Insights into the Underlying Mechanism of the Piriformospora indica-Enhanced Drought Tolerance in Blueberry
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. PCR Detection of P. indica Colonization in Blueberry Roots
2.3. Measurement of Photosynthetic Parameters, and Chlorophyll and Carotenoids Contents in Blueberry Leaves
2.4. Determinations of Root Activity and Antioxidant Ability Related Parameters
2.5. RNA-Seq Library Construction and Transcriptome Sequencing
2.6. Identification and Enrichment Analysis of Differentially Expressed Genes (DEGs)
2.7. MapMan Annotation and Weighted Gene Co-Expression Network Analysis (WGCNA) of DEGs
2.8. Quantitative Real-Time PCR Analysis
2.9. Statistic Analysis
3. Results
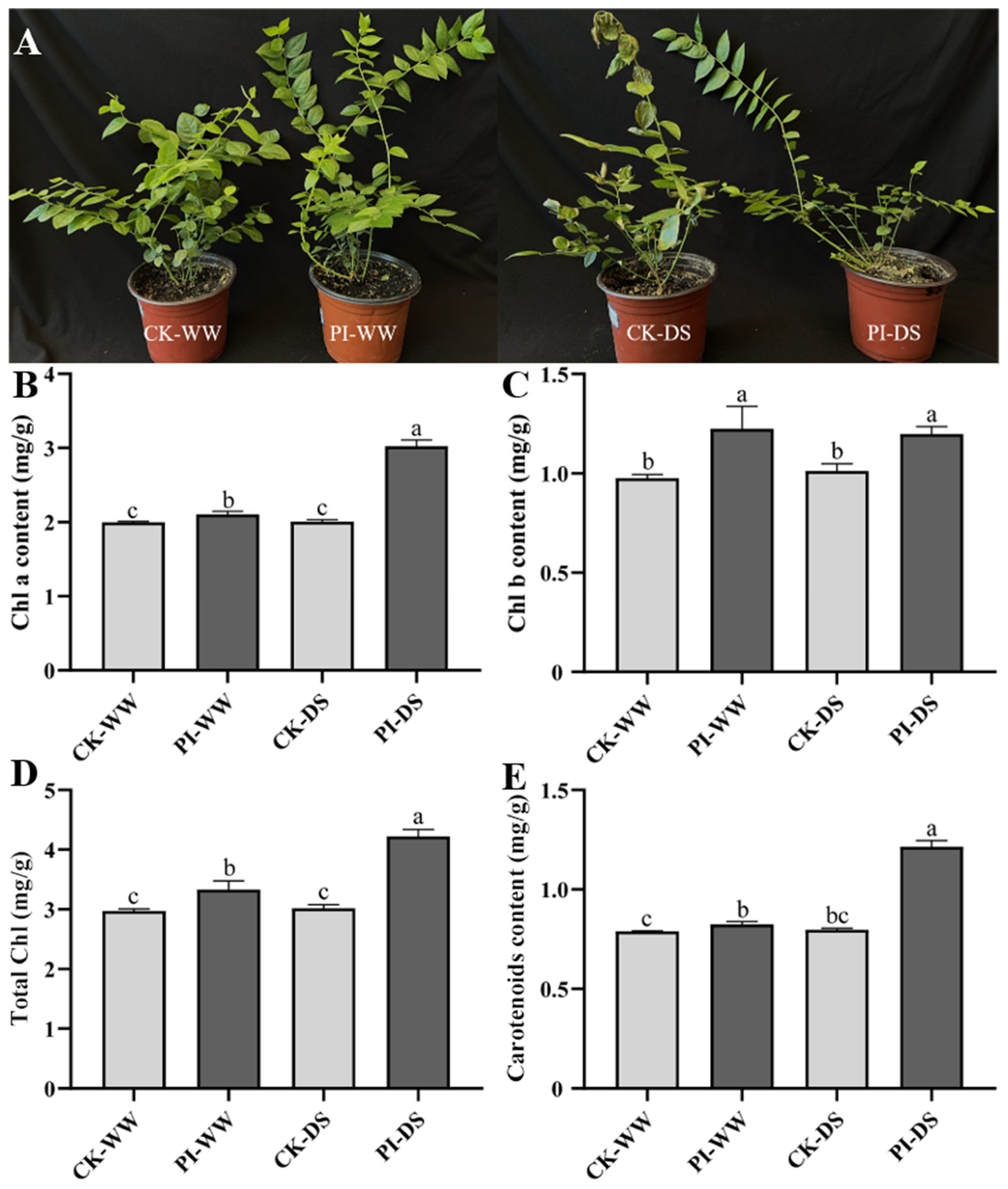
3.1. Influences of P. indica Colonization on Blueberry Drought Tolerance, Leaf Chlorophyll and Carotenoids Accumulations
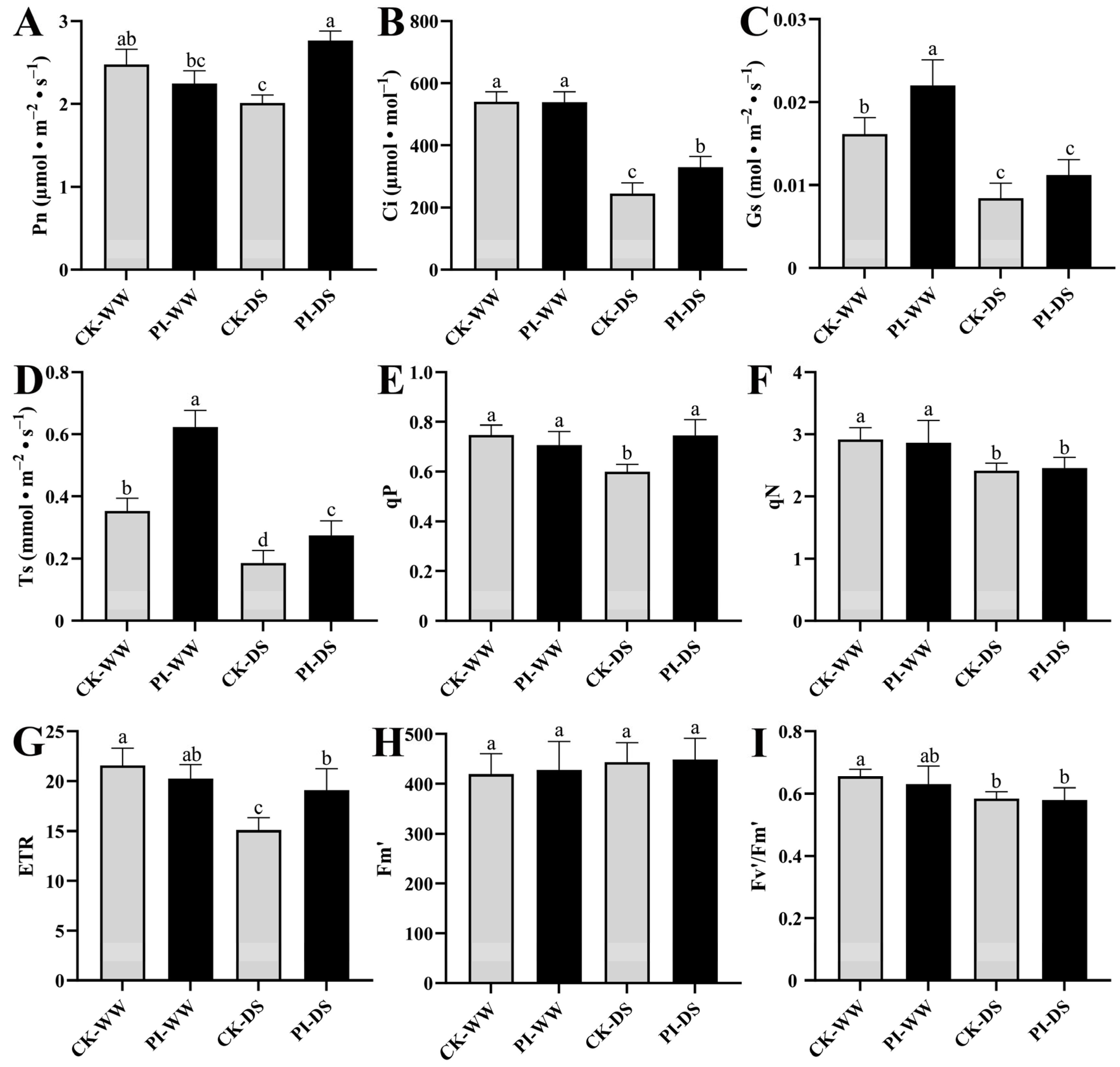
3.2. The Influences of P. indica Colonization on Blueberry Photosynthetic Capacity
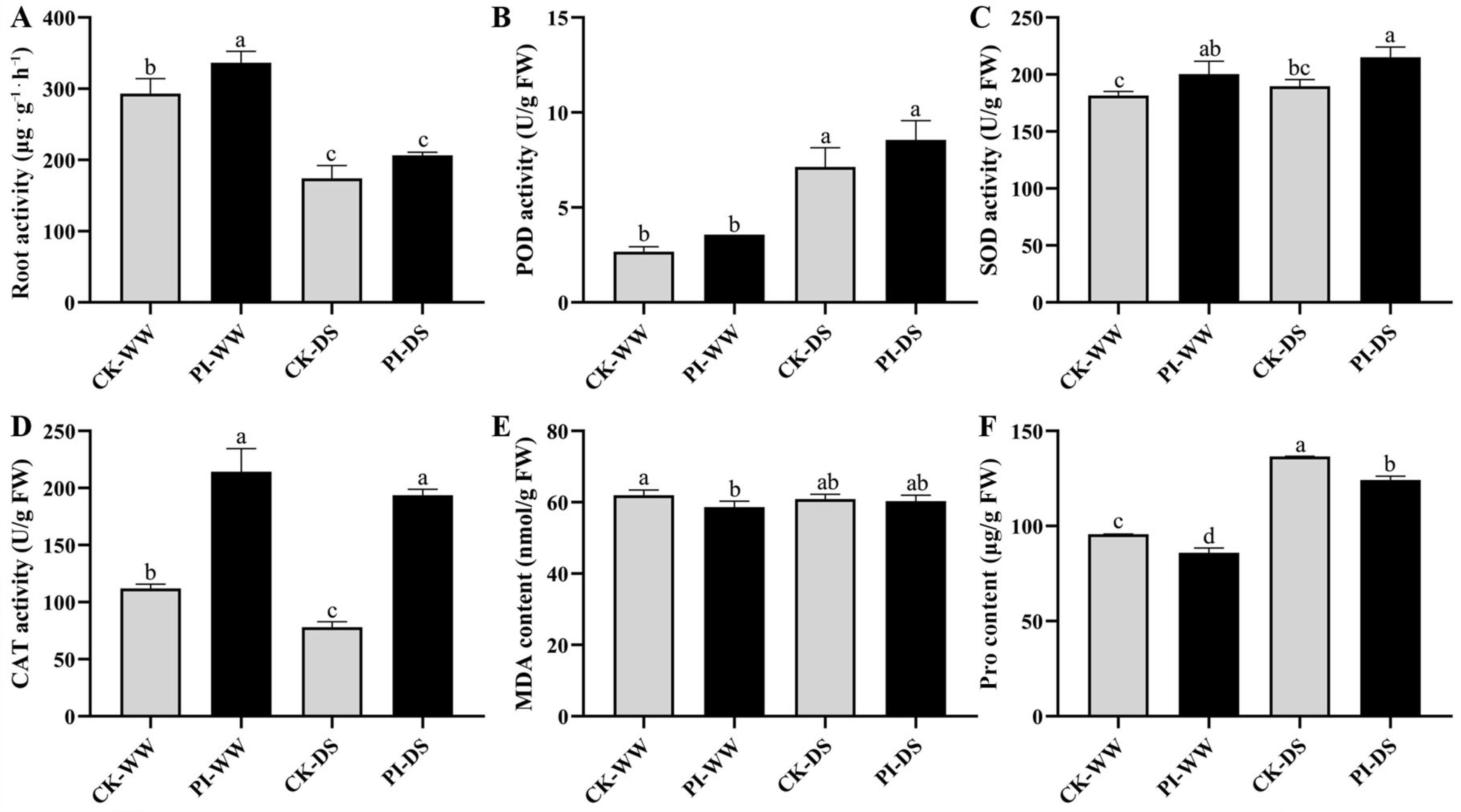
3.3. Influences of P. indica Colonization on Blueberry Root Activity
3.4. Influences of P. indica Colonization on Antioxidant Ability-Related Parameters in Blueberry Roots
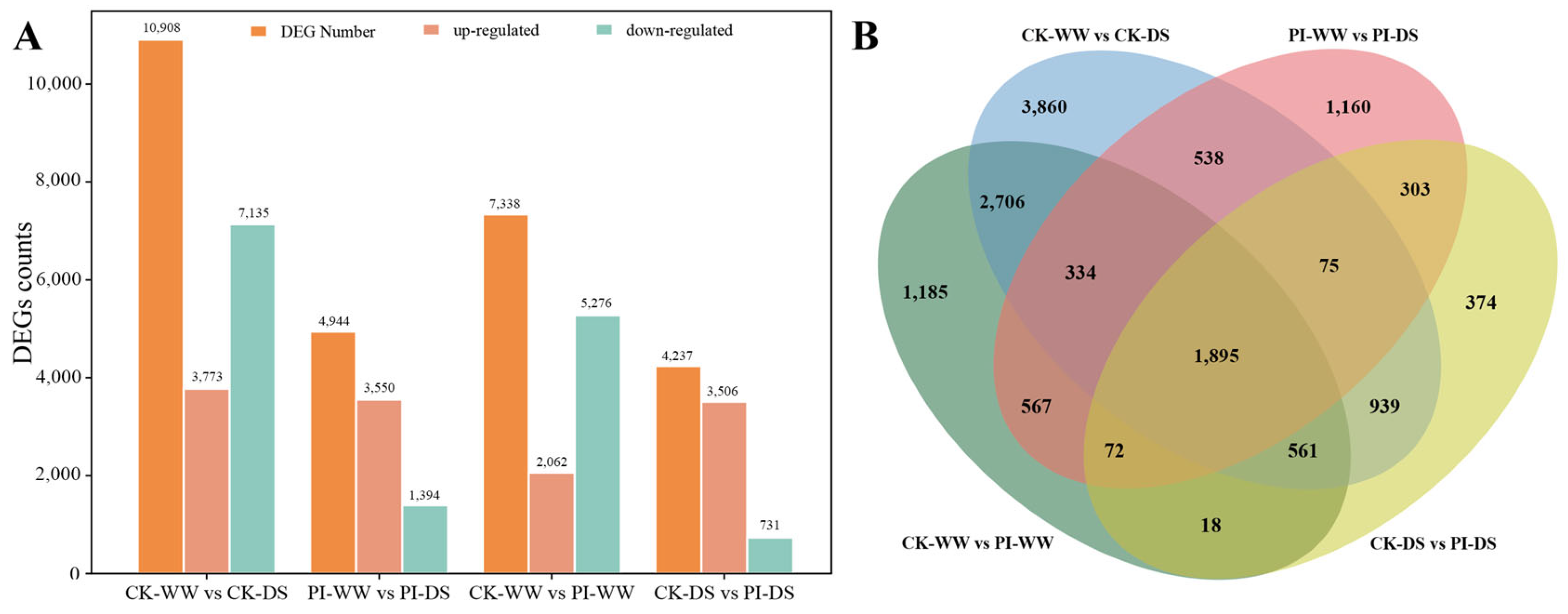
3.5. Transcriptome Sequencing and DEGs Analysis Results
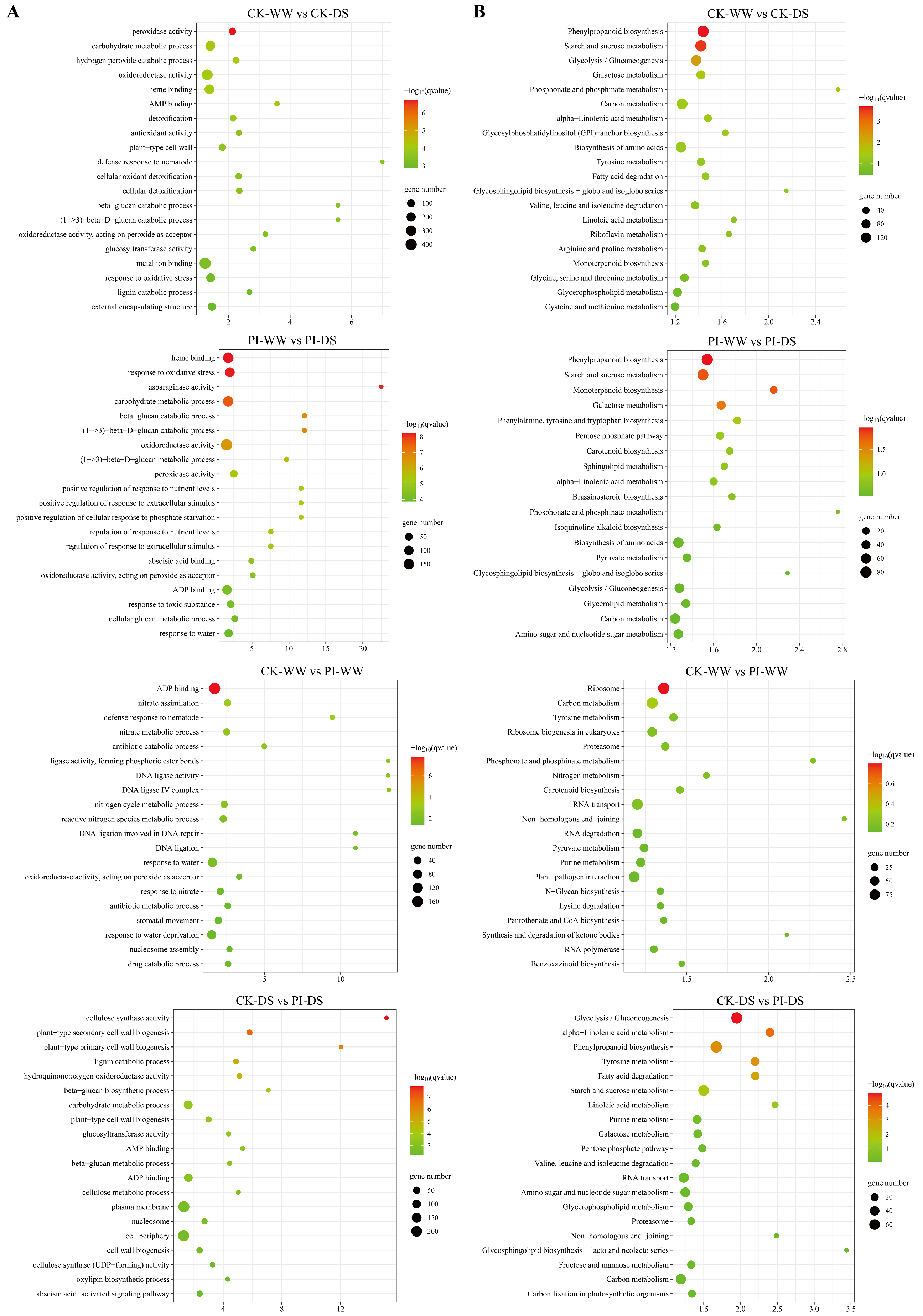
3.6. Enrichment Analysis Results of DEGs
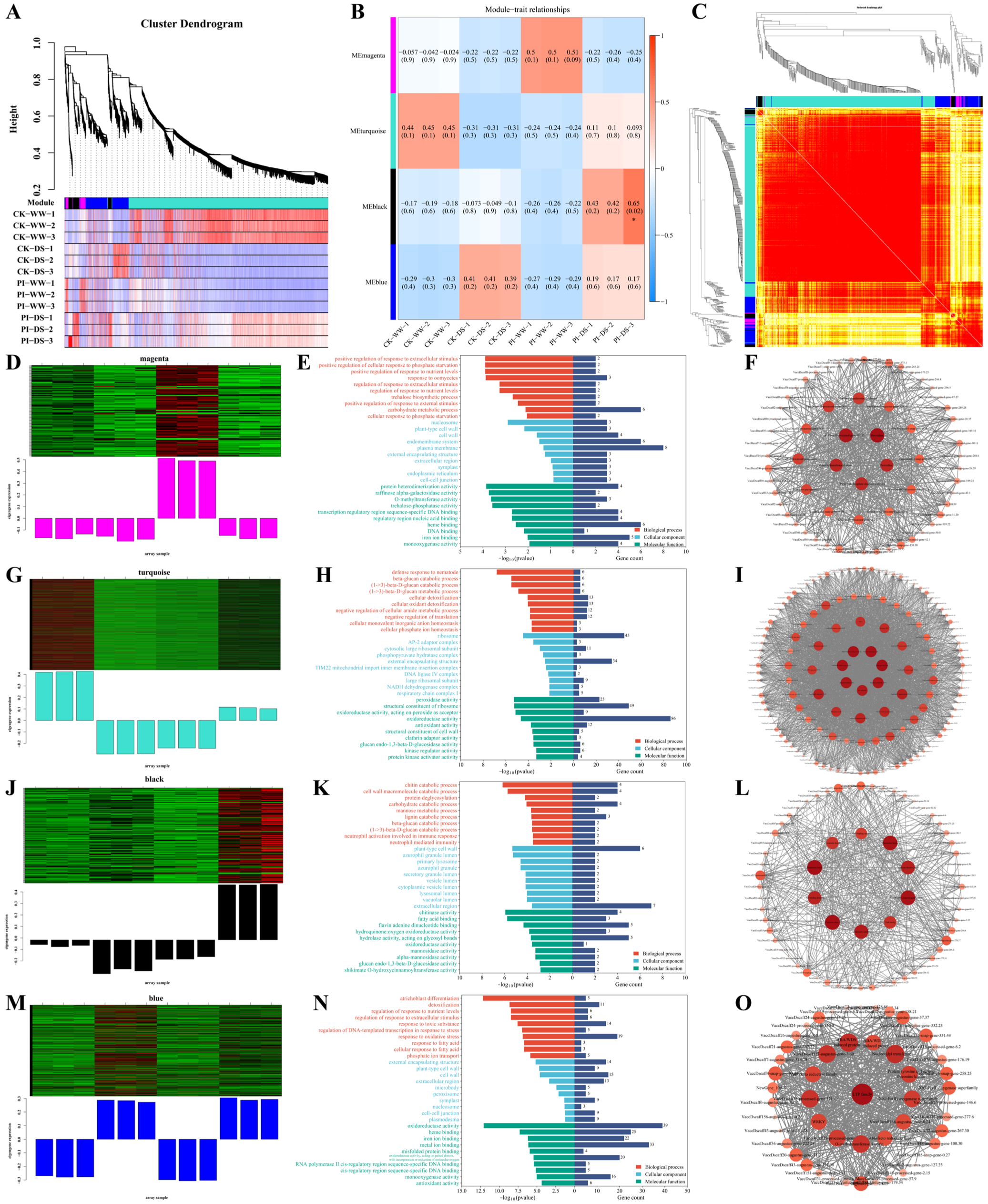
3.7. WGCNA Results
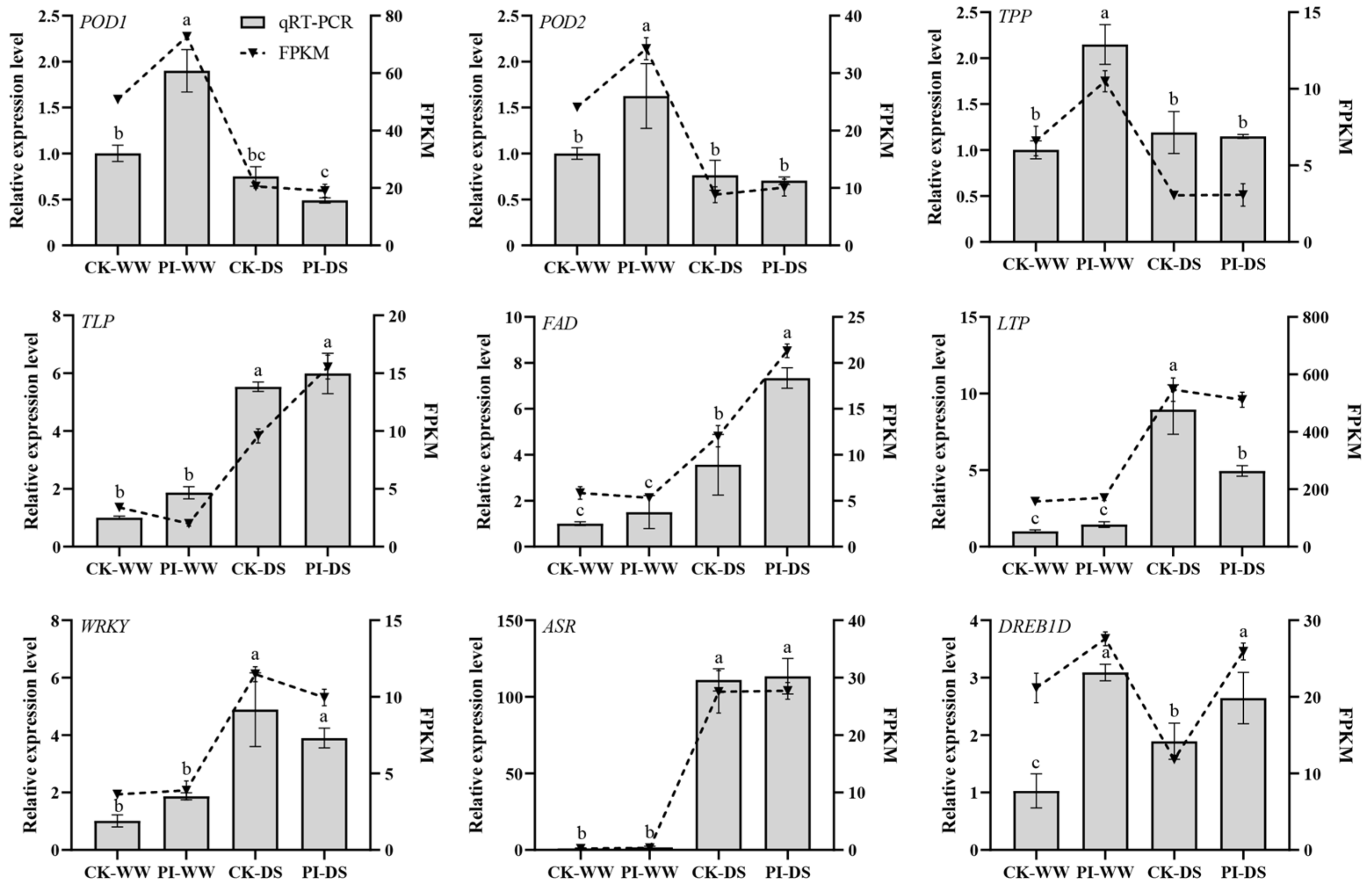
3.8. Quantitative Real-Time PCR Verification Results
4. Discussion
4.1. P. indica Colonization Improved the Photosynthetic Capacity and Mediated the Antioxidant Defense System of Blueberry Plants Under Drought Stress
4.2. P. indica Colonization Mitigates the Suppression of Drought on Metabolism- and Stress-Related Genes in Blueberry Roots
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| ASR | Abscisic stress ripening protein |
| CAT | Catalase |
| Ci | Intercellular CO2 concentration |
| DEG | Differentially expressed gene |
| DREB1D | dehydration-responsive element-binding protein 1B-like |
