Abstract
The large-scale disposal of loquat (Eriobotrya japonica Lindl.) flowers during fruit thinning represents a significant waste of bioactive resources. This study systematically evaluated how three processing methods—fresh (FS), heat-dried (HD), and freeze-dried (FD) treatments—affect the flavonoid composition and antioxidant capacity of loquat flower extracts, with the aim of developing value-added, sugar-free functional tea ingredients. Using UPLC-MS/MS and DPPH assays, we analyzed both pre-(FS/HD/FD) and post-extraction samples (FSP/HDP/FDP) to assess processing-specific metabolic signatures and extraction efficiency. The results revealed that heat-dried powder (HDP) exhibited the highest total flavonoid content and DPPH scavenging capacity (615.24 µg Trolox/g), attributed to enhanced release of stable compounds like quercetin. Freeze-dried powder (FDP) better preserved heat-sensitive flavonoids, such as catechin-(4α→8)-gallocatechin and naringenin, but showed lower overall antioxidant activity. Multivariate analysis confirmed distinct clustering patterns, with heat-drying favoring flavonoid extractability while freeze-drying maintained metabolic diversity. These findings demonstrate that processing methods significantly influence bioactive compound retention and functionality, with heat-drying offering optimal balance between yield and practicality for industrial applications. This work provides a scientific foundation for upcycling loquat flowers into standardized nutraceutical ingredients, addressing both agricultural waste reduction and the growing demand for natural functional foods.
1. Introduction
The agricultural sector generates vast amounts of biomass byproducts, many of which remain underutilized despite their rich bioactive potential [1,2]. Among these, loquat (Eriobotrya japonica Lindl.) flowers represent a significant yet overlooked resource. During commercial fruit production, farmers routinely thin loquat flowers to improve fruit size and quality, leading to the large-scale disposal of these floral materials [3,4,5]. This practice not only results in economic wastage but also discards a valuable source of phytochemicals with potential applications in functional foods and nutraceuticals. Loquat flowers possess a distinctive aromatic profile reminiscent of the fruit, negligible sugar content, and a diverse array of bioactive compounds (particularly flavonoids), making them ideal candidates for sugar-free health-promoting products such as herbal teas [5]. However, the transition from agricultural waste to standardized nutraceutical ingredients requires the systematic evaluation of processing methods to maximize bioactive retention and functionality.
Flavonoids, a major class of polyphenolic compounds, are well-documented for their antiradical activities, anti-inflammatory, and cardioprotective properties [6,7,8,9,10]. In loquat flowers, these compounds contribute not only to potential health benefits but also to sensory qualities such as aroma and flavor. However, the stability and extractability of flavonoids are highly dependent on post-harvest processing conditions [3,5]. Conventional drying methods, such as oven drying, may degrade heat-sensitive compounds, while freeze-drying, though gentler, is often cost-prohibitive for large-scale applications. Furthermore, hot-water extraction (the most common method for tea preparation) can selectively solubilize certain metabolites while leaving others behind, altering the final product’s bioactivity [11,12,13]. Thus, optimizing processing protocols is crucial to balance compound retention, antioxidant capacity, and economic feasibility.
Previous studies have explored the phytochemical composition of loquat leaves and fruits, revealing high levels of triterpenes, phenolics, and flavonoids with therapeutic potential [3,4,5,14,15]. However, research on loquat flowers remains limited, despite their unique metabolic profile. Unlike leaves, which may contain bitter or astringent compounds, loquat flowers offer a milder, more palatable flavor, making them particularly suitable for tea formulations [16]. Additionally, their low sugar content aligns with growing consumer demand for sugar-free functional beverages that cater to diabetic and health-conscious populations [15,16]. Despite these advantages, no comprehensive study has yet evaluated how different processing methods (such as fresh use, heat-drying, or freeze-drying) affect the flavonoid composition and antioxidant capacity of loquat flower extracts.
The large-scale annual disposal of loquat flowers during thinning represents both an economic loss and a wasted opportunity for value-added product development [17]. However, the transformation of this agricultural byproduct into consistent, high-quality ingredients requires the systematic evaluation of processing methods and their impact on critical phytochemicals [5,12,18,19]. This study therefore investigates how three key processing approaches (fresh, heat-dried, and freeze-dried treatments) influence the preservation and extractability of loquat flowers’ bioactive flavonoids, with particular emphasis on flavonoid diversity and antioxidant capacity. By analyzing both pre- and post-extraction samples (FS/HD/FD vs. FSP/HDP/FDP), we aim to (1) identify processing-specific metabolic signatures and their associated health benefits, (2) quantify the efficiency of hot-water extraction for recovering active compounds from differently treated flowers, and (3) establish optimal processing protocols that balance compound retention, antioxidant potential, and practical scalability. The findings will provide a scientific foundation for upcycling loquat flowers into standardized nutraceutical ingredients, addressing both agricultural waste reduction and the growing demand for natural, sugar-free functional foods. Ultimately, this work bridges the gap between traditional agricultural practices and modern food science by transforming an overlooked biomass into a sustainable source of health-promoting compounds.
2. Materials and Methods
2.1. Development and Utilization of Active Ingredients in Loquat Flowers
The loquat flowers were collected from northern Yunnan, selecting mature buds with partial blooms to ensure consistency. Using sterilized scissors, the flowers were gently harvested to preserve their structure, then placed in clean collection bags to avoid damage. Back in the lab, the flowers underwent thorough cleaning—first soaked in deionized water with gentle agitation to remove dust, then patted dry with sterile gauze and air-dried. The cleaned samples were divided into three pre-extraction groups: fresh loquat flowers (FS) stored at 4 °C, oven (heat)-dried loquat flowers (HD) at 60 °C for 6 h until completely dry, and freeze-dried loquat flowers (FD) pre-frozen at −20 °C, then vacuum-dried at −50 °C for 48 h. Drying parameters were optimized via preliminary tests against literature standards for floral tissues [20,21], selecting conditions that maximized flavonoid recovery [22,23].
For post-extraction, three pre-extraction groups of loquat flowers (FS, HD, FD) were subjected to hot-water extraction (FSP: fresh loquat powder; HDP: heat-dried powder; FDP: freeze-dried powder) at 90 °C for 30 min using a 1:20 flower-to-water ratio, followed by a 6 h settling period to separate the supernatant. This extract was then freeze-dried into a fine powder by pre-freezing at −40 °C and vacuum-drying for another 48 h, resulting in a stable, easily stored form. Throughout the process, precision instruments like a constant-temperature drying oven, vacuum freeze-dryer, and ultra-low freezer were used to maintain consistency, while sterile materials ensured sample integrity. The final products—fresh, oven-dried, and freeze-dried extracts—were sealed and labeled for further analysis, providing a reliable foundation for studying their flavonoid compounds.
2.2. Determination of DPPH Free Radical Scavenging Ability of Loquat
The DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging assay was employed to evaluate the antioxidant capacity of differently processed loquat flower samples, including fresh, oven-dried, freeze-dried, and their hot-water-extracted powders [24]. For extraction, 0.1 g of each sample was homogenized in 1 mL of 80% methanol, subjected to ultrasonic-assisted extraction at 60 °C for 30 min (with intermittent shaking), and centrifuged at 12,000 rpm to obtain the supernatant. The assay was performed by mixing 150 µL of supernatant with 150 µL of DPPH working solution (prepared in ethanol) in a microtube. The control reaction contained 150 µL of methanol (without sample) and 150 µL of DPPH solution. After 30 min of incubation in the dark, absorbance was measured at 517 nm using a microplate reader. The antioxidant capacity was further quantified as Trolox (0–25 µg/mL) equivalents (µg/g) using a standard curve.
