Abstract
Cold stress adversely impacts tomato (Solanum lycopersicum) production, particularly in temperate regions, by impairing growth, development, and yield. Abscisic acid (ABA), a key phytohormone, plays a central role in mediating tomato’s response to cold stress through a complex crosstalk network with other hormones and signaling molecules. This review examines ABA’s interactions with hormones such as ethylene, jasmonates, auxin, gibberellins, salicylic acid, brassinosteroids, and strigolactones, as well as signaling molecules like hydrogen peroxide, nitric oxide, hydrogen sulfide, and calcium. These interactions regulate various physiological processes, including osmolyte accumulation, membrane stability, and oxidative stress mitigation, and influence the expression of cold-responsive genes, such as CBFs, COR, and LEA. Critical knowledge gaps remain, particularly in understanding ABA’s context-specific interactions with other hormones and the integration of calcium signaling with ABA pathways under cold stress. By synthesizing current research, this review enhances our understanding of tomato’s cold stress response and provides insights for genetically improving cold tolerance, supporting sustainable tomato production amid climate challenges.
1. Introduction
Tomato (Solanum lycopersicum), belonging to Solanaceae family, is widely grown across the globe and is well-known as a crop of enormous industrial and nutritional value [1,2]. Being native to tropical and subtropical regions, tomato is highly susceptible to low temperatures [3]. Cold stress is a major threat to the global tomato industry, particularly in temperate regions. It adversely affects growth and development across the entire life cycle in tomato, leading to substantial agricultural losses [4]. Cold stress during the seedling stage suppresses root and shoot development, resulting in stunted growth, leaf curling, and chlorosis [5,6,7]. In severe cases, cold exposure triggers cotyledon abscission and plant death. During the reproductive stage, cold stress disrupts pollen development by inhibiting pollen tube elongation and reducing pollen viability. It also impairs pistil function [8]. This leads to pollination failures, flower and fruit drop, and a reduction in fruit set. Cold stress during fruit ripening slows the color change and softening of the fruit. It also reduces the accumulation of soluble solids, carotenoids, and ascorbic acid, thereby decreasing the fruit’s nutritional value and marketability [9,10]. At the physiological level, cold stress severely impairs photosynthesis in tomato plants by damaging thylakoid membranes, reducing photosystem II activity, and lowering light energy utilization [4,11]. Additionally, it suppresses Rubisco enzyme function and depletes Calvin cycle intermediates [12]. Chilling also destabilizes membrane systems by causing lipid peroxidation, as indicated by increased levels of malondialdehyde. This compromises the integrity of plasma and organelle membranes, leading to electrolyte leakage [13,14]. These effects are exacerbated by a burst of reactive oxygen species (ROS). Excessive ROS accumulation leads to oxidative damage, including protein carbonylation, DNA strand breaks, and accelerated cellular degradation [15,16]. At the molecular level, cold stress triggers complex responses in tomato plants, such as reprogramming of gene expression and activation of key signaling pathways. These pathways involve calcium signaling [17,18], mitogen-activated protein kinase (MAPK) cascades [19,20], and transcription factors [21,22,23,24]. These signaling pathways enhance the expression of cold-responsive genes, such as COR (cold-regulated), LEA (late embryogenesis abundant), and P5CS (involved in proline synthesis), which allow tomato plants to cope with cold conditions. Table 1 summarizes the representative effects of cold stress on tomato plants across various stages and levels of organization (Table 1).

Table 1.
Effects of cold stress on tomato plants.
Abscisic acid (ABA) plays a central role in mediating plant adaptation to diverse abiotic stresses, including drought, salinity, and cold [25]. Its biosynthesis, catabolism, and signaling pathways are tightly regulated to ensure precise spatiotemporal control of stress responses [26]. ABA is synthesized in plastids, starting with the cleavage of carotenoid precursors, such as violaxanthin, by 9-cis-epoxycarotenoid dioxygenases (NCEDs). In tomato, the enzymes SlNCED1 and SlNCED2 are critical, exhibiting increased activity during cold stress, which elevates ABA levels [14,27]. The process continues with ABA-aldehyde oxidase (AAO3) converting ABA-aldehyde into active ABA. Conversely, ABA is catabolized by cytochrome P450 enzymes, which transform it into inactive forms [28,29]. Synthesis occurs primarily in vascular tissues and root tips, followed by transport to guard cells and other target tissues via ATP-binding cassette (ABC) transporters. In tomato, the ABA efflux carrier SlABCG40 is critical for root-to-shoot ABA translocation under stress conditions [30]. The ABA signaling cascade is initiated by receptor binding and operates through a linear PYR/PYL-PP2C-SnRK2 module [31,32]. It starts with ABA binding to soluble PYR/PYL/RCAR receptors (e.g., SlPYL4 in tomato), which undergo conformational changes to inhibit clade A protein phosphatases 2C (PP2Cs, e.g., SlHAB1). This inhibition relieves the repression of subclass III SnRK2s (e.g., SlSnRK2.4/2.6) [33]. Activated SnRK2s then phosphorylate ABRE-binding transcription factors (e.g., SlAREB1/SlABF2), which promote the expression of stress-responsive genes such as RD29B, LEA, and P5CS.
ABA plays a vital role in how plants respond to cold stress [34,35]. The application of exogenous ABA mitigates low-temperature damage to cell membranes, boosts endogenous ABA levels, and enhances cold tolerance [36,37]. Under cold stress, the ABA biosynthesis gene NCED is upregulated, while the degradation gene CYP707A, encoding ABA 8′-hydroxylase, is downregulated. This regulation increases endogenous ABA levels, improving cold tolerance in plants [38]. In melon, overexpression of NCED3 elevates ABA concentrations and activates ABRE-binding factors (ABFs), further bolstering cold tolerance [39]. Similarly, in potato, both exogenous and endogenous ABA enhance the transcription of ScAREB4, a key ABA signaling factor, thereby improving cold resilience [40]. In tomato plants, ABA production markedly increases under cold stress, conferring cold tolerance. However, suppression of SlNCED2 significantly reduces ABA accumulation and diminishes cold tolerance [14], underscoring the critical role of ABA in the tomato cold response.
In this review, we summarize the roles of ABA and various hormones, as well as signaling molecules, in enhancing cold tolerance in plants, with a particular focus on tomatoes. We highlight the interactions between ABA and other hormones and signaling molecules in tomatoes under cold stress conditions. This comprehensive review improves our understanding of the mechanisms underlying tomatoes’ response to cold stress and provides valuable insights for genetically enhancing cold tolerance in tomatoes.
2. Crosstalk of ABA with Hormonal Networks in Tomato Cold Stress Response
2.1. Crosstalk of ABA with Ethylene
Ethylene, a gaseous plant hormone, plays a multifaceted role in regulating plant growth, development, and responses to environmental stresses, including cold stress. Recent studies have highlighted ethylene’s dual influence on plant responses to cold stress. In some cases, ethylene enhances cold tolerance. For instance, in apple seedlings, it upregulates the expression of the CBF1 gene, which is critical for cold adaptation [41]. Similarly, when applied to citrus fruits, ethylene reduces damage from low temperatures by increasing the activity of antioxidant enzymes [42]. Additionally, in grape seedlings (Vitis vinifera), cold exposure triggers ethylene production, which subsequently decreases their sensitivity to cold stress [43]. Conversely, ethylene can impair cold tolerance in certain species. For example, it has been shown to reduce the ability of Medicago truncatula, Arabidopsis thaliana, and Glycine max to withstand low temperatures [44,45,46]. In tomato, ethylene plays a positive role in cold stress tolerance. Cold stress induces ethylene production by stimulating the expression of biosynthesis genes SlACS1 and SlACO1 in tomato leaves. Furthermore, the application of ACC, an ethylene precursor, promotes cold tolerance, whereas the application of AVG, an ethylene biosynthesis inhibitor, abates it [47].
The interaction of ABA and ethylene can be either antagonistic or synergistic, depending on the context. It is widely recognized that ABA and ethylene frequently counteract each other with respect to synthesis and signaling transduction pathways [48]. Previous studies have shown that applying ABA externally can suppress ethylene production [49], suggesting that ABA acts as a negative regulator of ethylene biosynthesis. In ABA-deficient mutant aba2-1, ethylene synthesis is significantly elevated compared to in wild-type plants [50], reinforcing the inhibitory effect of ABA on ethylene production. However, ABA and ethylene can also act synergistically. For example, Luo et al. demonstrated that ABA activates calcium-dependent protein kinases (CPK4 and CPK11), which phosphorylate the C-terminal region of 1-aminocyclopropane-1-carboxylate synthase 6 (ACS6). This phosphorylation stabilizes ACS6, thereby increasing ethylene production [51]. A more recent study suggests that the interplay between ABA and ethylene is dynamically integrated through protein–protein interaction networks, enabling plants to fine-tune their responses to environmental cues [52].
The interaction between ABA and ethylene in tomato cold-stress tolerance remains underexplored. However, a recent study demonstrates that ABA and ethylene act synergistically to enhance cold tolerance in tomatoes [53]. The expression of ERF15, an ethylene response factor gene, is induced by both ABA and cold stress, and ABA-mediated cold tolerance is impaired in erf15 mutants. This finding highlights the potential interplay between ABA and ethylene in tomato plants under cold stress, suggesting that their combined action may play a key role in adapting to low-temperature conditions.
2.2. Crosstalk of ABA with Jasmonates
Jasmonates (JAs), initially identified as defense hormones against biotic stress, have been shown to regulate the response to abiotic stress, including cold stress [54,55]. In response to low temperatures, JA production increases in plants, suggesting its potential role in enhancing cold tolerance. Under cold-stress conditions, JA accumulation is markedly enhanced in both Arabidopsis and tomato, as evidenced by previous studies [15,56]. Consistent with this increase, low temperatures induce the expression of JA biosynthesis genes. For example, in tomato, cold stress boosts the expression of JA biosynthesis genes such as SlLOXD (lipoxygenase), SlAOS (allene oxide synthase), SlAOC (allene oxide cyclase), and SlOPR3 (12-oxo-phytodienoic acid reductase), resulting in elevated endogenous JA levels [47]. Moreover, the application of exogenous methyl jasmonate (MeJA) enhances cold tolerance across a variety of species, including tomato [57,58,59,60,61]. The importance of JA in the cold response is further supported by studies involving mutant or transgenic plants with altered JA biosynthesis. For instance, Arabidopsis plants carrying mutations in AOS and LOX2 exhibit impaired JA biosynthesis and heightened sensitivity to low temperatures [56]. Additionally, a genetic study in rice revealed that HAN1, a gene encoding an oxidase that converts the active JA-Ile into its inactive form, 12OH-JA-Ile, negatively regulates cold tolerance, underscoring the critical role of JA signaling [62]. Interestingly, a recent study on grafted tomato seedlings using Solanum habrochaites as rootstock demonstrated that this rootstock enhanced the cold tolerance of cultivated tomato scions by strengthening JA biosynthesis and signaling [63]. Collectively, these findings highlight the essential role of JA in plant cold tolerance.
ABA and JA interact across multiple plant processes, including biosynthesis, signaling, development, and stress responses [64,65]. Their crosstalk involves key molecular players. For example, MYC2, a central regulator of JA signaling, interacts with the ABA receptor PYL6 to cope with stress conditions [66]. Additionally, the transcription factor WRKY57, which regulates the expression of the ABA signaling component ABI5, is degraded by JAZ proteins, negative regulators of the JA signaling complex [67]. Another integrator, mediator complex subunit 25 (MED25), fine-tunes both the ABA and JA pathways by differently regulating genes tied to each hormone [68]. This interconnected regulation sets the stage for their combined action during cold stress.
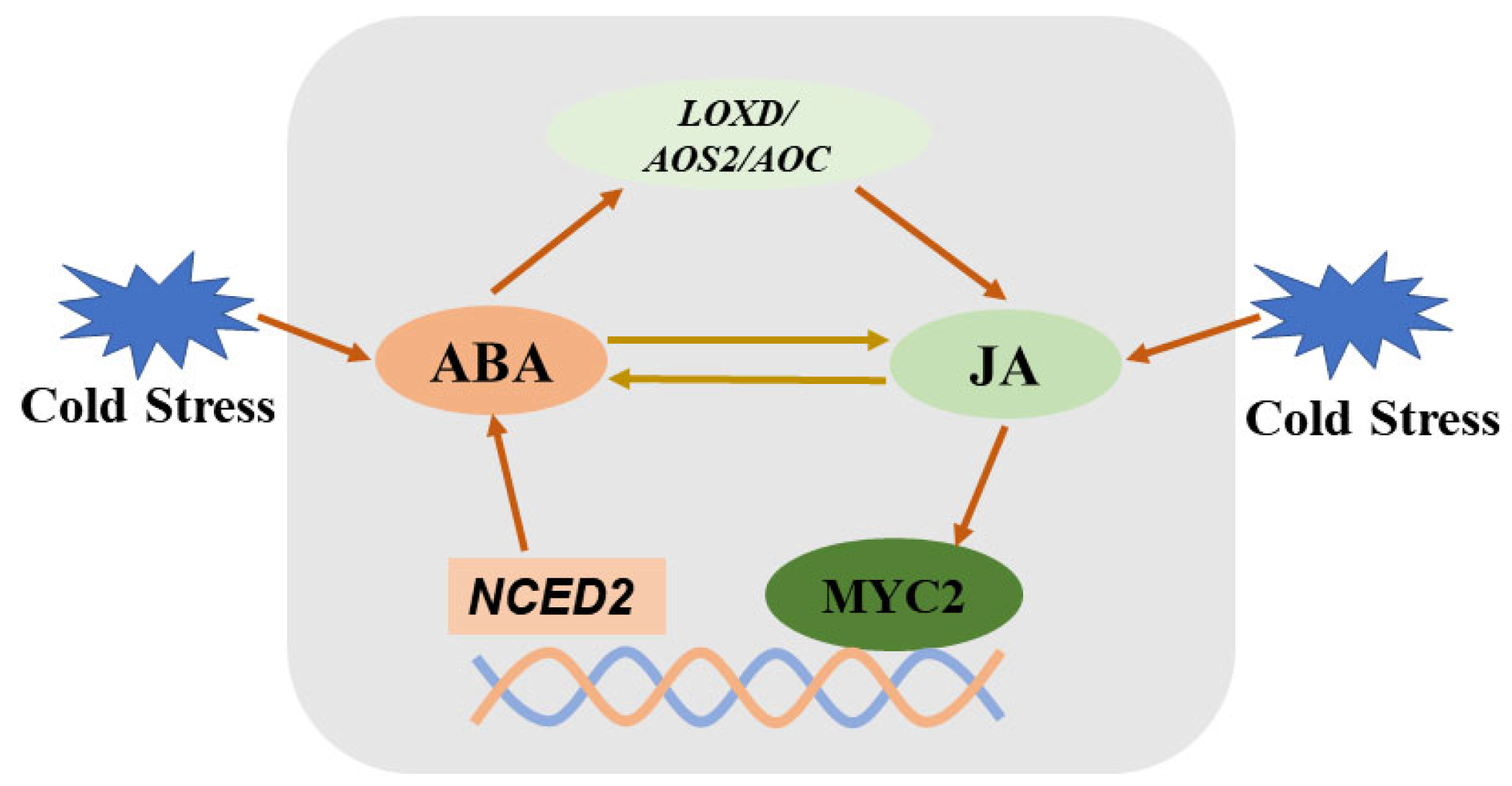
Both ABA and JA play roles in regulating the cold-stress response in plants, suggesting potential crosstalk between these two hormones during cold stress. Several studies have investigated their interactions in this context. For instance, one study demonstrates that JA acts downstream of ABA to activate the expression of C-repeat binding factors (CBFs), thereby conferring cold tolerance in tomato [69]. Supporting this finding, exogenous methyl jasmonate enhanced the cold tolerance of an ABA-deficient mutant, whereas exogenous ABA did not improve the cold tolerance of a JA-deficient mutant. Additionally, ABA induced the expression of JA biosynthesis genes, resulting in increased JA accumulation [69]. Interestingly, a recent study revealed that JA-induced cold tolerance in tomato seedlings is partially dependent on ABA, indicating a more complex relationship. Specifically, JA is found to increase endogenous ABA accumulation under cold stress, with MYC2, a key transcription factor in the JA signaling pathway, targeting the promoter of SlNCED2, an ABA biosynthesis gene, to activate its expression [14]. This reciprocal influence highlights a complex, synergistic crosstalk where both hormones amplify each other’s effects to enhance cold adaptation (Figure 1).
Figure 1.
Simplified diagram showing crosstalk between ABA and jasmonates in tomato plants under cold stress. Cold stress leads to increased levels of ABA, which enhances JA biosynthesis by promoting expression of LOXD, AOS2, and AOC. JA, in turn, promotes ABA biosynthesis through MYC2-targeted NCED2. ABA: abscisic acid; JA: jasmonate; LOXD: lipoxygenase; AOS: allene oxide synthase; AOC: allene oxide cyclase; MYC2: myelocytomatosis 2.
