1. Introduction
The tea (
Camellia sinensis) plant belongs to the Theaceae family and is a broad-leaved evergreen shrub or small tree that is traditionally grown in tropical and subtropical regions of more than 60 countries [
1]. The Assam type of tea has a planophile leaf type with a leaf angle >70° and benefits more from shading than the China type, which has an erectophile leaf type with a leaf angle <50° [
2,
3]. Tea is the second most popular beverage worldwide, with 6.5 million tons consumed in 2022 [
4]. The United States imports a total of 104,300 tons of tea annually and is the third largest importer of tea after Russia and Pakistan [
5]. China and India account for 50% and 21% of global tea production, making them the first and second largest tea-producing countries, respectively [
4].
Tea plants thrive in warm, humid climates, and the optimal growing conditions are 18 to 25 °C and 70% to 85% relative humidity (RH) with evenly distributed annual rainfall of 1800 to 3000 mm [
3,
6]. Tea requires well-drained acidic soils of pH 4.5 to 5.5 with high organic matter content [
7].
Tea plants are mostly grown in tropical and subtropical regions of the world, but over the last 25 to 100 years, production has been established in temperate zones, including England and South America. In the United States, tea is commercially grown on a small scale in Alabama, California, Georgia, Hawaii, Louisiana, Mississippi, Oregon, South Carolina, and Washington. In the continental United States, there are no published guidelines for tea vegetative propagation or published research on optimizing propagation of adapted tea cultivars. For commercial production worldwide, tea plants are mainly propagated by cuttings, as this vegetative propagation method overcomes heterozygosity and processing quality variability that occurs with seed propagation [
6]. Propagation with cuttings is used in all the major tea-producing regions worldwide [
3,
6,
8]. The formation of adventitious roots on a cutting is the prerequisite for successful propagation. Several factors that affect adventitious root formation include the cultivar, time of cutting collection, cutting position from the shoot, age of the donor plant, growth medium, use of rooting hormones, RH, and temperature [
6,
9,
10,
11,
12].
Cuttings from young plants generally have improved rooting due to a higher content of endogenous auxins and other rooting promoters compared to those from mature plants [
11,
13]. Higher rooting success for tea plants has been obtained from recently matured shoots containing slightly reddened bark [
6]. Additionally, tea cuttings from basal and median positions on a shoot had the greatest rooting success and lowest mortality [
14]. A single-node cutting with the leaf intact is the standard for tea plant vegetative propagation [
15]. However, two-node cuttings, with the leaves intact, have been used for in-ground tea plant propagation [
16]. Once the cuttings have been collected and rooting hormone applied, rooting success depends on the outdoor or grow-room environment. In tropical areas, rooting is carried out in protected beds outside. In temperate climates, some propagators have had good success propagating tea cuttings indoors using precisely timed misting systems, while others have been successful using manual watering once or twice a day [
15].
There is a lack of tea plant availability in the United States due, in part, to limited knowledge and skill for tea plant propagation. In particular, there is a lack of knowledge of the timing of cutting collection, and limited experience and understanding of successful rooting and plant establishment techniques. Preliminary research studies in Mississippi and Washington had low survival rates of tea cuttings due to too much shade applied (approximately 90% shade), low humidity, high pH of the potting media and water, and long duration when shipping cuttings. Successful plant propagation methods are needed for nurseries to supply plants to growers so that tea plants can be further explored as an alternative crop in the United States. The objective of this study was to determine the best time of year to collect tea cuttings, and the section of the shoot and number of nodes per cutting for successful propagation while optimizing the efficient use of greenhouse space. The goal of this research is to further develop and optimize protocols for vegetative propagation of tea utilizing stem cuttings under the temperate environmental conditions of western Washington.
2. Materials and Methods
Stock Plants. Approximately 20 stock plants of tea (cv. Minto Pacific) were grown by a local grower in Burlington, WA, USA, located about 16 km from the Washington State University, Northwestern Washington Research and Extension Center (WSU NWREC), Mount Vernon, WA, USA. This cultivar was originally from the Ilahee Hills Tea Farm in Salem, OR, USA, which became Minto Island Growers in 2020, and several plants were moved to Burlington, Washington, in 2000. The stock plants were 22 years old in 2022 when this study was initiated, and this cultivar has high adaptability to the western Washington climate. Due to the small number of stock plants, there was a limited supply of cuttings for these experiments. The cuttings were bulked from the mother plants, and the timing of cutting collection followed the recommendations of the local grower based on his extensive experience.
