Interactive Effects of Rootstock and Training System on Photosynthesis, Biochemical Responses, and Yield in Vitis labrusca Under Subtropical Climate Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Climatic Conditions of the Experimental Area
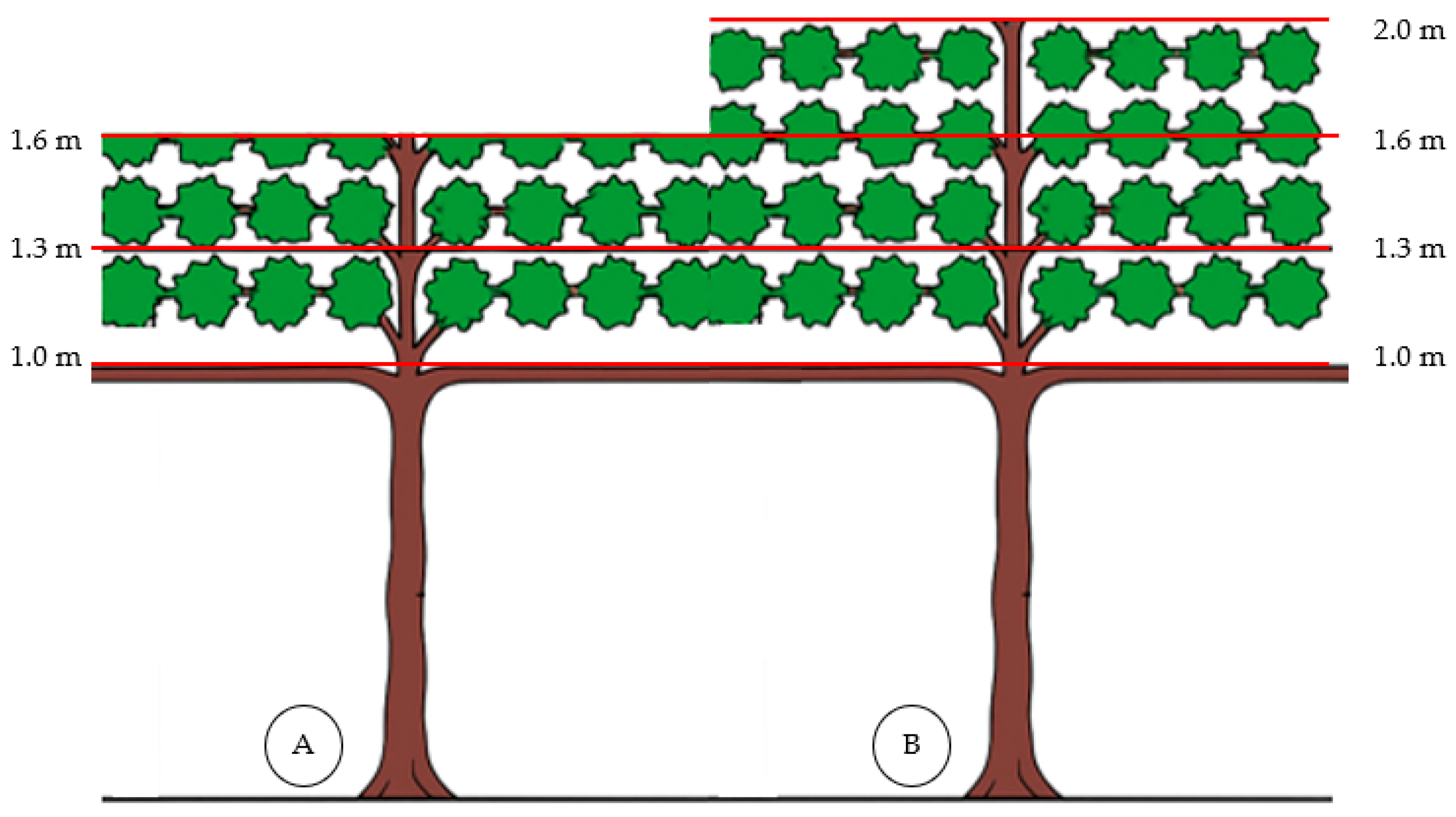
2.2. Experimental Design and Treatments
2.3. Sample Collection
2.3.1. Photosynthetic Evaluation
- Maximum quantum efficiency of photosystem II (Fv/Fm) [10];
- Effective quantum yield of PSII (ΦPSII) [11];
- Photochemical quenching (qP) [12];
- Non-photochemical quenching (NPQ) [13];
- Electron transport rate (ETR) [14];
- Non-regulated energy dissipation in PSII (ΦNO) [14];
- Regulated non-photochemical energy dissipation in PSII (ΦNPQ) [14].