2.3. Crosstalk of ABA with Auxin
Auxin is a critical phytohormone that plays an essential role in plant growth and development, regulating processes such as cell division, elongation, and differentiation. Emerging evidence suggests that, beyond its contributions to growth, auxin is also a key factor in plant responses to abiotic stresses, including drought, salinity, and extreme temperatures [70]. The regulatory functions of auxin are primarily mediated through its metabolism, transport, and signal transduction pathways. In wheat and rice, auxin production increases under cold stress [71,72]. Additionally, the application of auxin analogs, such as 1-[2-chloroethoxycarbonylmethyl]-4-naphthalenesulfonic acid calcium salt and 1-[2-dimethylaminoethoxycarbonylmethyl]naphthalene chlormethylate, promotes the accumulation of proline, sucrose, and glucose, thereby enhancing cold tolerance in Brassica napus [73]. Cold stress also alters the expression of genes involved in auxin transport and signaling [74,75,76]. For instance, in rice, the expression of YUCCA family genes, which are linked to auxin biosynthesis, is upregulated under cold stress [72]. Further evidence of auxin’s role in cold-stress responses comes from studies on Arabidopsis mutants defective in the auxin transcriptional repressor INDOLE 3-ACETIC ACID 14 (IAA14), which exhibit hypersensitivity to cold stress [77]. In the horticultural crop cucumber, overexpression of the auxin transcription factor gene AUXIN RESPONSE FACTOR5 (CsARF5) enhances chilling tolerance [78,79]. Similarly, in tomato, auxin application improves fruit set under cold stress [80]. Cold stress can also trigger the abscission of floral organs, and one study has shown that the expression of auxin synthesis genes, such as SlFLOOZY2 (SlFZY2), SlFZY3, SlFZY4-1, and SlFZY5, is elevated in tomato floral organs, underscoring auxin’s importance in pollen development in response to cold stress [81]. These findings collectively reinforce the pivotal role of auxin in plant responses to cold stress. However, its specific mechanisms in tomato under cold stress remain underexplored and merit further investigation.
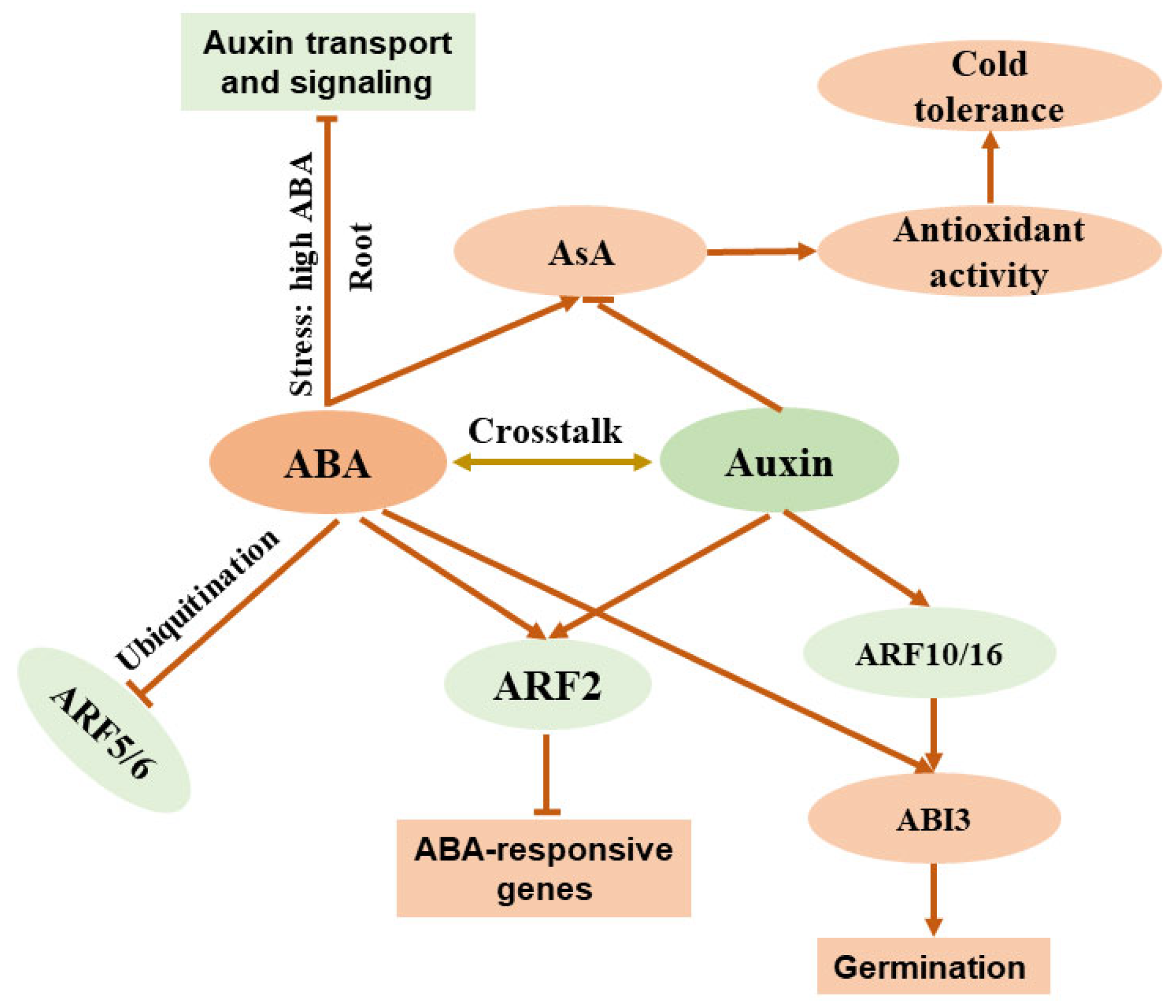
Recent studies have highlighted the intricate crosstalk between ABA and auxin, two pivotal phytohormones that regulate plant growth, development, and stress responses. The interaction is mediated by a network of genes and signaling pathways that influence key processes such as seed germination, stomatal regulation, and root development. [65,82,83]. ABA modulates auxin signaling by influencing the expression of auxin response factors (ARFs). For instance, it downregulates ARF5, ARF6, and ARF10 via ubiquitination, while upregulating ARF2, which governs floral bud abscission and leaf senescence and enhances ABA sensitivity during root growth and germination in Arabidopsis [84,85,86]. Auxin, in turn, affects ABA signaling. It indirectly upregulates ABSCISIC ACID INSENSITIVE3 (ABI3) expression through ARF16 and ARF10 to control germination and delays fruit ripening by altering ABA, salicylic acid, and cytokinin signals via ARF2. ARF2 suppresses ABA-responsive genes, promoting germination and root growth, and collaborates with PIN-FORMED (PIN) proteins and PLETHORAs (PLTs) to regulate meristem activity [87,88,89]. In primary root development, low ABA levels under non-stress conditions enhance auxin transport and signaling, whereas high ABA levels during stress suppress auxin transport gene expression. Overexpression of YUCCA4, an auxin biosynthesis gene, influences the ABA–auxin interplay through ABI4, impacting germination and root development. Mutants with impaired auxin responses (e.g., iaa7/axr2-1) resist the negative effects of high ABA levels, while auxin efflux mutants (e.g., pin2) show heightened sensitivity to low ABA levels, underscoring the role of auxin transporters in the interaction between ABA and auxin [90,91,92]. These findings collectively illustrate the complex and dynamic interplay between ABA and auxin, orchestrated through a multitude of genes and regulatory mechanisms (Figure 2).
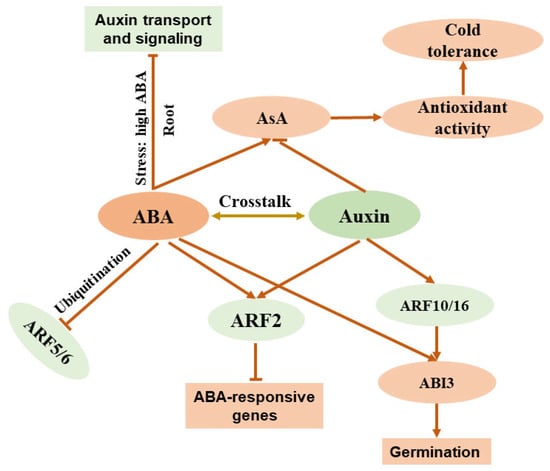
Figure 2.
Simplified diagram illustrating crosstalk between ABA and auxin in plants. ABA: abscisic acid; AsA: ascorbic acid; ARF: auxin response factor; ABI3: ABSCISIC ACID INSENSITIVE3.
The interplay between ABA and auxin is also evident in tomato plants. Under salt stress, ABA indirectly modulates auxin distribution by stimulating nitric oxide (NO) production in tomato roots, indicating a coordinated interaction among ABA, NO, and IAA signaling that influences root morphological changes [93]. Additionally, ABA regulates root growth during soil drying and subsequent recovery, a process that is partially reliant on auxin responses. Compared to ABA-deficient tomato mutants (not), wild-type plants exhibit enhanced primary root growth and increased lateral root formation. Exogenous IAA application partially alleviates root growth inhibition in not mutants, underscoring auxin’s compensatory role in downstream ABA signaling in tomato [94]. Furthermore, ABA and auxin exert antagonistic effects on ascorbic acid (AsA) production in tomato. ABA treatment boosts AsA accumulation under drought stress, whereas auxin suppresses it [95,96]. Their signaling pathways also intersect during tomato development through the interaction of type 2C protein phosphatase (SlPP2C2), flavin monooxygenase FZY (an IAA biosynthetic enzyme), and small auxin-upregulated RNA (SAUR), an IAA signaling protein [97]. These findings highlight the intricate crosstalk between ABA and auxin in tomato. However, their role in cold tolerance remains underexplored. Given that ABA and auxin antagonistically regulate AsA, an antioxidant widely linked to cold tolerance, these hormones may differentially influence tomato’s response to cold stress, warranting further investigation.
2.4. Crosstalk of ABA with Gibberellin
Gibberellins (GAs) are plant hormones that promote growth and regulate a wide array of developmental processes, such as seed germination, seedling growth, flower induction and development, and fruit expansion [98,99]. Cold stress impacts both GA metabolism and signaling pathways. Under cold stress conditions, the levels of bioactive GA decrease, resulting in stunted growth and delayed flowering in plants like tobacco, Arabidopsis, and tomato [100,101]. However, these effects can be reversed by the application of exogenous GAs. Correspondingly, cold stress triggers an increased accumulation of DELLA proteins, which act as negative regulators of GA signaling [100]. Alterations in GA biosynthesis and signaling influence how plants respond to cold stress. For example, mutants with impaired GA production exhibit greater cold tolerance compared to wild-type plants [101,102]. Similarly, the Arabidopsis gai mutant, which is insensitive to GA, demonstrates enhanced cold tolerance. In contrast, DELLA knockout mutants, such as gai-t6 rga-24, display heightened sensitivity to cold stress [100]. These findings suggest that GAs negatively regulate cold tolerance in plants.
ABA and GA play opposing roles in regulating developmental processes, such as seed dormancy and germination. Maintaining an optimal balance between these hormones is crucial for plant development. This balance is dynamically adjusted through changes in their metabolism and signaling pathways in response to developmental stages and environmental conditions [98]. ABA and GA exhibit crosstalk in response to abiotic stresses and during plant growth. Under unfavorable conditions, seeds exhibit high ABA and low GA levels, which promote dormancy [103]. ABA levels rise progressively from embryogenesis to maturation, inhibiting water uptake and cell-wall loosening, while inducing Late Embryogenesis Abundant (LEA) genes by activating the transcription factor ABSCISIC ACID INSENSITIVE 5 (ABI5) [104]. LEA proteins enhance tolerance to multiple stresses, including cold and drought. ABI5, along with ABI3, represses germination via ABRE elements [105,106]. Conversely, favorable conditions like light, warmth, and moisture trigger GA biosynthesis, counteracting ABA’s inhibitory effects.
Recent studies highlight how light and temperature modulate GA and ABA dynamics. PIL5, a light-sensitive transcription factor, inhibits germination by repressing GA biosynthesis genes, such as gibberellin3-oxidase1 (GA3ox1) and GA3ox2, and activating GA catabolism, while promoting ABA biosynthesis and repressing its catabolism in Arabidopsis [105,107,108]. Downstream of PIL5, DELAY OF GERMINATION 1 (DOG1) enhances dormancy by suppressing GA biosynthesis and activating ABI3 and ABI5. Similarly, SOMNUS (SOM), regulated by PHYTOCHROME INTERACTING FACTOR3-LIKE5 (PIL5), inhibits light-dependent germination [109]. Low temperatures disrupt the ABA-GA balance by boosting ABA levels while suppressing GA biosynthesis, thus delaying germination. Similarly, the transcription factor SPATULA (SPT), which controls the germination response to cold, represses GA biosynthesis genes (GA3ox1 and GA3ox2), but promotes the expression of ABI5 [110,111]. Salt stress also reveals DELLA’s role in ABA signaling, with DELLA accumulation linked to growth inhibition and ABA sensitivity. Studies on DELLA mutants and proteins like PROCERA in tomatoes underscore their influence on ABA-dependent processes [112]. Collectively, these interactions illustrate a sophisticated regulatory network where ABA and GA antagonistically govern seed dormancy and germination, adapting plants to their environment.
2.5. Crosstalk of ABA with Salicylic Acid
Salicylic acid (SA) is a widely recognized phytohormone linked to plant defense against biotic stress [113]. In recent years, numerous studies have demonstrated that, in addition to its role in combating biotic challenges, SA also plays a pivotal role in plant responses to abiotic stresses [114,115,116]. This defense hormone has been specifically associated with cold tolerance in plants. Under cold stress conditions, SA accumulation increases across various plant species, including Arabidopsis and wheat [117,118]. Furthermore, the exogenous application of SA has been shown to enhance cold tolerance in crops such as maize, potato, and rice [119]. In tomato plants, lower concentrations of SA and acetylsalicylic acid (ASA) have proven effective in mitigating cold stress. These findings underscore the significant connection between SA and plant responses to cold stress.
The mechanisms by which SA exerts its protective effects under stress conditions have been explored. The role of exogenous SA in alleviating cold stress has been attributed to enhanced protein synthesis and increased enzyme activities triggered by SA signaling [113,120]. Additionally, SA mitigates cold-induced damage by elevating the levels of total soluble sugars. Studies have shown that SA upregulates the expression of sucrose synthesis genes, including Sucrose Phosphate Synthase 4 (SPS4), Sucrose Synthase 2 (SuSy2), and Sucrose Transporter 1 (SUT1) [121]. Importantly, SA induces the expression of C-repeat Binding Factors (CBFs) and Inducer of CBF expression 1 (ICE1), which encode master transcription factors in the cold-signaling pathway [122].
The interaction between SA and ABA is crucial for plant tolerance to abiotic stress, generally exhibiting a positive correlation. SA treatment can elevate ABA levels by enhancing the expression of ABA biosynthesis genes (NCED1 and NCED2), as observed in tomato, barley, and wheat, thereby improving tolerance to the stresses of salinity and low temperatures [123,124,125]. However, ABA can also inhibit SA signaling, particularly in the systemic acquired resistance (SAR) pathway in Arabidopsis, indicating a complex regulatory relationship [126]. Under drought stress, ABA facilitates cuticular wax biosynthesis through the MYB96 transcription factor, which subsequently increases SA production [127]. Additionally, the small ubiquitin-like modifier E3 Ligase 1 (SIZ1) protein negatively regulates both ABA and SA pathways; mutations in SIZ1 result in elevated levels of both hormones [128,129]. Recent research highlights the integration of SA and ABA signaling in stomatal guard cells during drought, mediated by the CBK pathway [130,131]. Although the full extent of their interaction remains unclear, it is evident that SA and ABA play significant roles in plant stress tolerance. Further investigation into this relationship could yield new strategies for enhancing plant resilience to environmental stressors.
