Spice Defense: Resistance, Capsaicin, and Photosynthesis in Diverse Capsicum Genotypes Under Root-Knot Nematode Stress
Abstract
1. Introduction
2. Materials and Methods
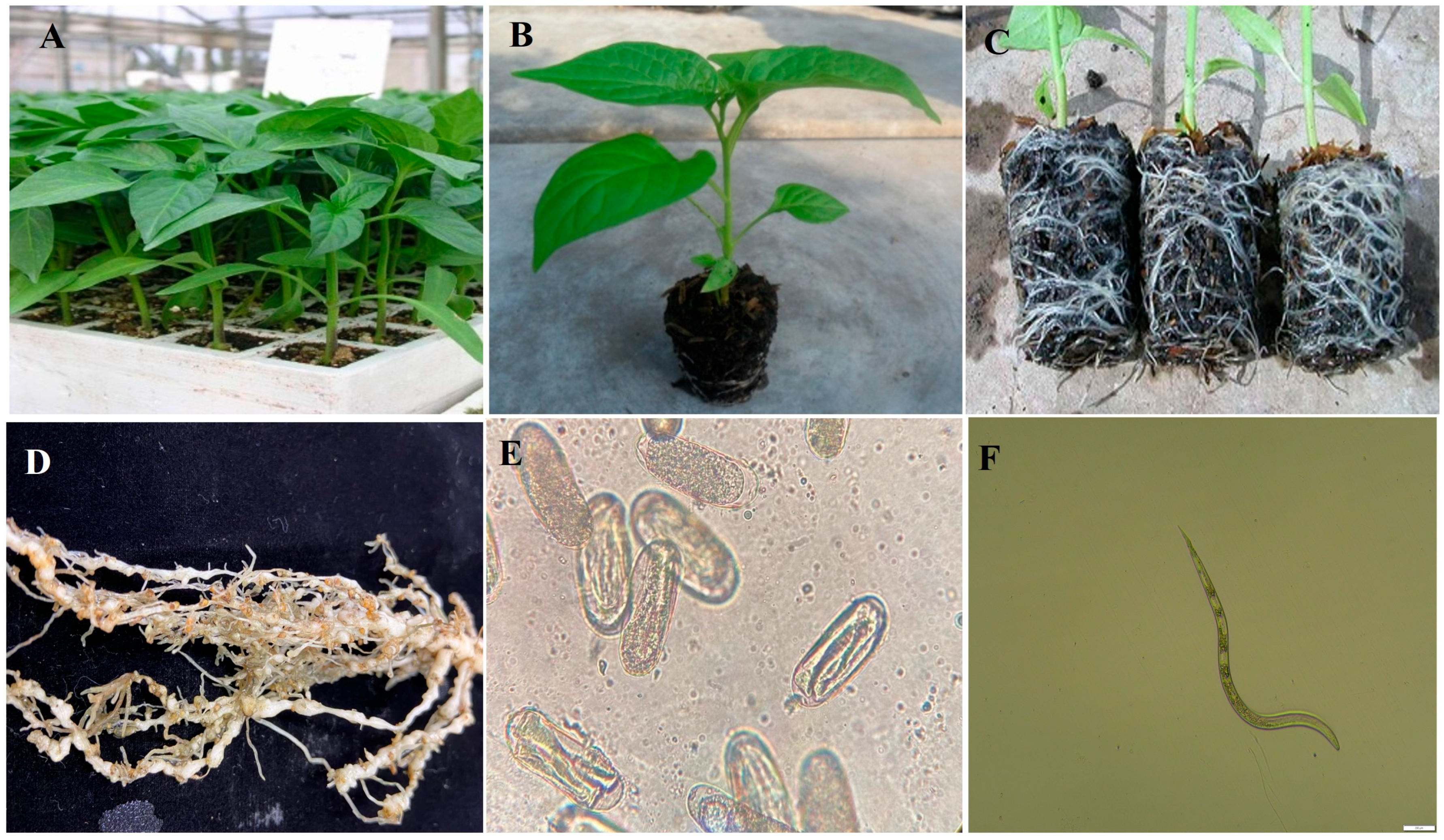
2.1. Seedling Preparation of Pepper
2.2. Meloidogyne enterolbiii Preparation
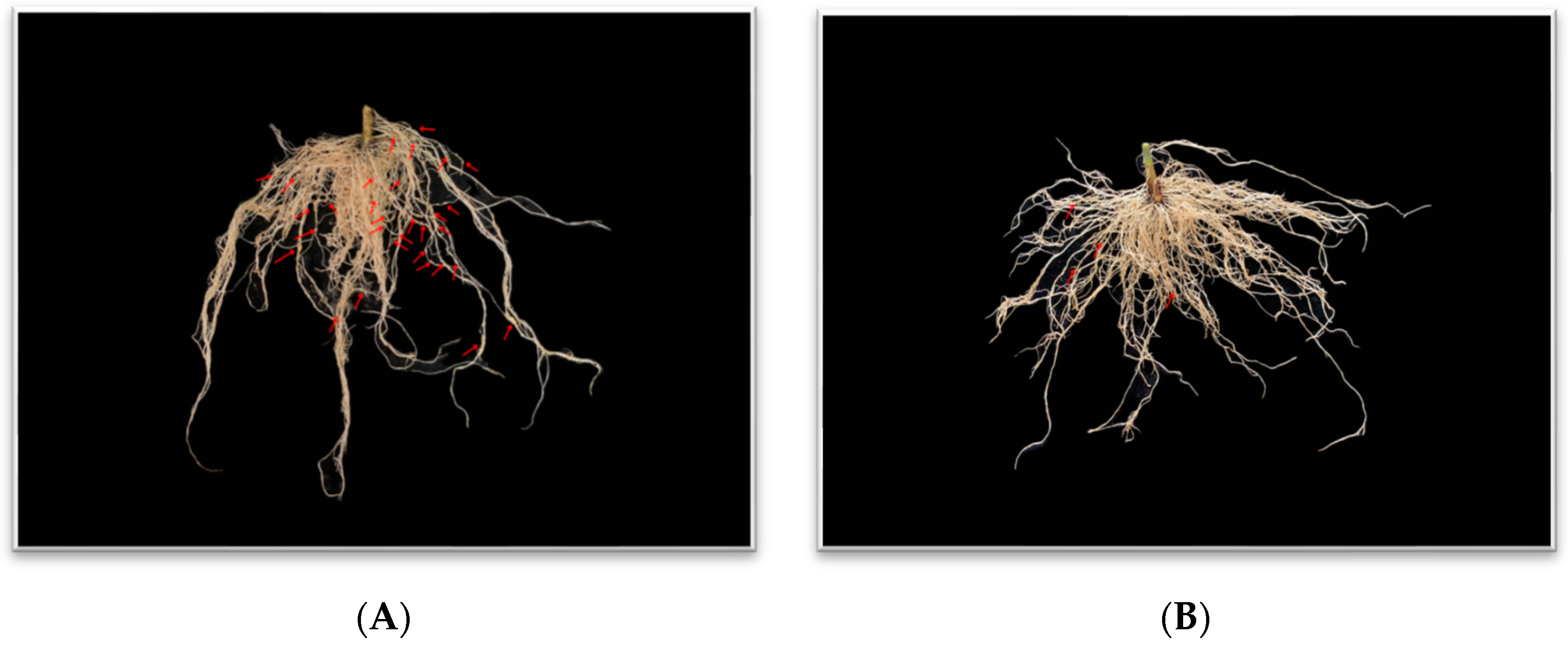
2.3. Resistance Screening of Pepper Cultivars to M. enterolobii
2.4. Changes in Capsaicin Content in Peppers in Response to M. enterolobii Infection
2.5. Photosynthetic Performance of Pepper Accessions with M. enterolobii Infection
2.6. Statistical Analysis
3. Results
3.1. Resistance Screening of Pepper Accessions to M. enterolobii
3.2. Changes in Peppers’ Capsaicin Content in Response to M. enterolobii Infection
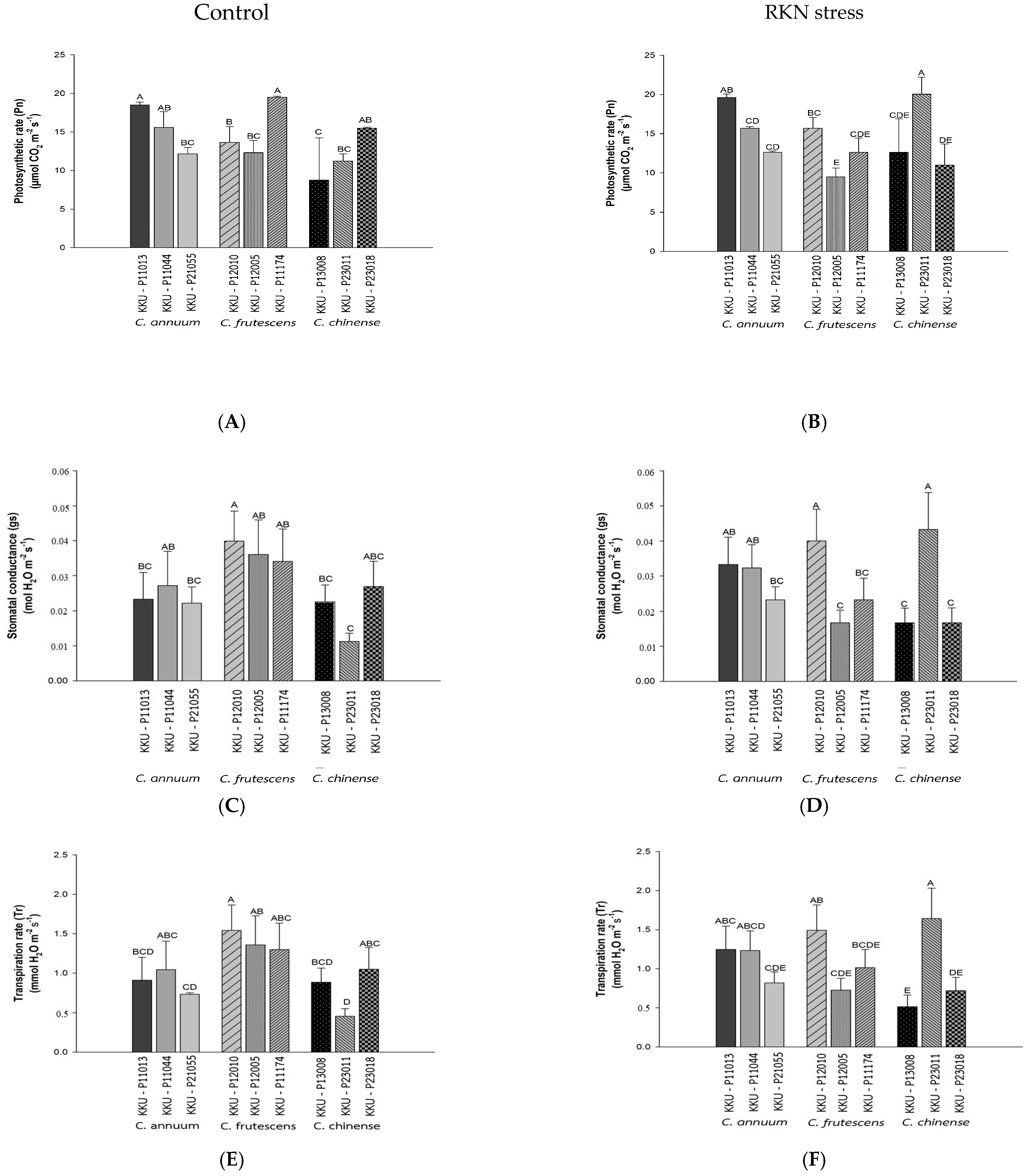
3.3. Photosynthetic Performance of Pepper Accessions with M. enterolobii Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kraikruan, W.; Sukprakarn, S.; Mongkolporn, O.; Wasee, S. Capsaicin and Dihydrocapsaicin contents of Thai Chili Cultivars. Kasetsart J. 2008, 42, 611–616. [Google Scholar]