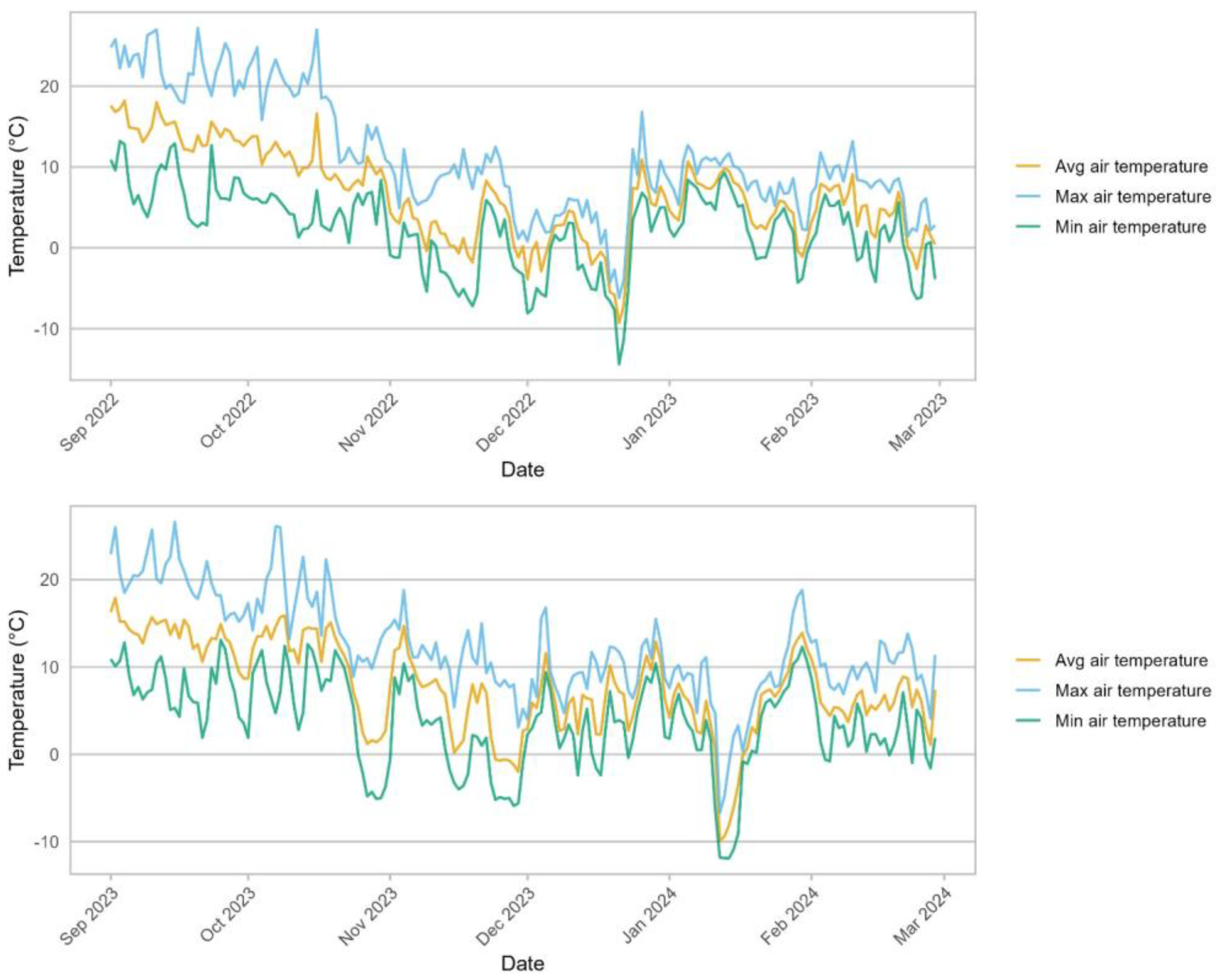
Environmental Conditions. Mount Vernon, WA, USA (lat. 48.4396, long. −122.3864, elevation 15 m) has a warm Mediterranean climate (Köppen climate classification: Csb) with 10 °C annual average air temperature (average maximum 15 °C and average minimum 6 °C), annual average precipitation of 1960 mm, and annual average RH of 82% (20-year average) [
17]. The specific temperatures during the period when cuttings were collected for this study are presented in
Figure 1.
Plant Culture and Experimental Design. Two experiments were conducted at WSU NWREC using shoots collected from the same stock plants to evaluate tea-cutting methods developed through preliminary studies for this location. Both experiments were carried out in 2022/23 and repeated in 2023/24. For both experiments, the shoots collected for the experiments were healthy, with no discernible diseases or pests, and with an initiated axillary bud. The cut shoots were placed in a 20 L bucket with approximately 12 cm of water and brought to the WSU NWREC greenhouse, where the shoots were immediately processed into cuttings in a shaded, cool area (
Figure 2A). Each cutting was scored, using a knife to remove a 3 to 5 cm length of bark to expose the inner tissue on one or both sides of the cutting’s base to encourage adventitious root formation [
18]. The scoring was carried out by two people who worked together to complete one treatment at a time. After scoring, the bottom end of the cutting was dipped into rooting hormone (indole-3-butyric acid and 1-naphthaleneacetic acid; Dip’n Grow, Clackamas, OR, USA) for 5 to 10 s, placed into a treepot filled with propagation media (peatmoss, vermiculite, and perlite in 5:3:2 ratio, pH 4.5) such that the node with a leaf rested on the soil line and was watered well.