2.3. Flavonoid Profiling of Loquat Flowers
Flavonoid profiling of loquat flowers (dry weight) was conducted utilizing ultra-performance liquid chromatography coupled with tandem mass spectrometry (UPLC-MS/MS) methodology [25]. Flower samples underwent lyophilization for 63 h, followed by mechanical pulverization at 30 Hz for 1.5 min. Flavonoid extraction was performed using 70% methanol supplemented with internal standards (30 mg of powdered sample per 1.5 mL of solvent) at −20 °C. The extraction mixture was subjected to intermittent vortexing (six 30 s intervals) and subsequently centrifuged at 12,000 rpm for 3 min. The resulting supernatants were passed through 0.22 μm filters prior to instrumental analysis.
Chromatographic separation was achieved on an Agilent SB-C18 column (2.1 × 100 mm, 1.8 μm particle size) employing a binary gradient system consisting of 0.1% formic acid in water (mobile phase A) and acetonitrile (mobile phase B). The gradient program increased from 5% to 95% B over 9 min, with a flow rate of 0.35 mL/min at a column temperature of 40 °C. Flavonoid identification was accomplished through comparison with a customized MWDB database, implementing a three-tiered confidence classification system—Level 1 (confirmed standards), Level 2 (match scores between 0.5–0.7), and Level 3 (consistency in Q1/Q3 transitions, retention time, declustering potential, and collision energy parameters, Supplementary Table S1)—following established protocols [25].
2.4. Statistical Analysis
For the multivariate analysis of flavonoid profiles, statistical approaches were implemented to identify distinct patterns across samples. Raw metabolomic data underwent normalization procedures prior to performing hierarchical cluster analysis (HCA). Both HCA and principal component analysis (PCA) were executed according to methodologies described by Chanana and colleagues (2020) [26]. The visualization of results and calculation of standard deviations/errors were accomplished using Microsoft Excel software (Excel 2024, Microsoft Corporation, Redmond, WA, USA). To assess statistical differences in antioxidant activities between loquat flower extract samples, the Least Significant Difference (LSD) test was applied. All biochemical parameters were statistically analyzed using Statistix 8.1 software (Analytical Software, Tallahassee, FL, USA), with experiments conducted in triplicate to ensure reproducibility. For the visualization of overlapping features between datasets, Venn diagrams were constructed using the EVenn online platform (https://www.bic.ac.cn/EVenn/#/ accessed on 11 April 2025).
3. Results
3.1. Loquat Flowers Morphology, Processing, and Extraction
Fresh loquat flowers were harvested at optimal maturity, with buds fully developed and a small percentage beginning to bloom, then immediately processed to preserve phytochemical integrity (Figure 1A). The flowers exhibit characteristic small, fragrant, creamy-white inflorescences (Figure 1A). For sample preparation, flowers underwent one of three processing methods: (1) immediate use as fresh samples (FS), (2) conventional heat-drying (HD), or (3) freeze-drying (FD) (Figure 1B,C). The freeze-drying involved pre-freezing at −20 °C followed by primary drying at −50 °C under vacuum for 48 h, producing stable, porous lyophilizates that were vacuum-sealed in moisture-proof bags and stored at −80 °C to prevent degradation (Figure 1B). All three sample types were then subjected to hot-water extraction followed by secondary lyophilization to generate comparable powdered extracts: FSP (fresh sample powder), HDP (heat-dried powder), and FDP (freeze-dried powder) (Figure 1C). This standardized preparation protocol ensured consistent starting materials for subsequent comparative analyses of flavonoid profiles and antioxidant capacities while maintaining the stability of both heat-stable and thermolabile bioactive compounds.

Figure 1.
Processing methods and morphology of loquat flowers. (A): Floral anatomy: Schematic showing key fragrant, creamy-white loquat flower structures of clustered petals (multiple stamens, central pistil, and protective calyx), highlighting their rich bioactive potential. (B): Illustration of the freeze-drying process. (C): Hot-water extraction: Visual representation of optimal boiling conditions for lyophilized powder preparation that maximizes soluble antioxidant release while maintaining structural integrity of flower-derived compounds.
3.2. Clustering and Grouping of Flavonoids from Loquat Flowers Extracts
The HCA revealed distinct clustering patterns of flavonoid compounds across the six extraction methods (FS, HD, FD, FSP, HDP, FDP), highlighting variations in flavonoid profiles due to processing techniques. Fresh samples (FSs) and their powdered extracts (FSPs) exhibited higher levels of certain flavonoids, such as (+)-Catechin and Cyanidin 3-O-glucoside*, suggesting that minimal processing preserves these compounds. Heat-dried samples (HD, HDP) showed elevated concentrations of compounds like tricin and quercetin-3-O-rhamnosyl(1→2)arabinoside, likely due to thermal degradation or the transformation of precursors during drying. Additionally, the HDP samples showed higher abundance of cyanidin-3-O-galactoside, 6-Hydroxykaempferol 7-rutinoside, 2′,3′,4′,5,7-pentahydroxyflavone, quercetin, 8-Hydroxykaempferol, homomangiferin, (-)-Epigallocatechin, and peonidin-3-O-rutinoside compound as compared to other processing extracts. Several flavonoids compounds showed higher concentration of mangiferin, equol, phellatin, isoorientin, 7,3′-dimethyl ether, arcapillin, peonidin-3-O-sambubioside, cyanidin-3-O-rutinoside (keracyanin), naringenin, pinobanksin, quercetin-4′-O-glucuronide, lancerin, tricetin 3′-glucuronide, 4,2′,3′,4′-Tetrahydroxychalcone, Naringenin chalcone, pseudosindorin, pelargonin, palasitrin, malonyl glucosyl amurensin, catechin-(α→8)-gallocatechin, and quercetin-3-O-(2″-O-Rhamnosyl)rutinoside in the powdered extracts (FSP, HDP, FDP) (Figure 2A).
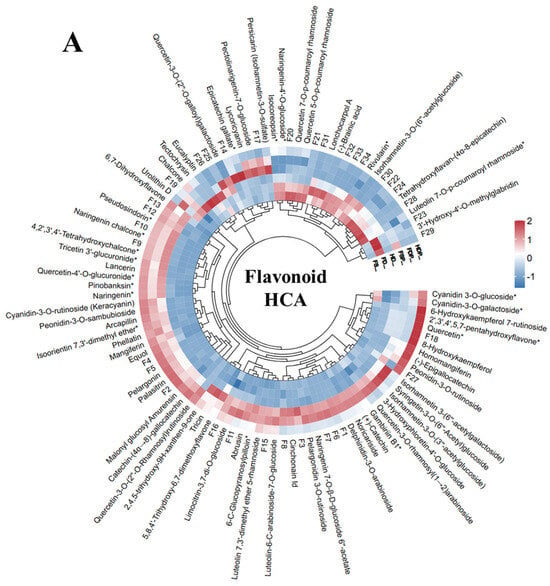
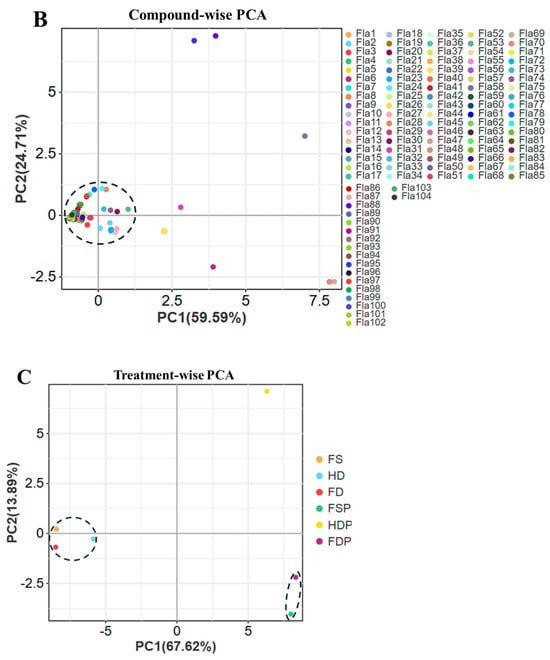
Figure 2.
