1–2; Figure S6: Regeneration efficiency of regenerated leaves and roots on medium 3–5; Figure S7: Regeneration efficiency of regenerated calluses, shoots and stems on medium 1–2; Figure S8: Regeneration efficiency of regenerated calluses, shoots and stems on medium 3–5; Figure S9: Number of anomalies on medium 1–2; Figure S10: Number of anomalies on medium 3–5; Figure S11: Percentage of phenolic oxidation on medium 1–2; Figure S12: Percentage of phenolic oxidation on medium 3–5; Figure S13: Photos of regenerated tissue); Table S1: Data temperature and light; Table S2: Data disinfection experiment; Table S3: Data micropropagation experiment—regeneration; Table S4: Data micropropagation experiment—phenotypic anomalies.
Author Contributions
Conceptualization, M.B. and D.C.A.; methodology, M.B. and D.C.A.; software, M.B.; validation, M.B. and D.C.A.; formal analysis, M.B.; investigation, M.B.;; data curation, M.B.; writing—original draft preparation, M.B.; writing—review and editing, M.B. and D.C.A.; visualization, M.B.; supervision, D.C.A.; project administration, M.B. and D.C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We are very grateful to Elke Haase, CEO of Piccoplant Mikrovermehrungen GmbH, for the great opportunity to carry out this project there and for the support of the employees during the project! We would also like to thank Christoph Hahn, who gave us helpful tips for working with kale and supported us with data acquisition during the project.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BAP | 6-benzylaminopurine |
| Co | cotyledon |
| D | days |
| GR | germination rate |
| GS | germination speed |
| Hy | hypocotyl |
| IR | infection rate |
| IS | infection speed |
| M1 | medium 1 |
| M2 | medium 2 |
| M3 | medium 3 |
| M4 | medium 4 |
| M5 | medium 5 |
| MS | Murashige and Skoog media |
| NA | not available |
| NAA | α-naphthaleneacetic acid |
| NGS | number of germinated seeds |
| NIS | number of infected seeds |
| NREx | number of explants showing regeneration |
| RE | regeneration efficiency |
| Ro | root |
| Se | intact seedling |
| TNEx | total number of explants |
| TS | total number of seeds |
References
- Cohen, J.E. Population and climate change. Proc. Am. Philos. Soc. 2010, 154, 158–182. [Google Scholar] [PubMed]
- Zhou, S.; Hu, Z.; Zhu, M.; Zhang, B.; Deng, L.; Pan, Y.; Chen, G. Biochemical and molecular analysis of a temperature-sensitive albino mutant in kale named “White Dove”. Plant Growth Regul. 2013, 71, 281–294. [Google Scholar] [CrossRef]
- Körber-Grohne, U. Nutzpflanzen in Deutschland: Kulturgeschichte und Biologie; Konrad Theiss Verlag GmbH: Stuttgart, Germany, 1987. [Google Scholar]
- Hagen, S.F.; Borge, G.I.A.; Solhaug, K.A.; Bengtsson, G.B. Effect of cold storage and harvest date on bioactive compounds in curly kale (Brassica oleracea L. var. acephala). Postharvest Biol. Technol. 2009, 51, 36–42. [Google Scholar] [CrossRef]
- Šamec, D.; Urlić, B.; Salopek-Sondi, B. Kale (Brassica oleracea var. acephala) as a superfood: Review of the scientific evidence behind the statement. Crit. Rev. Food Sci. Nutr. 2019, 59, 2411–2422. [Google Scholar] [CrossRef] [PubMed]
- Vinterhalter, D.; Sretenović-Rajičić, T.; Vinterhalter, B.; Ninković, S. Genetic transformation of Brassica oleracea vegetables. Transgenic Plant J. 2007, 1, 340–355. [Google Scholar]