Experiment 1 (Expt. 1) was arranged in a randomized complete split-plot design with 9 main plot treatments, 3 subplot treatments, and 3 replications. The main plot treatment comprised different times of shoot collection: early and late September, early and late October, early and late November, early December, and early January and early February. The shoot cuttings were collected only once in December, January, and February due to the limited availability of suitable shoots from the stock plants and axillary buds developing into lateral shoots, which is undesirable for propagation as these divert nutrients and energy away from root development [
6]. The subplot treatment was the section of the shoot taken as a cutting: top, mid, or bottom section (
Figure 2B). Each subplot had 10 cuttings. All the cuttings were 3-node, with the cut made 3 to 4 cm below the third node, and the leaf attached to the third (bottom) node was removed, keeping the top two leaves intact. The bottom node was buried in the potting medium.
Experiment 2 (Expt. 2) had a randomized complete block design with 3 treatments, 3 replications, and 10 cuttings per plot. Treatments included 1-node, 2-node, and 3-node cuttings and were collected in early November. For the 1-node cuttings, the leaf was intact. For the 2-node cuttings, the leaf at the first node was intact, and the leaf at the bottom/second node was removed; the bottom node of the cutting was buried in the potting medium (
Figure 2C). The 3-node cuttings were processed as described in Expt. 1. These treatments were selected to ascertain if root and shoot growth varied due to the number of nodes per cutting.
Propagation Environment. For both experiments, the treepots with cuttings were placed in a chamber constructed with PVC pipe and covered with a clear plastic and shade cloth (50% shade) on the greenhouse bench, with misters inside the chambers (model #549, Mist Timer II, Drips Inc., Concho, AZ, USA) (
Figure 2D). The greenhouse temperature was set at 20 to 23 °C. The chamber was set to mist for 20 s every 30 min using the municipal water supply, with a pH of 8.0. Supplemental bottom heat (Jump start seedling heat mat, 20 × 48, 107 Watts, Johnny’s Selected Seeds, Fairfield, ME, USA) was provided, and the RH inside the chamber was 70% to 80%.
Data Collection. For both experiments, data were collected for plant survival, plant height, number of new leaves, overall plant health, and root number and length for each subplot or plot. Plant survival was measured as the total number of living cuttings, indicated by the presence of either intact mature leaves or the presence of new buds or leaves. Plant survival was recorded at 1, 3, and 5 months after the cutting collection date. Plant height was measured for three randomly selected plants, as the height of the cuttings from the node resting on the potting medium surface to the tip of the cutting. The number of new leaves was counted for the same three plants. Overall plant health was recorded visually for the same three plants on a 5-point scale where 1 = very poor (cuttings appeared severely wilted, with extensive browning or discoloration and leaves were mostly dropped or severely damaged); 2 = poor (cuttings showed noticeable wilting, yellowing or browning on several leaves, with moderate leaf drop or evident stress symptoms); 3 = average (cuttings had mild to moderate stress symptoms, such as slight wilting or minor leaf yellowing); 4 = good (cuttings appeared healthy, with minimal stress indicators, and leaves were predominantly green with occasional mild imperfections); and 5 = very good (cuttings looked vigorous, fresh, and fully hydrated, with bright green, healthy foliage and no visible signs of stress). The data for plant height, number of new leaves, and overall plant health were collected in the spring (April) and summer (July) following the cutting collection. The root number and length were destructively measured for an additional set of three plants; the number of roots that emerged from the base of the stem and the length from the base to the tip of the longest root, respectively, were recorded. The data on root number and length were collected in June, following the cutting collection for all the cutting collection dates.
Data Analysis. All the data were analyzed using statistical analysis software (R version 1.4.1106-5 for Windows; RStudio 2024.12.1, Boston, MA, USA; SAS version 9.4 for Windows; SAS Institute, Cary, NC, USA). The assumptions of normality and homogeneity of variance were tested using the Shapiro–Wilk test (α = 0.05) and Levene’s test (α = 0.05), respectively, and the data were subjected to an analysis of variance. The means were separated using Tukey’s honestly significant difference test at a significance level of α < 0.05.
3. Results
Experiment 1. Plant survival, plant height, number of new leaves, overall plant health, and root number and length differed due to the year and collection time (
p < 0.02). The survival of tea cuttings collected from late September through early February in year 1 was 99% and 97%, on average, 1 and 5 months after cutting collection, respectively (
p < 0.0001), and in year 2 was, respectively, 98% and 86% on average (
p > 0.06) (
Table 1). The survival of the tea cuttings did not differ by shoot section in year 1 and declined slightly from 98%, on average, 1 month after cutting collection to 94%, on average, 5 months after cutting collection (
p > 0.12) (
Table 1). In year 2, tea cuttings collected from the top-shoot section had the greatest survival rate at 3 and 5 months after cutting collection, respectively, of 95% and 93% (
p < 0.007) (
Table 1). The survival for mid and bottom sections was 88% and 83%, on average, at 3 and 5 months after cutting collection (
Table 1). There was no interaction between the date of cutting collection and the shoot section for plant survival.