Hierarchical cluster analysis (HCA) and of flavonoid compounds in loquat flower extracts. (A): The HCA dendrogram illustrates the clustering of flavonoid across six processing methods. The F1-F34 numbers are consistent with Table S2 numbering, (*) means isomers. (B): Compound-wise PCA, the Fla1-Fla104 are consistent with Table S3 numbering. (C): Treatment-wise PCA. Abbreviations: Fresh sample (FS), heat-dried (HD), freeze-dried (FD), and their respective hot-water extracts (FSP, HDP, FDP).
Fresh sample powder and freeze-dried samples (FSP, FDPs) showed increased abundance of several flavonoids and anthocyanin compounds such as isorhamnetin 3-(6″-acetylgalactoside), syringetin-3-O-(6″-Acetyl)glucoside, isorhamnetin-3-O-(3″-acetylglucoside), 3-Hydroxyphloretin-4′-O-glucoside, quercetin-3-O-rhamnosyl(1→2)arabinoside, gambiriin B1, (+)-Catechin, noricariside, delphinidin-3-O-arabinoside, naringenin 7-O-β-D-glucoside 6″-acetate, pelargonidin 3-O-rutinoside, cinchonain Id, luteolin-6-C-arabinoside-7-O-glucoside, luteolin 7,3′-dimethyl ether 5-rhamnoside, 6-C-glucopyranosylpilloin, abrusin, 5,8,4′-Trihydroxy-6,7-dimethoxyflavone, tricin, 2,4,5-trihydroxy-9H-xanthen-9-one, and limocitrin-3,7-di-O-glucoside, indicating that fresh sample powder and freeze-drying powder better preserves heat-sensitive flavonoids (Figure 2A). The powdered extracts (FSP, HDP, FDP) generally displayed reduced flavonoid diversity compared to their non-extracted counterparts (FS, HD, FD), suggesting that hot-water extraction and lyophilization may lead to the partial loss or degradation of certain compounds. Notably, FDP samples clustered closely with FD, implying that freeze-drying post-extraction maintains flavonoid integrity better than heat-based methods. In contrast, HDP samples showed a distinct profile, with higher levels of thermally stable flavonoids like Isorhamnetin derivatives, which may withstand boiling and drying.
The PCA plot reveals distinct clustering patterns among the loquat flower samples processed using different methods (FS, HD, FD, FSP, HDP, FDP), highlighting variations in their flavonoid profiles (Figure 2B). Flavonoid compounds such as Fla6 (Catechin-(4α→8)-gallocatechin), Fla15 (Epimesquitol-(4α→6)-epimesquitol-4beta-ol), and Fla22 (Naringenin) are positioned farther from the origin along PC1 and PC2, indicating higher concentrations in specific samples (Figure 2B). For instance, Fla6 and Fla15 are strongly associated with freeze-dried samples FD and FDP, suggesting these methods better preserve certain flavonoids compared to heat-dried (HD/HDP) or fresh (FS/FSP) samples, respectively (Figure 2B). Conversely, Fla8 (Cyanidin 3-O-glucoside) and Fla69 (Quercetin, a potent antioxidant) are higher in fresh samples and heat-dried (HD and HDP) samples, respectively, implying their stability to degradation during processing.
The separation along PC1 (59.59% variance) primarily reflects differences between fresh/extracted samples, while PC2 (24.71% variance) distinguishes drying methods (Figure 2B). Eight flavonoid compounds cluster separately, likely due to their retention of heat-sensitive flavonoids or due to partial degradation of compounds during oven drying (Figure 2B). The hot-water extracts (P-samples) show intermediate positions, indicating selective extraction of flavonoids into the tea. Notably, Fla22 and Fla69 are prominent in extracts, suggesting their solubility and stability in hot water, which aligns with their potential health benefits.
The PCA plot highlights distinct clustering patterns among the six loquat flower samples (FS, HD, FD, FSP, HDP, FDP), revealing how different processing methods influence flavonoid composition (Figure 2C). The first principal component (PC1, 67.62% variance) strongly separates hot-water extracts (FSP, HDP, FDP) from their raw counterparts (FS, HD, FD), suggesting significant metabolic changes during extraction. Notably, freeze-dried powder (FDP, purple) and heat-dried powder (HDP, yellow) are positioned farthest along PC1, indicating higher concentrations of certain flavonoids in these extracts (Figure 2C).
Freeze-drying (FD/FDP) appears superior for preserving antioxidant flavonoids, as FDP is distinctly separated along both PC1 and PC2 (13.89% variance), likely due to minimal thermal degradation (Figure 2C). Heat-dried samples (HD/HDP) show moderate flavonoid retention, while fresh and freeze-dried samples (FS/FD) exhibit the least separation, suggesting that drying (especially freeze-drying) enhances flavonoid stability and extractability (Figure 2C). The positioning of HDP near the top-right quadrant implies it retains unique or higher quantities of antioxidant compounds (e.g., flavonoid and its derivatives) compared to FDP and FSP (Figure 2C).
3.3. Analysis of Processing Methods on Loquat Flower Extracts and Their Antioxidant Flavonoid Content
The pairwise comparisons of loquat flower samples (fresh, heat-dried, and freeze-dried, both before and after hot-water extraction) reveal significant differences in their active flavonoid profiles. Among the pre-extraction samples, heat-dried powder (HDP) showed the highest number of upregulated flavonoids (57 out of 100) compared to fresh sample powder (FSP), suggesting that heat-drying may enhance certain bioactive compounds. However, FDP exhibited fewer upregulated flavonoids (only 5 vs. FSP), indicating that freeze-drying might preserve a more natural composition. Interestingly, FD vs. HD samples showed a balanced up/downregulation (21 up, 34 down), implying that freeze-drying retains different antioxidants compared to heat-drying.
For tea extraction, hot-water extraction (P samples) generally led to a high proportion of insignificant changes (e.g., 77 out of 98 for FDP vs. FSP), meaning that many flavonoids remained stable. However, heat-dried powder extracts (HDPs) still showed more upregulated compounds than freeze-dried extracts (FDPs) when compared to fresh samples. This suggests that heat-drying followed by hot-water extraction may be more effective in concentrating certain flavonoids, whereas freeze-drying better preserves the original fresh-like profile. Since flavonoids are heat-sensitive, moderate heat-drying could enhance extraction efficiency, but excessive heat may degrade some antioxidants. Thus, a combination of gentle heat-drying followed by hot-water extraction may maximize flavonoid yield while maintaining antioxidant quality.
3.4. Analysis of Flavonoid Content in Loquat Flower Extracts: Impact of Processing Methods
The Venn diagram analysis revealed distinct patterns in flavonoid composition between the processing methods on loquat flower extracts (Figure 3A). The HDP vs. FSP and HDP vs. HD comparison exhibited the largest number of significantly altered flavonoids (n = 100), followed by FDP vs. FSP (n = 96), while the HD vs. FS showed the lowest number of altered flavonoids (n = 87). Notably, 73 flavonoids were shared across all six comparison groups, while 15 and 9 flavonoid compounds were shared across five and four different comparison groups, respectively (Figure 3A). These overlaps and distinct results highlight the influence of processing methods-specific flavonoid variation.