- Zou, J.; Zou, X.; Gong, Z.; Song, G.; Ren, J.; Feng, H. Thidiazuron promoted microspore embryogenesis and plant regeneration in curly kale (Brassica oleracea L. convar. acephala var. sabellica). Horticulturae 2023, 9, 327. [Google Scholar] [CrossRef]
- Bhalla, P.L.; Singh, M.B. Agrobacterium-mediated transformation of Brassica napus and Brassica oleracea. Nat. Protoc. 2008, 3, 181–189. [Google Scholar] [CrossRef]
- Kumar, N.; Reddy, M. In vitro plant propagation: A review. J. For. Environ. Sci. 2011, 27, 61–72. [Google Scholar]
- George, E.F.; Hall, M.A.; De Klerk, G.-J. Plant Propagation by Tissue Culture: Volume 1. the Background; Springer Science & Business Media: Dordrecht, The Netherlands, 2007; Volume 1. [Google Scholar]
- Abdalla, N.; El-Ramady, H.; Seliem, M.K.; El-Mahrouk, M.E.; Taha, N.; Bayoumi, Y.; Shalaby, T.A.; Dobránszki, J. An academic and technical overview on plant micropropagation challenges. Horticulturae 2022, 8, 677. [Google Scholar] [CrossRef]
- Sivanandhan, G.; Moon, J.; Sung, C.; Bae, S.; Yang, Z.H.; Jeong, S.Y.; Choi, S.R.; Kim, S.-G.; Lim, Y.P. L-Cysteine increases the transformation efficiency of Chinese cabbage (Brassica rapa ssp. pekinensis). Front. Plant Sci. 2021, 12, 767140. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, S.; Zhang, Y.; Shi, F.; Liu, X.; Du, S.; Feng, H. Establishment of an efficient shoot regeneration system in vitro in Brassica rapa. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 977–986. [Google Scholar] [CrossRef]
- Cardoza, V.; Stewart, C.N. Brassica biotechnology: Progress in cellular and molecular biology. Vitr. Cell. Dev. Biol.-Plant 2004, 40, 542–551. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Y.; Liu, Q. Establishment of an efficient regeneration system using heading leaves of Chinese cabbage (Brassica rapa L.) and its application in genetic transformation. Hortic. Environ. Biotechnol. 2018, 59, 583–596. [Google Scholar] [CrossRef]
- Sparrow, P.; Townsend, T.; Morgan, C.; Dale, P.; Arthur, A.; Irwin, J. Genetic analysis of in vitro shoot regeneration from cotyledonary petioles of Brassica oleracea. Theor. Appl. Genet. 2004, 108, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.A.T.; Winarto, B.; Dobránszki, J.; Zeng, S. Disinfection procedures for propagation of purple basil Ocimum basilicum L. ‘red rubin’ at different levels of salts, charcoal, sucrose and potassium iodine. Folia Hortic. 2015, 27, 3–14. [Google Scholar]
- Williams, J.; Pink, D.; Biddington, N. Effect of silver nitrate on long-term culture and regeneration of callus from Brassica oleracea var. gemmifera. Plant Cell Tissue Organ Cult. 1990, 21, 61–66. [Google Scholar] [CrossRef]
- Wright, K.M.; Marshall, J.; Wright, P.J.; Holden, N.J. Vacuolar localisation of anthocyanin pigmentation in microgreen cotyledons of basil, cabbage and mustard greens does not impact on colonisation by Shiga-toxigenic Escherichia coli O157:H7. Food Microbiol. 2023, 116, 104367. [Google Scholar] [CrossRef]
- Hundleby, P.; Chhetry, M. Brassica oleracea transformation. In Genetic Transformation in Crops; IntechOpen: London, UK, 2020. [Google Scholar]
- Kumar, P.; Srivastava, D. High frequency organogenesis in hypocotyl, cotyledon, leaf and petiole explants of broccoli (Brassica oleracea L. var. italica), an important vegetable crop. Physiol. Mol. Biol. Plants 2015, 21, 279–285. [Google Scholar] [CrossRef]
- Smith, N.A.; Bhalla, P.L. Comparison of shoot regeneration potential from seedling explants of Australian cauliflower (Brassica oleracea var. botrytis) varieties. Aust. J. Agric. Res. 1998, 49, 1261–1266. [Google Scholar] [CrossRef]