2.3.2. Biochemical Analyses
2.3.3. Harvest, Yield, and Must Quality
2.4. Statistical Analyses
3. Results and Discussion
3.1. Physiological and Biochemical Performance of ‘Bordô’ Grapevines
3.2. Yield and Quality of ‘Bordô’ Grapevines
3.3. Physiological and Biochemical Performance of ‘Isabel’ Grapevines
3.4. Yield and Quality of ‘Isabel’ Grapevines
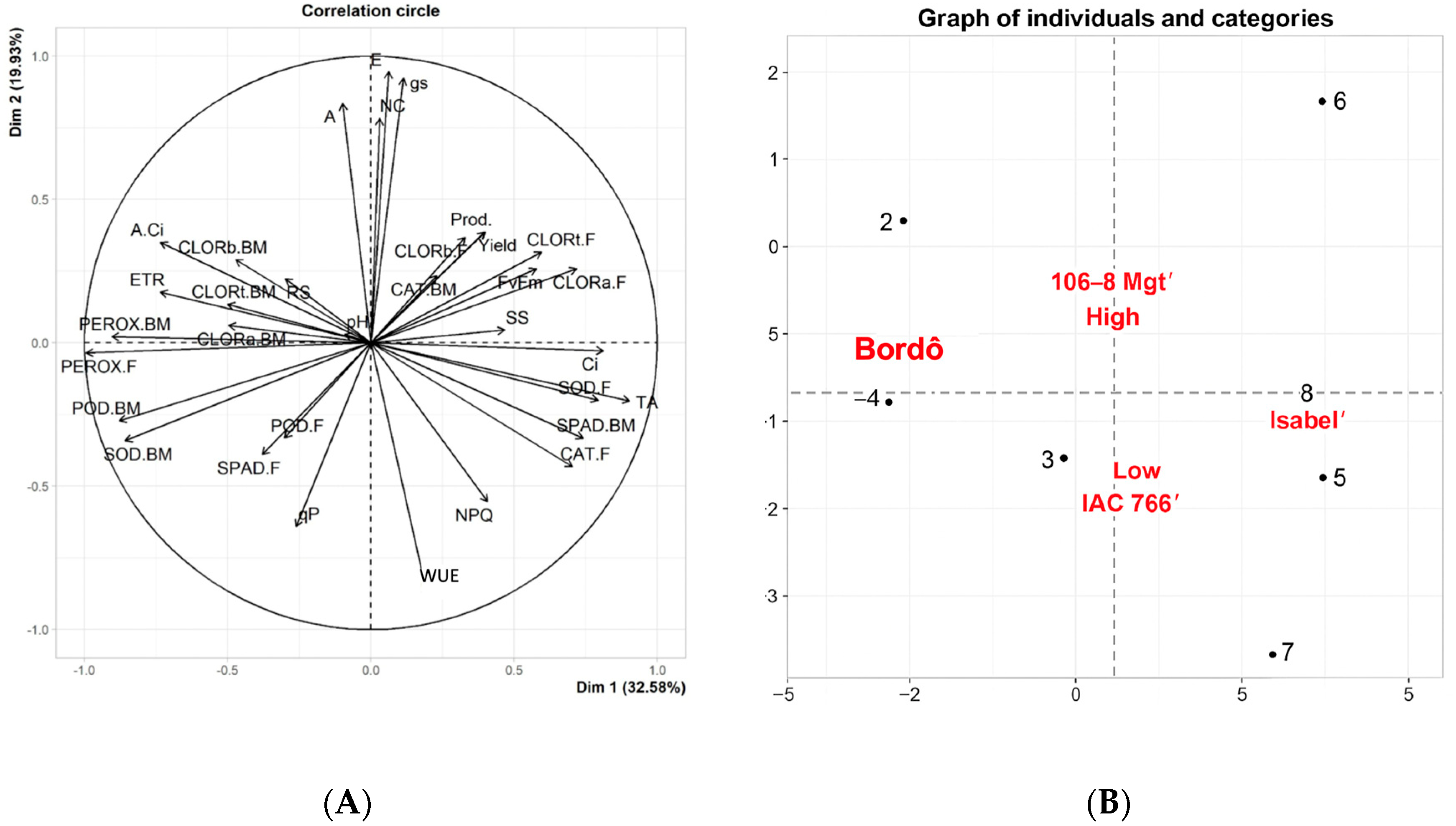
3.5. Principal Component Analysis of Vitis labrusca Grapevines
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Atak, A. Vitis species for stress tolerance/resistance. Genet. Resour. Crop Evol. 2024, 72, 2425–2444. [Google Scholar] [CrossRef]
- Domingues Neto, F.J.; Tecchio, M.A.; Borges, C.V.; Rodrigues, J.D.; Ono, E.O.; Lima, G.P.P.; Moura, M.F.; Hernandes, J.L.; Silva, M.d.S.; Leonel, M. Yield performance and quality assessment of Brazilian hybrid grapes influenced by rootstocks and training systems. Horticulturae 2024, 10, 909. [Google Scholar] [CrossRef]
- Santarosa, E.; Dutra de Souza, P.V.; de Araujo Mariath, J.E.; Lourosa, G.V. Physiological interaction between rootstock-scion: Effects on xylem vessels in Cabernet Sauvignon and Merlot grapevines. Am. J. Enol. Vitic. 2016, 67, 65–76. [Google Scholar] [CrossRef]
- Biasuz, E.C.; Kalcsits, L. Rootstocks induce functional differences that affect carbon isotope discrimination and water relations in the apple scion. Authorea Preprints 2021. [Google Scholar]
- Prinsi, B.; Simeoni, F.; Galbiati, M.; Meggio, F.; Tonelli, C.; Scienza, A.; Espen, L. Grapevine rootstocks differently affect physiological and molecular responses of the scion under water deficit condition. Agronomy 2021, 11, 289. [Google Scholar] [CrossRef]
- Domingues Neto, F.J.; Pimentel Junior, A.; Modesto, L.R.; Moura, M.F.; Putti, F.F.; Boaro, C.S.F.; Ono, E.O.; Rodrigues, J.D.; Tecchio, M.A. Photosynthesis, biochemical and yield performance of grapevine hybrids in two rootstock and trellis height. Horticulturae 2023, 9, 596. [Google Scholar] [CrossRef]
- Del Zozzo, F.; Poni, S. Climate change affects choice and management of training systems in the grapevine. Aust. J. Grape Wine Res. 2024, 2024, 7834357. [Google Scholar] [CrossRef]
- Pimentel Junior, A.; Domingues Neto, F.J.; Basílio, L.P.; Monteiro, G.C.; Lima, G.P.P.; Tecchio, M.A. Training systems improved agronomic characteristics and quality of ‘Niagara Rosada’ table grapes. Bragantia 2023, 82, 7834357. [Google Scholar] [CrossRef]