- Zhang, Y.; Zhang, H.; Liu, Y.; Wang, J.; Li, X.; Chen, Q. A comprehensive review of capsaicin: Biosynthesis, industrial applications, and pharmacological properties. Heliyon 2024, 10, e15752. [Google Scholar]
- Azlan, A.; Sultana, S.; Huei, C.S.; Razman, M.R. Antioxidant, Anti-Obesity, Nutritional and Other Beneficial Effects of Different Chili Pepper: A Review. Molecules 2022, 27, 898. [Google Scholar] [CrossRef]
- Prabaningrum, L.; Moekasan, T.K.; Hasyim, A.; Setiawati, W.; Murtiningsih, R.; Udiarto, B.; Sulastrini, I.; Korlina, E.; Gunaeni, N.; Wulandari, A.; et al. Diversity of insect pests and their natural enemies in hot pepper (Capsicum annuum L.) ecosystem of Indonesia. Appl. Ecol. Environ. Res. 2022, 20, 3367–3377. [Google Scholar] [CrossRef]
- Nalla, M.K.; Schafleitner, R.; Pappu, H.R.; Barchenger, D.W. Current status, breeding strategies and future prospects for managing chilli leaf curl virus disease and associated begomoviruses in chilli (Capsicum spp.). Front. Plant Sci. 2023, 14, 1223982. [Google Scholar] [CrossRef]
- Tangchitsomkid, N. Root-Knot Disease Management in Chili; Department of Agriculture: Bangkok, Thailand, 2007.