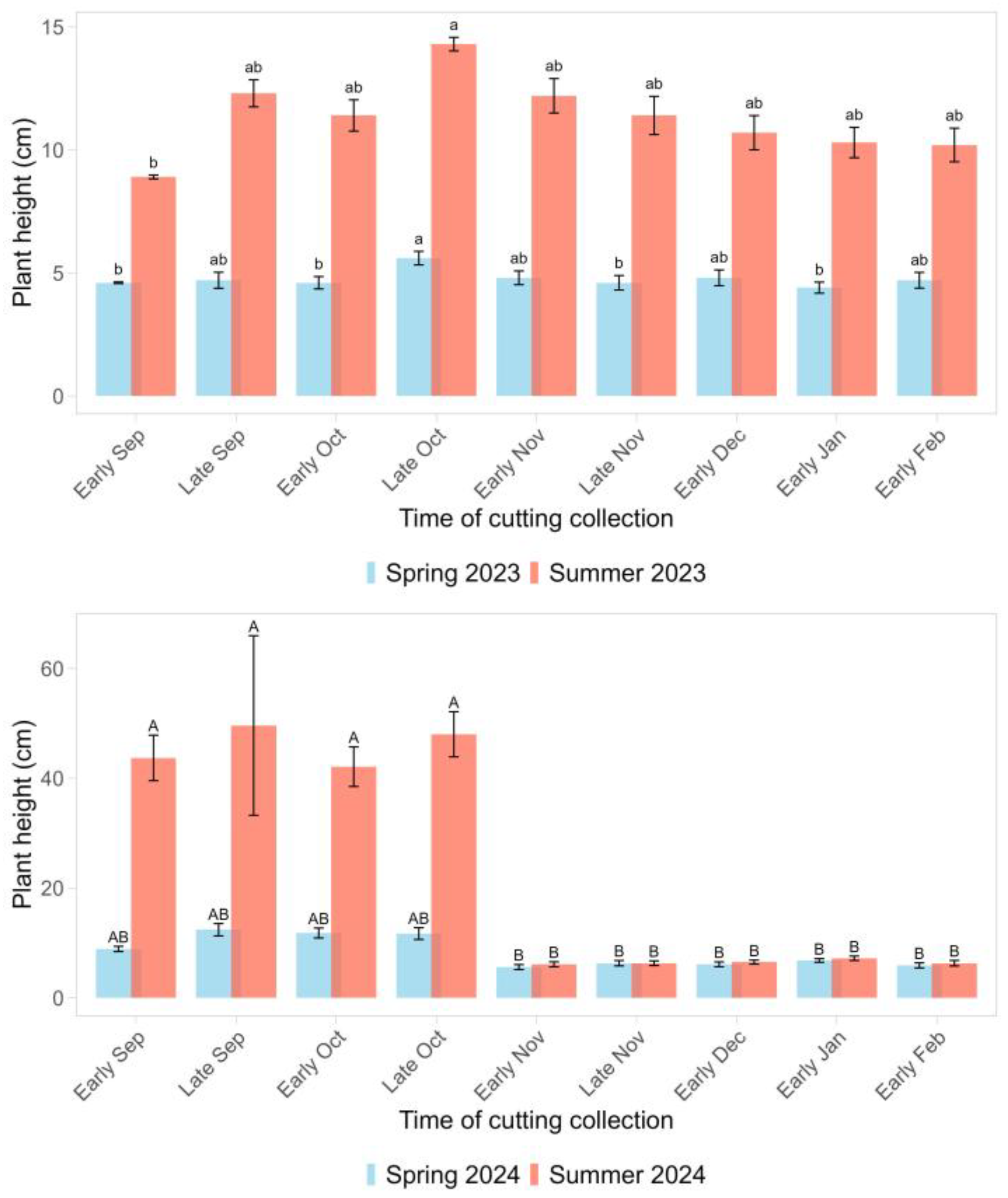
The plant height in year 1 was greatest for the cuttings collected in late October and was 5.6 cm the following spring and 14.3 cm in the summer (
p = 0.02) (
Figure 3). Cuttings collected in early September had the lowest plant height, 4.6 cm in spring and 8.9 cm in summer. In year 2, the cuttings collected from early September through late October had the greatest plant height the following spring (11.2 cm on average) and summer (45.9 cm on average) (
p < 0.0001) (
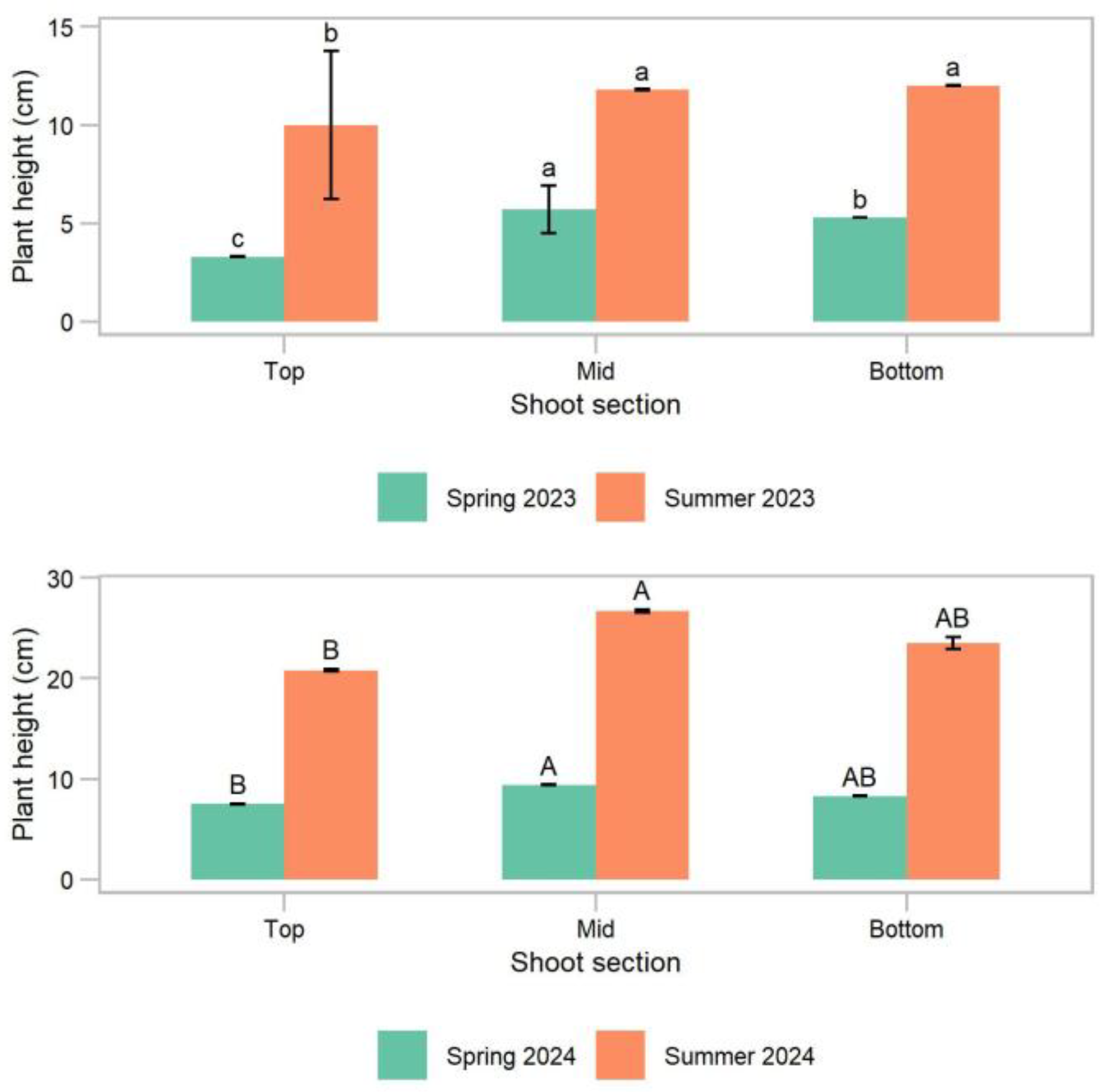
Figure 3). The plant height for all the other treatments was 6.1 cm, on average, in spring and 6.5 cm, on average, in summer. The plant height in the following spring and summer of both years was lowest for the cuttings from the top section of the shoot: 3.3 cm and 7.5 cm in the spring, and 10.0 cm and 20.8 cm in the summer of years 1 and 2, respectively (
p < 0.01) (
Figure 4). In year 1, cuttings from the mid-section of the shoot had the greatest plant height the following spring (5.7 cm), and in the summer, the plant height of the mid and bottom sections was similar, 11.9 cm on average. In year 2, the plant height of the cuttings from the mid and bottom sections of the shoot was similar: 8.9 cm on average in spring and 25.0 cm on average in summer.
The number of new leaves in year 1 was greatest for the cuttings collected in late October and was 14.0 in the following summer (
p < 0.0001) (
Table S1). Cuttings collected in early September had the lowest number of new leaves, 5.4 in summer. In year 2, the cuttings collected from early September through late October had the greatest number of new leaves the following summer (27.0 on average) (
p < 0.0001) (
Table S1). The number of new leaves for all the other treatments was 4.5, on average, in the summer. The number of new leaves in the following summer of both years was greatest for the cuttings from the mid-section of the shoot (9.7 in year 1 and 16.4 in year 2) and lowest for cuttings from the top section of the shoot (8.6 in year 1 and 13.1 in year 2) (
p = 0.001) (
Table S1).