- Von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–387. [Google Scholar] [CrossRef]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Schreiber, U.; Schliwa, U.; Bilger, W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 1986, 10, 51–62. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Teisseire, H.; Guy, V. Copper-induced changes in antioxidant enzymes activities in fronds of duckweed (Lemna minor). Plant Sci. 2000, 153, 65–72. [Google Scholar] [CrossRef]
- Peixoto, P.H.P.; Cambria, J.; Sant’Anna, R.; Mosquim, P.R.; Moreira, M.A. Aluminium effects on lipid peroxidation and the activities of enzymes of oxidative metabolism in sorghum. Rev. Bras. Fisiol. Veg. 1999, 11, 137–143. [Google Scholar]
- Rama Devi, S.; Prasad, M.N.V. Copper toxicity in Ceratophyllum demersum L. (Coontail), a free-floating macrophyte: Response of antioxidant enzymes and antioxidants. Plant Sci. 1986, 138, 157–165. [Google Scholar] [CrossRef]
- Nelson, N. A photometric adaptation of Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 135, 136–175. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciência E Agrotecnologia 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Tan, L.; Xu, W.; He, X.; Wang, J. The feasibility of Fv/Fm on judging nutrient limitation of marine algae through indoor simulation and in situ experiment. Estuar. Coast. Shelf Sci. 2019, 229, 106411. [Google Scholar] [CrossRef]
- Adams, W.W.; Muller, O.; Cohu, C.M.; Demmig-Adams, B. Photosystem II efficiency and non-photochemical fluorescence quenching in the context of source-sink balance. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Demmig-Adams, B., Garab, G., Adams, W., III, Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 503–529. [Google Scholar]
- Giovagnetti, V.; Ruban, A.V. Discerning the effects of photoinhibition and photoprotection on the rate of oxygen evolution in Arabidopsis leaves. J. Photochem. Photobiol. B Biol. 2015, 152 Pt B, 272–278. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y.; Hua, W.; Last, R.L. A new light on Photosystem II maintenance in oxygenic photosynthesis. Front. Plant Sci. 2019, 10, 975. [Google Scholar] [CrossRef]
- Bassi, R.; Dall’Osto, L. Dissipation of light energy absorbed in excess: The molecular mechanisms. Annu. Rev. Plant Biol. 2021, 72, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.; John, G.P.; Pan, R.; Bartlett, M.K.; Fletcher, L.R.; Scoffoni, C.; Sack, L. A stomatal safety-efficiency trade-off constrains responses to leaf dehydration. Nat. Commun. 2019, 10, 3398. [Google Scholar] [CrossRef]