2.6. Crosstalk of ABA with Brassinosteroids
Brassinosteroids (BRs) are a class of plant-specific steroidal hormones, with over 70 identified members. They play essential roles in regulating plant growth, development, and environmental adaptability [132]. In cereal crops, for example, BRs are critical regulators of key agronomic traits, including plant height, lamina bending, tiller number, grain size, and yield [133,134]. Beyond cereals, BRs also influence economically significant traits in other species, such as fruit size, color, and ripening, as well as leaf morphology in vegetable crops [135,136]. Initially identified as growth-promoting phytohormones, BRs have since been shown to play a vital role in plant responses to various stresses, particularly cold stress [137,138,139]. Previous studies have demonstrated that endogenous BR levels increase in tomato plants under cold stress conditions. Specifically, three BRs, brassinolide (BL), castasterone (CS), and 28-norCS, exhibit elevated levels in response to low temperatures [140]. Additionally, the exogenous application of BRs enhances cold tolerance in species such as tomato, Arabidopsis, and cucumber [141,142,143]. Genetic studies further highlight their importance. Overexpression of the BR biosynthesis gene DWARF in tomato increases the endogenous BR content, thereby enhancing cold tolerance, while suppression of DWARF reduces BR levels and diminishes cold tolerance [142]. Similarly, overexpression of SlCYP90B3, another BR biosynthesis gene in tomato, improves cold tolerance in tomato fruits by upregulating SICBF1 expression and boosting antioxidant enzyme activity [144]. Collectively, these findings highlight the critical role of BRs in mediating plant responses to cold stress.
The interaction between ABA and BRs is complex, particularly in the context of plant responses to cold stress. In the BR signaling pathway, BIN2 negatively regulates cold tolerance by phosphorylating SnRK2.2 and SnRK2.3, which are key positive regulators of the ABA signaling pathway [145]. Additionally, BZR1, another component of the BR signaling pathway, positively regulates cold tolerance by directly activating the expression of CBF1/DREB1B, CBF2/DREB1C, and other cold-responsive (COR) genes [146]. Furthermore, BIN2 interacts with SnRK2.6/OST1, indicating that it influences the ABA signaling pathway through interactions with multiple proteins, including SnRK2.2, SnRK2.3, and SnRK2.6/OST1 [145]. In tomato, low temperatures significantly increase ABA content. Moreover, BR-treated plants show a sharper increase in ABA levels under cold stress compared to control plants [147]. Recent studies have shown that the cold tolerance induced by BRs relies on ABA biosynthesis. Specifically, in tomato, cold stress increases endogenous BR levels, which, in turn, reduce BIN2 abundance. This reduction activates BZR1, promoting ABA biosynthesis by upregulating the expression of the ABA biosynthesis gene NCED1 [148]. Interestingly, BR-induced increased BZR1 stability activates NBR1-dependent selective autophagy, thus conferring cold tolerance in tomato [149]. These findings demonstrate that ABA and BRs can act synergistically to enhance the cold stress response in plants.
2.7. Crosstalk of ABA with Strigolactones
Strigolactones (SLs), derived from carotenoids, are plant hormones that were initially discovered as stimulants for the germination of parasitic witchweed seeds [150]. They play a critical role in regulating plant architecture by modulating shoot branching and root development [151,152]. In Arabidopsis, SL biosynthesis involves the MORE AXILLARY GROWTH3 (MAX3) and MAX4 genes, which encode carotenoid cleavage dioxygenases, and MAX1, which encodes a cytochrome P450 monooxygenase [153,154]. The SL signaling pathway involves the receptor D14, which, upon binding SLs, interacts with the F-box protein MAX2 to promote the degradation of SUPPRESSOR OF MAX2-LIKE (SMXL) proteins, thereby triggering downstream responses [155,156,157]. Beyond their developmental functions, SLs also contribute to plant adaptation to abiotic stresses, including drought, salinity, heat, and cold [158,159,160].
SLs have shown to be associated with cold tolerance in plants [161]. Cold stress triggers the upregulation of SL biosynthesis genes, such as Carotenoid Cleavage Dioxygenase 7 (CCD7), CCD8, and MAX1, and the signaling gene (MAX2) in tomato roots, leading to enhanced levels of solanacol, which is a key SL [160]. SLs mitigate cold-stress damage in plants by enhancing the accumulation of osmoregulatory substances, such as soluble sugars and proline, which are vital for maintaining water balance and cold tolerance [162]. For example, GR24 treatment increases proline and soluble sugar levels in mung bean seedlings under cold stress, reducing water deficits [163]. Similarly, GR24 pretreatment enhances cell activity in rape leaves, lowers oxidative stress markers such as H2O2 and MDA, and reduces relative conductivity, indicating less membrane damage [164]. Another synthetic SL analog, GR245Ds, decreases relative electrolyte leakage in tomatoes under cold stress [160]. Moreover, SL-deficient (max3, max4) and SL-response (max2) Arabidopsis mutants exhibit heightened sensitivity to freezing, with reduced survival and increased electrolyte leakage [158]. Additionally, tomato mutants deficient in SL biosynthesis exhibited sensitivity to cold stress with increased accumulation of ubiquitinated proteins, while exogenous application of GR245Ds reduced ubiquitinated proteins and increased cold tolerance [165]. These findings underscore SLs’ critical role in enhancing plant cold tolerance through osmoregulation and oxidative-stress reduction.
SLs have been demonstrated to interact extensively with ABA in plants, particularly under various stress conditions, highlighting their coordinated roles in stress tolerance. Under salt stress, the SL and ABA contents in mycorrhizal Glomus intraradices plants change concurrently, highlighting their interplay [166]. Specifically, studies have shown that both hormones often increase in response to salt stress, indicating their potential roles in mediating stress-tolerance mechanisms, such as osmotic adjustment and antioxidant defense. This interplay underscores the importance of both hormones in coordinating plant responses to environmental challenges. Further evidence of their interaction comes from experiments involving exogenous ABA application. Exogenous ABA inhibits growth more in wild-type (WT) seedlings than in SL-deficient (max3, max4) and SL-response (max2) mutants [159]. This suggests that SLs may be necessary for the full expression of ABA’s inhibitory effects on growth, possibly by enhancing ABA sensitivity or by mediating downstream signaling pathways. In other words, the reduced sensitivity to ABA in SL mutants implies that SLs play a critical role in modulating the plant’s response to ABA, particularly in growth regulation under stress. Under osmotic stress, SL-depleted Ljccd7 plants show higher stomatal conductance than WT plants [167]. Since ABA typically promotes stomatal closure to prevent water loss, the impaired response in SL-depleted plants indicates that SLs are necessary for proper ABA-mediated stomatal regulation. This further supports the idea that SLs enhance the plant’s ability to respond to ABA under stress conditions. Interestingly, mutants such as smxl6,7,8, which are defective in SMXL proteins that act as repressors of SL signaling, exhibit a stronger ABA response. For example, these mutants show lower cotyledon opening rates under ABA treatment compared to WT plants [168]. Moreover, ABA-treated max mutants, which are SL-deficient or SL-insensitive, display slower stomatal closure, higher stomatal density, and larger stomatal apertures, leading to greater water loss compared to WT plants [159]. This reinforces the notion that SLs are essential for the full stomatal closure response induced by ABA. Without functional SL signaling, plants are less capable of regulating stomatal behavior in response to ABA, further emphasizing the role of SLs in modulating ABA’s effects. The interconnection between SLs and ABA is also evident in studies involving the overexpression of OsD27 in rice under drought conditions. OsD27 encodes an enzyme involved in the early steps of SL biosynthesis, and its overexpression leads to increased ABA levels [169]. This suggests that enhanced SL production can stimulate ABA accumulation, directly linking the SL biosynthetic pathway to ABA regulation. Such findings highlight the potential for SLs to influence ABA levels, thereby affecting stress responses.
Conversely, ABA also affects SL function, as demonstrated in ABA-deficient not tomato mutants under cold stress. These mutants exhibited severe wilting, reduced photosystem II efficiency, and increased relative electrolyte leakage compared to WT plants. Notably, in these ABA-deficient mutants, the SL analog GR24 failed to trigger ABA accumulation or the expression of stress-related genes [163]. This indicates that ABA is necessary for SLs to exert their effects on stress responses, suggesting a dependency of SL function on the presence of ABA. Thus, the relationship between SLs and ABA is reciprocal, with each hormone influencing the other’s role in mediating plant responses to stress.
3. Crosstalk of ABA with Signaling Molecules in Tomato Cold-Stress Response
3.1. Crosstalk of ABA with H2O2
Reactive oxygen species (ROS) are generated in cells as byproducts of respiration and photosynthesis [170]. Hydrogen peroxide (H2O2), a type of ROS, plays a key role in the cold-stress response in plants. The role of H2O2 in cold stress can be understood in two distinct ways. On one hand, excessive H2O2 accumulation triggers lipid peroxidation and causes oxidative stress. On the other hand, H2O2 serves as a signaling molecule that regulates the cold stress response. Plants with impaired H2O2 biosynthesis exhibit reduced cold tolerance. For instance, the Arabidopsis mutant frostbite1 (fro1), which has a defective mitochondrial complex I, shows lower H2O2 levels, leading to decreased expression of cold-stress-responsive genes and, consequently, reduced cold tolerance [171]. Additionally, H2O2 has been shown to regulate cold-acclimation-induced chilling tolerance in tomato plants. Cold acclimation modestly elevates H2O2 levels by enhancing the expression of respiratory burst oxidase homolog 1 (RBOH1) and NADPH oxidase activity. This, in turn, promotes the expression of genes encoding antioxidant enzymes. Conversely, treating plants with an NADPH oxidase inhibitor or H2O2 scavenger abolishes cold-acclimation-induced chilling tolerance in tomato [172]. Moreover, exogenous application of H2O2 to tomato roots enhances cold tolerance by increasing the accumulation of anthocyanin and proline, while maintaining the relative water content [173]. Additionally, applying H2O2 exogenously prior to chilling stress leads to enhanced activities of antioxidant enzymes and enhanced relative expressions of SlMAPK1/2/3 and SlCBF1 in tomato plants [174]. These findings underscore the critical role of H2O2 in conferring cold tolerance in tomatoes.
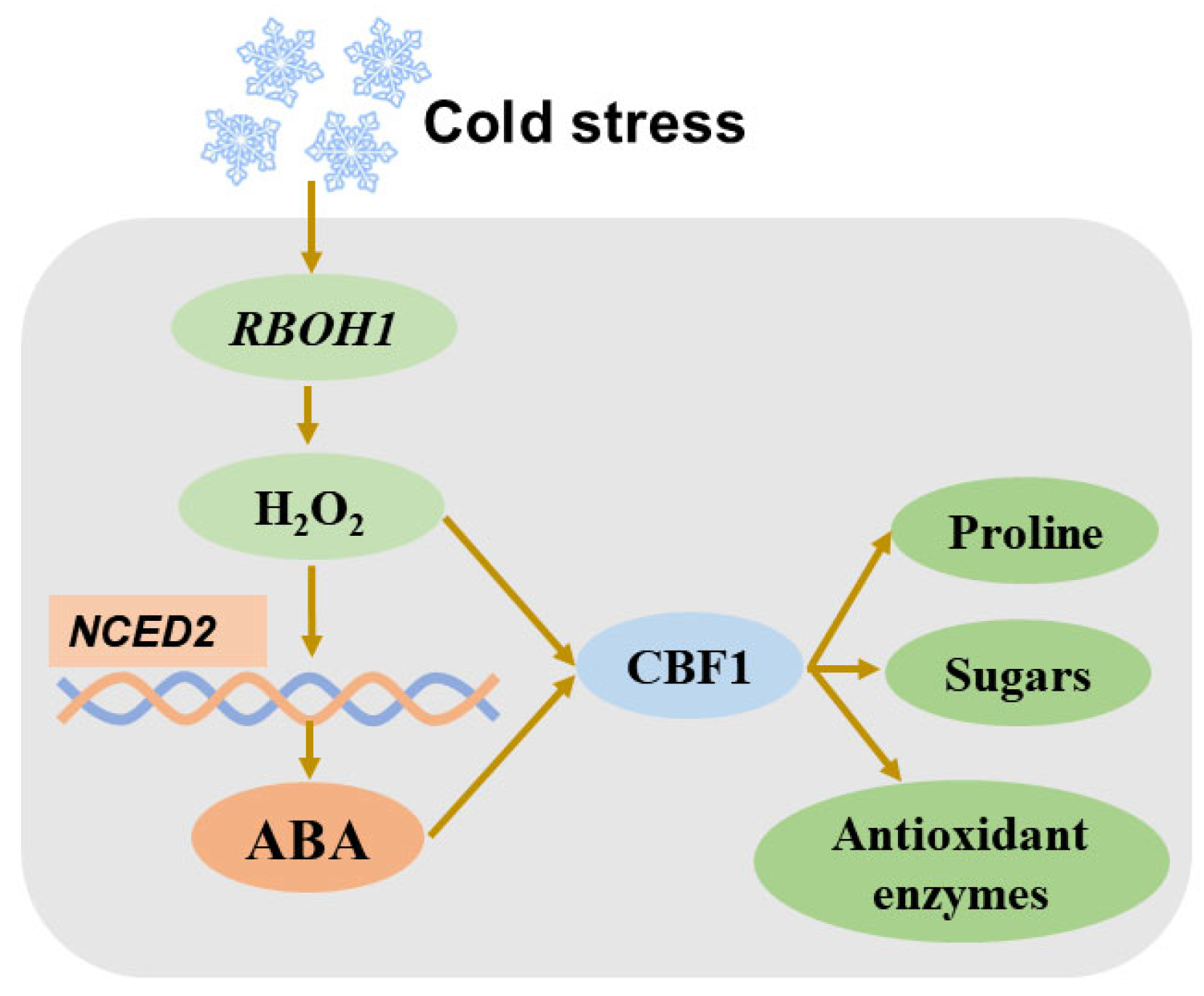
As a signaling molecule, H2O2 interacts with ABA during the cold-stress response. A study has demonstrated that H2O2 and ABA regulate proline homeostasis by forming a bidirectional closed loop in melon seedlings under cold stress [175]. Another study reveals that H2O2, acting as a downstream signal, is involved in ABA-induced cold tolerance in grafted cucumber seedlings [176]. Furthermore, treatment with fluridone, an ABA biosynthesis inhibitor, reduces H2O2 accumulation by inhibiting NADPH oxidase, which subsequently decreases freezing tolerance in wheat [124]. In tomato plants, RBOH1-mediated production of H2O2 triggers enhanced ABA production by upregulating NCED1 under cold stress. Both H2O2 and ABA boost the expression of ICE1 and CBF1, which, in turn, enhance the accumulation of proline and soluble sugars, while stimulating the activity of antioxidant enzymes [177] (Figure 3). These studies collectively highlight the intricate interplay between H2O2 and ABA in plants under cold stress.
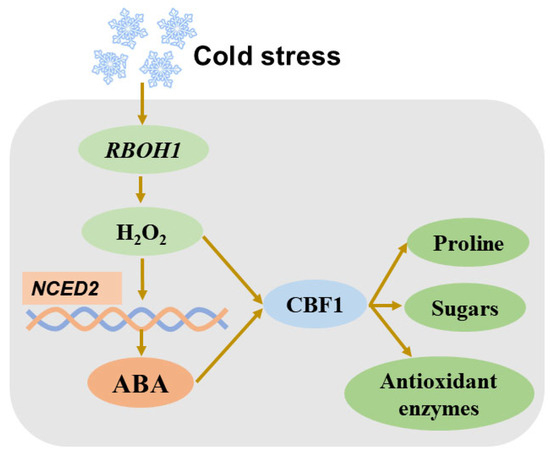
Figure 3.
Simplified diagram showing crosstalk between H2O2 and ABA in tomato cold-stress response. ABA: abscisic acid; RBOH1: respiratory burst oxidase homolog 1; CBF1: C-repeat binding factor.
3.2. Crosstalk of ABA with Nitric Oxide
Nitric oxide (NO), a free-radical-generating reactive nitrogen species (RNS), serves as a key signaling molecule in plants. NO is involved in diverse physiological processes, such as seed germination, root growth, stomatal closure, senescence, and abiotic stress responses [178]. Its role in cold-stress tolerance has been well-documented. Cold acclimation boosts endogenous NO production, which enhances proline synthesis in Arabidopsis, aiding freezing tolerance [179]. In walnut seedlings, cold stress elevates NO levels, and exogenous NO supplementation improves cold tolerance by increasing soluble sugars, proline, and phenols, and reducing electrolyte leakage, lipid peroxidation, and photosynthetic damage [180]. Similarly, in bermudagrass, NO mitigates cold-induced damages by lowering MDA, enhancing antioxidant enzymes (SOD, POD, CAT), and upregulating cold-responsive genes [181]. NO production during cold acclimation involves NOS-like and NR-dependent pathways in tomato, elevating the levels of polyamine, such as putrescine and spermidine [182]. Silencing S-nitrosoglutathione reductase (GSNOR), which maintains NO balance, increases NO and MPK1/2 activation and enhances chilling tolerance in tomato plants [183]. In yellow alfalfa, NO donors induce MfSAMS1 (S-adenosylmethionine synthetase) expression, raising S-adenosylmethionine and polyamine levels and boosting H2O2-mediated antioxidant protection against cold stress [184]. NO has been shown to regulate C-repeat Binding Factor (CBF) genes, which are critical for cold acclimation. Cold-evoked NO is essential for the expression of CBF1 and CBF3, as demonstrated by their reduced levels in NO-depleted conditions, such as in the nia1nia2 mutant or when treated with the NO scavenger 2-4-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) [185]. Moreover, exogenous application of sodium nitroprusside (SNP), a NO donor, enhances SlCBF1 expression in tomato fruits, whereas L-NNA, a NO synthase inhibitor, blocks cold-triggered SlCBF1 expression [186]. Additionally, NO modulates the expression of genes involved in the metabolism of compatible compounds, such as proline (Pro) and myo-inositol. Cold-induced Pro accumulation results from the upregulation of P5CS1 and downregulation of proline dehydrogenase (ProDH). NO is pivotal in this process: P5CS1 expression drops in NO-depleted conditions, while NO donors like SNP enhance it [179]. These results underscore NO’s significance in coordinating gene expression and signaling during cold acclimation.