| DS | Drought stress |
| ETR | Electron transport rate |
| Fm’ | The maximum chlorophyll fluorescence yield under illumination |
| FPKM | Fragments Per Kilobase of transcript per Million mapped reads |
| Fv’/Fm’ | capture efficiency of excitation energy |
| GO | Gene ontology |
| GR | Glutathione reductase |
| Gs | Stomatal conductance |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LTP | Lipid-transfer protein |
| MDA | Malondialdehyde |
| OS | Oxidative stress |
| Pn | Net photosynthetic rate |
| POD | Peroxidase |
| PPO | Polyphenol oxidase |
| Pro | Proline |
| qN | Non-photochemical quenching |
| qP | Photochemical quenching |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| TLP | Thaumatin-like protein |
| TPP | Trehalose-phosphate phosphatase |
| Ts | Transpiration rate |
| WGCNA | Weighted gene co-expression network analysis |
| WW | Well-watered |
References
- Duan, Y.; Tarafdar, A.; Chaurasia, D.; Singh, A.; Bhargava, P.C.; Yang, J.; Li, Z.; Ni, X.; Tian, Y.; Li, H.; et al. Blueberry fruit valorization and valuable constituents: A review. Int. J. Food Microbiol. 2022, 381, 109890. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent research on the health benefits of blueberries and their anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Xiao, Y.; Jia, C.; Zhang, H.; Gan, Z.; Li, X.; Yang, M.; Yin, Y.; Zhang, G.; Hao, J.; et al. Physiological and biochemical changes during fruit maturation and ripening in highbush blueberry (Vaccinium corymbosum L.). Food Chem. 2023, 410, 135299. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, Y.; Tighe-Neira, R.; Inostroza-Blancheteau, C.; Soto-Cerda, B.; González-Villagra, J. Melatonin improves plant water status, photosynthetic performance, and antioxidant defense system in highbush blueberry (Vaccinium corymbosum L.) plants subjected to drought stress. Sci. Hortic. 2024, 323, 112528. [Google Scholar] [CrossRef]
- Balboa, K.; Ballesteros, G.I.; Molina-Montenegro, M.A. Integration of physiological and molecular traits would help to improve the insights of drought resistance in highbush blueberry cultivars. Plants 2020, 9, 1457. [Google Scholar] [CrossRef]