- FAO; OIV. Table and Dried Grapes: World Data Available. FAO-OIV Focus 2017. Available online: http://www.oiv.int/js/lib/pdfjs/web/viewer.html?file=/public/medias/5268/fao-oiv-focus-2025.pdf (accessed on 3 March 2025).
- Frölech, D.B.; de Assis, A.M.; Nadal, M.C.; de Mello, L.L.; dos Santos Oliveira, B.A.; Schuch, M.W. Chemical and sensory analysis of juices and cuts of ‘Bordô’ and ‘Niágara Rosada’ grapes. Rev. Bras. Frutic. 2019, 41, e-141. [Google Scholar] [CrossRef]
- Brasil. Instrução Normativa n° 01, de 07 de janeiro de 2000. Regulamento técnico geral para fixação dos padrões de identidade e qualidade para polpa de fruta. Diário Of. República Fed. Bras. 2000, 1, 54–58. [Google Scholar]
- Bender, A.; de Souza, A.L.K.; Malgarim, M.B.; Caliari, V.; Kaltbach, P.; Costa, V.B. Physicochemical and sensory properties of grape juices produced from different cultivars and extraction systems. Semin. Ciências Agrárias 2021, 42 (Suppl. S1), 1615–1634. [Google Scholar] [CrossRef]
- Domingues Neto, F.J.; Pimentel Junior, A.; Borges, C.V.; Rodrigues, J.D.; Figueira, R.; Moura, M.F.; Minatel, I.O.; Nunes, A.; Lima, G.P.P.; Tecchio, M.A. Polyphenolic profile and antioxidant activity of whole grape juices from Vitis labrusca and Brazilian hybrid grapes in two training systems. Antioxidants 2024, 13, 1132. [Google Scholar] [CrossRef] [PubMed]
- Villalobos-González, L.; Alarcón, N.; Bastías, R.; Pérez, C.; Sanz, R.; Peña-Neira, Á.; Pastenes, C. Photoprotection is achieved by photorespiration and modification of the leaf incident light, and their extent is modulated by the stomatal sensitivity to water deficit in grapevines. Plants 2022, 11, 1050. [Google Scholar] [CrossRef] [PubMed]
- Haworth, M.; Marino, G.; Centritto, M. An introductory guide to gas exchange analysis of photosynthesis and its application to plant phenotyping and precision irrigation to enhance water use efficiency. J. Water Clim. Change 2018, 9, 786–808. [Google Scholar] [CrossRef]
- Wingler, A.; Lea, P.J.; Quick, W.P.; Leegood, R.C. Photorespiration: Metabolic pathways and their role in stress protection. Philos. Trans. R. Soc. B 2000, 355, 1517–1529. [Google Scholar] [CrossRef]
- Bhutta, M.A.; Bibi, A.; Ahmad, N.H.; Kanwal, S.; Amjad, Z.; Rehman, H.; Farooq, U.; Khalid, M.N.; Nayab, S.F. Molecular mechanisms of photoinhibition in plants: A review. Sarhad J. Agric. 2023, 39, 340–345. [Google Scholar] [CrossRef]