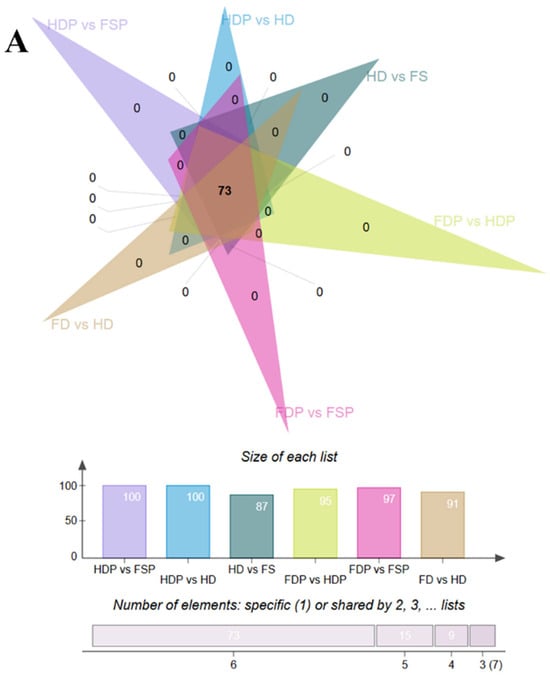
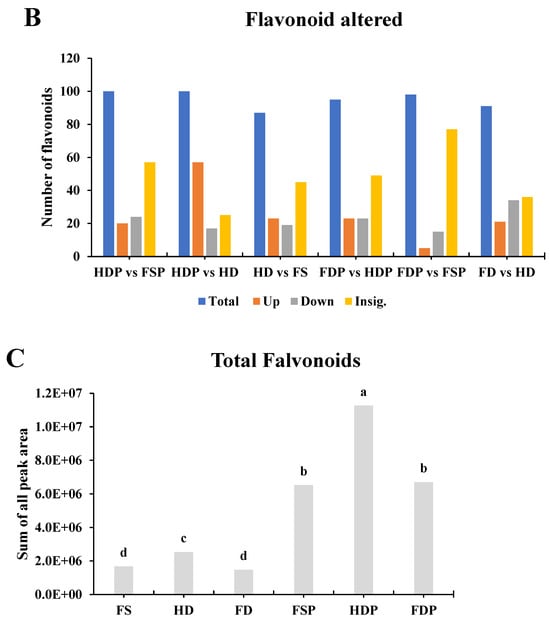
Figure 3.
Interactive Venn matrix and significantly altered flavonoids across processing methods. (A): The interactive diagram visualizes overlaps and unique flavonoids between six comparison groups. (B): Bar graphs represent the number of upregulated, downregulated, and insignificant flavonoids in pairwise comparisons between different processing methods. (C): Bar chart comparing total flavonoid peak areas (LC-MS/MS) across different processing methods. Statistical significance (p < 0.05) between different sample groups (with three replicates, n = 3) was determined using Fisher’s Least Significant Difference (LSD) test, with lowercase letters indicating distinct groupings where significant differences exist for individual compounds. Abbreviations: (FS: fresh sample, HD: heat-drying, FD: freeze-drying, FSP: fresh sample powder, HDP: heat-dried powder, FDP: freeze-dried powder).
The LC-MS/MS analysis of flavonoid peak areas reveals significant differences in antioxidant content based on processing methods. Heat-dried powder exhibited the highest flavonoid levels (11,268, 993.62 peak area), followed by FDP (6700, 324.418) and fresh sample powder (FSP, 6521, 278.008). This suggests that heat-drying before extraction significantly enhances flavonoid recovery, likely due to cell wall breakdown and improved compound release during hot-water extraction. Interestingly, freeze-dried (FD) samples showed lower flavonoid levels (1479, 830.344) compared to fresh samples (FS, 1682, 580.553), indicating that freeze-drying alone may not concentrate flavonoids as effectively as heat-drying. However, once extracted into powder form (FDP), freeze-dried samples still retain substantial flavonoids, suggesting that hot-water extraction is crucial for flavonoid recovery regardless of drying method.
For tea production, heat-dried loquat flowers processed into HDP appear to be the most effective method for maximizing flavonoid content. The nearly twofold higher flavonoid levels in HDP compared to FDP indicate that thermal processing enhances extractability, possibly by degrading cell structures and increasing solubility. However, since excessive heat can degrade some sensitive antioxidants, optimizing drying temperature and duration could further improve yield. Freeze-drying, while gentler, may be preferable if preserving heat-sensitive compounds is a priority, though it results in lower overall flavonoid recovery. These findings support using heat-dried loquat flower HDP for antioxidant-rich tea, balancing high yield with practical processing feasibility.
3.5. Impact of Processing Methods on Key Flavonoid Compounds in Loquat Flower Extracts
The LC-MS/MS analysis revealed distinct patterns in flavonoid preservation and enhancement across different processing methods (Figure 4). FDP showed the highest levels of catechin-(4α→8)-gallocatechin (10.21%), suggesting that freeze-drying better preserves this specific antioxidant compared to heat-dried powder (7.92%). However, heat-dried samples (HD and HDP) dominated in quercetin content (HD: 18.11%, HDP: 17.47%), a potent antioxidant, indicating that thermal processing enhances its extractability (Figure 4). Interestingly, fresh samples (FSs) retained higher levels of cyanidin-3-O-galactoside (5.30%) and lonchocarpol A (12.86%), but these compounds sharply declined after hot-water extraction, likely due to heat sensitivity. This suggests that while fresh samples contain certain beneficial flavonoids, hot-water extraction significantly alters their composition, favoring more stable compounds like quercetin and catechin derivatives.
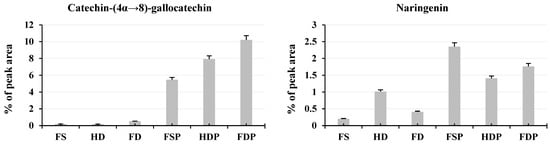
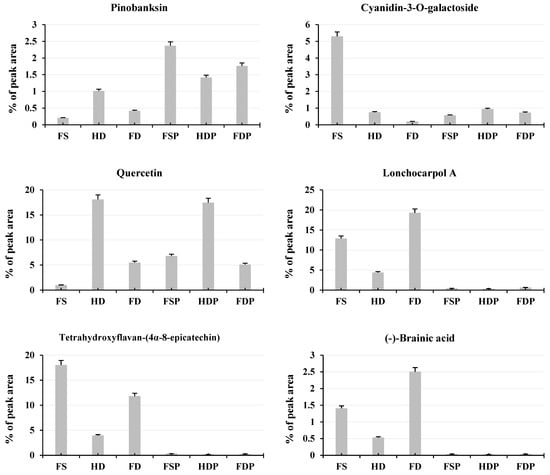
Figure 4.
Relative abundance (%) of major flavonoids in loquat flower samples. Column bars compare key flavonoids across processing methods (FS, HD, FD, FSP, HDP, FDP). All quantitative results were derived from LC-MS/MS analyses performed in triplicate to ensure reliability. The error bars displayed in figures represent the standard deviation (±SD) across these three biological replicates. Abbreviations: FS: fresh sample, HD: heat-drying, FD: freeze-drying, FSP: fresh sample powder, HDP: heat-dried powder, FDP: freeze-dried powder.