- Lobos, G.A.; Hancock, J.F. Breeding blueberries for a changing global environment: A review. Front. Plant Sci. 2015, 6, 782. [Google Scholar] [CrossRef]
- Balbontín, C.; Reyes, M.; Yáñez, M.A.; Parra-Palma, C.; Morales-Quintana, L.; Ramos, P. Enhancing blueberry drought resilience: ABA and MeJA hormonal formulations unveil water-saving strategies. Hortic. Environ. Biotechnol. 2025, 66, 99–110. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, L.; Guo, H.; Wang, Y.; Yuan, H.; Yan, D.; Zheng, B. Spermidine induces physiological and biochemical changes in southern highbush vlueberry under drought stress. Braz. J. Bot. 2017, 40, 841–851. [Google Scholar] [CrossRef]
- Lenart, A.; Wrona, D.; Krupa, T. Biostimulators with marine algae extracts and their role in increasing tolerance to drought stress in highbush blueberry cultivation. PLoS ONE 2024, 19, e0306831. [Google Scholar] [CrossRef]
- Acuña-Rodríguez, I.S.; Ballesteros, G.I.; Atala, C.; Gundel, P.E.; Molina-Montenegro, M.A. Hardening blueberry plants to face drought and cold events by the application of fungal endophytes. Agronomy 2022, 12, 1000. [Google Scholar] [CrossRef]
- Balbontín, C.; Flores, S.; Reyes, M.; Urrutia, V.; Parra-Palma, C.; Morales-Quintana, L.; Ramos, P. Antarctic fungal inoculation enhances drought tolerance and modulates fruit physiology in blueberry plants. Curr. Plant Biol. 2025, 42, 100462. [Google Scholar] [CrossRef]
- Mu, D.; Du, N.; Zwiazek, J.J. Inoculation with Ericoid mycorrhizal associations alleviates drought stress in lowland and upland velvetleaf blueberry (Vaccinium myrtilloides) seedlings. Plants 2021, 10, 2786. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Guo, Y.; Gu, L.; Shi, X.; Zhou, Y.; Wu, F.; Wang, L. A novel growth-promoting dark septate endophytic fungus improved drought tolerance in blueberries by modulating phytohormones and non-structural carbohydrates. Tree Physiol. 2024, 44, tpae105. [Google Scholar] [CrossRef]