The overall plant health rating in year 1 was similar for the cuttings collected from early October through February in the following spring (average 4.5 out of 5) and summer (average 4.4 out of 5) and was lowest for the early and late September collections (average 3.0 out of 5 in spring and average 2.7 out of 5 in summer) (
p < 0.0001) (
Table S2). In year 2, the overall plant health rating was similar for all the treatments in the spring (average 4.8 out of 5) except for the cuttings collected in late October (3.9) and January (3.8) (
p < 0.01) (
Table S2). In the summer, the overall plant health rating was similar for all the treatments (average 4.5 out of 5) except for cuttings collected in December (3.4) and January (3.3). The overall plant health rating was greatest in the summer of year 1 for the top and mid-sections of the shoot (average 4.0 out of 5) and for the mid-section in year 2 (4.4 out of 5) (
Table S2).
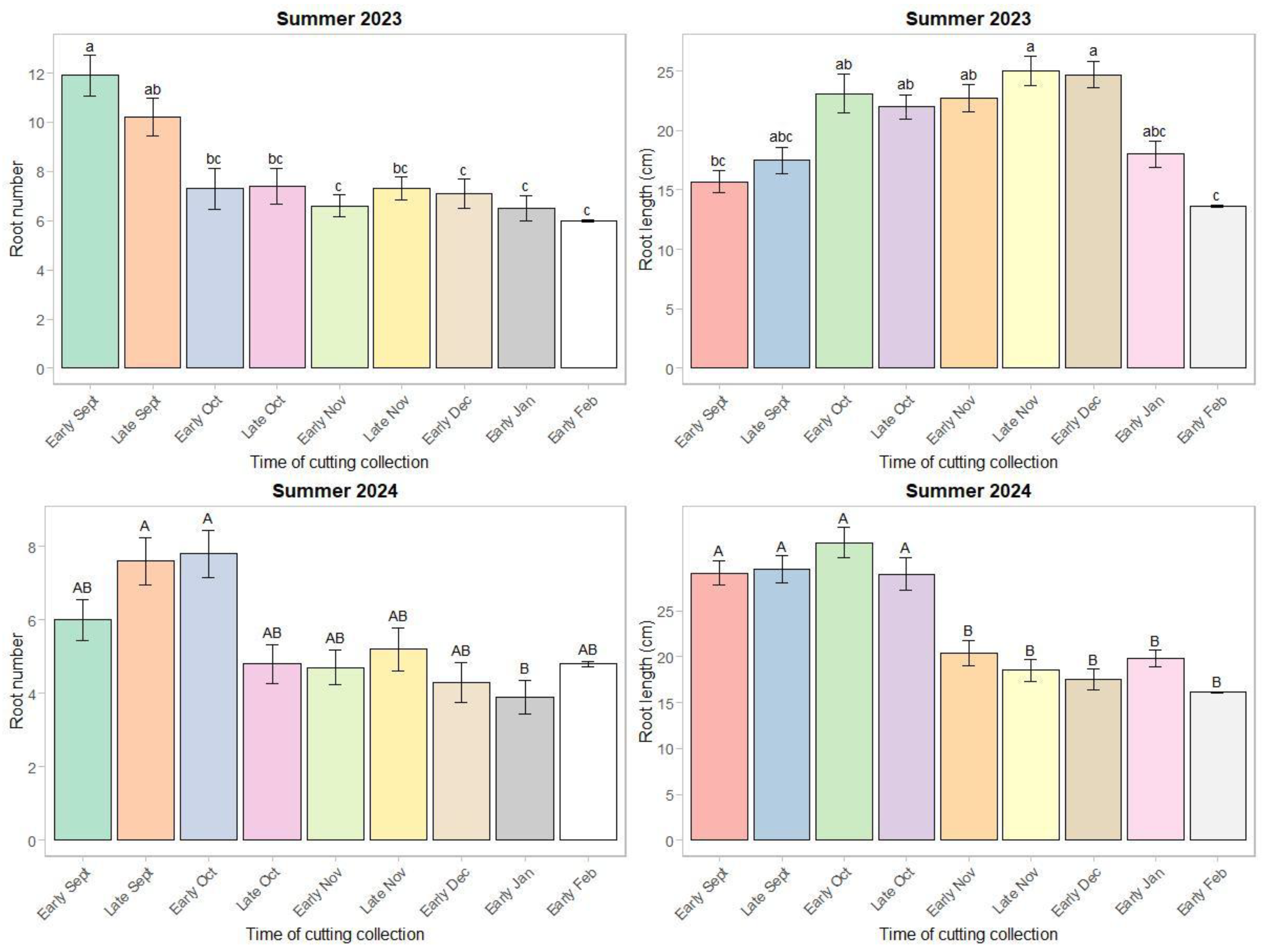
The root number in year 1 was greater for the early and late September cuttings (11.0 on average), and the root length was greatest for the cuttings collected from early October through December (23.5 cm on average) (
p < 0.0009) (
Figure 5). In year 2, all the treatments except the January collection had a similar root number (5.7 on average), while the early September through late October collections had the greatest root length (30.0 cm on average) (
p < 0.01) (
Figure 5). The root number in year 1 was similar for all three section cuttings (7.8 on average) but was greater for the top section in year 2 (6.2). The root length in year 1 was greater for mid-section cuttings (21.4 cm), while all the treatments were similar in year 2 (23.6 cm on average).