- Monteiro, A.I.; Malheiro, A.C.; Bacelar, E.A. Morphology, Physiology and Analysis Techniques of Grapevine Bud Fruitfulness: A Review. Agriculture 2021, 11, 127. [Google Scholar] [CrossRef]
- Şen, A.; Atak, A. Bud fertility determination of some new table grape cultivars (Vitis vinifera). Bahçe 2020, 49, 43–49. [Google Scholar]
- Biasi, R.; Brunori, E.; Vanino, S.; Bernardini, A.; Catalani, A.; Farina, R.; Bruno, A.; Chilosi, G. Soil–Plant Interaction Mediated by Indigenous AMF in Grafted and Own-Rooted Grapevines under Field Conditions. Agriculture 2023, 13, 1051. [Google Scholar] [CrossRef]
- Silva, M.J.R.; Paiva, A.P.M.; de Souza, J.F.; da Padilha, C.V.S.; Basílio, L.S.P.; dos Lima, M.S.; Pereira, G.E.; Corrêa, L.C.; Vianello, F.; Lima, G.P.P.; et al. Phytochemical profile of Brazilian grapes (Vitis labrusca and hybrids) grown on different rootstocks. PLoS ONE 2022, 17, e0275489. [Google Scholar] [CrossRef]
| Flowering | ||||
|---|---|---|---|---|
| Variables | Trellis System | Rootstocks | CV (%) | |
| ‘IAC 766 Campinas’ | ‘106-8 Mgt’ | |||
| Fv/Fm | Low | 0.87 ± 0.002 aB | 0.91 ± 0.005 aA | 0.38 |
| High | 0.82 ± 0.003 bB | 0.91 ± 0.004 aA | ||
| qP | Low | 0.43 ± 0.01 bB | 0.50 ± 0.004 aA | 3.19 |
| High | 0.62 ± 0.02 aA | 0.52 ± 0.016 aB | ||
| NPQ | Low | 3.10 ± 0.13 aB | 3.24 ± 0.19 aA | 5.68 |
| High | 2.31 ± 0.13 bB | 2.94 ± 0.09 bA | ||
| ETR (μmol m−2s−1 electrons) | Low | 101.70 ± 3.86 bA | 106.72 ± 5.46 bA | 4.66 |
| High | 147.54 ± 9.37 aA | 117.25 ± 4.06 aB | ||
| gs (mol m−2s−1) | Low | 0.11 ± 0.01 aB | 0.13 ± 0.01 aA | 4.81 |
| High | 0.10 ± 0.001 bB | 0.13 ± 0.001 aA | ||
| E (mmol m−2s−1 water vapor) | Low | 4.15 ± 0.19 aB | 5.93 ± 0.24 aA | 3.22 |
| High | 3.42 ± 0.10 bB | 5.79 ± 0.05 aA | ||
| WUE (μmol CO2 (mmol H2O)−1) | Low | 7.40 ± 0.44 bA | 5.94 ± 0.28 aB | 2.03 |
| High | 7.83 ± 0.29 aA | 6.03 ± 0.28 aB | ||
| A/Ci | Low | 0.21 ± 0.01 aB | 0.29 ± 0.01 bA | 3.65 |
| High | 0.18 ± 0.01 bB | 0.32 ± 0.02 aA | ||
| Chl a (mg 100 g−1 leaves) | Low | 29.75 ± 0.79 aA | 25.68 ± 2.68 bB | 5.99 |
| High | 28.91 ± 1.74 aB | 38.83 ± 1.97 aA | ||
| Chl b (mg 100 g−1 leaves) | Low | 13.07 ± 0.21 aA | 11.43 ± 0.92 bB | 5.18 |
| High | 12.97 ± 1.29 aB | 26.55 ± 0.50 aA | ||
| Chl total (mg 100 g−1 leaves) | Low | 42.82 ± 0.81 aA | 37.12 ± 3.59 bB | 5.39 |
| High | 41.88 ± 2.78 aB | 65.39 ± 2.44 aA | ||
| SOD (mg U−1 protein) | Low | 2793.95 ± 55.74 bA | 2858.31 ± 48.46 aA | 10.30 |