- Verma, S.; Varma, A.; Rexer, K.-H.; Hassel, A.; Kost, G.; Sarbhoy, A.; Bisen, P.; Bütehorn, B.; Franken, P. Piriformospora indica, gen. et sp. nov., a new root-colonizing fungus. Mycologia 1998, 90, 896–903. [Google Scholar] [CrossRef]
- Mensah, R.A.; Li, D.; Liu, F.; Tian, N.; Sun, X.; Hao, X.; Lai, Z.; Cheng, C. Versatile Piriformospora indica and its potential applications in horticultural crops. Hortic. Plant J. 2020, 6, 111–121. [Google Scholar] [CrossRef]
- Saleem, S.; Sekara, A.; Pokluda, R. Serendipita indica—A review from agricultural point of view. Plants 2022, 11, 3417. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Feng, Y.; Qi, F.; Hao, R. Research progress of Piriformospora indica in improving plant growth and stress resistance to plant. J. Fungi 2023, 9, 965. [Google Scholar] [CrossRef]
- Swetha, S.; Padmavathi, T. Mitigation of drought stress by Piriformospora indica in Solanum melongena L. cultivars. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 585–593. [Google Scholar] [CrossRef]
- Azizi, M.; Fard, E.M.; Ghabooli, M. Piriformospora indica affect drought tolerance by regulation of genes expression and some morphophysiological parameters in tomato (Solanum lycopersicum L.). Sci. Hortic. 2021, 287, 110260. [Google Scholar] [CrossRef]
- Tsai, H.-J.; Shao, K.-H.; Chan, M.-T.; Cheng, C.-P.; Yeh, K.-W.; Oelmüller, R.; Wang, S.-J. Piriformospora indica symbiosis improves water stress tolerance of rice through regulating stomata behavior and ROS scavenging systems. Plant Signal. Behav. 2020, 15, 1722447. [Google Scholar] [CrossRef]
- Yaghoubian, I.; Modarres-Sanavy, S.A.M.; Smith, D.L. Plant growth promoting microorganisms (PGPM) as an eco-friendly option to mitigate water deficit in soybean (Glycine max L.): Growth, physio-biochemical properties and oil content. Plant Physiol. Biochem. 2022, 191, 55–66. [Google Scholar] [CrossRef]
- Cao, J.-L.; He, W.-X.; Zou, Y.-N.; Wu, Q.-S. An endophytic fungus, Piriformospora indica, enhances drought tolerance of trifoliate orange by modulating the antioxidant defense system and composition of fatty acids. Tree Physiol. 2023, 43, 452–466. [Google Scholar] [CrossRef]