The interaction between NO and ABA has been observed under different abiotic stress conditions. Their interaction enhances plant resilience by modulating gene expression, activating signaling pathways, and mitigating stress-induced damage. NO is essential for ABA-induced stomatal closure, a process critical for plant adaptation to stress [187]. ABA triggers NO production in guard cells, which regulates calcium ion release and inward-rectifying potassium channels, leading to stomatal closure [188]. Studies using NO scavengers like cPTIO demonstrate that NO is indispensable in this ABA-mediated signaling pathway [187]. Furthermore, NO interacts with protein phosphatase 2C (PP2C), which stabilizes the ABA–receptor complex [189]. However, PP2C mutants produce NO in response to ABA, but fail to close stomata, suggesting that PP2C functions downstream of NO. NO also engages the GC/cGMP pathway with ABI1, influencing ABA signaling [190]. Beyond stomatal regulation, NO mediates post-translational modifications, such as S-nitrosylation of ABI5, promoting its degradation to facilitate seed germination, and tyrosine nitration of ABA receptors, modulating cellular ABA sensitivity under stress [191]. During drought or UV-B exposure, NO and ABA crosstalk enhances adaptive responses, including stomatal closure and antioxidant defenses. In bromeliads, this interaction regulates crassulacean acid metabolism, supporting survival in water- and nutrient-scarce environments [192]. Thus, NO acts as a key modulator in ABA-driven physiological responses. However, the crosstalk between NO and ABA in regulating plant responses to cold stress, particularly in tomatoes, remains poorly characterized and warrants further investigation.
3.3. Crosstalk of ABA with Hydrogen Sulfide
Hydrogen sulfide (H2S) is an emerging signaling molecule in plants, playing a key role in physiological processes like cell division, seed germination, senescence, and stress responses, particularly to low temperatures [193,194]. In plants like grape (Vitis vinifera L.) and bermudagrass (Cynodon dactylon), cold stress boosts endogenous H2S levels and the activity of H2S-producing enzymes, such as L-/D-cysteine desulfhydrase (L/DCD). Applying sodium hydrosulfide (NaHS), an H2S donor, enhances cold tolerance by increasing antioxidant enzyme activities, such as superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and glutathione reductase (GR). This reduces harmful reactive oxygen species (ROS) and malondialdehyde (MDA), stabilizing plasma membranes and mitigating oxidative damage [195,196]. In high-altitude environments, such as the Northern Tibetan Plateau, plants like Lamiophlomis rotata produce more H2S to cope with cold stress. Elevated H2S levels correlate with higher activity of proteins and enzymes involved in its biosynthesis. This adaptation enhances antioxidant defenses, reduces ROS and reactive nitrogen species (RNS) damage, and promotes the accumulation of proline and soluble sugars, aiding osmotic regulation and stress tolerance [197]. Transgenic Arabidopsis plants overexpressing LCD or DCD genes, which boost H2S production, show improved cold tolerance. These plants exhibit lower levels of hydrogen peroxide, superoxide anions, and oxidized glutathione, alongside higher antioxidant enzyme activity and reduced glutathione levels, maintaining an appropriate redox state under cold stress [198]. H2S regulates cold responses via different molecular pathways. In Arabidopsis, it upregulates mitogen-activated protein kinase (MAPK) genes, especially MPK4, which is crucial for cold-stress responses [199]. In cucumber, H2S induces cucurbitacin C synthesis, enhancing stress tolerance, and increases indole-3-acetic acid (IAA) levels by boosting flavin monooxygenase (FMO) activity and YUCCA2 expression [200,201]. This reduces electrolyte leakage and ROS buildup and enhances photosynthesis under cold conditions. Emerging evidence in tomato demonstrates that exogenous H2S treatment markedly elevates the expression of CBF1 and ICE1 under cold-stress conditions [19,202]. Subsequent experiments reveal that H2S modulates CBF transcriptional activation through phosphorylation-dependent stimulation of the MPK4 signaling cascade. Crucially, Virus-Induced Gene Silencing (VIGS)-mediated silencing of SlMPK4 abolishes the H2S-induced upregulation of both CBF1 and ICE1, unequivocally identifying MPK4 as a critical downstream mediator in H2S signaling [19]. These findings establish that H2S enhances cold stress tolerance in tomato plants via an MPK4-CBF regulatory module. Thus, H2S is a vital signaling molecule that enhances plant cold tolerance by strengthening antioxidant defenses, regulating gene expression, and interacting with signaling pathways. Its multifaceted role underscores its significance in helping plants to adapt to low-temperature stress.
The interaction between H2S and ABA significantly influences plant physiological processes, particularly stomatal movement, and stress responses. In species like Vicia faba and Arabidopsis thaliana, ABA enhances H2S production by activating enzymes such as LCD and DCD, leading to stomatal closure. This effect is partially reversed by the H2S scavenger hypotaurine, as observed in Impatiens walleriana [203,204]. Conversely, in Capsicum annuum, H2S donors like NaHS promote stomatal opening by reducing ABA-dependent nitric oxide levels [205]. Studies with Arabidopsis mutants, such as abi1 (ABA-insensitive) and des1 (lacking L-cysteine desulfhydrase), reveal that disruptions in H2S production or ABA signaling alter stomatal responses, underscoring their complex interplay [206,207]. Under drought stress in wheat (Triticum aestivum), H2S enhances ABA accumulation by modulating its biosynthesis and catabolism, activating antioxidant systems to reduce oxidative damage [208]. Additionally, H2S triggers persulfidation, a post-translational modification, of the ABA receptors PYR1 and PYL1, influencing their activity [209,210]. The relationship is bidirectional. ABA can stimulate H2S production to bolster stress tolerance. In tobacco (Nicotiana tabacum) suspension cells, ABA increases H2S levels, enhancing heat tolerance, an effect partially blocked by H2S inhibitors like hypotaurine and dl-propargylglycine [211]. Similarly, H2S regulates ABA-mediated stomatal closure by persulfidating SnRK2.6, a key signaling protein. These findings highlight a mutual regulatory mechanism where H2S and ABA interact to modulate plant responses to environmental stresses. However, the crosstalk between the H2S and ABA signaling pathways during cold-stress adaptation in plants, particularly in tomato, remains a critical knowledge gap, and warrants further investigations.
3.4. Crosstalk of ABA with Calcium Signaling
Calcium (Ca2+) serves as a critical signal transducer in plants, mediating responses to abiotic stresses, including cold stress. When challenged by cold stress, plants accumulate high levels of cytosolic Ca2+ rapidly, forming specific Ca2+ transients known as Ca2+ signatures [212]. These signatures, defined by changes in amplitude, duration, and frequency, act as early indicators of environmental stress. The Ca2+ signal transduction pathway consists of three key phases: generation of the Ca2+ signature, signal recognition, and signal transduction. Normally, cytosolic Ca2+ remains low, but cold stress triggers a swift increase through influx across the plasma membrane and release from internal stores, such as the vacuole [213]. This elevation is detected by Ca2+ sensors, including calmodulins (CaMs), CaM-like proteins (CMLs), Ca2+-dependent protein kinases (CPKs), and calcineurin B-like proteins (CBLs). These sensors translate the Ca2+ signal into downstream responses, such as kinase activation, transcriptional reprogramming, and the production of reactive oxygen species or nitric oxide [214].
In Arabidopsis and rice, cold-induced Ca2+ transients are well-documented. For example, in Arabidopsis, CALCIUM EXCHANGER 1 (CAX1), a vacuolar Ca2+/H+ antiporter, regulates Ca2+ homeostasis by restoring resting levels after stress. Mutants lacking CAX1 show enhanced cold tolerance due to altered expression of cold-responsive genes, such as CBF/DREB1 [215]. Cyclic nucleotide-gated channels (CNGCs) are also important in this process. In rice, OsCNGC9 mediates Ca2+ influx, and its overexpression enhances cold tolerance, while Oscngc9 mutants are cold-sensitive [216]. Additionally, the cold sensor COLD1 in rice, localized on the plasma membrane and endoplasmic reticulum, interacts with G-protein signaling to facilitate Ca2+ influx, boosting cold resilience [217]. Ca2+ sensors drive phosphorylation events that are critical to the cold response. In Arabidopsis, CRLK1, a Ca2+/CaM-regulated kinase, enhances cold tolerance by influencing MAPK-mediated pathways [218]. CBLs, paired with CBL-interacting protein kinases (CIPKs), relay Ca2+ signals to downstream targets, upregulating genes like COR15a and KIN1. CPKs also play a key role. OsCPK17 regulates channel activity and sugar metabolism, while OsCPK24 increases proline and glutathione levels, improving cold tolerance in rice [219]. In tomato plants, SlCPK27 promotes cold tolerance by integrating ROS, NO, or MAPK pathways [220]. SlCML37 interacts with the proteasome maturation factor SlUMP1 and enhances cold tolerance in tomato fruits [221]. The Ca2+ sensor also plays a negative role in cold response. SlCaM6, a tomato calmodulin, negatively regulates cold tolerance by attenuating ICE1-dependent COR gene expression [18]. These findings suggest that calcium signaling is essential for plant adaptation to cold stress. The rapid rise in cytosolic Ca2+, its recognition by diverse sensors, and the activation of downstream pathways enable plants to cope with low temperatures.
Ca2+ and ABA signaling pathways converge through shared target proteins, especially in abiotic stress responses. Proteins such as reactive burst oxidases (RBOHs), S-type anion channels (SLAC1/SLAH3), and ABA-responsive transcription factors (ABFs) are phosphorylated by both Ca2+-dependent kinases (e.g., CPKs, CBL-CIPKs) and ABA-activated SnRK2.6 [222,223,224,225]. Additionally, ABA-inhibited PP2Cs dephosphorylate these targets, such as SLAC1/SLAH3 and ABI5, reversing kinase activity [222,226]. This interplay, driven by coordinated phosphorylation and dephosphorylation, integrates Ca2+ and ABA signaling to regulate stress responses effectively. However, the role of this interplay during the cold stress response in tomato remains largely underexplored and warrants further studies.
4. Conclusions and Future Perspectives
In this review, we have explored the pivotal role of ABA in enhancing cold-stress tolerance in tomato, with a particular emphasis on its intricate crosstalk with various hormones and signaling molecules. ABA acts as a central regulator, orchestrating a sophisticated network that integrates hormonal and molecular responses to mitigate the adverse effects of low temperatures. This network involves dynamic interactions with phytohormones such as ethylene, jasmonates, auxin, gibberellins, salicylic acid, brassinosteroids, and strigolactones, as well as signaling molecules including hydrogen peroxide, nitric oxide, hydrogen sulfide, and calcium. These interactions collectively modulate various processes, such as osmolyte accumulation, antioxidant defense, and gene expression reprogramming, and enable tomatoes to adapt to cold stress effectively. Table 2 provides a summary of the hormones and signaling molecules that interact with ABA in tomato cold stress tolerance, encapsulating their roles and key molecular players (Table 2).

Table 2.
Summary of hormones and signaling molecules interacting with ABA in tomato cold-stress tolerance.
Despite significant advances, several aspects of ABA’s crosstalk with other phytohormones and signaling molecules in tomato cold-stress tolerance remain underexplored. The molecular mechanisms governing ABA’s interactions with ethylene, auxin, and strigolactones under cold stress are not fully understood, particularly the context-dependent nature of their synergistic or antagonistic effects. Moreover, the role of emerging hormones like strigolactones in cold tolerance and their interplay with ABA is an area requiring investigation, given their recent identification in stress responses. Furthermore, the precise integration of Ca2+ signaling with ABA pathways during cold-stress adaptation in tomatoes lacks detailed characterization, limiting our understanding of early stress-signaling events. Finally, the spatiotemporal dynamics of hormone and signaling molecule accumulation under cold stress remain largely uncharted, hindering a holistic view of whole-plant coordination.
Future studies should leverage advanced omics technologies, including transcriptomics, proteomics, and metabolomics, to map the signaling networks and identify key regulatory hubs involved in ABA-mediated cold tolerance. Genetic approaches, such as the use of CRISPR-edited mutants and transgenic tomato lines, will be instrumental in dissecting the functional roles of specific genes and pathways involved in ABA crosstalk. For instance, manipulating genes like SlNCED2 (ABA biosynthesis), SlERF15 (ethylene signaling), or SlMPK4 (H2S signaling) could reveal their contributions to cold resilience. Additionally, exploring the temporal and spatial patterns of hormone and signaling molecule distribution using imaging techniques or biosensors could provide insights into how tomatoes orchestrate systemic responses to cold stress.
From a practical standpoint, understanding ABA’s crosstalk offers promising avenues for improving tomato cold tolerance, a critical trait for sustainable tomato production amid climate change. Genetic strategies could involve overexpressing ABA biosynthesis genes (e.g., SlNCED1/2) or enhancing the expression of downstream mediators like CBFs and ICE1 to bolster cold resilience. Alternatively, modulating the interactions between ABA and other hormones, such as upregulating JA or BR signaling, could yield tomato varieties that are better equipped to withstand low temperatures. Agronomic interventions, such as applying exogenous compounds (e.g., MeJA, BRs, or H2S donors like NaHS), could also mimic or amplify these signaling pathways, providing a cost-effective means to mitigate cold-stress damage in field conditions.
In summary, ABA’s role in tomato cold stress tolerance is deeply embedded within a complex web of hormonal and signaling interactions. This review underscores its centrality in orchestrating adaptive responses, while highlighting the need for deeper mechanistic insights. By unraveling these relationships and translating them into practical applications, we can enhance tomato resilience to cold stress, supporting global food security in an era of unpredictable climate challenges. Continued research into ABA’s multifaceted crosstalk will not only advance our fundamental understanding of plant stress biology, but also pave the way for innovative solutions in tomato cultivation.