- Phanbut, P.; Chairin, T.; Chinnasri, B. Identification of root-knot nematodes (Meloidogyne spp.) in chili from Sisaket Province. Khon Kaen Agric. J. Suppl. 2022, 50, 550–555. [Google Scholar]
- Boonrin, C.; Beesa, N.; Jindapunnapat, K.; Sasnarukkit, A.; Chen, P.J.; Chinnasri, B. Morphological and Molecular Identification of Meloidogyne enterolobii Populations from Different Chili-cultivated Areas in Ubon Ratchathani Province, Thailand. Trends Sci. 2024, 21, 7816. [Google Scholar] [CrossRef]
- Jindapunnapat, K.; Chinnasri, B.; Kwankuae, S. Biological control of root-knot nematodes (Meloidogyne enterolobii) in guava by the fungus Trichoderma harzianum. J. Dev. Sus. Agr. 2013, 8, 110–118. [Google Scholar]
- Jindapunnapat, K.; Chinnasri, B.; Beesa, N.; Chomphuphuang, N. Molecular phylogeny and morphological studies reveal a 30-year-old rain tree (Samanea saman) maintains populations of Meloidogyne enterolobii, a new host plant in Thailand. J. Phytopathol. 2023, 171, 409–420. [Google Scholar] [CrossRef]
- Beesa, N.; Suwanngam, A.; Puttawong, K.; Phanbut, P.; Jindapunnapat, K.; Sasnarukkit, A.; Chinnasri, B. First report of the root-knot nematode Meloidogyne graminicola on shallot (Allium cepa var. aggregatum) in Thailand. New Dis. Rep. 2023, 47, e12158. [Google Scholar] [CrossRef]
- Long, H.; Sun, Y.; Chen, Y.; Pei, Y.; Feng, T.; Che, H. Occurrence of Root-Knot Nematode (Meloidogyne spp.) on Peppers in Hainan, China, and M. enterolobii and M. incognita Resistance of Common Cultivars. Plant Dis. 2023, 107, 3148–3154. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.S.A.; Gomes, V.M.; Robaina, R.R.; Valim, R.H.; Rodrigues, R.; Aranha, F.M. Resistance to root-knot nematode (Meloidogyne enterolobii) in Capsicum spp. accessions. Agraria 2014, 9, 49–52. [Google Scholar] [CrossRef]
- Salazar-Mesta, R.J.; Carrillo-Fasio, J.A.; Retes-Manjarrez, J.E.; Garcia-Estrada, R.S.; Leon-Felix, J.; Tovar-Pedraza, J.M. Characterization of resistance responses to Meloidogyne enterolobii in Capsicum annuum landraces from Mexico. Chil. J. Agric. Res. 2024, 84, 301–310. [Google Scholar] [CrossRef]
- Pinheiro, J.B.; Boiteux, L.S.; Almeida, M.R.A.; Pereira, R.B.; Galhardo, L.C.; Carneiro, R.M.D.G. First report of Meloidogyne enterolobii in Capsicum rootstocks carrying the Me1 and Me3/Me7 genes in central Brazil. Nematropica 2015, 45, 184–188. [Google Scholar]
- Pinheiro, J.B.; Olegario da Silva, G.; Macêdo, A.G.; Biscaia, D.; Ragassi, C.F.; Ribeiro, C.S.C.; Carvalho, S.I.C.; Reifschneider, F.J.B. New resistance sources to root-knot nematode in Capsicum pepper. Hortic. Bras. 2020, 38, 33–44. [Google Scholar] [CrossRef]
- Techawongstien, S. Chili Pepper: Innovation from the Theory of Plant Breeding to Practical Utilization; Klangnanawitthaya Printing Partnership: Khon Kaen, Thailand, 2014; 300p. [Google Scholar]
- Holterman, M.; van der Wurff, A.; van den Elsen, S.; van Megen, H.; Bongers, T.; Holovachov, O.; Bakker, J.; Helder, J. Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Mol. Biol. Evol. 2006, 23, 1792–1880. [Google Scholar] [CrossRef]
- Powers, T.O.; Harris, T.S. A polymerase chain reaction method for identification of five major Meloidogyne species. J. Nematol. 1993, 25, 1–6. [Google Scholar]
- Gómez-González, G.; Cruz-Lachica, I.; Márquez-Zequera, I.; Valdez-Torres, J.B.; Tovar-Pedraza, J.M.; Osuna-García, L.A.; García-Estrada, R.S. Meloidogyne enterolobii egg extraction in NaOCl versus infectivity of inoculum on cucumber. J. Nematol. 2021, 53, e2021-57. [Google Scholar] [CrossRef]
- Barker, K.R.; Carter, C.C.; Sasser, J.N. (Eds.) Nematode extraction and bioassays. In An Advanced Treatise on Meloidogyne Volume II: Methodology; Department of Plant Pathology, North Carolina State University: Raleigh, NC, USA, 1985. [Google Scholar]