- Hosseini, F.; Mosaddeghi, M.R.; Dexter, A.R.; Sepehri, M. Maize water status and physiological traits as affected by root endophytic fungus Piriformospora indica under combined drought and mechanical stresses. Planta 2018, 247, 1229–1245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, J.; Xu, L.; Wang, A.; Huang, L.; Du, H.; Qiu, L.; Oelmüller, R. Drought stress responses in maize are diminished by Piriformospora indica. Plant Signal. Behav. 2017, 13, e1414121. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.R.; Mirzaei, M.; Ghabooli, M.; Khatabi, B.; Wu, Y.; Zabet-Moghaddam, M.; Mohammadi-Nejad, G.; Haynes, P.A.; Hajirezaei, M.R.; Sepehri, M.; et al. Root endophytic fungus Piriformospora indica improves drought stress adaptation in barley by metabolic and proteomic reprogramming. Environ. Exp. Bot. 2019, 157, 197–210. [Google Scholar] [CrossRef]
- Liu, B.; Jing, D.; Liu, F.; Ma, H.; Liu, X.; Peng, L. Serendipita indica alleviates drought stress responses in walnut (Juglans regia L.) seedlings by stimulating osmotic adjustment and antioxidant defense system. Appl. Microbiol. Biotechnol. 2021, 105, 8951–8968. [Google Scholar] [CrossRef] [PubMed]
- Trzewik, A.; Marasek-Ciolakowska, A.; Orlikowska, T. Protection of highbush blueberry plants against Phytophthora cinnamomi using Serendipita indica. Agronomy 2020, 10, 1598. [Google Scholar] [CrossRef]
- Qu, P.; Zhang, Z.; Li, R.; Liu, R.; Zhang, Y.; Cheng, C. Insights into the rooting and growth-promoting effects of endophytic fungus Serendipita indica in blueberry (Vaccinium corymbosum). J. Plant Growth Regul. 2024. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, F.; Wang, B.; Qu, P.; Liu, J.; Zhang, Y.; Liu, W.; Tong, Z.; Deng, G. Influences of Serendipita indica and Dictyophorae echinovolvata on the growth and fusarium wilt disease resistance of banana. Biology 2022, 11, 393. [Google Scholar] [CrossRef]
- Bütehorn, B.; Rhody, D.; Franken, P. Isolation and characterisation of pitef1 encoding the translation elongation factor ef-1α of the root endophyte Piriformospora indica. Plant Biol. 2000, 2, 687–692. [Google Scholar] [CrossRef]