Author Contributions
F.D. and Z.S. designed the review and wrote the manuscript; F.D., X.F., R.T., M.W. and Z.S. collected the references and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (3247180862), the Shandong Provincial Natural Science Foundation (ZR202103070240), and Liaocheng University (318042402).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sato, S.; Tabata, S.; Hirakawa, H.; Asamizu, E.; Shirasawa, K.; Isobe, S.; Kaneko, T.; Nakamura, Y.; Shibata, D.; Aoki, K.; et al. The Tomato Genome Sequence Provides Insights into Fleshy Fruit Evolution. Nature 2012, 485, 635–641. [Google Scholar] [CrossRef]
- Liu, W.; Liu, K.; Chen, D.; Zhang, Z.; Li, B.; El-Mogy, M.M.; Tian, S.; Chen, T. Solanum lycopersicum, a Model Plant for the Studies in Developmental Biology, Stress Biology and Food Science. Foods 2022, 11, 2402. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shi, Y.; Yang, S. Advances and Challenges in Uncovering Cold Tolerance Regulatory Mechanisms in Plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, S.; Ding, F. Melatonin Mitigates Chilling-Induced Oxidative Stress and Photosynthesis Inhibition in Tomato Plants. Antioxidants 2020, 9, 218. [Google Scholar] [CrossRef]
- Liu, T.; Jiao, X.; Yang, S.; Zhang, Z.; Ye, X.; Li, J.; Qi, H.; Hu, X. Crosstalk between GABA and ALA to Improve Antioxidation and Cell Expansion of Tomato Seedling under Cold Stress. Environ. Exp. Bot. 2020, 180, 104228. [Google Scholar] [CrossRef]
- Dai, T.; Ban, S.; Han, L.; Li, L.; Zhang, Y.; Zhang, Y.; Zhu, W. Effects of Exogenous Glycine Betaine on Growth and Development of Tomato Seedlings under Cold Stress. Front. Plant Sci. 2024, 15, 1332583. [Google Scholar] [CrossRef]
- Wang, M.; Ding, F.; Zhang, S. Mutation of SlSBPASE Aggravates Chilling-Induced Oxidative Stress by Impairing Glutathione Biosynthesis and Suppressing Ascorbate-Glutathione Recycling in Tomato Plants. Front. Plant Sci. 2020, 11, 565701. [Google Scholar] [CrossRef]
- Sharma, K.D.; Nayyar, H. Regulatory Networks in Pollen Development under Cold Stress. Front. Plant Sci. 2016, 7, 402. [Google Scholar] [CrossRef]
- Shu, P.; Sheng, J.; Qing, Y.; Shen, L. Metabolomic Profiling Unveils Metabolites That Are Co-Regulated by the Tomato Fruit Ripening and the Cold Stress Response. Postharvest Biol. Technol. 2025, 224, 113473. [Google Scholar] [CrossRef]
- Mitalo, O.W.; Kang, S.W.; Tran, L.T.; Kubo, Y.; Ariizumi, T.; Ezura, H. Transcriptomic Analysis in Tomato Fruit Reveals Divergences in Genes Involved in Cold Stress Response and Fruit Ripening. Front. Plant Sci. 2023, 14, 1227349. [Google Scholar] [CrossRef]
- Martin, B.; Ort, D.R.; Boyer, J.S. Impairment of Photosynthesis by Chilling-Temperatures in Tomato. Plant Physiol. 1981, 68, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Wang, M.; Zhang, S.; Ai, X. Changes in SBPase Activity Influence Photosynthetic Capacity, Growth, and Tolerance to Chilling Stress in Transgenic Tomato Plants. Sci. Rep. 2016, 6, 132741. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Liu, B.; Zhang, S. Exogenous Melatonin Ameliorates Cold-Induced Damage in Tomato Plants. Sci. Hortic. 2017, 219, 264–271. [Google Scholar] [CrossRef]
- Ding, F.; Wang, X.; Li, Z.; Wang, M. Jasmonate Positively Regulates Cold Tolerance by Promoting ABA Biosynthesis in Tomato. Plants 2023, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Wang, C.; Zhang, S.; Wang, M. A Jasmonate-Responsive Glutathione S-Transferase Gene SlGSTU24 Mitigates Cold-Induced Oxidative Stress in Tomato Plants. Sci. Hortic. 2022, 303, 111231. [Google Scholar] [CrossRef]
- Nie, S.; Zhao, R.; Yang, W.; Li, J.; Wang, D. Overexpression of SlMYB1R1 Improves Chilling Stress Tolerance in Tomato. Sci. Hortic. 2024, 338, 113662. [Google Scholar] [CrossRef]
- Saand, M.A.; Xu, Y.P.; Munyampundu, J.P.; Li, W.; Zhang, X.R.; Cai, X.Z. Phylogeny and Evolution of Plant Cyclic Nucleotide-Gated Ion Channel (CNGC) Gene Family and Functional Analyses of Tomato CNGCs. DNA Res. 2015, 22, 471–483. [Google Scholar] [CrossRef]
- Lin, R.; Song, J.; Tang, M.; Wang, L.; Yu, J.; Zhou, Y. CALMODULIN6 Negatively Regulates Cold Tolerance by Attenuating ICE1-Dependent Stress Responses in Tomato. Plant Physiol. 2023, 193, 2105–2121. [Google Scholar] [CrossRef]
- Wu, G.; Niu, X.; Chen, J.; Wu, C.; Li, Y.; Li, Y.; Cui, D.; He, X.; Wang, F.; Li, S. Hydrogen Sulfide Alleviates Oxidative Damage under Chilling Stress through Mitogen-Activated Protein Kinase in Tomato. Antioxidants 2024, 13, 323. [Google Scholar] [CrossRef]
- Yu, L.; Yan, J.; Yang, Y.; He, L.; Zhu, W. Enhanced Tolerance to Chilling Stress in Tomato by Overexpression of a Mitogen-Activated Protein Kinase, SlMPK7. Plant Mol. Biol. Rep. 2016, 34, 76–88. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, G.; Xu, R.; Jiao, Z.; Yang, J.; Lin, T.; Wang, Z.; Huang, S.; Chong, L.; Zhu, J.K. A Natural Promoter Variation of SlBBX31 Confers Enhanced Cold Tolerance during Tomato Domestication. Plant Biotechnol. J. 2023, 21, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, Z.; Du, H.; Tang, M.; Fan, P.; Yu, J.; Zhou, Y. SEC1-C3H39 Module Fine-Tunes Cold Tolerance by Mediating Its Target MRNA Degradation in Tomato. New Phytol. 2023, 237, 870–884. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, X.; Zhang, Y.; Yan, J.; Ahammed, G.J.; Bu, X.; Sun, X.; Liu, Y.; Xu, T.; Qi, H.; et al. SlFHY3 and SlHY5 Act Compliantly to Enhance Cold Tolerance through the Integration of Myo-Inositol and Light Signaling in Tomato. New Phytol. 2022, 233, 2127–2143. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Yang, F.; Zhu, L.; Wang, L.; Li, Z.; Qi, Z.; Fotopoulos, V.; Yu, J.; Zhou, J. Loss of Cold Tolerance Is Conferred by Absence of the WRKY34 Promoter Fragment during Tomato Evolution. Nat. Commun. 2024, 15, 6667. [Google Scholar] [CrossRef]
- de Zelicourt, A.; Colcombet, J.; Hirt, H. The Role of MAPK Modules and ABA during Abiotic Stress Signaling. Trends Plant Sci. 2016, 21, 677–685. [Google Scholar] [CrossRef]
- Trivedi, D.K.; Gill, S.S.; Tuteja, N. Abscisic Acid (ABA): Biosynthesis, Regulation, and Role in Abiotic Stress Tolerance. In Abiotic Stress Response in Plants; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Liu, Z.; Chen, Y.; Xiang, Q.; Wang, T.; Gao, L.; Zhang, W. Tomato SlMETS1 Positively Regulates Cold Stress Tolerance by Involving ABA Biosynthesis. Veg. Res. 2023, 3, 28. [Google Scholar] [CrossRef]
- Ji, K.; Kai, W.; Zhao, B.; Sun, Y.; Yuan, B.; Dai, S.; Li, Q.; Chen, P.; Wang, Y.; Pei, Y.; et al. SlNCED1 and SlCYP707A2: Key Genes Involved in ABA Metabolism during Tomato Fruit Ripening. J. Exp. Bot. 2014, 65, 5243–5255. [Google Scholar] [CrossRef]
- Pandian, B.A.; Sathishraj, R.; Djanaguiraman, M.; Prasad, P.V.V.; Jugulam, M. Role of Cytochrome P450 Enzymes in Plant Stress Response. Antioxidants 2020, 9, 454. [Google Scholar] [CrossRef]
- Han, K.; Zhao, Y.; Sun, Y.; Li, Y. NACs, Generalist in Plant Life. Plant Biotechnol. J. 2023, 21, 2433–2457. [Google Scholar] [CrossRef]
- Weiner, J.J.; Peterson, F.C.; Volkman, B.F.; Cutler, S.R. Structural and Functional Insights into Core ABA Signaling. Curr. Opin. Plant Biol. 2010, 13, 495–502. [Google Scholar] [CrossRef]
- Raghavendra, A.S.; Gonugunta, V.K.; Christmann, A.; Grill, E. ABA Perception and Signalling. Trends Plant Sci. 2010, 15, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Sun, Y.F.; Kai, W.B.; Liang, B.; Zhang, Y.S.; Zhai, X.W.; Jiang, L.; Du, Y.W.; Leng, P. Interactions of ABA Signaling Core Components (SlPYLs, SlPP2Cs, and SlSnRK2s) in Tomato (Solanum lycopersicon). J. Plant Physiol. 2016, 205, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; He, L.; Li, F. Understanding Cold Stress Response Mechanisms in Plants: An Overview. Front. Plant Sci. 2024, 15, 1443317. [Google Scholar] [CrossRef]
- Liang, K.; Fan, X.; Liu, Y.; Tian, R.; Wang, M.; Sun, Z.; Ding, F. ABA Positively Regulates SlAPX2-Mediated Tolerance to Heat and Cold in Tomato Plants. Agronomy 2025, 15, 1206. [Google Scholar] [CrossRef]
- Huang, X.; Chen, M.H.; Yang, L.T.; Li, Y.R.; Wu, J.M. Effects of Exogenous Abscisic Acid on Cell Membrane and Endogenous Hormone Contents in Leaves of Sugarcane Seedlings under Cold Stress. Sugar Tech 2015, 17, 59–64. [Google Scholar] [CrossRef]
- Li, M.; Wang, C.; Shi, J.; Zhang, Y.; Liu, T.; Qi, H. Abscisic Acid and Putrescine Synergistically Regulate the Cold Tolerance of Melon Seedlings. Plant Physiol. Biochem. 2021, 166, 1054–1064. [Google Scholar] [CrossRef]
- Shen, J.; Liu, J.; Yuan, Y.; Chen, L.; Ma, J.; Li, X.; Li, J. The Mechanism of Abscisic Acid Regulation of Wild Fragaria Species in Response to Cold Stress. BMC Genom. 2022, 23, 670. [Google Scholar] [CrossRef]
- Li, M.; Duan, X.; Gao, G.; Liu, T.; Qi, H. CmABF1 and CmCBF4 Cooperatively Regulate Putrescine Synthesis to Improve Cold Tolerance of Melon Seedlings. Hortic. Res. 2022, 9, uhac002. [Google Scholar] [CrossRef]
- Liu, T.; Wang, J.; Chen, L.; Liu, S.; Liu, T.; Yu, L.; Guo, J.; Chen, Y.; Zhang, Y.; Song, B. ScAREB4 Promotes Potato Constitutive and Acclimated Freezing Tolerance Associated with Enhancing Trehalose Synthesis and Oxidative Stress Tolerance. Plant Cell Environ. 2023, 46, 3839–3857. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, H.; Mao, Z.; Liu, W.; Jiang, S.; Xu, H.; Su, M.; Zhang, J.; Wang, N.; Zhang, Z.; et al. Ethylene Increases the Cold Tolerance of Apple via the MdERF1B–MdCIbHLH1 Regulatory Module. Plant J. 2021, 106, 379–393. [Google Scholar] [CrossRef]
- Lafuente, M.T.; Sala, J.M.; Zacarias, L. Active Oxygen Detoxifying Enzymes and Phenylalanine Ammonia-Lyase in the Ethylene-Induced Chilling Tolerance in Citrus Fruit. J. Agric. Food Chem. 2004, 52, 3606–3611. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhao, T.; Gan, S.; Ren, X.; Fang, L.; Karungo, S.K.; Wang, Y.; Chen, L.; Li, S.; Xin, H. Ethylene Positively Regulates Cold Tolerance in Grapevine by Modulating the Expression of ETHYLENE RESPONSE FACTOR 057. Sci. Rep. 2016, 6, 24066. [Google Scholar] [CrossRef] [PubMed]
- Robison, J.D.; Yamasaki, Y.; Randall, S.K. The Ethylene Signaling Pathway Negatively Impacts CBF/DREB-Regulated Cold Response in Soybean (Glycine max). Front. Plant Sci. 2019, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Tian, S.; Hou, L.; Huang, X.; Zhang, X.; Guo, H.; Yang, S. Ethylene Signaling Negatively Regulates Freezing Tolerance by Repressing Expression of CBF and Type-A ARR Genes in Arabidopsis. Plant Cell 2012, 24, 2578–2595. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, W.; Xia, X.; Wang, T.; Zhang, W.H. Cold Acclimation-Induced Freezing Tolerance of Medicago truncatula Seedlings Is Negatively Regulated by Ethylene. Physiol. Plant. 2014, 152, 115–129. [Google Scholar] [CrossRef]
- Ding, F.; Wang, C.; Xu, N.; Wang, M. The Ethylene Response Factor SlERF.B8 Triggers Jasmonate Biosynthesis to Promote Cold Tolerance in Tomato. Environ. Exp. Bot. 2022, 203, 105073. [Google Scholar] [CrossRef]
- Müller, M. Foes or Friends: Aba and Ethylene Interaction under Abiotic Stress. Plants 2021, 10, 448. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Yu, Y.; Quan, R.; Zhang, Z.; Zhang, H.; Huang, R. The Ethylene Response Factor AtERF11 That Is Transcriptionally Modulated by the BZIP Transcription Factor HY5 Is a Crucial Repressor for Ethylene Biosynthesis in Arabidopsis. Plant J. 2011, 68, 88–99. [Google Scholar] [CrossRef]
- LeNoble, M.E.; Spollen, W.G.; Sharp, R.E. Maintenance of Shoot Growth by Endogenous ABA: Genetic Assessment of the Involvement of Ethylene Suppression. Proc. J. Exp. Bot. 2004, 55, 237–245. [Google Scholar] [CrossRef]
- Luo, X.; Chen, Z.; Gao, J.; Gong, Z. Abscisic Acid Inhibits Root Growth in Arabidopsis through Ethylene Biosynthesis. Plant J. 2014, 79, 44–55. [Google Scholar] [CrossRef]
- Altmann, M.; Altmann, S.; Rodriguez, P.A.; Weller, B.; Elorduy Vergara, L.; Palme, J.; Marín-de la Rosa, N.; Sauer, M.; Wenig, M.; Villaécija-Aguilar, J.A.; et al. Extensive Signal Integration by the Phytohormone Protein Network. Nature 2020, 583, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Wang, M.; Zhu, C.; Wu, S.; Li, J.; Yu, J.; Hu, Z. A Transcriptional Regulation of ERF15 Contributes to ABA-Mediated Cold Tolerance in Tomato. Plant Cell Environ. 2024, 47, 1334–1347. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fan, X.; Ding, F. Jasmonate: A Hormone of Primary Importance for Temperature Stress Response in Plants. Plants 2023, 12, 4080. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, T.; Ding, F. Exogenous Melatonin Delays Methyl Jasmonate-Triggered Senescence in Tomato Leaves. Agronomy 2019, 9, 795. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, L.; Wang, F.; Yu, D. Jasmonate Regulates the INDUCER OF CBF EXPRESSION-C-REPEAT BINDING FACTOR/DRE BINDING FACTOR1 Cascade and Freezing Tolerance in Arabidopsis. Plant Cell 2013, 25, 2907–2924. [Google Scholar] [CrossRef]
- Ding, F.; Ren, L.; Xie, F.; Wang, M.; Zhang, S. Jasmonate and Melatonin Act Synergistically to Potentiate Cold Tolerance in Tomato Plants. Front. Plant Sci. 2022, 12, 763284. [Google Scholar] [CrossRef]
- Ding, F.; Wang, C.; Xu, N.; Zhang, S.; Wang, M. SlMYC2 Mediates Jasmonate-Induced Tomato Leaf Senescence by Promoting Chlorophyll Degradation and Repressing Carbon Fixation. Plant Physiol. Biochem. 2022, 180, 27–34. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Tiznado-Hernández, M.E.; Zavaleta-Gatica, R.; Martínez-Téllez, M.A. Methyl Jasmonate Treatments Reduce Chilling Injury and Activate the Defense Response of Guava Fruits. Biochem. Biophys. Res. Commun. 2004, 313, 694–701. [Google Scholar] [CrossRef]