- Hajihassani, A.; Rutter, W.B.; Luo, X. Resistant pepper carrying N, Me1, and Me3 have different effect on penetration and reproduction of four major Meloidogyne species. J. Nematol. 2019, 51, e2019-20. [Google Scholar] [CrossRef]
- Collins, M.; Wasmund, L.M.; Bosland, P.W. Improved method for quantifying capsaicinoids in Capsicum using high-performance liquid chromatography. HortScience 1995, 30, 137–139. [Google Scholar] [CrossRef]
- Erwin, J.; Hussein, T.; Baumler, D.J. Pepper photosynthesis, stomatal conductance, transpiration, and water use efficiency differ with variety, indigenous habitat, and species of origin. HortScience 2019, 54, 1662–1666. [Google Scholar] [CrossRef]
- Brito, J.A.; Stanley, J.D.; Mendes, M.L.; Cetintas, R.; Dickson, D.W. Host status of selected cultivated plants to Meloidogyne mayaguensis in Florida. Nematropica 2007, 37, 65–71. [Google Scholar]
- Castagnone-Sereno, P. Meloidogyne enterolobii (=M. mayaguensis): Profile of an emerging, highly pathogenic, root-knot nematode species. Nematology 2012, 14, 133–138. [Google Scholar] [CrossRef]
- Philbrick, A.N.; Adhikari, T.B.; Louws, F.J.; Gorny, A.M. Meloidogyne enterolobii: A major threat to tomato production: Current status and future prospects for its management. Front. Plant Sci. 2020, 11, 606395. [Google Scholar] [CrossRef]
- Suwanngam, A.; Schiffer, P.H.; Sasnarukkit, A.; Siripattanapipong, S.; Jindapunnapat, K.; Chinnasri, B.; Ruang-areerate, T. Development of colorimetric and fluorescent closed tube LAMP assay using simplified extraction for diagnosis of Meloidogyne enterolobii in root tissues. Sci. Rep. 2025, 15, 160. [Google Scholar] [CrossRef]
- Sikandar, A.; Jia, L.; Wu, H.; Yang, S. Meloidogyne enterolobii risk to agriculture, its present status and future prospective for management. Front. Plant Sci. 2023, 13, 1093657. [Google Scholar] [CrossRef]
- Anwar, S.A.; McKenry, M.V. Incidence and reproduction of Meloidogyne incognita on vegetable crop genotypes. Pak. J. Zool. 2010, 42, 135–141. [Google Scholar]
- Moens, M.; Perry, R.N.; Starr, J.L. Meloidogyne species—A diverse group of novel and important plant parasites. In Root-Knot Nematodes, 1st ed.; Perry, R.N., Moens, M., Starr, J.L., Eds.; CABI Publishing: Wallingford, UK, 2009; pp. 1–17. [Google Scholar] [CrossRef]
- Dong, W.; Holbrook, C.C.; Timper, P.; Brenneman, T.B.; Mullinix, B.G. Comparison of Methods for Assessing Resistance to Meloidogyne arenaria in Peanut. J. Nematol. 2007, 39, 169–175. [Google Scholar]
- Wood, C.W.; Pilkington, B.L.; Vaidya, P.; Biel, C.; Stinchcombe, J.R. Genetic conflict with a parasitic nematode disrupts the legume–rhizobia mutualism. Evol. Lett. 2018, 2, 233–245. [Google Scholar] [CrossRef]
- Bridge, J.; Page, S.L.J. Estimation of root-knot nematode infestation levels on roots using a rating chart. Trop. Pest Manag. 1980, 26, 296–298. [Google Scholar] [CrossRef]
- Williamson, V.M.; Hussey, R.S. Nematode pathogenesis and resistance in plants. Plant Cell 1996, 8, 1735–1745. [Google Scholar] [PubMed]
- Sato, K.; Kadota, Y.; Shirasu, K. Plant Immune Responses to Parasitic Nematodes. Front Plant Sci. 2019, 10, 1165. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.R.; Ng, J.L.P.; Mathesius, U. Interaction of symbiotic rhizobia and parasitic root-knot nematodes in legume roots: From molecular regulation to field application. Mol. Plant-Microbe Interact. 2021, 34, 470–490. [Google Scholar] [CrossRef] [PubMed]
- Kankam, F.; Sowley, E.N.K.; Adomako, J.; Boateng, A. Variations in the level of resistance to root-knot nematodes (Meloidogyne spp.) infestation among ten cowpeas (Vigna unguiculata L. Walp.) genotypes. Ghana J. Agric. Sci. 2019, 54, 68–78. [Google Scholar] [CrossRef]
- Fudali, S.L.; Wang, C.; Williamson, V.M. Ethylene signaling pathway modulates attractiveness of host roots to the root-knot nematode Meloidogyne hapla. Mol. Plant-Microbe Interact. 2013, 26, 75–86. [Google Scholar] [CrossRef]