| High | 3526.43 ± 52.66 aA | 2577.19 ± 50.38 aB | ||
| Berry Ripening | ||||
| Variables | Trellis System | Rootstocks | CV (%) | |
| ‘IAC 766 Campinas’ | ‘106-8 Mgt’ | |||
| Chl a (mg 100 g−1 leaves) | Low | 29.15 ± 3.91 bB | 42.47 ± 0.85 bA | 4.74 |
| High | 48.44 ± 0.67 aA | 50.54 ± 0.30 aA | ||
| Chl total (mg 100 g−1 leaves) | Low | 43.50 ± 5.32 bB | 61.32 ± 1.25 bA | 6.04 |
| High | 69.03 ± 1.24 aA | 74.94 ± 3.75 aA | ||
| POD (µmol mg−1 min−1 protein) | Low | 19.46 ± 3.03 bB | 23.91 ± 1.02 aA | 7.68 |
| High | 24.68 ± 1.56 aA | 18.74 ± 0.71 bB | ||
| SOD (mg U−1 protein) | Low | 4036.05 ± 54.17 aA | 3824.72 ± 56.26 aA | 3.74 |
| High | 4183.20 ± 50.14 aA | 3499.31 ± 55.22 bB | ||
| CAT (µg mKat−1 protein) | Low | 18.78 ± 1.54 aB | 30.01 ± 3.37 aA | 10.64 |
| High | 21.68 ± 1.36 aA | 11.01 ± 0.71 bB | ||
| Flowering | |||||
|---|---|---|---|---|---|
| Variables | Trellis System | Rootstocks | CV (%) | ||
| Low | High | ‘IAC 766 Campinas’ | ‘106-8 Mgt’ | ||
| A (μmol m−2s−1 CO2) | 29.59 ± 4.04 a | 27.47 ± 3.71 b | 25.12 ± 1.60 b | 31.94 ± 1.86 a | 5.26 |
| Ci (μmol mol−1 CO2) | 117.06 ± 18.09 a | 117.15 ± 18.69 a | 133.87 ± 1.67 a | 100.35 ± 5.61 b | 2.76 |
| SPAD | 39.06 ± 2.29 b | 40.34 ± 3.32 a | 42.05 ± 1.39 a | 37.36 ± 1.66 b | 2.27 |
| POD (µmol mg−1 min−1 protein) | 27.96 ± 2.47 a | 27.19 ± 3.97 a | 29.34 ± 3.27 a | 25.82 ± 2.10 b | 10.69 |
| CAT (µg mKat−1 protein) | 4.74 ± 0.62 a | 3.98 ± 0.49 b | 4.43 ± 0.72 a | 4.29 ± 0.66 a | 11.49 |
| Berry Ripening | |||||
| Variables | Trellis System | Rootstocks | CV (%) | ||
| Low | High | ‘IAC 766 Campinas’ | ‘106-8 Mgt’ | ||
| SPAD | 40.92 ± 1.65 a | 41.98 ± 2.02 a | 42.56 ± 0.93 a | 40.35 ± 1.94 b | 3.86 |
| Chl b (mg 100 g−1 leaves) | 16.59 ± 2.87 b | 22.50 ± 3.12 a | 21.62 ± 3.90 a | 17.47 ± 3.58 b | 12.71 |
| Variables | Trellis System | Rootstocks | CV (%) | ||
|---|---|---|---|---|---|
| Low | High | ‘IAC 766 Campinas’ | ‘106-8 Mgt’ | ||
| Number of clusters per plant | 18.25 ± 3.78 a | 19.61 ± 3.01 a | 20.07 ± 3.67 a | 17.79 ± 2.85 b | 17.66 |
| Yield (kg per plant) | 2.10 ± 0.82 a | 2.27 ± 0.71 a | 2.62 ± 0.74 a | 1.76 ± 0.50 b | 24.13 |
| Productivity (t ha⁻1) | 8.42 ± 3.26 a | 9.11 ± 2.86 a | 10.49 ± 2.98 a | 7.04 ± 1.98 b | 24.14 |
| Soluble solids (°Brix) | 15.94 ± 0.65 b | 16.88 ± 0.71 a | 16.28 ± 0.94 a | 16.54 ± 0.69 a | 4.09 |
| pH | 3.56 ± 0.10 a | 3.59 ± 0.05 a | 3.57 ± 0.08 a | 3.58 ± 0.08 a | 2.15 |