- Fan, X.; Lin, H.; Ding, F.; Wang, M. Jasmonates Promote β-Amylase-Mediated Starch Degradation to Confer Cold Tolerance in Tomato Plants. Plants 2024, 13, 1055. [Google Scholar] [CrossRef]
- Ding, F.; Wang, C.; Xu, N.; Wang, M.; Zhang, S. Jasmonic Acid-Regulated Putrescine Biosynthesis Attenuates Cold-Induced Oxidative Stress in Tomato Plants. Sci. Hortic. 2021, 288, 110373. [Google Scholar] [CrossRef]
- Mao, D.; Xin, Y.; Tan, Y.; Hu, X.; Bai, J.; Liu, Z.Y.; Yu, Y.; Li, L.; Peng, C.; Fan, T.; et al. Natural Variation in the HAN1 Gene Confers Chilling Tolerance in Rice and Allowed Adaptation to a Temperate Climate. Proc. Natl. Acad. Sci. USA 2019, 116, 3494–3501. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, B.; Chen, G.; Chen, H.; Peng, Y.; Sohail, H.; Geng, S.; Luo, G.; Xu, D.; Ouyang, B.; et al. The Essential Role of Jasmonate Signaling in Solanum habrochaites Rootstock-Mediated Cold Tolerance in Tomato Grafts. Hortic. Res. 2022, 10, uhac227. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalks Between Jasmonic Acid and Other Plant Hormone Signaling Highlight the Involvement of Jasmonic Acid as a Core Component in Plant Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef] [PubMed]
- Parwez, R.; Aftab, T.; Gill, S.S.; Naeem, M. Abscisic Acid Signaling and Crosstalk with Phytohormones in Regulation of Environmental Stress Responses. Environ. Exp. Bot. 2022, 199, 104885. [Google Scholar] [CrossRef]
- Aleman, F.; Yazaki, J.; Lee, M.; Takahashi, Y.; Kim, A.Y.; Li, Z.; Kinoshita, T.; Ecker, J.R.; Schroeder, J.I. An ABA-Increased Interaction of the PYL6 ABA Receptor with MYC2 Transcription Factor: A Putative Link of ABA and JA Signaling. Sci. Rep. 2016, 6, 28941. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, G.; Yang, S.; Yu, D. Arabidopsis WRKY57 Functions as a Node of Convergence for Jasmonic Acid- and Auxin-Mediated Signaling in Jasmonic Acid-Induced Leaf Senescence. Plant Cell 2014, 26, 230–245. [Google Scholar] [CrossRef]
- Skubacz, A.; Daszkowska-Golec, A.; Szarejko, I. The Role and Regulation of ABI5 (ABA-Insensitive 5) in Plant Development, Abiotic Stress Responses and Phytohormone Crosstalk. Front. Plant Sci. 2016, 7, 1884. [Google Scholar] [CrossRef]
- Wang, F.; Guo, Z.; Li, H.; Wang, M.; Onac, E.; Zhou, J.; Xia, X.; Shi, K.; Yu, J.; Zhou, Y. Phytochrome A and B Function Antagonistically to Regulate Cold Tolerance via Abscisic Acid-Dependent Jasmonate Signaling. Plant Physiol. 2016, 170, 459–471. [Google Scholar] [CrossRef]
- Jing, H.; Wilkinson, E.G.; Sageman-Furnas, K.; Strader, L.C. Auxin and Abiotic Stress Responses. J. Exp. Bot. 2023, 74, 7000–7014. [Google Scholar] [CrossRef]
- Wang, R.; Yu, M.; Xia, J.; Ren, Z.; Xing, J.; Li, C.; Xu, Q.; Cang, J.; Zhang, D. Cold Stress Triggers Freezing Tolerance in Wheat (Triticum aestivum L.) via Hormone Regulation and Transcription of Related Genes. Plant Biol. 2023, 25, 308–321. [Google Scholar] [CrossRef]
- Du, H.; Liu, H.; Xiong, L. Endogenous Auxin and Jasmonic Acid Levels Are Differentially Modulated by Abiotic Stresses in Rice. Front. Plant Sci. 2013, 4, 397. [Google Scholar] [CrossRef] [PubMed]
- Gavelienė, V.; Novickienė, L.; Pakalniškytė, L. Effect of Auxin Physiological Analogues on Rapeseed (Brassica napus) Cold Hardening, Seed Yield and Quality. J. Plant Res. 2013, 126, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Hannah, M.A.; Heyer, A.G.; Hincha, D.K. A Global Survey of Gene Regulation during Cold Acclimation in Arabidopsis thaliana. PLoS Genet. 2005, 1, e26. [Google Scholar] [CrossRef]
- Jain, M.; Khurana, J.P. Transcript Profiling Reveals Diverse Roles of Auxin-Responsive Genes during Reproductive Development and Abiotic Stress in Rice. FEBS J. 2009, 276, 3148–3162. [Google Scholar] [CrossRef]
- Shibasaki, K.; Uemura, M.; Tsurumi, S.; Rahman, A. Auxin Response in Arabidopsis under Cold Stress: Underlying Molecular Mechanisms. Plant Cell 2009, 21, 3823–3838. [Google Scholar] [CrossRef]
- Aslam, M.; Sugita, K.; Qin, Y.; Rahman, A. Aux/Iaa14 Regulates Microrna-mediated Cold Stress Response in Arabidopsis Roots. Int. J. Mol. Sci. 2020, 21, 8441. [Google Scholar] [CrossRef]
- Sharif, R.; Su, L.; Chen, X.; Qi, X. Involvement of Auxin in Growth and Stress Response of Cucumber. Veg. Res. 2022, 2, 13. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, X.; Liu, F.; Wang, Y.; Bi, H.; Ai, X. Hydrogen Sulfide Improves the Cold Stress Resistance through the CsARF5-CsDREB3 Module in Cucumber. Int. J. Mol. Sci. 2021, 22, 13229. [Google Scholar] [CrossRef]
- Ramin, A.A. Effects of Auxin Application on Fruit Formation in Tomato Growing under Stress Temperatures in the Field. J. Hortic. Sci. Biotechnol. 2003, 78, 706–710. [Google Scholar] [CrossRef]
- Meng, S.; Xiang, H.; Yang, X.; Ye, Y.; Han, L.; Xu, T.; Liu, Y.; Wang, F.; Tan, C.; Qi, M.; et al. Effects of Low Temperature on Pedicel Abscission and Auxin Synthesis Key Genes of Tomato. Int. J. Mol. Sci. 2023, 24, 9186. [Google Scholar] [CrossRef]
- Emenecker, R.J.; Strader, L.C. Auxin-Abscisic Acid Interactions in Plant Growth and Development. Biomolecules 2020, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Essemine, J.; Pang, X.; Chen, H.; Jin, J.; Cai, W. Abscisic Acid Regulates the Root Growth Trajectory by Reducing Auxin Transporter PIN2 Protein Levels in Arabidopsis Thaliana. Front. Plant Sci. 2021, 12, 632676. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hua, D.; He, J.; Duan, Y.; Chen, Z.; Hong, X.; Gong, Z. Auxin Response Factor2 (ARF2) and Its Regulated Homeodomain Gene HB33 Mediate Abscisic Acid Response in Arabidopsis. PLoS Genet. 2011, 7, e1002172. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.M.; Nagpal, P.; Young, J.C.; Hagen, G.; Guilfoyle, T.J.; Reed, J.W. AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 Regulate Senescence and Floral Organ Abscission in Arabidopsis thaliana. Development 2005, 132, 4563–4574. [Google Scholar] [CrossRef]
- Li, K.; Wang, S.; Wu, H.; Wang, H. Protein Levels of Several Arabidopsis Auxin Response Factors Are Regulated by Multiple Factors and Aba Promotes Arf6 Protein Ubiquitination. Int. J. Mol. Sci. 2020, 21, 9437. [Google Scholar] [CrossRef]
- Breitel, D.A.; Chappell-Maor, L.; Meir, S.; Panizel, I.; Puig, C.P.; Hao, Y.; Yifhar, T.; Yasuor, H.; Zouine, M.; Bouzayen, M.; et al. AUXIN RESPONSE FACTOR 2 Intersects Hormonal Signals in the Regulation of Tomato Fruit Ripening. PLoS Genet. 2016, 12, e1005903. [Google Scholar] [CrossRef]
- Ali, F.; Qanmber, G.; Li, F.; Wang, Z. Updated Role of ABA in Seed Maturation, Dormancy, and Germination. J. Adv. Res. 2022, 35, 199–214. [Google Scholar] [CrossRef]
- Promchuea, S.; Zhu, Y.; Chen, Z.; Zhang, J.; Gong, Z. ARF2 Coordinates with PLETHORAs and PINs to Orchestrate ABA-Mediated Root Meristem Activity in Arabidopsis. J. Integr. Plant Biol. 2017, 59, 30–43. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Forde, B.G.; Davies, W.J. The Biphasic Root Growth Response to Abscisic Acid in Arabidopsis Involves Interaction with Ethylene and Auxin Signalling Pathways. Front. Plant Sci. 2017, 8, 1493. [Google Scholar] [CrossRef]
- Munguía-Rodríguez, A.G.; López-Bucio, J.S.; Ruiz-Herrera, L.F.; Ortiz-Castro, R.; Guevara-García, Á.A.; Marsch-Martínez, N.; Carreón-Abud, Y.; López-Bucio, J.; Martínez-Trujillo, M. YUCCA4 Overexpression Modulates Auxin Biosynthesis and Transport and Influences Plant Growth and Development via Crosstalk with Abscisic Acid in Arabidopsis thaliana. Genet. Mol. Biol. 2020, 43, e20190221. [Google Scholar] [CrossRef]
- Sun, L.R.; Wang, Y.B.; He, S.B.; Hao, F.S. Mechanisms for Abscisic Acid Inhibition of Primary Root Growth. Plant Signal. Behav. 2018, 13, e1500069. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.P.; Zandonadi, D.B.; de Sá, A.F.L.; Costa, E.P.; de Oliveira, C.J.L.; Perez, L.E.P.; Façanha, A.R.; Bressan-Smith, R. Abscisic Acid-Nitric Oxide and Auxin Interaction Modulates Salt Stress Response in Tomato Roots. Theor. Exp. Plant Physiol. 2020, 32, 301–313. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, W.; Wang, Q.; Cao, Y.; Xu, F.; Dodd, I.C.; Xu, W. ABA Regulation of Root Growth during Soil Drying and Recovery Can Involve Auxin Response. Plant Cell Environ. 2022, 45, 871–883. [Google Scholar] [CrossRef]
- Xu, X.; Huang, B.; Fang, X.; Zhang, Q.; Qi, T.; Gong, M.; Zheng, X.; Wu, M.; Jian, Y.; Deng, J.; et al. SlMYB99-Mediated Auxin and Abscisic Acid Antagonistically Regulate Ascorbic Acids Biosynthesis in Tomato. New Phytol. 2023, 239, 949–963. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Q.; Gao, X.; Wu, G.; Wu, M.; Yuan, Y.; Zheng, X.; Gong, Z.; Hu, X.; Gong, M.; et al. Auxin and Abscisic Acid Antagonistically Regulate Ascorbic Acid Production via the SlMAPK8–SlARF4–SlMYB11 Module in Tomato. Plant Cell 2022, 34, 4409–4427. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Yin, Z.; Pan, Y.; Mao, W.; Peng, L.; Guo, X.; Li, B.; Leng, P. SlPP2C2 Interacts with FZY/SAUR and Regulates Tomato Development via Signaling Crosstalk of ABA and Auxin. Plant J. 2024, 119, 1073–1090. [Google Scholar] [CrossRef]
- Vishal, B.; Kumar, P.P. Regulation of Seed Germination and Abiotic Stresses by Gibberellins and Abscisic Acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef]
- Shohat, H.; Eliaz, N.I.; Weiss, D. Gibberellin in Tomato: Metabolism, Signaling and Role in Drought Responses. Mol. Hortic. 2021, 1, 15. [Google Scholar] [CrossRef]
- Achard, P.; Gong, F.; Cheminant, S.; Alioua, M.; Hedden, P.; Genschika, P. The Cold-Inducible CBF1 Factor-Dependent Signaling Pathway Modulates the Accumulation of the Growth-Repressing DELLA Proteins via Its Effect on Gibberellin Metabolism. Plant Cell 2008, 20, 2117–2129. [Google Scholar] [CrossRef]
- Richter, R.; Bastakis, E.; Schwechheimer, C. Cross-Repressive Interactions between SOC1 and the GATAs GNC and GNL/CGA1 in the Control of Greening, Cold Tolerance, and Flowering Time in Arabidopsis. Plant Physiol. 2013, 162, 1992–2004. [Google Scholar] [CrossRef]
- Richter, R.; Behringer, C.; Müller, I.K.; Schwechheimer, C. The GATA-Type Transcription Factors GNC and GNL/CGA1 Repress Gibberellin Signaling Downstream from DELLA Proteins and Phytochrome-Interacting Factors. Genes Dev. 2010, 24, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Karssen, C.M.; Brinkhorst-van der Swan, D.L.C.; Breekland, A.E.; Koornneef, M. Induction of Dormancy during Seed Development by Endogenous Abscisic Acid: Studies on Abscisic Acid Deficient Genotypes of Arabidopsis thaliana (L.) Heynh. Planta 1983, 157, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R.R.; Lynch, T.J. The Arabidopsis Abscisic Acid Response Gene ABI5 Encodes a Basic Leucine Zipper Transcription Factor. Plant Cell 2000, 12, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, N.; Kim, W.; Lim, S.; Choi, G. ABI3 and PIL5 Collaboratively Activate the Expression of SOMNUS by Directly Binding to Its Promoter in Imbibed Arabidopsis Seeds. Plant Cell 2011, 23, 1404–1415. [Google Scholar] [CrossRef]
- Lee, S.C.; Lee, M.Y.; Kim, S.J.; Jun, S.H.; An, G.; Kim, S.R. Characterization of an Abiotic Stress-Inducible Dehydrin Gene, OsDhn1, in Rice (Oryza sativa L.). Mol. Cells 2005, 19, 212–218. [Google Scholar] [CrossRef]
- Oh, E.; Yamaguchi, S.; Hu, J.; Yusuke, J.; Jung, B.; Paik, I.; Lee, H.S.; Sun, T.P.; Kamiya, Y.; Choi, G. PIL5, a Phytochrome-Interacting BHLH Protein, Regulates Gibberellin Responsiveness by Binding Directly to the GAI and RGA Promoters in Arabidopsis Seeds. Plant Cell 2007, 19, 1192–1208. [Google Scholar] [CrossRef]
- Finkelstein, R.; Reeves, W.; Ariizumi, T.; Steber, C. Molecular Aspects of Seed Dormancy. Annu. Rev. Plant Biol. 2008, 59, 387–415. [Google Scholar] [CrossRef]
- Dong, H.K.; Yamaguchi, S.; Lim, S.; Oh, E.; Park, J.; Hanada, A.; Kamiya, Y.; Choi, G. SOMNUS, a CCCH-Type Zinc Finger Protein in Arabidopsis, Negatively Regulates Light-Dependent Seed Germination Downstream of PIL5. Plant Cell 2008, 20, 1260–1277. [Google Scholar] [CrossRef]
- Vaistij, F.E.; Gan, Y.; Penfield, S.; Gilday, A.D.; Dave, A.; He, Z.; Josse, E.M.; Choi, G.; Halliday, K.J.; Graham, I.A. Differential Control of Seed Primary Dormancy in Arabidopsis Ecotypes by the Transcription Factor SPATULA. Proc. Natl. Acad. Sci. USA 2013, 110, 10866–10871. [Google Scholar] [CrossRef]
- Penfield, S.; Josse, E.M.; Kannangara, R.; Gilday, A.D.; Halliday, K.J.; Graham, I.A. Cold and Light Control Seed Germination through the BHLH Transcription Factor SPATULA. Curr. Biol. 2005, 15, 1998–2006. [Google Scholar] [CrossRef]
- Nir, I.; Shohat, H.; Panizel, I.; Olszewski, N.; Aharoni, A.; Weiss, D. The Tomato DELLA Protein PROCERA Acts in Guard Cells to Promote Stomatal Closure. Plant Cell 2017, 29, 3186–3197. [Google Scholar] [CrossRef] [PubMed]
- Elsisi, M.; Elshiekh, M.; Sabry, N.; Aziz, M.; Attia, K.; Islam, F.; Chen, J.; Abdelrahman, M. The Genetic Orchestra of Salicylic Acid in Plant Resilience to Climate Change Induced Abiotic Stress: Critical Review. Stress Biol. 2024, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhou, Z.; Chu, Z. Emerging Roles of Salicylic Acid in Plant Saline Stress Tolerance. Int. J. Mol. Sci. 2023, 24, 3388. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Fariduddin, Q.; Castroverde, C.D.M. Salicylic Acid: A Key Regulator of Redox Signalling and Plant Immunity. Plant Physiol. Biochem. 2021, 168, 381–397. [Google Scholar] [CrossRef]
- Boamah, S.; Ojangba, T.; Zhang, S.; Zhu, N.; Osei, R.; John Tiika, R.; Boakye, T.A.; Khurshid, A.; Inayat, R.; Effah, Z.; et al. Evaluation of Salicylic Acid (SA) Signaling Pathways and Molecular Markers in Trichoderma-Treated Plants under Salinity and Fusarium Stresses. A Review. Eur. J. Plant Pathol. 2023, 166, 259–274. [Google Scholar] [CrossRef]