- Kyndt, T.; Vieira, P.; Gheysen, G.; de Almeida Engler, J. Nematode feeding sites: Unique organs in plant roots. Plant Physiol. 2012, 160, 807–818. [Google Scholar] [CrossRef]
- Branch, C.; Hwang, C.F.; Navarre, D.A.; Williamson, V.M. Salicylic acid is part of the Mi-1–mediated defense response to root-knot nematode in tomato. Mol. Plant-Microbe Interact. 2004, 17, 351–356. [Google Scholar] [CrossRef]
- Seo, E.; Kim, S.; Yeom, S.I.; Choi, D. Genome-wide comparative analyses reveal the dynamic evolution of nucleotide-binding leucine-rich repeat gene family among Solanaceae plants. Front. Plant Sci. 2020, 11, 1057. [Google Scholar] [CrossRef]
- Shukla, N.; Awasthi, R.P.; Rawat, L.; Kumar, J. Transcriptome analysis provides insight into the root transcriptome and its regulation under nematode stress in resistant and susceptible chickpea genotypes. Sci. Rep. 2018, 8, 4883. [Google Scholar]
- Djian-Caporalino, C.; Molinari, S.; Palloix, A.; Ciancio, A.; Fazari, A.; Marteu, N.; Ris, N.; Castagnone-Sereno, P. The reproductive potential of the root-knot nematode Meloidogyne incognita is affected by selection for virulence against major resistance genes from tomato and pepper. Eur. J. Plant Pathol. 2011, 131, 431–440. [Google Scholar] [CrossRef]
- Colla, P.; Gillardi, G.; Gullino, M.L. A review and critical analysis of the European situation of soilborne disease management in the vegetable sector. Phytoparasitica 2012, 40, 515–523. [Google Scholar] [CrossRef]
- Sánchez-Solana, F.; Ros, C.; Guerrero, M.M.; Lacasa, C.M.; Sánchez-López, E.; Lacasa, A. New pepper accessions proved to be suitable as a genetic resource for use in breeding nematode-resistant rootstocks. Plant Genet. Resour. 2016, 14, 28–34. [Google Scholar] [CrossRef]
- Mazourek, M.; Pujar, A.; Borovsky, Y.; Paran, I.; Mueller, L.; Jahn, M.M. A dynamic interface for capsaicinoid systems biology. Plant Physiol. 2009, 150, 1806–1821. [Google Scholar] [CrossRef]
- Sanatombi, K.; Kabita, K.C.; Adhikari, A.; Roy, D.; Hossain, Z.; Sharma, S.K. Identification of differentially expressed miRNAs and target genes in a highly pungent pepper (Capsicum chinense Jacq.). J. Plant Growth Regul. 2024, 17, 1281–1296. [Google Scholar] [CrossRef]
- Pereira, T.d.S.; Paula, A.M.d.; Ferrari, L.H.; Silva, J.d.; Pinheiro, J.B.; Cajamarca, S.M.N.; Jindo, K.; Santos, M.P.; Zandonadi, D.B.; Busato, J.G. Trichoderma-enriched vermicompost extracts reduces nematode biotic stress in tomato and bell pepper crops. Agronomy 2021, 11, 1165. [Google Scholar] [CrossRef]
- Tikoria, R.; Kaur, A.; Ohri, P. Modulation of various phytoconstituents in tomato seedling growth and Meloidogyne incognita–induced stress alleviation by vermicompost. Front. Environ. Sci. 2022, 10, 891195. [Google Scholar] [CrossRef]
- Ji, W.; Luo, H.; Song, Y.; Hong, E.; Li, Z.; Lin, B.; Fan, C.; Wang, H.; Song, X.; Jin, S.; et al. Changes in photosynthetic characteristics of Paeonia suffruticosa under high temperature stress. Agronomy 2022, 12, 1203. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S. Impact of combined stress of high temperature and water deficit on growth and seed yield of soybean. Physiol. Mol. Biol. Plants 2017, 24, 37–50. [Google Scholar] [CrossRef]

| No | Code | Capsicum Species | Gall Index | Egg/g Root | Rf | Resistance Level |
|---|---|---|---|---|---|---|
| 1 | KKU-P11013 | C. annuum | 1.25 ± 0.25 | 1762.45 ± 1115.21 | 36.18 ± 27.47 | HS |
| 2 | KKU-P11216 | C. annuum | 1.25 ± 0.25 | 1170.55 ± 478.86 | 22.42 ± 12.91 | HS |
| 3 | KKU-P11027 | C. annuum | 0.75 ± 0.25 | 1319.40 ± 301.14 | 16.23 ± 2.71 | HS |
| 4 | KKU-P31075 | C. annuum | 1.5 ± 0.29 | 732.04 ± 250.51 | 15.34 ± 5.48 | HS |
| 5 | KKU-P21004 | C. annuum | 0.75 ± 0.25 | 1890.40 ± 517.82 | 13.72 ± 13.72 | HS |
| 6 | KKU-P21003 | C. annuum | 1.25 ± 0.25 | 984.03 ± 289.92 | 8.88 ± 2.75 | HS |
| 7 | KKU-P21009 | C. annuum | 0.75 ± 0.25 | 427.33 ± 239.83 | 7.86 ± 3.55 | HS |