- Evans, J.R.; Santiago, L.S. PrometheusWiki Gold Leaf Protocol: Gas exchange using LI-COR 6400. Funct. Plant Biol. 2014, 41, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yang, Y.; Hu, Y.; Yin, L.; Qu, P.; Wang, P.; Mu, X.; Zhang, S.; Xie, P.; Cheng, C.; et al. Comparison of bioactive compounds and antioxidant activities in differentially pigmented cerasus humilis fruits. Molecules 2023, 28, 6272. [Google Scholar] [CrossRef]
- Ghazanfar, B.; Cheng, Z.; Ahmad, I.; Khan, A.R.; Liu, H.; Haiyan, D.; Fang, C. Synergistic and individual effect of Glomus etunicatum root colonization and acetyl salicylic acid on root activity and architecture of tomato plants under moderate NaCl stress. Pak. J. Bot. 2015, 47, 2047–2054. [Google Scholar]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Vonapartis, E.; Mohamed, D.; Li, J.; Pan, W.; Wu, J.; Gazzarrini, S. CBF4/DREB1D represses XERICO to attenuate ABA, osmotic and drought stress responses in Arabidopsis. Plant J. 2022, 110, 961–977. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F.; Wang, B.; Wu, H.; Wu, J.; Liu, J.; Sun, Y.; Cheng, C.; Qiu, D. Identification, characterization and expression analysis of anthocyanin biosynthesis-related bHLH genes in blueberry (Vaccinium corymbosum L.). Int. J. Mol. Sci. 2021, 22, 13274. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, J.-L.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.-S. Serendipita indica mitigates drought-triggered oxidative burst in trifoliate orange by stimulating antioxidant defense systems. Front. Plant Sci. 2023, 14, 1247342. [Google Scholar] [CrossRef]
- Hosseini, F.; Mosaddeghi, M.R.; Dexter, A.R. Effect of the fungus Piriformospora indica on physiological characteristics and root morphology of wheat under combined drought and mechanical stresses. Plant Physiol. Bioch. 2017, 118, 107–120. [Google Scholar] [CrossRef]
- Yaghoubian, Y.; Goltapeh, E.M.; Pirdashti, H.; Esfandiari, E.; Feiziasl, V.; Dolatabadi, H.K.; Varma, A.; Hassim, M.H. Effect of Glomus mosseae and Piriformospora indica on growth and antioxidant defense responses of wheat plants under drought stress. Agr. Res. 2014, 3, 239–245. [Google Scholar] [CrossRef]