- Kosová, K.; Prášil, I.T.; Vítámvás, P.; Dobrev, P.; Motyka, V.; Floková, K.; Novák, O.; Turečková, V.; Rolčik, J.; Pešek, B.; et al. Complex Phytohormone Responses during the Cold Acclimation of Two Wheat Cultivars Differing in Cold Tolerance, Winter Samanta and Spring Sandra. J. Plant Physiol. 2012, 169, 567–576. [Google Scholar] [CrossRef]
- Scott, I.M.; Clarke, S.M.; Wood, J.E.; Mur, L.A.J. Salicylate Accumulation Inhibits Growth at Chilling Temperature in Arabidopsis. Plant Physiol. 2004, 135, 1040–1049. [Google Scholar] [CrossRef]
- Janda, T.; Szalai, G.; Tari, I.; Páldi, E. Hydroponic Treatment with Salicylic Acid Decreases the Effects of Chilling Injury in Maize (Zea mays L.) Plants. Planta 1999, 208, 175–180. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, G.; Liu, C.; Li, H.; Chen, X.; Fu, Q.; Lin, Y.; Guo, B. Salicylic Acid, a Multifaceted Hormone, Combats Abiotic Stresses in Plants. Life 2022, 12, 886. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, C.; Brummell, D.A.; Qi, S.; Lin, Q.; Bi, J.; Duan, Y. Salicylic Acid Treatment Mitigates Chilling Injury in Peach Fruit by Regulation of Sucrose Metabolism and Soluble Sugar Content. Food Chem. 2021, 358, 129867. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q.; Janda, T. Multifaceted Role of Salicylic Acid in Combating Cold Stress in Plants: A Review. J. Plant Growth Regul. 2021, 40, 464–485. [Google Scholar] [CrossRef]
- Szepesi, Á.; Csiszár, J.; Gémes, K.; Horváth, E.; Horváth, F.; Simon, M.L.; Tari, I. Salicylic Acid Improves Acclimation to Salt Stress by Stimulating Abscisic Aldehyde Oxidase Activity and Abscisic Acid Accumulation, and Increases Na+ Content in Leaves without Toxicity Symptoms in Solanum lycopersicum L. J. Plant Physiol. 2009, 166, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Hydrogen Peroxide and Abscisic Acid Mediate Salicylic Acid-Induced Freezing Tolerance in Wheat. Front. Plant Sci. 2018, 9, 1137. [Google Scholar] [CrossRef] [PubMed]
- Torun, H.; Novák, O.; Mikulík, J.; Pěnčík, A.; Strnad, M.; Ayaz, F.A. Timing-Dependent Effects of Salicylic Acid Treatment on Phytohormonal Changes, ROS Regulation, and Antioxidant Defense in Salinized Barley (Hordeum vulgare L.). Sci. Rep. 2020, 10, 13886. [Google Scholar] [CrossRef]
- Yasuda, M.; Ishikawa, A.; Jikumaru, Y.; Seki, M.; Umezawa, T.; Asami, T.; Maruyama-Nakashita, A.; Kudo, T.; Shinozaki, K.; Yoshida, S.; et al. Antagonistic Interaction between Systemic Acquired Resistance and the Abscisic Acid-Mediated Abiotic Stress Response in Arabidopsis. Plant Cell 2008, 20, 1678–1692. [Google Scholar] [CrossRef]
- Seo, P.J.; Lee, S.B.; Suh, M.C.; Park, M.J.; Park, C.M. The MYB96 Transcription Factor Regulates Cuticular Wax Biosynthesis under Drought Conditions in Arabidopsis. Plant Cell 2011, 23, 1138–1152. [Google Scholar] [CrossRef]
- Xu, E.; Vaahtera, L.; Brosché, M. Roles of Defense Hormones in the Regulation of Ozone-Induced Changes in Gene Expression and Cell Death. Mol. Plant 2015, 8, 1776–1794. [Google Scholar] [CrossRef]
- Miura, K.; Okamoto, H.; Okuma, E.; Shiba, H.; Kamada, H.; Hasegawa, P.M.; Murata, Y. SIZ1 Deficiency Causes Reduced Stomatal Aperture and Enhanced Drought Tolerance via Controlling Salicylic Acid-Induced Accumulation of Reactive Oxygen Species in Arabidopsis. Plant J. 2013, 73, 91–104. [Google Scholar] [CrossRef]
- Li, X.C.; Chang, C.; Pei, Z.M. Reactive Oxygen Species in Drought-Induced Stomatal Closure: The Potential Roles of NPR1. Plants 2023, 12, 3194. [Google Scholar] [CrossRef]
- Prodhan, M.Y.; Munemasa, S.; Nahar, M.N.E.N.; Nakamura, Y.; Murata, Y. Guard Cell Salicylic Acid Signaling Is Integrated into Abscisic Acid Signaling via the Ca2+/CPK-Dependent Pathway. Plant Physiol. 2018, 178, 441–450. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Zabed, H.M.; Li, J.; Xiong, M.; Shi, H.; Li, J. Manipulating Brassinosteroid Signaling Pathway to Genetically Improve Horticultural Plants. aBIOTECH 2025. [Google Scholar] [CrossRef]
- Zhang, C.; Bai, M.Y.; Chong, K. Brassinosteroid-Mediated Regulation of Agronomic Traits in Rice. Plant Cell Rep. 2014, 33, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Chu, C. Functional Specificities of Brassinosteroid and Potential Utilization for Crop Improvement. Trends Plant Sci. 2018, 23, 1016–1028. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, W.; Liu, M.; Li, X.; Li, J.; Lu, Y.; Li, G.; Zhang, S.; Feng, D.; Wang, Y.; et al. OCTOPUS Regulates BIN2 to Control Leaf Curvature in Chinese Cabbage. Proc. Natl. Acad. Sci. USA 2022, 119, e2208978119. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhou, Y.; Wang, Z.; Zhang, Z.; Xi, Z.; Wang, X. Emerging Roles of Brassinosteroids and Light in Anthocyanin Biosynthesis and Ripeness of Climacteric and Non-Climacteric Fruits. Crit. Rev. Food Sci. Nutr. 2023, 63, 4541–4553. [Google Scholar] [CrossRef]
- Yu, M.H.; Zhao, Z.Z.; He, J.X. Brassinosteroid Signaling in Plant–Microbe Interactions. Int. J. Mol. Sci. 2018, 19, 4091. [Google Scholar] [CrossRef]
- Yao, T.; Xie, R.; Zhou, C.; Wu, X.; Li, D. Roles of Brossinosteroids Signaling in Biotic and Abiotic Stresses. J. Agric. Food Chem. 2023, 71, 7947–7960. [Google Scholar] [CrossRef]
- Ramirez, V.E.; Poppenberger, B. Modes of Brassinosteroid Activity in Cold Stress Tolerance. Front. Plant Sci. 2020, 11, 583666. [Google Scholar] [CrossRef]
- Fang, P.; Yan, M.; Chi, C.; Wang, M.; Zhou, Y.; Zhou, J.; Shi, K.; Xia, X.; Foyer, C.H.; Yu, J. Brassinosteroids Act as a Positive Regulator of Photoprotection in Response to Chilling Stress. Plant Physiol. 2019, 180, 2061–2076. [Google Scholar] [CrossRef]
- Anwar, A.; Liu, Y.; Dong, R.; Bai, L.; Yu, X.; Li, Y. The Physiological and Molecular Mechanism of Brassinosteroid in Response to Stress: A Review. Biol. Res. 2018, 51, 46. [Google Scholar] [CrossRef]
- Xia, X.J.; Fang, P.P.; Guo, X.; Qian, X.J.; Zhou, J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Brassinosteroid-Mediated Apoplastic H2O2-Glutaredoxin 12/14 Cascade Regulates Antioxidant Capacity in Response to Chilling in Tomato. Plant Cell Environ. 2018, 41, 1052–1064. [Google Scholar] [CrossRef] [PubMed]
- Kagale, S.; Divi, U.K.; Krochko, J.E.; Keller, W.A.; Krishna, P. Brassinosteroid Confers Tolerance in Arabidopsis thaliana and Brassica napus to a Range of Abiotic Stresses. Planta 2007, 225, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, T.; Shao, Z.; Meng, F.; Chen, H.; Wang, Q.; Zheng, J.; Liu, L. Brassinosteroid Biosynthetic Gene SlCYP90B3 Alleviates Chilling Injury of Tomato (Solanum lycopersicum) Fruits during Cold Storage. Antioxidants 2022, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, J.; Wang, H.; Yang, C.; Chen, Y.; Li, Y.; Pan, S.; Dong, R.; Tang, G.; De Dios Barajas-Lopez, J.; et al. GSK3-like Kinases Positively Modulate Abscisic Acid Signaling through Phosphorylating Subgroup III SnRK2s in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 9651–9656. [Google Scholar] [CrossRef]
- Li, H.; Ye, K.; Shi, Y.; Cheng, J.; Zhang, X.; Yang, S. BZR1 Positively Regulates Freezing Tolerance via CBF-Dependent and CBF-Independent Pathways in Arabidopsis. Mol. Plant 2017, 10, 545–559. [Google Scholar] [CrossRef]
- Heidari, P.; Entazari, M.; Ebrahimi, A.; Ahmadizadeh, M.; Vannozzi, A.; Palumbo, F.; Barcaccia, G. Exogenous Ebr Ameliorates Endogenous Hormone Contents in Tomato Species under Low-Temperature Stress. Horticulturae 2021, 7, 84. [Google Scholar] [CrossRef]
- An, S.; Liu, Y.; Sang, K.; Wang, T.; Yu, J.; Zhou, Y.; Xia, X. Brassinosteroid Signaling Positively Regulates Abscisic Acid Biosynthesis in Response to Chilling Stress in Tomato. J. Integr. Plant Biol. 2022, 65, 10–24. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Fang, P.; Xia, X.; Shi, K.; Zhou, Y.; Zhou, J.; Yu, J. Brassinosteroids Act as a Positive Regulator of NBR1-Dependent Selective Autophagy in Response to Chilling Stress in Tomato. J. Exp. Bot. 2020, 71, 1092–1106. [Google Scholar] [CrossRef]
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of Witchweed (Striga lutea Lour.): Isolation and Properties of a Potent Stimulant. Science 1966, 154, 1189–1190. [Google Scholar] [CrossRef]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of Shoot Branching by New Terpenoid Plant Hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef]
- Rasmussen, A.; Mason, M.G.; de Cuyper, C.; Brewer, P.B.; Herold, S.; Agusti, J.; Geelen, D.; Greb, T.; Goormachtig, S.; Beeckman, T.; et al. Strigolactones Suppress Adventitious Rooting in Arabidopsis and Pea. Plant Physiol. 2012, 158, 1976–1987. [Google Scholar] [CrossRef] [PubMed]
- Booker, J.; Sieberer, T.; Wright, W.; Williamson, L.; Willett, B.; Stirnberg, P.; Turnbull, C.; Srinivasan, M.; Goddard, P.; Leyser, O. MAX1 Encodes a Cytochrome P450 Family Member That Acts Downstream of MAX3/4 to Produce a Carotenoid-Derived Branch-Inhibiting Hormone. Dev. Cell 2005, 8, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.T.; Gutjahr, C.; Bennett, T.; Nelson, D.C. Strigolactone Signaling and Evolution. Annu. Rev. Plant Biol. 2017, 68, 291–322. [Google Scholar] [CrossRef]
- Soundappan, I.; Bennett, T.; Morffy, N.; Liang, Y.; Stanga, J.P.; Abbas, A.; Leyser, O.; Nelsona, D.C. SMAX1-LIKE/D53 Family Members Enable Distinct MAX2-Dependent Responses to Strigolactones and Karrikins in Arabidopsis. Plant Cell 2015, 27, 3143–3159. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Jiang, L.; Liu, X.; Li, X.; Lu, Z.; Meng, X.; Wang, Y.; Smith, S.M.; Lia, J. Strigolactone Signaling in Arabidopsis Regulates Shoot Development by Targeting D53-like SMXL Repressor Proteins for Ubiquitination and Degradation. Plant Cell 2015, 27, 3128–3142. [Google Scholar] [CrossRef]
- Yao, R.; Ming, Z.; Yan, L.; Li, S.; Wang, F.; Ma, S.; Yu, C.; Yang, M.; Chen, L.; Chen, L.; et al. DWARF14 Is a Non-Canonical Hormone Receptor for Strigolactone. Nature 2016, 536, 469–473. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Shi, Y.; Liu, Z.; Zhang, X.; Gong, Z.; Yang, S. Strigolactones Promote Plant Freezing Tolerance by Releasing the WRKY41-mediated Inhibition of CBF/DREB1 Expression. EMBO J. 2023, 42, e112999. [Google Scholar] [CrossRef]
- Van Ha, C.; Leyva-Gonzalez, M.A.; Osakabe, Y.; Tran, U.T.; Nishiyama, R.; Watanabe, Y.; Tanaka, M.; Seki, M.; Yamaguchi, S.; Van Dong, N.; et al. Positive Regulatory Role of Strigolactone in Plant Responses to Drought and Salt Stress. Proc. Natl. Acad. Sci. USA 2014, 111, 851–856. [Google Scholar] [CrossRef]
- Chi, C.; Xu, X.; Wang, M.; Zhang, H.; Fang, P.; Zhou, J.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Strigolactones Positively Regulate Abscisic Acid-Dependent Heat and Cold Tolerance in Tomato. Hortic. Res. 2021, 8, 237. [Google Scholar] [CrossRef]
- Qi, J.; Mao, Y.; Cui, J.; Lu, X.; Xu, J.; Liu, Y.; Zhong, H.; Yu, W.; Li, C. The Role of Strigolactones in Resistance to Environmental Stress in Plants. Physiol. Plant. 2024, 176, e14419. [Google Scholar] [CrossRef]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and Drought Stresses in Crop Plants: Implications, Cross Talk, and Potential Management Opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Omoarelojie, L.O.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Strigolactone Analog (Rac-GR24) Enhances Chilling Tolerance in Mung Bean Seedlings. S. Afr. J. Bot. 2021, 140, 173–181. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Sun, Y.; Zheng, S.; Wang, J.; Zhang, T. Hydrogen Peroxide Is Involved in Strigolactone Induced Low Temperature Stress Tolerance in Rape Seedlings (Brassica rapa L.). Plant Physiol. Biochem. 2020, 157, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Chen, X.; Zhu, C.; Cao, J.; Li, H.; Fu, Y.; Qin, G.; Zhao, J.; Yu, J.; Zhou, J. Strigolactones Positively Regulate HY5-Dependent Autophagy and the Degradation of Ubiquitinated Proteins in Response to Cold Stress in Tomato. New Phytol. 2024, 245, 1106–1123. [Google Scholar] [CrossRef]
- Aroca, R.; Ruiz-Lozano, J.M.; Zamarreño, Á.M.; Paz, J.A.; García-Mina, J.M.; Pozo, M.J.; López-Ráez, J.A. Arbuscular Mycorrhizal Symbiosis Influences Strigolactone Production under Salinity and Alleviates Salt Stress in Lettuce Plants. J. Plant Physiol. 2013, 170, 47–55. [Google Scholar] [CrossRef]
- Liu, J.; He, H.; Vitali, M.; Visentin, I.; Charnikhova, T.; Haider, I.; Schubert, A.; Ruyter-Spira, C.; Bouwmeester, H.J.; Lovisolo, C.; et al. Osmotic Stress Represses Strigolactone Biosynthesis in Lotus japonicus Roots: Exploring the Interaction between Strigolactones and ABA under Abiotic Stress. Planta 2015, 241, 1435–1451. [Google Scholar] [CrossRef]
- Li, W.; Nguyen, K.H.; Tran, C.D.; Watanabe, Y.; Tian, C.; Yin, X.; Li, K.; Yang, Y.; Guo, J.; Miao, Y.; et al. Negative Roles of Strigolactone-Related SMXL6, 7 and 8 Proteins in Drought Resistance in Arabidopsis. Biomolecules 2020, 10, 607. [Google Scholar] [CrossRef]
- Haider, I.; Andreo-Jimenez, B.; Bruno, M.; Bimbo, A.; Floková, K.; Abuauf, H.; Ntui, V.O.; Guo, X.; Charnikhova, T.; Al-Babili, S.; et al. The Interaction of Strigolactones with Abscisic Acid during the Drought Response in Rice. J. Exp. Bot. 2018, 69, 2403–2414. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, S.; Zhang, M.; Jiao, S.; Guo, Y.; Jiang, T. The Role of Reactive Oxygen Species in Plant-Virus Interactions. Plant Cell Rep. 2024, 43, 197. [Google Scholar] [CrossRef]
- Lee, B.H.; Lee, H.; Xiong, L.; Zhu, J.K. A Mitochondrial Complex I Defect Impairs Cold-Regulated Nuclear Gene Expression. Plant Cell 2002, 14, 1235–1251. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Shi, K.; Xia, X.J.; Zhou, Y.H.; Yu, J.Q. Hydrogen Peroxide Is Involved in the Cold Acclimation-Induced Chilling Tolerance of Tomato Plants. Plant Physiol. Biochem. 2012, 60, 141–149. [Google Scholar] [CrossRef] [PubMed]