| 8 | KKU-P31117 | C. annuum | 0.75 ± 0.48 | 773.74 ± 255.97 | 7.09 ± 1.84 | HS |
| 9 | KKU-P11037 | C. annuum | 1.25 ± 0.25 | 635.61 ± 880.36 | 6.64 ± 2.04 | MS |
| 10 | KKU-P28004 | C. annuum | 2.50 ± 0.29 | 658.51 ± 223.33 | 6.40 ± 1.71 | MS |
| 11 | KKU-P28013 | C. annuum | 0.25 ± 0.25 | 650.86 ± 68.78 | 6.35 ± 0.45 | MS |
| 12 | KKU-P11032 | C. annuum | 0.75 ± 0.25 | 317.89 ± 170.52 | 6.04 ± 4.06 | MS |
| 13 | KKU-P31078 | C. annuum | 0.50 ± 0.50 | 488.48 ± 164.44 | 6.00 ± 2.55 | MS |
| 14 | KKU-P21002 | C. annuum | 1.75 ± 0.25 | 468.32 ± 86.85 | 5.93 ± 1.26 | MS |
| 15 | KKU-P41012 | C. annuum | 2.00 ± 0.41 | 691.62 ± 100.44 | 5.82 ± 0.71 | MS |
| 16 | KKU-P21036 | C. annuum | 0.75 ± 0.25 | 368.17 ± 102.65 | 5.82 ± 1.97 | MS |
| 17 | KKU-P28003 | C. annuum | 1.00 ± 0.00 | 445.39 ± 219.28 | 5.43 ± 1.76 | MS |
| 18 | KKU-P11241 | C. annuum | 1.25 ± 0.25 | 486.39 ± 180.86 | 5.38 ± 1.67 | MS |
| 19 | local | C. annuum | 1.25 ± 0.73 | 330.58 ± 49.13 | 5.30 ± 0.25 | MS |
| 20 | KKU-P28010 | C. annuum | 1.00 ± 0.41 | 439.52 ± 198.3 | 4.89 ± 1.67 | MS |
| 21 | KKU-P28009 | C. annuum | 1.00 ± 0.41 | 233.07 ± 74.32 | 4.86 ± 2.17 | MS |
| 22 | Huysiton | C. annuum | 0.5 ± 0.29 | 216.13 ± 40.60 | 4.85 ± 4.85 | MS |
| 23 | JD16 | C. annuum | 1.00 ± 0.00 | 263.15 ± 55.06 | 4.50 ± 1.18 | MS |
| 24 | KKU-P11043 | C. annuum | 0.75 ± 0.25 | 341.92 ± 127.05 | 4.25 ± 1.45 | MS |
| 25 | KKU-P28007 | C. annuum | 0.75 ± 0.25 | 420.04 ± 86.65 | 4.18 ± 4.18 | MS |
| 26 | KKU-P24001 | C. annuum | 1.00 ± 0.00 | 222.74 ± 100.58 | 4.10 ± 1.58 | MS |
| 27 | KKU-P21010 | C. annuum | 0.75 ± 0.25 | 618.27 ± 97.43 | 4.04 ± 0.84 | MS |
| 28 | Mundam KKU | C. annuum | 0.25 ± 0.25 | 377.78 ± 78.61 | 3.60 ± 1.45 | S |
| 29 | Bushy hot | C. annuum | 0.75 ± 0.25 | 302.33 ± 113.44 | 3.51 ± 1.27 | S |
| 30 | KKU-P31115 | C. annuum | 1.75 ± 0.25 | 264.23 ± 64.69 | 3.29 ± 0.76 | S |
| 31 | KKU-P31105 | C. annuum | 0.25 ± 0.25 | 411.74 ± 106.48 | 3.27 ± 0.76 | S |
| 32 | KKU-P11015 | C. annuum | 1.00 ± 0.00 | 187.67 ± 27.64 | 3.22 ± 0.40 | S |
| 33 | KKU-P28011 | C. annuum | 1.25 ± 0.25 | 165.20 ± 41.54 | 3.15 ± 0.71 | S |
| 34 | KKU-P31032 | C. annuum | 1.00 ± 0.00 | 180.67 ± 64.35 | 2.64 ± 0.86 | VS |
| 35 | KKU-P10003 | C. annuum | 0.50 ± 0.29 | 237.70 ± 108.57 | 2.32 ± 1.13 | VS |
| 36 | KKU-P11036 | C. annuum | 1.75 ± 0.75 | 90.15 ± 23.53 | 2.18 ± 2.18 | VS |
| 37 | KKU-P11010 | C. annuum | 0.75 ± 0.25 | 109.15 ± 12.96 | 2.11 ± 0.72 | VS |
| 38 | KKU-P11051 | C. annuum | 1.25 ± 0.25 | 157.46 ± 61.28 | 2.10 ± 1.27 | VS |
| 39 | KKU-P28002 | C. annuum | 1.25 ± 0.25 | 89.90 ± 28.62 | 2.03 ± 0.48 | VS |
| 40 | KKU-P21005 | C. annuum | 1.50 ± 0.50 | 119.52 ± 54.04 | 1.99 ± 1.26 | R |
| 41 | KKU-P11012 | C. annuum | 1.50 ± 0.29 | 106.43 ± 35.1 | 1.97 ± 0.62 | R |
| 42 | KKU-P31069 | C. annuum | 0.75 ± 0.25 | 151.43 ± 21.77 | 1.88 ± 0.37 | R |
| 43 | KKU-P11045 | C. annuum | 2.25 ± 0.75 | 174.97 ± 77.9 | 1.83 ± 0.42 | R |
| 44 | KKU-P31048 | C. annuum | 0.00 ± 0.00 | 442.55 ± 73.62 | 1.60 ± 0.70 | R |
| 45 | KKU-P11217 | C. annuum | 1.00 ± 0.00 | 106.53 ± 31.43 | 1.48 ± 0.49 | R |
| 46 | KKU-P28005 | C. annuum | 0.00 ± 0.00 | 222.74 ± 63.78 | 1.31 ± 0.32 | R |
| 47 | KKU-P31118 | C. annuum | 2.00 ± 0.41 | 98.42 ± 19.79 | 1.21 ± 0.40 | R |
| 48 | KKU-P21056 | C. annuum | 0.75 ± 0.25 | 102.86 ± 21.83 | 1.20 ± 0.2 | R |
| 49 | KKU-P21080 | C. annuum | 1.00 ± 0.00 | 74.08 ± 17.56 | 1.15 ± 0.37 | R |
| 50 | KKU-P28008 | C. annuum | 1.75 ± 0.48 | 119.08 ± 75 | 1.11 ± 0.69 | R |
| 51 | KKU-P31082 | C. annuum | 1.75 ± 0.48 | 37.26 ± 10.82 | 1.00 ± 0.30 | R |
| 52 | KKU-P11135 | C. annuum | 0.75 ± 0.25 | 68.81 ± 17.32 | 0.98 ± 0.3 | VR |
| 53 | KKU-P11007 | C. annuum | 1.00 ± 0.26 | 50.18 ± 12.19 | 0.97 ± 0 | VR |
| 54 | Red sun-Esan | C. annuum | 0.50 ± 0.29 | 84.29 ± 14.38 | 0.97 ± 0.97 | VR |
| 55 | KKU-P21006 | C. annuum | 1.25 ± 0.25 | 175.85 ± 49.50 | 0.93 ± 0.33 | VR |