- Ghabooli, M.; Khatabi, B.; Ahmadi, F.S.; Sepehri, M.; Mirzaei, M.; Amirkhani, A.; Jorrín-Novo, J.V.; Salekdeh, G.H. Prote-omics study reveals the molecular mechanisms underlying water stress tolerance induced by Piriformospora indica in barley. J. Proteom. 2013, 94, 289–3017. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef]
- Garipova, S.R.; Markova, O.V.; Fedorova, K.A.; Dedova, M.A.; Kamaletdinova, A.A.; Lastochkina, O.V.; Pusenkova, L.I. Malondialdehyde and proline content in bean cultivars following the inoculation with endophytic bacteria. Acta Physiol. Plant 2022, 44, 89. [Google Scholar] [CrossRef]
- Xu, L.; Wang, A.; Wang, J.; Wei, Q.; Zhang, W. Piriformospora indica confers drought tolerance on Zea mays L. through enhancement of antioxidant activity and expression of drought-related genes. Crop J. 2017, 5, 251–258. [Google Scholar] [CrossRef]
- Wang, A.; Wang, L.; Liu, K.; Liang, K.; Yang, S.; Cao, Y.; Zhang, L.; Wang, A.; Wang, L.; Liu, K.; et al. Comparative transcriptome profiling reveals the defense pathways and mechanisms in the leaves and roots of blueberry to drought stress. Fruit Res. 2022, 2, 18. [Google Scholar] [CrossRef]
- Fan, X.; Lin, B.; Yin, Y.; Zong, Y.; Li, Y.; Zhu, Y.; Guo, W. Unraveling the molecular mechanisms of blueberry root drought tolerance through yeast functional screening and metabolomic profiling. Plants 2024, 13, 3528. [Google Scholar] [CrossRef]
- Feng, X.; Bai, S.; Zhou, L.; Song, Y.; Jia, S.; Guo, Q.; Zhang, C. Integrated analysis of transcriptome and metabolome provides insights into flavonoid biosynthesis of blueberry leaves in response to drought stress. Int. J. Mol. Sci. 2024, 25, 11135. [Google Scholar] [CrossRef]