- Dai, X.; Shi, X.; Ye, Y.; Fu, Q.; Bao, M. High frequency plant regeneration from cotyledon and hypocotyl explants of ornamental kale. Biol. Plant. 2009, 53, 769–773. [Google Scholar] [CrossRef]
- Jaiswal, S.; Hammatt, N.; Bhojwani, S.; Cocking, E.; Davey, M. Plant regeneration from cotyledon protoplasts of Brassica carinata. Plant Cell Tissue Organ Cult. 1990, 22, 159–165. [Google Scholar] [CrossRef]
- Wright, K.M.; Holden, N.J. Quantification and colonisation dynamics of Escherichia coli O157:H7 inoculation of microgreens species and plant growth substrates. Int. J. Food Microbiol. 2018, 273, 1–10. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055. [Google Scholar] [CrossRef] [PubMed]
- Timson, J. New method of recording germination data. Nature 1965, 207, 216–217. [Google Scholar] [CrossRef]
- Khan, M.A.; Ungar, I.A. The effect of salinity and temperature on the germination of polymorphic seeds and growth of Atriplex triangularis Willd. Am. J. Bot. 1984, 71, 481–489. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Farooq, N.; Nawaz, M.A.; Mukhtar, Z.; Ali, I.; Hundleby, P.; Ahmad, N. Investigating the in vitro regeneration potential of commercial cultivars of Brassica. Plants 2019, 8, 558. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Wilcoxon, F. Individual comparisons by ranking methods. Biom. Bull. 1992, 1, 80–83. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 1st ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- FC, M.; Davis, T.; ggplot2 authors. ggpattern: ‘ggplot2’ Pattern Geoms. Available online: https://github.com/coolbutuseless/ggpattern (accessed on 1 September 2023).
- Luo, C.; Bai, S. Versatile Pie Charts, Ring Charts, Bar Charts and Box Plots using Patterns, Colors and Images; R Package Version 1.0.0. Available online: https://github.com/cran/patternplot (accessed on 1 September 2024).
- van den Brand, T. ggh4x: Hacks for ‘ggplot2’, R Package Version 0.2.3. Available online: https://github.com/teunbrand/ggh4x (accessed on 18 September 2023).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research; R Package Version 2.4.6; Northwestern University: Evanston, IL, USA, 2020; Volume 2. [Google Scholar]
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots; R Package Version 0.6.0. Available online: https://rpkgs.datanovia.com/ggpubr/ (accessed on 19 September 2024).
- Wickham, H. Reshaping data with the reshape package. J. Stat. Softw. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Rudis, B. Hrbrthemes: Additional Themes, Theme Components and Utilities for ‘ggplot2’; R Package Version 0.8.7; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Leopold, A.C.; Vertucci, C.W. Moisture as a regulator of physiological reaction in seeds. Seed Moisture 1989, 14, 51–67. [Google Scholar]
- Taylor, A.; Paine, D.; Paine, C. Sinapine leakage from Brassica seeds. J. Am. Soc. Hortic. Sci. 1993, 118, 546–550. [Google Scholar] [CrossRef]
- Duke, S.H.; Kakefuda, G.; Harvey, T.M. Differential leakage of intracellular substances from imbibing soybean seeds. Plant Physiol. 1983, 72, 919–924. [Google Scholar] [CrossRef]
- Mahajan, G.; Mutti, N.K.; Jha, P.; Walsh, M.; Chauhan, B.S. Evaluation of dormancy breaking methods for enhanced germination in four biotypes of Brassica tournefortii. Sci. Rep. 2018, 8, 17103. [Google Scholar] [CrossRef] [PubMed]