- İşeri, Ö.D.; Körpe, D.A.; Sahin, F.I.; Haberal, M. Hydrogen Peroxide Pretreatment of Roots Enhanced Oxidative Stress Response of Tomato under Cold Stress. Acta Physiol. Plant. 2013, 35, 1905–1913. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, R.; Zheng, Y.; Chen, L.; Li, R.; Ma, J.; Hong, X.; Ma, P.; Sheng, J.; Shen, L. SlMAPK1/2/3 and Antioxidant Enzymes Are Associated with H2O2-Induced Chilling Tolerance in Tomato Plants. J. Agric. Food Chem. 2017, 65, 6812–6820. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, W.; Du, Q.; Xiao, H.; Li, J.; Wang, J.; Shang, F. Abscisic Acid and Hydrogen Peroxide Regulate Proline Homeostasis in Melon Seedlings under Cold Stress by Forming a Bidirectional Closed Loop. Environ. Exp. Bot. 2023, 205, 105102. [Google Scholar] [CrossRef]
- Lv, C.; Li, F.; Ai, X.; Bi, H. H2O2 Participates in ABA Regulation of Grafting-Induced Chilling Tolerance in Cucumber. Plant Cell Rep. 2022, 41, 1115–1130. [Google Scholar] [CrossRef]
- Li, K.; Zhong, C.; Shi, Q.; Bi, H.; Gong, B. Cold Plasma Seed Treatment Improves Chilling Resistance of Tomato Plants through Hydrogen Peroxide and Abscisic Acid Signaling Pathway. Free Radic. Biol. Med. 2021, 172, 286–297. [Google Scholar] [CrossRef]
- Zhou, X.; Joshi, S.; Khare, T.; Patil, S.; Shang, J.; Kumar, V. Nitric Oxide, Crosstalk with Stress Regulators and Plant Abiotic Stress Tolerance. Plant Cell Rep. 2021, 40, 1395–1414. [Google Scholar] [CrossRef]
- Zhao, M.G.; Chen, L.; Zhang, L.L.; Zhang, W.H. Nitric Reductase-Dependent Nitric Oxide Production Is Involved in Cold Acclimation and Freezing Tolerance in Arabidopsis. Plant Physiol. 2009, 151, 755–767. [Google Scholar] [CrossRef]
- Dong, N.; Li, Y.; Qi, J.; Chen, Y.; Hao, Y. Nitric Oxide Synthase-Dependent Nitric Oxide Production Enhances Chilling Tolerance of Walnut Shoots in Vitro via Involvement Chlorophyll Fluorescence and Other Physiological Parameter Levels. Sci. Hortic. 2018, 230, 68–77. [Google Scholar] [CrossRef]
- Fan, J.; Chen, K.; Amombo, E.; Hu, Z.; Chen, L.; Fu, J. Physiological and Molecular Mechanism of Nitric Oxide (NO) Involved in Bermudagrass Response to Cold Stress. PLoS ONE 2015, 10, e0132991. [Google Scholar] [CrossRef]
- Diao, Q.; Song, Y.; Shi, D.; Qi, H. Interaction of Polyamines, Abscisic Acid, Nitric Oxide, and Hydrogen Peroxide under Chilling Stress in Tomato (Lycopersicon esculentum Mill.) Seedlings. Front. Plant Sci. 2017, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Ge, S.; Jalal Ahammed, G.; Xiang, X.; Guo, Z.; Yu, J.; Zhou, Y. Crosstalk between Nitric Oxide and MPK1/2 Mediates Cold Acclimation-Induced Chilling Tolerance in Tomato. Plant Cell Physiol. 2017, 58, 1963–1975. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Tan, J.; Zhuo, C.; Wang, C.; Xiang, B.; Wang, Z. Abscisic Acid, H2O2 and Nitric Oxide Interactions Mediated Cold-Induced S-Adenosylmethionine Synthetase in Medicago Sativa Subsp. Falcata That Confers Cold Tolerance through up-Regulating Polyamine Oxidation. Plant Biotechnol. J. 2014, 12, 601–612. [Google Scholar] [CrossRef]
- Cantrel, C.; Vazquez, T.; Puyaubert, J.; Rezé, N.; Lesch, M.; Kaiser, W.M.; Dutilleul, C.; Guillas, I.; Zachowski, A.; Baudouin, E. Nitric Oxide Participates in Cold-Responsive Phosphosphingolipid Formation and Gene Expression in Arabidopsis thaliana. New Phytol. 2011, 189, 415–427. [Google Scholar] [CrossRef]
- Zhao, R.; Sheng, J.; Lv, S.; Zheng, Y.; Zhang, J.; Yu, M.; Shen, L. Nitric Oxide Participates in the Regulation of LeCBF1 Gene Expression and Improves Cold Tolerance in Harvested Tomato Fruit. Postharvest Biol. Technol. 2011, 62, 121–126. [Google Scholar] [CrossRef]
- Neill, S.J.; Desikan, R.; Clarke, A.; Hancock, J.T. Nitric Oxide Is a Novel Component of Abscisic Acid Signaling in Stomatal Guard Cells. Plant Physiol. 2002, 128, 13–16. [Google Scholar] [CrossRef]
- Bright, J.; Desikan, R.; Hancock, J.T.; Weir, I.S.; Neill, S.J. ABA-Induced NO Generation and Stomatal Closure in Arabidopsis Are Dependent on H2O2 Synthesis. Plant J. 2006, 45, 113–122. [Google Scholar] [CrossRef]
- Santiago, J.; Rodrigues, A.; Saez, A.; Rubio, S.; Antoni, R.; Dupeux, F.; Park, S.Y.; Márquez, J.A.; Cutler, S.R.; Rodriguez, P.L. Modulation of Drought Resistance by the Abscisic Acid Receptor PYL5 through Inhibition of Clade A PP2Cs. Plant J. 2009, 60, 575–588. [Google Scholar] [CrossRef]
- Dubovskaya, L.V.; Bakakina, Y.S.; Kolesneva, E.V.; Sodel, D.L.; Mcainsh, M.R.; Hetherington, A.M.; Volotovski, I.D. CGMP-Dependent ABA-Induced Stomatal Closure in the ABA-Insensitive Arabidopsis Mutant Abi1-1. New Phytol. 2011, 191, 57–69. [Google Scholar] [CrossRef]
- Albertos, P.; Romero-Puertas, M.C.; Tatematsu, K.; Mateos, I.; Sánchez-Vicente, I.; Nambara, E.; Lorenzo, O. S-Nitrosylation Triggers ABI5 Degradation to Promote Seed Germination and Seedling Growth. Nat. Commun. 2015, 6, 8669. [Google Scholar] [CrossRef]
- Mioto, P.T.; Mercier, H. Abscisic Acid and Nitric Oxide Signaling in Two Different Portions of Detached Leaves of Guzmania monostachia with CAM Up-Regulated by Drought. J. Plant Physiol. 2013, 170, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Su, M.; Wang, L.; Zhang, T.; Wang, J.; Xie, H.; Wu, X.; Haq, S.I.U.; Qiu, Q.S. Small Signaling Molecules in Plant Response to Cold Stress. J. Plant Physiol. 2021, 266, 153534. [Google Scholar] [CrossRef] [PubMed]
- Aroca, A.; Gotor, C.; Romero, L.C. Hydrogen Sulfide Signaling in Plants: Emerging Roles of Protein Persulfidation. Front. Plant Sci. 2018, 9, 1369. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ye, T.; Chan, Z. Exogenous Application of Hydrogen Sulfide Donor Sodium Hydrosulfide Enhanced Multiple Abiotic Stress Tolerance in Bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol. Biochem. 2013, 71, 226–234. [Google Scholar] [CrossRef]
- Fu, P.; Wang, W.; Hou, L.; Liu, X. Hydrogen Sulfide Is Involved in the Chilling Stress Response in Vitis vinifera L. Acta Soc. Bot. Pol. 2013, 82, 295–302. [Google Scholar] [CrossRef]
- Ma, L.; Yang, L.; Zhao, J.; Wei, J.; Kong, X.; Wang, C.; Zhang, X.; Yang, Y.; Hu, X. Comparative Proteomic Analysis Reveals the Role of Hydrogen Sulfide in the Adaptation of the Alpine Plant Lamiophlomis rotata to Altitude Gradient in the Northern Tibetan Plateau. Planta 2015, 241, 887–906. [Google Scholar] [CrossRef]
- Shi, H.; Ye, T.; Han, N.; Bian, H.; Liu, X.; Chan, Z. Hydrogen Sulfide Regulates Abiotic Stress Tolerance and Biotic Stress Resistance in Arabidopsis. J. Integr. Plant Biol. 2015, 57, 628–640. [Google Scholar] [CrossRef]
- Du, X.; Jin, Z.; Liu, D.; Yang, G.; Pei, Y. Hydrogen Sulfide Alleviates the Cold Stress through MPK4 in Arabidopsis thaliana. Plant Physiol. Biochem. 2017, 120, 112–119. [Google Scholar] [CrossRef]
- Zhang, X.W.; Liu, F.J.; Zhai, J.; De Li, F.; Bi, H.G.; Ai, X.Z. Auxin Acts as a Downstream Signaling Molecule Involved in Hydrogen Sulfide-Induced Chilling Tolerance in Cucumber. Planta 2020, 251, 69. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Cao, C.; Liang, S.; Ma, Y.; Liu, X.; Pei, Y. The Role of H2S in Low Temperature-Induced Cucurbitacin C Increases in Cucumber. Plant Mol. Biol. 2019, 99, 535–544. [Google Scholar] [CrossRef]
- Cui, J.; Li, C.; Qi, J.; Yu, W.; Li, C. Hydrogen Sulfide in Plant Cold Stress: Functions, Mechanisms, and Challenge. Plant Mol. Biol. 2025, 115, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, X.; Che, Y.; Hou, L.; Liu, X.; Zhang, W. Extracellular ATP Mediates H2S-Regulated Stomatal Movements and Guard Cell K+ Current in a H2O2-Dependent Manner in Arabidopsis. Sci. Bull. 2015, 60, 419–427. [Google Scholar] [CrossRef]
- García-Mata, C.; Lamattina, L. Hydrogen Sulphide, a Novel Gasotransmitter Involved in Guard Cell Signalling. New Phytol. 2010, 188, 977–984. [Google Scholar] [CrossRef]
- Li, Z.G.; Xiang, R.H.; Wang, J.Q. Hydrogen Sulfide–Phytohormone Interaction in Plants Under Physiological and Stress Conditions. J. Plant Growth Regul. 2021, 40, 2476–2484. [Google Scholar] [CrossRef]
- Scuffi, D.; Álvarez, C.; Laspina, N.; Gotor, C.; Lamattina, L.; García-Mata, C. Hydrogen Sulfide Generated by L-Cysteine Desulfhydrase Acts Upstream of Nitric Oxide to Modulate Abscisic Acid-Dependent Stomatal Closure. Plant Physiol. 2014, 166, 2065–2076. [Google Scholar] [CrossRef]
- Jin, Z.; Xue, S.; Luo, Y.; Tian, B.; Fang, H.; Li, H.; Pei, Y. Hydrogen Sulfide Interacting with Abscisic Acid in Stomatal Regulation Responses to Drought Stress in Arabidopsis. Plant Physiol. Biochem. 2013, 62, 41–46. [Google Scholar] [CrossRef]
- Ma, D.; Ding, H.; Wang, C.; Qin, H.; Han, Q.; Hou, J.; Lu, H.; Xie, Y.; Guo, T. Alleviation of Drought Stress by Hydrogen Sulfide Is Partially Related to the Abscisic Acid Signaling Pathway in Wheat. PLoS ONE 2016, 11, e0163082. [Google Scholar] [CrossRef]
- Chen, S.; Jia, H.; Wang, X.; Shi, C.; Wang, X.; Ma, P.; Wang, J.; Ren, M.; Li, J. Hydrogen Sulfide Positively Regulates Abscisic Acid Signaling through Persulfidation of SnRK2.6 in Guard Cells. Mol. Plant 2020, 13, 732–744. [Google Scholar] [CrossRef]
- Aroca, A.; Benito, J.M.; Gotor, C.; Romero, L.C. Persulfidation Proteome Reveals the Regulation of Protein Function by Hydrogen Sulfide in Diverse Biological Processes in Arabidopsis. J. Exp. Bot. 2017, 68, 4915–4927. [Google Scholar] [CrossRef]
- Li, Z.G.; Jin, J.Z. Hydrogen Sulfide Partly Mediates Abscisic Acid-Induced Heat Tolerance in Tobacco (Nicotiana tabacum L.) Suspension Cultured Cells. Plant Cell Tissue Organ Cult. 2016, 125, 207–214. [Google Scholar] [CrossRef]
- Iqbal, Z.; Memon, A.G.; Ahmad, A.; Iqbal, M.S. Calcium Mediated Cold Acclimation in Plants: Underlying Signaling and Molecular Mechanisms. Front. Plant Sci. 2022, 13, 855559. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Pottosin, I.I.; Shabala, S.S.; Palmgren, M.G.; Shabala, S. Calcium Efflux Systems in Stress Signaling and Adaptation in Plants. Front. Plant Sci. 2011, 2, 85. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.N.; Ali, G.S.; Celesnik, H.; Day, I.S. Coping with Stresses: Roles of Calcium- and Calcium/Calmodulin-Regulated Gene Expression. Plant Cell 2011, 23, 2010–2032. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.H.; Pittman, J.K.; Barkla, B.J.; Shigaki, T.; Hirschi, K.D. The Arabidopsis Cax1 Mutant Exhibits Impaired Ion Homeostasis, Development, and Hormonal Responses and Reveals Interplay among Vacuolar Transporters. Plant Cell 2003, 15, 347–364. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Y.; Liu, X.; Luo, S.; Zhang, X.; Liu, X.; Lin, Q.; Zhu, S.; Wan, H.; Yang, Y.; et al. Transcriptional Activation and Phosphorylation of OsCNGC9 Confer Enhanced Chilling Tolerance in Rice. Mol. Plant 2021, 14, 315–329. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; et al. COLD1 Confers Chilling Tolerance in Rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef]
- Furuya, T.; Matsuoka, D.; Nanmori, T. Phosphorylation of Arabidopsis thaliana MEKK1 via Ca2+ Signaling as a Part of the Cold Stress Response. J. Plant Res. 2013, 126, 833–840. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, C.; Zhu, Y.; Zhang, L.; Chen, T.; Zhou, F.; Chen, H.; Lin, Y. The Calcium-Dependent Kinase OsCPK24 Functions in Cold Stress Responses in Rice. J. Integr. Plant Biol. 2018, 60, 173–188. [Google Scholar] [CrossRef]
- Lv, X.; Li, H.; Chen, X.; Xiang, X.; Guo, Z.; Yu, J.; Zhou, Y. The Role of Calcium-Dependent Protein Kinase in Hydrogen Peroxide, Nitric Oxide and ABA-Dependent Cold Acclimation. J. Exp. Bot. 2018, 69, 4127–4139. [Google Scholar] [CrossRef]
- Tang, M.; Xu, C.; Cao, H.; Shi, Y.; Chen, J.; Chai, Y.; Li, Z. Tomato Calmodulin-like Protein SlCML37 Is a Calcium (Ca2+) Sensor That Interacts with Proteasome Maturation Factor SlUMP1 and Plays a Role in Tomato Fruit Chilling Stress Tolerance. J. Plant Physiol. 2021, 258–259, 153373. [Google Scholar] [CrossRef]
- Brandt, B.; Brodsky, D.E.; Xue, S.; Negi, J.; Iba, K.; Kangasjärvi, J.; Ghassemian, M.; Stephan, A.B.; Hu, H.; Schroeder, J.I. Reconstitution of Abscisic Acid Activation of SLAC1 Anion Channel by CPK6 and OST1 Kinases and Branched ABI1 PP2C Phosphatase Action. Proc. Natl. Acad. Sci. USA 2012, 109, 10593–10598. [Google Scholar] [CrossRef] [PubMed]
- Demir, F.; Horntrich, C.; Blachutzik, J.O.; Scherzer, S.; Reinders, Y.; Kierszniowska, S.; Schulze, W.X.; Harms, G.S.; Hedrich, R.; Geiger, D.; et al. Arabidopsis Nanodomain-Delimited ABA Signaling Pathway Regulates the Anion Channel SLAH3. Proc. Natl. Acad. Sci. USA 2013, 110, 8296–8301. [Google Scholar] [CrossRef] [PubMed]
- Drerup, M.M.; Schlücking, K.; Hashimoto, K.; Manishankar, P.; Steinhorst, L.; Kuchitsu, K.; Kudla, J. The Calcineurin B-like Calcium Sensors CBL1 and CBL9 Together with Their Interacting Protein Kinase CIPK26 Regulate the Arabidopsis NADPH Oxidase RBOHF. Mol. Plant 2013, 6, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Dubiella, U.; Seybold, H.; Durian, G.; Komander, E.; Lassig, R.; Witte, C.P.; Schulze, W.X.; Romeis, T. Calcium-Dependent Protein Kinase/NADPH Oxidase Activation Circuit Is Required for Rapid Defense Signal Propagation. Proc. Natl. Acad. Sci. USA 2013, 110, 8744–8749. [Google Scholar] [CrossRef]
- Maierhofer, T.; Diekmann, M.; Offenborn, J.N.; Lind, C.; Bauer, H.; Hashimoto, K.; Al-Rasheid, K.A.S.; Luan, S.; Kudla, J.; Geiger, D.; et al. Site-and Kinase-Specific Phosphorylation-Mediated Activation of SLAC1, a Guard Cell Anion Channel Stimulated by Abscisic Acid. Sci. Signal. 2014, 7, ra86. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).