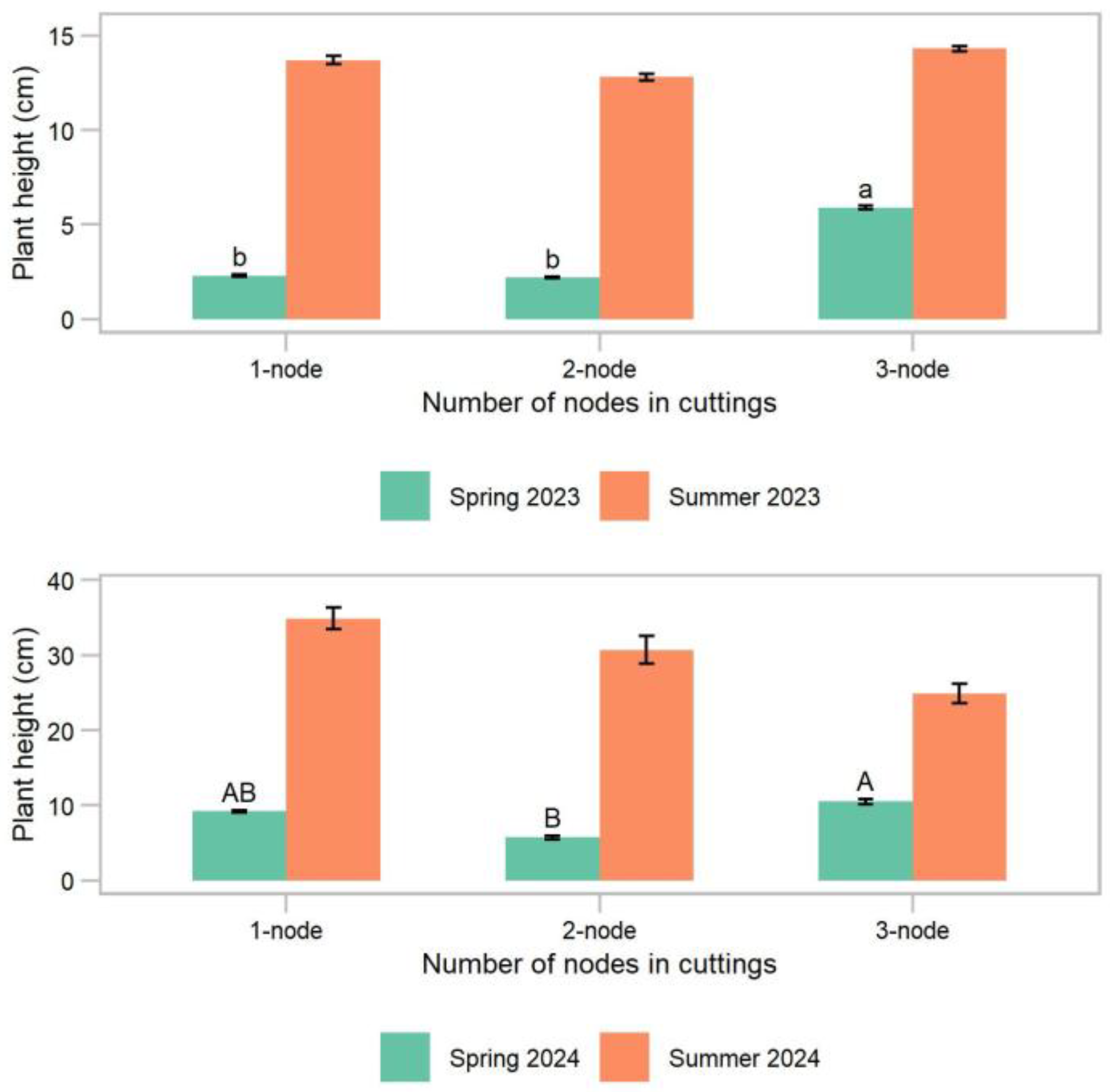
Experiment 2. Plant survival and the root number and length differed only by the year, while the plant height, number of new leaves, and overall plant health of the cuttings differed by both the year and number of nodes per cutting (
p < 0.02). In year 1, all the treatments had 100% plant survival at all times. In year 2, the plant survival of all the cuttings was 100% at 1 month after cutting collection and then declined to 92% and 87%, on average, at 3 and 5 months after the cutting collection, respectively (
p > 0.06) (
Table 2). The plant height in both years was greatest for the 3-node cuttings in the spring (5.9 cm in year 1 and 10.5 cm in year 2) (
p < 0.04) but was similar for all the treatments in the summer (13.6 cm average in year 1 and 30.2 cm average in year 2) (
p > 0.57) (
Figure 6). The number of new leaves in the following summer of year 1 was greatest for the 3-node cuttings (13.4), while the 1-node and 2-node cuttings were similar (9.0 on average) (
p = 0.001) (
Table S3). In the summer of year 2, there was no significant difference among the treatments in the number of new leaves (15.0 on average) (
p = 0.89). The overall plant health rating did not differ by the number of nodes per cutting in the spring and summer of year 1 and was 4.7 and 4.5 out of 5 on average, respectively (
p > 0.34) (
Table S4). In the summer of year 2, all the treatments had 5 out of 5, on average, for the overall plant health rating. The root number and length were similar for all the treatments in both years: average 6.0 and 23.6 cm, respectively, in year 1 and average 6.7 and 28.3 cm, respectively, in year 2 (
p > 0.07) (
Table S5).