| 56 | KKU-P11024 | C. annuum | 0.5 ± 0.29 | 132.70 ± 16.80 | 0.90 ± 0.20 | VR |
| 57 | KKU-P21008 | C. annuum | 1.00 ± 0.00 | 60.87 ± 31.56 | 0.87 ± 0.35 | VR |
| 58 | KKU-P18037 | C. annuum | 0.75 ± 0.25 | 69.71 ± 11.16 | 0.86 ± 0.17 | VR |
| 59 | KKU-P11231 | C. annuum | 1.00 ± 0.41 | 59.64 ± 35.11 | 0.80 ± 0.33 | VR |
| 60 | KKU-P28012 | C. annuum | 1.25 ± 0.25 | 53.86 ± 15.95 | 0.70 ± 0.17 | VR |
| 61 | KKU-P21055 | C. annuum | 0.5 ± 0.29 | 38.68 ± 4.97 | 0.61 ± 0.24 | VR |
| 62 | KKU-P21041 | C. annuum | 2.00 ± 0.41 | 42.14 ± 20.5 | 0.54 ± 0.54 | VR |
| 63 | KKU-P11084 | C. annuum | 0.25 ± 0.25 | 33.13 ± 2.35 | 0.49 ± 0.49 | VR |
| 64 | KKU-P18005 | C. annuum | 1.00 ± 0.00 | 75.98 ± 52.93 | 0.47 ± 0.47 | VR |
| 65 | Tepin | C. annuum | 1.50 ± 0.00 | 51.36 ± 8.55 | 0.45 ± 0.84 | VR |
| 66 | KKU-P31135 | C. annuum | 0.00 ± 0.00 | 48.43 ± 13.59 | 0.45 ± 0.11 | VR |
| 67 | KKU-P13004 | C. annuum | 0.00 ± 0.00 | 38.73 ± 9.56 | 0.35 ± 0.01 | VR |
| 68 | KKU-P11044 | C. annuum | 1.00 ± 0.00 | 50.42 ± 12.20 | 0.26 ± 0.06 | VR |
| 69 | KKU-P11034 | C. annuum | 1.00 ± 0.00 | 10.07 ± 1.26 | 0.11 ± 0.01 | VR |
| 70 | KKU-P11095 | C. annuum | 0.50 ± 0.29 | 11.64 ± 2.99 | 0.11 ± 0.04 | VR |
| 71 | KKU-P34004 | C. annuum | 1.00 ± 0.00 | 4.88 ± 1.62 | 0.11 ± 0.03 | VR |
| No | Accession Code | Capsicum Species | Gall Index | Egg/g Root | Rf | Resistance Level |
|---|---|---|---|---|---|---|
| 1 | KKU-P34014 | C. baccatum | 0.50± 0.29 b | 163.98 ± 33.60 a | 3.87 ± 1.23 a | S |
| 2 | KKU-P34011 | C. baccatum | 1.25 ± 0.25 b | 229.82 ± 65.13 a | 2.86 ± 0.66 a | VS |
| 3 | KKU-P24003 | C. baccatum | 2.25 ± 0.25 a | 210.51 ± 110.16 a | 2.21 ± 0.61 a | VS |
| 4 | KKU-P34012 | C. baccatum | 0.50 ± 0.29 b | 108.98 ± 53.87 a | 2.18 ± 0.76 a | VS |
| 5 | KKU-P34031 | C. baccatum | 0.75 ± 0.25 b | 80.26 ± 23.55 a | 1.96 ± 0.75 a | R |
| No | Accession Code | Capsicum Species | Gall Index | Egg/g Root | Rf | Resistance Level |
|---|---|---|---|---|---|---|
| 1 | KKU-P13008 | C. chinense | 0.75 ± 0.25 cd | 2001.98 ± 93.12 a | 20.37 ± 1.6 a | HS |
| 2 | KKU-P33022 | C. chinense | 2.50 ± 0.29 a | 761.34 ± 157.55 b | 11.42 ± 2.53 ab | HS |
| 3 | KKU-P31066 | C. chinense | 1.00 ± 0.00 bcd | 847.03 ± 247.03 b | 8.16 ± 1.74 bc | HS |
| 4 | KKU-P33012 | C. chinense | 1.00 ± 0.00 bcd | 845.42 ± 587.07 b | 7.34 ± 4.14 bc | HS |
| 5 | KKU-P31137 | C. chinense | 0.75 ± 0.75 cd | 377.15 ± 148.17 bcd | 5.61 ± 1.71 bc | MS |
| 6 | KKU-P11124 | C. chinense | 2.25 ± 0.48 ab | 385.42 ± 126.54 bcd | 5.56 ± 0.11 bc | MS |
| 7 | Akanee pirote | C. chinense | 2.25 ± 0.48 ab | 205.11 ± 120.01 cd | 5.20 ± 0.11 bc | MS |
| 8 | KKU-P34019 | C. chinense | 1.50 ± 0.50 abc | 367.49 ± 141.58 bcd | 5.20 ± 2.56 bc | MS |
| 9 | KKU-P37001 | C. chinense | 1.00 ± 0.00 bcd | 213.01 ± 59.03 cd | 2.87 ± 0.54 c | VS |
| 10 | KKU-P13004 | C. chinense | 1.00 ± 0.41 bcd | 79.80 ± 55.26 d | 2.63 ± 0.79 c | VS |
| 11 | Habanero | C. chinense | 0.25 ± 0.25 cd | 250.70 ± 57.23 cd | 2.38 ± 0.64 c | VS |
| 12 | KKU-P23026 | C. chinense | 2.25 ± 0.25 ab | 124.46 ± 35.13 d | 1.85 ± 0.36 c | R |
| 13 | PBC932 | C. chinense | 0.75 ± 0.25 cd | 168.05 ± 77.48 cd | 1.70 ± 0.59 c | R |
| 14 | KKU-P33013 | C. chinense | 1.00 ± 0.00 bcd | 121.57 ± 10.88 d | 1.39 ± 0.33 c | R |
| 15 | KKU-P33030 | C. chinense | 1.25 ± 0.25 bcd | 89.99 ± 28.62 d | 1.15 ± 0.48 c | R |
| 16 | KKU-P23011 | C. chinense | 0.25 ± 0.25 cd | 43.87 ± 10.09 d | 0.43 ± 0.08 c | VR |
| 17 | KKU-P13001 | C. chinense | 0.00 ± 0.00 d | 23.90 ± 4.65 d | 0.33 ± 0.06 c | VR |
| 18 | KKU-P18021 | C. chinense | 1.00 ± 0.00 bcd | 20.70 ± 3.90 d | 0.23 ± 0.03 c | VR |
| 19 | KKU-P23018 | C. chinense | 0.50 ± 0.29 cd | 8.77 ± 4.95 d | 0.05 ± 0.02 c | HR |
| No | Accession Code | Capsicum Species | Gall Index | Egg/g Root | Rf | Resistance Level |
|---|---|---|---|---|---|---|