For naringenin and pinobanksin, heat-dried samples before extraction (HD) showed higher levels (1.02% and 1.02%, respectively) compared to freeze-dried (FD: 0.41% and 0.42%), but after extraction, FDP outperformed HDP (1.76% vs. 1.41% for naringenin). This implies that freeze-drying may better retain these compounds post-extraction. Conversely, tetrahydroxyflavan and (-)-brainic acid were most abundant in fresh and freeze-dried samples (FS/FD) but drastically reduced in powdered extracts (FSP/HDP/FDP), indicating they may degrade during hot-water processing (Figure 4). These findings highlight a trade-off: heat-drying boosts certain flavonoids (e.g., quercetin) but degrades others (e.g., cyanidin-3-O-galactoside), while freeze-drying better preserves some heat-sensitive compounds but may yield lower overall extraction efficiency for others.
Optimal Processing Method for Antioxidant-Rich Tea
For maximum flavonoid diversity and antioxidant potential, freeze-dried powder appears superior for catechin-(4α→8)-gallocatechin, naringenin, and pinobanksin, while HDP is better for quercetin extraction (Figure 4). If the goal is a high-quercetin tea (valuable for anti-inflammatory effects), HDP is the clear choice. However, if preserving a broader flavonoid profile is desired, FDP may be preferable despite its lower quercetin content. Fresh samples, though rich in certain compounds, are impractical for tea production due to rapid spoilage and significant flavonoid loss during extraction. Thus, the optimal method depends on the target flavonoids: heat-drying for quercetin-rich tea or freeze-drying for a more balanced, heat-sensitive antioxidant profile.
3.6. Antioxidant Capacity of Loquat Flower Extracts: Evaluating Processing Methods
The DPPH scavenging capacity results reveal significant differences in antioxidant activity among the processed loquat flower samples (Figure 5). HDP demonstrated the highest antioxidant capacity (615.24 μg Trolox/g), followed by FDP (487.73 μg Trolox/g) and fresh sample powder (FSP, 403.38 μg Trolox/g). This indicates that hot-water extraction significantly enhances antioxidant activity, likely due to improved solubility and release of bioactive compounds. Among the pre-extraction samples, HD flowers showed stronger antioxidant potential (269.32 μg Trolox/g) than fresh (FS, 211.45 μg Trolox/g) or freeze-dried (FD, 197.17 μg Trolox/g) samples, suggesting that heat-drying alone can boost antioxidant properties, possibly by concentrating certain compounds or breaking down cell walls for better extraction.
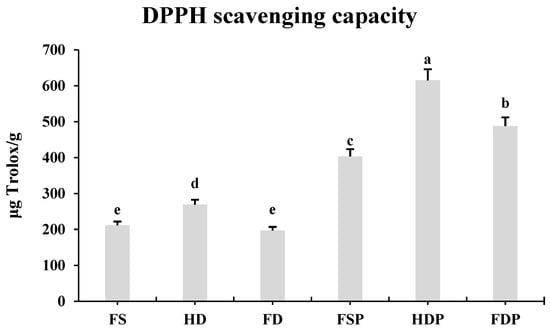
Figure 5.
DPPH scavenging capacity of loquat flower extracts. Column bars compare antioxidant activity (μg Trolox/g) across processing methods. The error bars displayed in figures represent the standard deviation (±SD) across these three biological replicates. Statistical significance (p < 0.05) between different sample groups was determined using Fisher’s Least Significant Difference (LSD) test, with lowercase letters (a, b, c, d, e) indicating distinct groupings where significant differences exist for individual compounds.
For maximum antioxidant yield in tea production, HDP is clearly the most effective method, offering nearly 50% higher activity than FDP. While freeze-drying preserves some heat-sensitive antioxidants (as seen in previous flavonoid analysis), the overall radical-scavenging capacity is superior to heat processing (Figure 5). This aligns with the earlier observation that heat-dried samples contain higher levels of potent antioxidants like quercetin, which are likely to contribute to this enhanced activity (Figure 4 and Figure 5). However, FDP still outperforms FSP, indicating that even without heat, the extraction process itself improves antioxidant availability. Thus, for applications prioritizing maximum antioxidant potency, heat-drying followed by hot-water extraction (HDP) is optimal, while freeze-drying (FDP) remains a viable alternative for preserving a broader range of heat-sensitive compounds.
4. Discussion
Our study systematically evaluated the impact of different processing methods on the flavonoid composition and antioxidant capacity of loquat flower extracts. Our findings demonstrate that processing techniques significantly influence bioactive compound retention, extractability, and overall functionality, with heat-dried powder exhibiting the highest total flavonoid content and DPPH radical scavenging activity, while freeze-dried powder better preserved certain heat-sensitive flavonoids [15,17,27,28,29]. These results align with the growing interest in agricultural byproduct valorization and provide practical insights for developing functional loquat flower-based products, particularly sugar-free health teas [11,30,31,32,33,34,35].
4.1. Impact of Processing Methods on Flavonoid Profiles
Flavonoids are highly sensitive to thermal degradation [20,21], yet our data indicates that moderate heat-drying (60 °C) enhanced the extractability of certain compounds, such as quercetin and its derivatives, in HDP samples. This observation is consistent with previous studies on other plant materials, where controlled heat exposure was found to disrupt cell wall structures, facilitating the release of bound phenolics [36,37,38]. However, excessive heat can degrade thermolabile flavonoids [39,40], as seen in the reduced levels of cyanidin-3-O-galactoside and lonchocarpol A in HDP compared to fresh samples (FSs). Previously, similar trends were observed that showed that high-temperature drying of herbal teas led to the partial degradation of anthocyanins while increasing the concentration of more stable flavonoids like quercetin [22,23].
Freeze-drying, known for its ability to preserve heat-sensitive compounds, resulted in higher retention of catechin-(4α→8)-gallocatechin and naringenin in FDP. This aligns with previous findings, which demonstrated that freeze-dried plant materials retained greater phenolic diversity compared to conventional drying methods [41,42]. However, our study also revealed that freeze-drying alone (FD) did not significantly enhance flavonoid content unless followed by hot-water extraction (FDP), suggesting that the extraction step is crucial for compound solubilization. This finding supports previous findings, which showed that freeze-drying preserves cellular integrity but may require additional processing (e.g., extraction) to maximize bioactive release [43,44].
4.2. Antioxidant Capacity: Correlation with Processing and Flavonoid Composition
The DPPH assay confirmed that HDP possessed the highest antioxidant activity (615.24 μg Trolox/g), followed by FDP (487.73 μg Trolox/g) and FSP (403.38 μg Trolox/g). This trend correlates with the elevated levels of quercetin and other stable flavonoids in HDP [22,23], which are known for their potent radical-scavenging properties [45,46,47,48]. The superior antioxidant performance of heat-dried samples may also be attributed to Maillard reaction products formed during drying, which can contribute to additional reducing capacity [48,49,50]. However, the fact that FDP still outperformed FSP suggests that freeze-drying, despite being a low-temperature process, does not necessarily compromise antioxidant potential when combined with efficient extraction.
Interestingly, fresh samples (FSs) exhibited lower DPPH activity compared to their processed counterparts, contradicting the assumption that minimal processing always retains the highest bioactivity. This could be due to the presence of bound phenolics in fresh tissues that are not fully extracted without prior drying [48,51,52]. The comparatively lower antioxidant activity in fresh samples may reflect both the incomplete release of bound phenolics and potential enzymatic degradation during the initial extraction phase before thermal inactivation [53,54,55,56]. Future studies should include enzyme inhibition controls (e.g., immediate blanching or liquid nitrogen freezing) to isolate these effects. Our results thus emphasize that some degree of processing (either heat- or freeze-drying) is necessary to optimize antioxidant yield, particularly for tea applications where hot-water extraction is standard.