4. Discussion
This study demonstrates that in the temperate climate of western Washington and utilizing greenhouse structures that are common in the region without specialized facilities, tea cuttings collected from early September through February have similar plant survival rates. For other plant growth variables such as plant height and number of new leaves, the cuttings collected in late October outperformed cuttings collected on other dates (
Figure 7). The late October collection also had the highest overall health rating. Root number and length were highest for cuttings collected in September and October. These results indicate that despite a later start, cuttings collected in late October catch up and surpass the growth of cuttings collected earlier, which can reduce the use of resources such as greenhouse space, labor, and water. The mid-shoot section outperformed the other shoot sections for plant growth, including plant height, number of new leaves, and root length, while maintaining a high rate of plant survival and overall health rating.
There is a lack of published studies focusing on tea vegetative propagation that evaluate the time of year, shoot section, and number of nodes; thus, published studies from other plants with similar propagation methods and considerations are also presented. In a study conducted by Lima et al. (2013) in Brazil, the greatest rooting success and lowest mortality with tea cuttings were from basal and median positions on a shoot [
14]. Studies conducted by de Andrés et al. (2004) showed that the rooting capacity of different
Colutea istria cutting sections depends on the time of year the cuttings were collected [
19]. In those studies, in winter (January), medial and basal cuttings showed a greater rooting capacity than apical cuttings, whereas in autumn (October), medial and apical cuttings showed a greater rooting capacity than basal cuttings [
19]. It is noteworthy that medial cuttings had a greater rooting capacity in both winter and autumn. Cuttings taken from different positions of the shoot show variation in rooting ability, which in turn affects the subsequent growth of rooted stem cuttings [
20,
21,
22,
23]. The shoot mid-section might have a better balance of endogenous root-promoting substances and carbohydrate reserves than the top and bottom sections.
The general recommendation for
Camellia spp. propagation is for semi-hardwood cuttings containing slightly reddened bark with mature leaves [
6,
24]. Semi-hardwood cuttings are usually collected from partially mature wood of the current season’s growth, just after a flush of growth. In the northern hemisphere, this type of cutting normally is made from mid-July to early fall, depending on the location and environmental conditions [
24]. The tea cuttings collected in the current study in late October were partially matured and slightly hardened but still pliable.
Plant survival, plant growth, overall health, and rooting success were similar among 1-node, 2-node, and 3-node cuttings, indicating that 1-node cuttings can be used successfully for vegetative propagation of tea. When there is a limited supply of cuttings, 1-node cuttings can be used to maximize the number of cuttings. However, Duc et al. (2019) reported a higher rooting percentage for 2-node cuttings, with both leaves intact, compared with 2-node cuttings, with the first leaf intact and the second leaf removed for
C. impressinervis (golden camellia) [
25]. Thus, genotype (species and cultivar) and environmental factors may need to be taken into consideration. In our study, root data were collected in June, following the cutting collection in early November. The longer time duration may have contributed to similar root growth for all the 1-node, 2-node, and 3-node cuttings, especially as the shoot growth (plant height and number of leaves) was similar among treatments by summer. In stem-cutting propagation, greater photosynthetic surface area and resultant higher carbohydrate supply likely increase rooting success rate until other factors such as evapotranspiration stress become limiting [
26]. None of the treatments in our study showed signs of wilting or other indications of evapotranspiration stress, likely due to the stable temperature and high humidity maintained in the misting chamber in our experiments.
Overall, lower plant survival for both experiments in the second year was due to the malfunctioning of some misters in the chamber, causing pots placed at the edges of the misting chamber to become dry, and hence those cuttings wilted and died. The higher plant growth rate noted in the second year was likely due to the bottom heat remaining on for several weeks longer than in the first year.