- Muhammad Aslam, M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of abscisic acid-mediated drought stress responses in plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Zareen, S.; Ali, A.; Yun, D.-J. Significance of ABA biosynthesis in plant adaptation to drought stress. J. Plant Biol. 2024, 67, 175–184. [Google Scholar] [CrossRef]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, F.S.; Rodrigues, J.M.; Lima, L.L.; Mesquita, R.O.; Carpinetti, P.A.; Machado, J.P.B.; Vital, C.E.; Vidigal, P.M.; Ramos, M.E.S.; Maximiano, M.R.; et al. Remodeling of the cell wall as a drought-tolerance mechanism of a soybean genotype revealed by global gene expression analysis. aBIOTECH 2021, 2, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, X.; Zhang, J.; Huang, L.; Shi, Z.; Tian, Z.; Sha, A.; Lu, G. Thaumatin-like protein family genes VfTLP4-3 and VfTLP5 are critical for faba bean’s response to drought stress at the seedling stage. Plant Physiol. Bioch. 2024, 206, 108243. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, L.; Zhu, Y.; Pan, Y.; Zhang, X.; Han, X.; Li, M.; Chen, C.; Li, H.; Wang, C. BolTLP1, a thaumatin-like protein gene, confers tolerance to salt and drought stresses in broccoli (Brassica oleracea L. Var. italica). Int. J. Mol. Sci. 2021, 22, 11132. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Qu, P.; Zhang, J.; Li, R.; Liu, R.; Cheng, C. Insights into the Underlying Mechanism of the Piriformospora indica-Enhanced Drought Tolerance in Blueberry. Horticulturae 2025, 11, 605. https://doi.org/10.3390/horticulturae11060605
Zhang Y, Qu P, Zhang J, Li R, Liu R, Cheng C. Insights into the Underlying Mechanism of the Piriformospora indica-Enhanced Drought Tolerance in Blueberry. Horticulturae. 2025; 11(6):605. https://doi.org/10.3390/horticulturae11060605
Chicago/Turabian StyleZhang, Yongyan, Pengyan Qu, Junke Zhang, Ruide Li, Rui Liu, and Chunzhen Cheng. 2025. "Insights into the Underlying Mechanism of the Piriformospora indica-Enhanced Drought Tolerance in Blueberry" Horticulturae 11, no. 6: 605. https://doi.org/10.3390/horticulturae11060605
APA StyleZhang, Y., Qu, P., Zhang, J., Li, R., Liu, R., & Cheng, C. (2025). Insights into the Underlying Mechanism of the Piriformospora indica-Enhanced Drought Tolerance in Blueberry. Horticulturae, 11(6), 605. https://doi.org/10.3390/horticulturae11060605