4.3. Comparative Advantages of Heat-Drying vs. Freeze-Drying for Functional Tea Development
From an industrial perspective, the choice between heat-drying and freeze-drying involves trade-offs between flavonoid yield, compound stability, and economic feasibility. Heat-drying is more cost-effective and scalable, making it suitable for large-scale production of antioxidant-rich loquat flower tea. The higher quercetin content in HDP is particularly advantageous, as this flavonoid is associated with anti-inflammatory and cardioprotective effects [57,58,59,60,61,62]. However, if the goal is to preserve a broader spectrum of flavonoids—including heat-sensitive compounds like catechins and anthocyanins—freeze-drying may be preferable despite its higher operational costs.
Our HCA and PCA further highlighted these distinctions, with HDP and FDP forming separate clusters due to their divergent flavonoid profiles. This aligns with previous metabolomic studies on medicinal herbs, where processing methods were shown to induce distinct chemical signatures [63,64,65]. The intermediate positioning of FSP in PCA plots suggests that fresh extraction alone is less effective, reinforcing the need for pre-processing (drying) to enhance phytochemical recovery.
5. Conclusions, Practical Implications, and Future Direction
This study demonstrates that processing methods significantly influence the bioactive compound profile and antioxidant potential of loquat flower extracts. Heat-drying (60 °C) followed by hot-water extraction yielded the highest total flavonoid content (11.27 × 106 peak area) and DPPH scavenging capacity (615.24 μg Trolox/g), primarily due to the enhanced release of stable flavonoids like quercetin. In contrast, freeze-drying better preserves heat-sensitive compounds such as catechin-(4α→8)-gallocatechin, albeit with lower overall antioxidant activity. Multivariate analysis revealed distinct metabolic signatures for each processing method, with heat-dried samples showing superior extractability while freeze-dried samples maintained greater compound diversity. These findings provide critical insights for transforming an agricultural byproduct into value-added nutraceutical ingredients. For the industrial-scale production of functional loquat flower tea, heat-drying offers the optimal balance between bioactive yield and processing feasibility. This work not only addresses agricultural waste valorization but also contributes to the growing demand for natural, sugar-free functional foods with scientifically validated health benefits.
The findings of this study have direct implications for the nutraceutical and functional food industries. By converting loquat flowers—a currently wasted agricultural byproduct—into a value-added ingredient, farmers and processors can tap into the growing market for natural, sugar-free health products. Our results suggest that
- For maximum antioxidant yield: Heat-drying followed by hot-water extraction (HDP) is optimal.
- For preserving heat-sensitive flavonoids: Freeze-drying (FDP) should be prioritized, especially if targeting specific bioactive compounds like catechins.
- For industrial scalability: Heat-drying offers a practical balance between cost and bioactivity, though hybrid approaches (e.g., mild heat-drying combined with optimized extraction) could be explored further.
Future research will incorporate ABTS, FRAP, and ORAC assays to validate antioxidant mechanisms, quantify Maillard reaction products, and analyze volatile compounds via GC-MS to assess sensory properties (aroma profiles) of loquat flower teas produced via different methods, as consumer acceptance is critical for commercialization. These steps will further optimize processing protocols for both bioactive retention and sensory quality. Additionally, in vitro and in vivo studies could validate the health benefits (e.g., anti-diabetic, anti-inflammatory effects) of these extracts to strengthen their functional food applications.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/horticulturae11070766/s1. Table S1: Raw metabolic data; Table S2: Figure 2A numbering (F1–F34) are consistent with Table S2; Table S3: Figure 2B numbering (Fla1–Fla104) are consistent with Table S3 numbering.
Author Contributions
Conceptualization, M.J.R. and S.Y.; methodology, M.D. and M.J.R.; software, M.D.; investigation, M.D., X.W. (Xi Wang), J.F., X.X., L.Z., S.H., L.M. and X.W. (Xue Wang); resources, S.Y. and M.D.; writing—original draft preparation, M.D. and M.J.R.; writing—review and editing, all authors; funding acquisition, M.D. and S.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was jointly funded by Yunnan Provincial First-Class Discipline Construction Project: Cold Highland Characteristic Crops and Biological Resources Science, Grant number: 202210; The project of Scientific research start-up funds for doctoral talents of Zhaotong University -Mingzheng Duan, Grant number: 202406; Young Talent Project of Talent Support Program for the Development of Yunnan. Grant number: 210604199008271015; Team Project of the “Xingzhao Talent Support Plan” in Zhaotong City. Grant No.: ZhaodangRencai[2023]No.3.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rao, M.J.; Duan, M.; Yang, M.; Fan, H.; Shen, S.; Hu, L.; Wang, L. Novel Insights into Anthocyanin Metabolism and Molecular Characterization of Associated Genes in Sugarcane Rinds Using the Metabolome and Transcriptome. Int. J. Mol. Sci. 2022, 23, 338. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Cao, S.; Yang, Z.; Zheng, Y. MeJA Regulates Enzymes Involved in Ascorbic Acid and Glutathione Metabolism and Improves Chilling Tolerance in Loquat Fruit. Postharvest Biol. Technol. 2011, 59, 324–326. [Google Scholar] [CrossRef]
- Shah, H.M.S.; Khan, A.S.; Singh, Z.; Ayyub, S. Postharvest Biology and Technology of Loquat (Eriobotrya japonica Lindl.). Foods 2023, 12, 1329. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S.; Bhardwaj, R.; Vyas, N. Bioactive Compounds of Loquat (Eriobotrya japonica (Thunb.) L.). In Bioactive Compounds in Underutilized Fruits and Nuts; Springer: Berlin/Heidelberg, Germany, 2020; pp. 123–143. [Google Scholar]
- Dhiman, A.; Suhag, R.; Thakur, D.; Gupta, V.; Prabhakar, P.K. Current Status of Loquat (Eriobotrya japonica Lindl.): Bioactive Functions, Preservation Approaches, and Processed Products. Food Rev. Int. 2022, 38, 286–316. [Google Scholar] [CrossRef]
- Nile, S.H.; Keum, Y.S.; Nile, A.S.; Jalde, S.S.; Patel, R. V Antioxidant, Anti-inflammatory, and Enzyme Inhibitory Activity of Natural Plant Flavonoids and Their Synthesized Derivatives. J. Biochem. Mol. Toxicol. 2018, 32, e22002. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michael, M. Flavonoids as Antioxidants: Determination of Radical Scavenging Efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar]