- Carmello, C.R.; Cardoso, J.C. Effects of plant extracts and sodium hypochlorite on lettuce germination and inhibition of Cercospora longissima in vitro. Sci. Hortic. 2018, 234, 245–249. [Google Scholar] [CrossRef]
- Shu, F.; Zhao-xia, L.; Jin-zhi, C.; Geng-xiao, S.; Cui-ying, S.; Jin, C.; Guang, Y. Optimization of Seed Sterilization and Rooting Medium for Regeneration of Brassica napus. Fujian J. Agric. Sci. 2019, 34, 1371–1378. [Google Scholar]
- Gilbert, G.S.; Diaz, A.; Bregoff, H.A. Seed disinfestation practices to control seed-borne fungi and bacteria in home production of sprouts. Foods 2023, 12, 747. [Google Scholar] [CrossRef] [PubMed]
- Azis, N.; Hasbullah, N.; Rasad, F.; Daud, N.; Amin, M.; Lassim, M. Organogenesis and growth response of Brassica oleracea var. italica through in vitro culture. In Proceedings of the International Conference on Agricultural, Ecological and Medical Sciences, Sabah, Malaysia, 15–17 April 2015; pp. 7–8. [Google Scholar]
- Cui, J.; Li, M.; Qiu, L.; Cao, J.; Huang, L. Stable expression of exogenous imported sporamin in transgenic Chinese cabbage enhances resistance against insects. Plant Growth Regul. 2017, 81, 543–552. [Google Scholar] [CrossRef]
- Zhao, J.; Liang, A.; Zhu, Z.; Tang, Y. Regeneration of Chinese cabbage transgenic plants expressing antibacterial peptide gene and cowpea trypsin inhibitor gene. Euphytica 2006, 150, 397–406. [Google Scholar] [CrossRef]
- Skoog, F. Chemical regulation of growth and organ formation in plant tissue cultured in vitro. Proc. Symp. Soc. Exp. Biol. 1957, 11, 118–131. [Google Scholar]
- Jain, S.; Nidhi, N.; Kale, S.; Rathod, M.; Dhurve, L.; Mehara, H. A comprehensive review on role of bio-regulators in the growth and development of fruit and vegetable crops. Int. J. Environ. Clim. Chang. 2023, 13, 2879–2892. [Google Scholar] [CrossRef]
- Tahoori, F.; Majd, A.; Nejadsattari, T.; Ofoghi, H.; Iranbakhsh, A. Effects of silver nitrate (AgNO3) on growth and anatomical structure of vegetative organs of liquorice (Glycyrrhiza glabra L.) under in vitro condition. Plant Omics 2018, 11, 153–160. [Google Scholar] [CrossRef]
- Tang, G.; Zhou, W.; Li, H.; Mao, B.; He, Z.; Yoneyama, K. Medium, explant and genotype factors influencing shoot regeneration in oilseed Brassica spp. J. Agron. Crop Sci. 2003, 189, 351–358. [Google Scholar] [CrossRef]
- Naaz, A.; Shahzad, A.; Anis, M. Effect of adenine sulphate interaction on growth and development of shoot regeneration and inhibition of shoot tip necrosis under in vitro condition in adult Syzygium cumini L.—A multipurpose tree. Appl. Biochem. Biotechnol. 2014, 173, 90–102. [Google Scholar] [CrossRef]
- Doğru, S.; Murata, M.; Balkaya, A.; Kurtar, E.S. In vitro micropropagation of maintainer white head cabbage lines using cotyledon and hypocotyl explants. Black Sea J. Agric. 2022, 5, 7–8. [Google Scholar]
- Cristea, T.; Leonte, C.; Brezeanu, C.; Brezeanu, M.; Ambarus, S.; Calin, M.; Prisecaru, M. Effect of AgNO3 on androgenesis of Brassica oleracea L. anthers cultivated in vitro. Afr. J. Biotechnol. 2012, 11, 13788–13795. [Google Scholar]
- Zhang, P.; Phansiri, S.; Puonti-Kaerlas, J. Improvement of cassava shoot organogenesis by the use of silver nitrate in vitro. Plant Cell Tissue Organ Cult. 2001, 67, 47–54. [Google Scholar] [CrossRef]
- Asgher, M.; Khan, M.I.R.; Anjum, N.A.; Verma, S.; Vyas, D.; Per, T.S.; Masood, A.; Khan, N.A. Ethylene and polyamines in counteracting heavy metal phytotoxicity: A crosstalk perspective. J. Plant Growth Regul. 2018, 37, 1050–1065. [Google Scholar] [CrossRef]