| 1 | KKU-P32024 | C. frutescens | 2.75 ± 0.63 a | 630.03 ± 179.90 a | 8.61 ± 3.33 a | HS |
| 2 | KKU-P12010 | C. frutescens | 2.25 ± 0.25 ab | 353.68 ± 139.42 bc | 7.91 ± 2.75 ab | HS |
| 3 | KKU-P34021 | C. frutescens | 1.00 ± 0.00 bc | 276.28 ± 95.77 bc | 4.54 ± 1.11 abc | MS |
| 4 | KKU-P22006 | C. frutescens | 1.00 ± 0.00 bc | 208.50 ± 75.43 bc | 3.81 ± 0.15 abc | S |
| 5 | KKU-P11039 | C. frutescens | 1.00 ± 0.00 bc | 99.19 ± 16.46 c | 2.21 ± 0.53 abc | VS |
| 6 | KKU-P12005 | C. frutescens | 0.50 ± 0.29 c | 80.35 ± 4.26 c | 1.17 ± 0.37 bc | R |
| 7 | KKU-P32041 | C. frutescens | 1.00 ± 0.00 bc | 52.89 ± 21.81 c | 0.59 ± 0.15 c | VR |
| 8 | KKU-P22001 | C. frutescens | 0.25 ± 0.25 c | 40.46 ± 8.33 c | 0.50 ± 0.11 c | VR |
| 9 | KKU-P11174 | C. frutescens | 0.50 ± 0.29 c | 23.80 ± 7.99 c | 0.31 ± 0.04 c | VR |
| 10 | KKU-P32012 | C. frutescens | 1.50 ± 0.29 abc | 26.03 ± 8.52 c | 0.17 ± 0.04 c | VR |
| Variables | Pearson Correlation | Sig. (2-Tailed) | 95% Confidence Interval (Lower–Upper) |
|---|---|---|---|
| Egg/g root vs. GI | 0.090 | 0.080 | –0.011 to 0.190 |
| Rf vs. GI | 0.071 | 0.172 | –0.031 to 0.170 |
| Egg/g root vs. GI | 0.803 | <0.001 | 0.763 to 0.836 |
| Code | Reaction | Control | Root-Knot Nematode Infection | ||||
|---|---|---|---|---|---|---|---|
| Capsaicin (SHU) | Dihydrocapsaicin (SHU) | Sum of Capsaicin and Dihydrocapsaicin (SHU) | Capsaicin (SHU) | Dihydrocapsaicin (SHU) | Sum of Capsaicin and Dihydrocapsaicin (SHU) | ||
| C. annuum | |||||||
| KKU-P11013 | Highly susceptible | 11,676.0 g | 6848.8 g | 18,524.8 g | 11,949.6 e | 7071.6 d | 19,021.2 e |
| KKU-P11044 | Very resistant | 15,505.4 f | 9425.5 f | 24,931.0 f | 16,452.1 e | 9704.2 d | 26,156.4 e |
| KKU-P21055 | Very resistant | 30,632.6 d | 22,973.7 b | 53,606.2 d | 25,981.2 d | 19,755.5 b | 45,736.7 cd |
| C. frutescens | |||||||
| KKU-P12010 | Highly susceptible | 37,644.4 c | 20,852.1 d | 58,496.6 c | 26,807.8 d | 15,504.2 c | 42,312.0 d |
| KKU-P12005 | Resistant | 42,363.4 b | 20,211.3 d | 62,574.7 b | 33,500.0 c | 19,758.4 b | 53,258.5 bc |
| KKU-P11174 | Very resistant | 51,016.2 a | 26,211.4 a | 77,227.6 a | 54,128.4 a | 35,077.7 a | 89,206.0 a |
| C. chinense | |||||||
| KKU-P13008 | Highly susceptible | 4824.0 h | 3976.8 h | 8800.8 h | 2567.8 f | 1955.7 e | 4523.5 f |
| KKU-P23011 | Very resistant | 20,575.2 e | 13,066.2 e | 33,641.3 e | 14,396.4 e | 7451.6 d | 21,848.1 e |
| KKU-P23018 | Highly resistant | 37,106.7 c | 21,878.1 c | 58,984.8 c | 39,864.6 b | 19,934.7 b | 59,799.3 b |
| F-test | ** | ** | ** | ** | ** | ** | |
| CV (%) | 2.1 | 3.5 | 2.3 | 11.0 | 11.1 | 10.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jindapunnapat, K.; Sroisai, P.; Auangaree, N.; Pornsopin, N.; Techawongstien, S.; Tarinta, T. Spice Defense: Resistance, Capsaicin, and Photosynthesis in Diverse Capsicum Genotypes Under Root-Knot Nematode Stress. Horticulturae 2025, 11, 607. https://doi.org/10.3390/horticulturae11060607
Jindapunnapat K, Sroisai P, Auangaree N, Pornsopin N, Techawongstien S, Tarinta T. Spice Defense: Resistance, Capsaicin, and Photosynthesis in Diverse Capsicum Genotypes Under Root-Knot Nematode Stress. Horticulturae. 2025; 11(6):607. https://doi.org/10.3390/horticulturae11060607
Chicago/Turabian StyleJindapunnapat, Kansiree, Pornthip Sroisai, Nichaphat Auangaree, Nawarat Pornsopin, Suchila Techawongstien, and Tanyarat Tarinta. 2025. "Spice Defense: Resistance, Capsaicin, and Photosynthesis in Diverse Capsicum Genotypes Under Root-Knot Nematode Stress" Horticulturae 11, no. 6: 607. https://doi.org/10.3390/horticulturae11060607
APA StyleJindapunnapat, K., Sroisai, P., Auangaree, N., Pornsopin, N., Techawongstien, S., & Tarinta, T. (2025). Spice Defense: Resistance, Capsaicin, and Photosynthesis in Diverse Capsicum Genotypes Under Root-Knot Nematode Stress. Horticulturae, 11(6), 607. https://doi.org/10.3390/horticulturae11060607