- Rao, M.J.; Zheng, B. The Role of Polyphenols in Abiotic Stress Tolerance and Their Antioxidant Properties to Scavenge Reactive Oxygen Species and Free Radicals. Antioxidants 2025, 14, 74. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y.; et al. Antioxidant Defense System in Plants: Reactive Oxygen Species Production, Signaling, and Scavenging During Abiotic Stress-Induced Oxidative Damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Wang, J.; Han, S.; Ma, L.; Mo, X.; Li, M.; Hu, L.; Wang, L. Transcriptomic and Widely Targeted Metabolomic Approach Identified Diverse Group of Bioactive Compounds, Antiradical Activities, and Their Associated Genes in Six Sugarcane Varieties. Antioxidants 2022, 11, 1319. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Huang, P.-H.; Shih, M.-K.; Wu, C.-C.; Hsieh, C.-W.; Chen, M.-H.; Hsieh, S.-L.; Hou, C.-Y. Functional Evaluation of Loquat (Eriobotrya japonica Lindl.) Flower Water Extracts and Its Potential Use in Tea. J. Food Process Preserv. 2023, 2023, 1188178. [Google Scholar] [CrossRef]
- Cioanca, O.; Lungu, I.-I.; Mita-Baciu, I.; Robu, S.; Burlec, A.F.; Hancianu, M.; Crivoi, F. Extraction and Purification of Catechins from Tea Leaves: An Overview of Methods, Advantages, and Disadvantages. Separations 2024, 11, 171. [Google Scholar] [CrossRef]
- Fu, Y.; Li, F.; Ding, Y.; Li, H.-Y.; Xiang, X.-R.; Ye, Q.; Zhang, J.; Zhao, L.; Qin, W.; Gan, R.-Y. Polysaccharides from Loquat (Eriobotrya japonica) Leaves: Impacts of Extraction Methods on Their Physicochemical Characteristics and Biological Activities. Int. J. Biol. Macromol. 2020, 146, 508–517. [Google Scholar] [CrossRef]
- Costa, B.P.; Ikeda, M.; de Melo, A.M.; Alves, F.E.S.B.; Carpiné, D.; Ribani, R.H. Eriobotrya japonica Fruits and Its By-Products: A Promising Fruit with Bioactive Profile and Trends in the Food Application–A Bibliometric Review. Food Biosci. 2022, 50, 102099. [Google Scholar] [CrossRef]
- Zhao, X. Comprehensive Review of Bioactive Compounds in Loquat and Their Pharmacological Mechanisms. Med. Plant Res. 2024, 14, 196–209. [Google Scholar] [CrossRef]
- Kumar, V.; Gupta, P.; Kumar, M. Loquat and Its Phytochemical Potential: A Promising Application in Food Technology. eFood 2024, 5, e158. [Google Scholar] [CrossRef]
- Lu, Z.M.; Wu, W.X.; Zhang, Z.L. Effect of Flower and Fruit Thinning on Yield and Quality of Loquat in Northern Margin of Loquat Distribution Area in China. In Proceedings of the III International Symposium on Loquat 887, Antakya, Turkey, 3–6 May 2010; pp. 165–169. [Google Scholar]
- Rao, M.J.; Duan, M.; Wei, X.; Zuo, H.; Ma, L.; Tahir Ul Qamar, M.; Li, M.; Han, S.; Hu, L.; Wang, L. LC–MS/MS-Based Metabolomics Approach Revealed Novel Phytocompounds from Sugarcane Rind with Promising Pharmacological Value. J. Sci. Food Agric. 2022, 102, 6632–6642. [Google Scholar] [CrossRef]
- Rao, M.J.; Tahir Ul Qamar, M.; Wang, D.; Ali, Q.; Ma, L.; Han, S.; Duan, M.; Hu, L.; Wang, L. A High-Throughput Lipidomics and Transcriptomic Approach Reveals Novel Compounds from Sugarcane Linked with Promising Therapeutic Potential against COVID-19. Front. Nutr. 2022, 9, 988249. [Google Scholar] [CrossRef]
- Ioannou, I.; Chekir, L.; Ghoul, M. Effect of the Processing Temperature on the Degradation of Food Flavonoids: Kinetic and Calorimetric Studies on Model Solutions. J. Food Eng. Technol. 2019, 8, 91–102. [Google Scholar] [CrossRef]
- Gao, Y.; Xia, W.; Shao, P.; Wu, W.; Chen, H.; Fang, X.; Mu, H.; Xiao, J.; Gao, H. Impact of Thermal Processing on Dietary Flavonoids. Curr. Opin. Food Sci. 2022, 48, 100915. [Google Scholar] [CrossRef]
- ElGamal, R.; Song, C.; Rayan, A.M.; Liu, C.; Al-Rejaie, S.; ElMasry, G. Thermal Degradation of Bioactive Compounds during Drying Process of Horticultural and Agronomic Products: A Comprehensive Overview. Agronomy 2023, 13, 1580. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Chuyen, H. Van Processing of Herbal Tea from Roselle (Hibiscus sabdariffa L.): Effects of Drying Temperature and Brewing Conditions on Total Soluble Solid, Phenolic Content, Antioxidant Capacity and Sensory Quality. Beverages 2020, 6, 2. [Google Scholar] [CrossRef]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef]
- Chanana, S.; Thomas, C.S.; Zhang, F.; Rajski, S.R.; Bugni, T.S. HCAPCA: Automated Hierarchical Clustering and Principal Component Analysis of Large Metabolomic Datasets in R. Metabolites 2020, 10, 297. [Google Scholar] [CrossRef]
- Yang, J.; Guo, C.; Chen, F.; Lv, B.; Song, J.; Ning, G.; He, Y.; Lin, J.; He, H.; Yang, Y.; et al. Heat-Induced Modulation of Flavonoid Biosynthesis via a LhMYBC2-Mediated Regulatory Network in Oriental Hybrid Lily. Plant Physiol. Biochem. 2024, 214, 108966. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Charbonnel, C.; Chekir, L.; Ghoul, M. Effect of Heat Processing on Thermal Stability and Antioxidant Activity of Six Flavonoids. J. Food Process Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Cui, M.; Liang, Z.; Liu, Y.; Sun, Q.; Wu, D.; Luo, L.; Hao, Y. Flavonoid Profile of Anoectochilus roxburghii (Wall.) Lindl. Under Short-Term Heat Stress Revealed by Integrated Metabolome, Transcriptome, and Biochemical Analyses. Plant Physiol. Biochem. 2023, 201, 107896. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Q.; Lai, C.; Jiang, F. Optimized Formulation of a Chewable Candy Containing Loquat Flower Tea. Fujian J. Agric. Sci. 2021, 36, 964–971. [Google Scholar]
- Zheng, M.; Li, J.; Feng, J.; Lu, S. Effect of Drying Methods on Sensory Qualities of Loquat Flower Tea and Its Toxicological Evaluation. Food Sci. 2018, 39, 116–121. [Google Scholar]
- Zheng, M.; Xia, Q.; Lu, S. Study on Drying Methods and Their Influences on Effective Components of Loquat Flower Tea. LWT-Food Sci. Technol. 2015, 63, 14–20. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, L.; Shan, Y.; Liu, Y.; Tian, Y.; Xia, T. Influence of Shade on Flavonoid Biosynthesis in Tea (Camellia sinensis (L.) O. Kuntze). Sci. Hortic. 2012, 141, 7–16. [Google Scholar] [CrossRef]
- Zubova, M.Y.; Nechaeva, T.L.; Kartashov, A.V.; Zagoskina, N. V Regulation of the Phenolic Compounds Accumulation in the Tea-Plant Callus Culture with a Separate and Combined Effect of Light and Cadmium Ions. Biol. Bull. 2020, 47, 593–604. [Google Scholar] [CrossRef]
- Zhao, X.; Li, P.; Zuo, H.; Peng, A.; Lin, J.; Li, P.; Wang, K.; Tang, Q.; Tadege, M.; Liu, Z. CsMYBL2 Homologs Modulate the Light and Temperature Stress-regulated Anthocyanin and Catechins Biosynthesis in Tea Plants (Camellia sinensis). Plant J. 2023, 115, 1051–1070. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound Phenolics in Foods, a Review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Liu, X.; Le Bourvellec, C.; Renard, C.M.G.C. Interactions between Cell Wall Polysaccharides and Polyphenols: Effect of Molecular Internal Structure. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3574–3617. [Google Scholar] [CrossRef]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of Grape Skins: Significance of Plant Cell-Wall Structural Components and Extraction Techniques for Phenol Release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Zhao, Y.-W.; Wang, C.-K.; Huang, X.-Y.; Hu, D.-G. Anthocyanin Stability and Degradation in Plants. Plant Signal Behav. 2021, 16, 1987767. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Kittibunchakul, S.; Temviriyanukul, P.; Chaikham, P.; Kemsawasd, V. Effects of Freeze Drying and Convective Hot-Air Drying on Predominant Bioactive Compounds, Antioxidant Potential and Safe Consumption of Maoberry Fruits. LWT 2023, 184, 114992. [Google Scholar] [CrossRef]