- Kumar, P.G.; Sivakumar, S.; Siva, G.; Vigneswaran, M.; Senthil Kumar, T.; Jayabalan, N. Silver nitrate promotes high-frequency multiple shoot regeneration in cotton (Gossypium hirsutum L.) by inhibiting ethylene production and phenolic secretion. Vitr. Cell. Dev. Biol.-Plant 2016, 52, 408–418. [Google Scholar] [CrossRef]
- Shyamali, S.; Hattori, K. Effect of polyamines and silver nitrate on the high frequency regeneration from cotyledon explants of bottle gourd (Lagenaria siceraria; sp. asiatica). Pak. J. Biol. Sci. 2007, 10, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, H.H.; Wang, H.; Song, J.; Li, X. Effects of genotypes and explants on garlic callus production and endogenous hormones. Sci. Rep. 2020, 10, 4867. [Google Scholar] [CrossRef]
- Ċosiċ, T.; Motyka, V.; Raspor, M.; Savić, J.; Cingel, A.; Vinterhalter, B.; Vinterhalter, D.; Trávníčková, A.; Dobrev, P.I.; Bohanec, B.; et al. In vitro shoot organogenesis and comparative analysis of endogenous phytohormones in kohlrabi (Brassica oleracea var. gongylodes): Effects of genotype, explant type and applied cytokinins. Plant Cell Tissue Organ Cult. 2015, 121, 741–760. [Google Scholar] [CrossRef]
- Christianson, M.; Warnick, D. Organogenesis in vitro as a developmental process. HortScience 1988, 23, 515–519. [Google Scholar] [CrossRef]
- Hoang, N.N.; Kitaya, Y.; Shibuya, T.; Endo, R. Effects of supporting materials in in vitro acclimatization stage on ex vitro growth of wasabi plants. Sci. Hortic. 2020, 261, 109042. [Google Scholar] [CrossRef]
- Sparrow, P.; Goldsack, C.; Østergaard, L. Transformation Technology in the Brassicaceae. In Genetics and Genomics of the Brassicaecea; Schmidt, R., Brncroft, I., Eds.; Springer: New York, NY, USA, 2010. [Google Scholar]
- José, D.P.; De Campos, J.M.S.; Viccini, L.F.; Alkimim, E.R.; de Oliveira Santos, M. Micropropagation and ploidy stability of Lippia lacunosa Mart. & Schauer: An endangered brazilian medicinal plant. Rev. De Agric. Neotrop. 2019, 6, 1–7. [Google Scholar]
- Gupta, S.K.; Khanuja, S. In vitro micropropagation of Lippia alba. Curr. Sci. 2001, 81, 206–210. [Google Scholar]
- Canonge, J.; Roby, C.; Hamon, C.; Potin, P.; Pfannschmidt, T.; Philippot, M. Occurrence of albinism during wheat androgenesis is correlated with repression of the key genes required for proper chloroplast biogenesis. Planta 2021, 254, 1–16. [Google Scholar] [CrossRef]
- Sreelekshmi, R.; Siril, E.A.; Muthukrishnan, S. Role of Biogenic Silver Nanoparticles on Hyperhydricity Reversion in Dianthus chinensis L. an In Vitro Model Culture. J. Plant Growth Regul. 2022, 41, 23–39. [Google Scholar] [CrossRef]
- Ha, N.T.M.; Manh Do, C.; Hoang, T.T.; Ngo, N.D.; Van Bui, L.; Nhut, D.T. The effect of cobalt and silver nanoparticles on overcoming leaf abscission and enhanced growth of rose (Rosa hybrida L. ‘Baby Love’) plantlets cultured in vitro. Plant Cell Tissue Organ Cult. (PCTOC) 2020, 141, 393–405. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Grosser, J.W.; Dutt, M. Silver compounds regulate leaf drop and improve in vitro regeneration from mature tissues of australian finger lime (Citrus australasica). Plant Cell Tissue Organ Cult. (PCTOC) 2020, 141, 455–464. [Google Scholar] [CrossRef]
- Isah, T. Adjustments to in vitro culture conditions and associated anomalies in plants. Acta Biol. Cracoviensia. Ser. Bot. 2015, 57, 9–28. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).