- Gat, Y.; Gawande, P. Freeze-Drying Effect on Nutrients and Their Stability. In Freeze Drying of Food Products: Fundamentals, Processes and Applications; Wiley Online Library: Hoboken, NJ, USA, 2024; pp. 179–201. [Google Scholar]
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G. Encapsulation and Delivery of Bioactive Compounds Using Spray and Freeze-Drying Techniques: A Review. Dry. Technol. 2020, 38, 235–258. [Google Scholar] [CrossRef]
- Kandasamy, S.; Naveen, R. A Review on the Encapsulation of Bioactive Components Using Spray-drying and Freeze-drying Techniques. J. Food Process Eng. 2022, 45, e14059. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Eman, M.; Yuan, H.; Sharma, A.; Zheng, B. Comparative Analysis of Citrus Species’ Flavonoid Metabolism, Gene Expression Profiling, and Their Antioxidant Capacity under Drought Stress. Antioxidants 2024, 13, 1149. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Feng, B.; Ahmad, M.H.; Tahir Ul Qamar, M.; Aslam, M.Z.; Khalid, M.F.; Hussain, S.B.; Zhong, R.; Ali, Q.; Xu, Q.; et al. LC-MS/MS-Based Metabolomics Approach Identified Novel Antioxidant Flavonoids Associated with Drought Tolerance in Citrus Species. Front. Plant Sci. 2023, 14, 1150854. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Duan, M.; Shad, M.A.; Aslam, M.Z.; Wang, J.; Wang, L. Widely Targeted LC-MS/MS Approach Provides Insights into Variations in Bioactive Flavonoid Compounds and Their Antioxidant Activities in Green, Red, and Purple Sugarcane. LWT Food Sci. Technol. 2024, 209, 116792. [Google Scholar] [CrossRef]
- Sun, M.-J.; Chiang, Y.-C.; Hou, C.-Y.; Ciou, J.-Y. Effect of Combining Moisture-Assisted Drying on Lemon (Citrus limon (L.) Brum.): Physicochemical Properties, Antioxidant Capacities, and Sensory Evaluations. Food Bioprocess Technol. 2025, 18, 508–518. [Google Scholar] [CrossRef]
- Lu, W.; Wang, S.; Liu, S.; Zhou, J.; Ke, L.; Rao, P. Increase in the Free Radical Scavenging Capability of Bitter Gourd by a Heat-Drying Process. Food Funct. 2013, 4, 1850–1855. [Google Scholar]
- Abbaspour-Gilandeh, Y.; Kaveh, M.; Fatemi, H.; Aziz, M. Combined Hot Air, Microwave, and Infrared Drying of Hawthorn Fruit: Effects of Ultrasonic Pretreatment on Drying Time, Energy, Qualitative, and Bioactive Compounds’ Properties. Foods 2021, 10, 1006. [Google Scholar] [CrossRef]
- Bhebhe, M.; Füller, T.N.; Chipurura, B.; Muchuweti, M. Effect of Solvent Type on Total Phenolic Content and Free Radical Scavenging Activity of Black Tea and Herbal Infusions. Food Anal. Methods 2016, 9, 1060–1067. [Google Scholar] [CrossRef]
- Mulinacci, N.; Innocenti, M.; Bellumori, M.; Giaccherini, C.; Martini, V.; Michelozzi, M. Storage Method, Drying Processes and Extraction Procedures Strongly Affect the Phenolic Fraction of Rosemary Leaves: An HPLC/DAD/MS Study. Talanta 2011, 85, 167–176. [Google Scholar] [CrossRef]
- Rocchetti, G.; Gregorio, R.P.; Lorenzo, J.M.; Barba, F.J.; Oliveira, P.G.; Prieto, M.A.; Simal-Gandara, J.; Mosele, J.I.; Motilva, M.; Tomas, M. Functional Implications of Bound Phenolic Compounds and Phenolics–Food Interaction: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 811–842. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total Phenolics, Flavonoids, Anthocyanins and Antioxidant Activity Following Simulated Gastro-Intestinal Digestion and Dialysis of Apple Varieties: Bioaccessibility and Potential Uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, X.; Zhang, J.; Zhang, W.; Hu, X.; Li, W.; Li, C.; Liu, S. Extraction Methods for the Releasing of Bound Phenolics from Rubus idaeus L. Leaves and Seeds. Ind. Crops Prod. 2019, 135, 1–9. [Google Scholar] [CrossRef]
- Pędziwiatr, D.; Lamadrid, M.C.; Wojdyło, A. Cookies Fortified with Polyphenols Extracts: Impact on Phenolic Content, Antioxidant Activity, Inhibition of α-Amylase and α-Glucosidase Enzyme, Colour and Sensory Attractiveness. Antioxidants 2024, 13, 1108. [Google Scholar] [CrossRef] [PubMed]
- Cizmarova, B.; Hubkova, B.; Birkova, A. Quercetin as an Effective Antioxidant against Superoxide Radical. Funct. Food Sci. 2023, 3, 15–25. [Google Scholar] [CrossRef]
- Wu, Q.; Needs, P.W.; Lu, Y.; Kroon, P.A.; Ren, D.; Yang, X. Different Antitumor Effects of Quercetin, Quercetin-3′-Sulfate and Quercetin-3-Glucuronide in Human Breast Cancer MCF-7 Cells. Food Funct. 2018, 9, 1736–1746. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S.; Kim, K.-M. Bioactivity and Therapeutic Potential of Kaempferol and Quercetin: New Insights for Plant and Human Health. Plants 2022, 11, 2623. [Google Scholar] [CrossRef]
- Erlund, I. Review of the Flavonoids Quercetin, Hesperetin, and Naringenin. Dietary Sources, Bioactivities, Bioavailability, and Epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Mamani-Matsuda, M.; Rambert, J.; Malvy, D.; Lejoly-Boisseau, H.; Daulouède, S.; Thiolat, D.; Coves, S.; Courtois, P.; Vincendeau, P.; Mossalayi, M.D. Quercetin Induces Apoptosis of Trypanosoma Brucei Gambiense and Decreases the Proinflammatory Response of Human Macrophages. Antimicrob. Agents Chemother. 2004, 48, 924–929. [Google Scholar] [CrossRef]
- Saviranta, N.M.M.; Veeroos, L.; Granlund, L.J.; Hassinen, V.H.; Kaarniranta, K.; Karjalainen, R.O. Plant Flavonol Quercetin and Isoflavone Biochanin A Differentially Induce Protection against Oxidative Stress and Inflammation in ARPE-19 Cells. Food Res. Int. 2011, 44, 109–113. [Google Scholar] [CrossRef]
- An, K.; Zhao, D.; Wang, Z.; Wu, J.; Xu, Y.; Xiao, G. Comparison of Different Drying Methods on Chinese Ginger (Zingiber officinale Roscoe): Changes in Volatiles, Chemical Profile, Antioxidant Properties, and Microstructure. Food Chem. 2016, 197, 1292–1300. [Google Scholar] [CrossRef]
- Chua, L.Y.W.; Chong, C.H.; Chua, B.L.; Figiel, A. Influence of Drying Methods on the Antibacterial, Antioxidant and Essential Oil Volatile Composition of Herbs: A Review. Food Bioprocess Technol. 2019, 12, 450–476. [Google Scholar] [CrossRef]
- Chumroenphat, T.; Somboonwatthanakul, I.; Saensouk, S.; Siriamornpun, S. Changes in Curcuminoids and Chemical Components of Turmeric (Curcuma longa L.) under Freeze-Drying and Low-Temperature Drying Methods. Food Chem. 2021, 339, 128121. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).