| Titratable acidity (g/L tartaric acid) | 6.1 ± 0.11 a | 4.2 ± 0.11 b | 5.8 ± 0.21 a | 5.7 ± 0.14 a | 2.98 |
| Reducing sugars (%) | 12.86 ± 1.70 b | 14.60 ± 1.16 a | 11.41 ± 1.06 a | 11.19 ± 0.76 a | 3.04 |
| Flowering | |||||
|---|---|---|---|---|---|
| Variables | Trellis System | Rootstocks | CV (%) | ||
| Low | High | ‘IAC 766 Campinas’ | ‘106-8 Mgt’ | ||
| Fv/Fm | 0.91 ± 0.01 b | 0.93 ± 0.01 a | 0.93 ± 0.01 a | 0.91 ± 0.01 b | 0.43 |
| qP | 0.55 ± 0.07 a | 0.46 ± 0.07 b | 0.57 ± 0.06 a | 0.44 ± 0.05 b | 3.79 |
| WUE (μmol CO2 (mmol H2O)−1) | 12.31 ± 1.80 a | 4.93 ± 1.98 b | 10.35 ± 3.86 a | 6.89 ± 4.07 b | 5.78 |
| Berry Ripening | |||||
| Variables | Trellis System | Rootstocks | CV (%) | ||
| Low | High | ‘IAC 766 Campinas’ | ‘106-8 Mgt’ | ||
| SPAD | 45.30 ± 2.74 a | 45.93 ± 2.27 a | 47.30 ± 1.45 a | 43.93 ± 2.09 b | 2.13 |
| Chl a (mg 100 g−1 leaves) | 40.07 ± 3.29 a | 37.02 ± 2.13 b | 39.78 ± 3.15 a | 37.32 ± 2.71 a | 6.17 |
| Chl b (mg 100 g−1 leaves) | 17.47 ± 2.77 a | 17.49 ± 0.83 a | 17.26 ± 1.55 a | 17.70 ± 2.42 a | 10.74 |
| Chl total (mg 100 g−1 leaves) | 57.54 ± 4.46 a | 54.52 ± 2.45 a | 57.04 ± 2.29 a | 55.02 ± 4.85 a | 6.51 |
| POD (µmol mg−1 min−1 protein) | 17.12 ± 1.98 a | 15.38 ± 1.34 b | 17.42 ± 1.70 a | 15.08 ± 1.20 b | 6.56 |
| Flowering | ||||
|---|---|---|---|---|
| Variables | Trellis System | Rootstocks | CV (%) | |
| ‘IAC 766 Campinas’ | ‘106-8 Mgt’ | |||
| NPQ | Low | 3.42 ± 0.03 aB | 4.24 ± 0.12 aA | 3.15 |
| High | 3.43 ± 0.15 aA | 2.39 ± 0.09 bB | ||
| ETR (μmol m−2s−1 electrons) | Low | 100.63 ± 3.54 aA | 65.03 ± 1.87 bB | 5.01 |
| High | 87.12 ± 5.44 bB | 103.57 ± 4.58 aA | ||
| gs (mol m−2s−1) | Low | 0.03 ± 0.001 bA | 0.04 ± 0.001 bA | 5.34 |
| High | 0.10 ± 0.01 aB | 0.31 ± 0.01 aA | ||
| E (mmol m−2s−1 water vapor) | Low | 1.67 ± 0.02 bA | 1.93 ± 0.13 bA | 3.49 |
| High | 4.25 ± 0.13 aB | 10.90 ± 0.25 aA | ||
| A/Ci | Low | 0.18 ± 0.01 aA | 0.10 ± 0.001 bB | 3.17 |
| High | 0.18 ± 0.001 aA | 0.17 ± 0.001 aA | ||
| A (μmol m−2s−1 CO2 | Low | 24.69 ± 1.07 bA | 22.44 ± 0.57 bB | 4.68 |
| High | 26.83 ± 1.05 aB | 35.13 ± 1.70 aA | ||
| Ci (μmol mol−1 CO2) | Low | 139.33 ± 2.96 aB | 208.17 ± 11.49 aA | 4.41 |
| High | 150.74 ± 4.32 aB | 177.86 ± 4.54 bA | ||
| SPAD | Low | 36.67 ± 1.89 bA | 35.95 ± 1.03 aA | 3.20 |
| High | 42.34 ± 0.57 aA | 34.89 ± 0.71 aB | ||
| Chl a (mg 100 g−1 leaves) | Low | 46.22 ± 3.92 aA | 33.55 ± 1.07 bB | 5.23 |
| High | 41.16 ± 1.96 bB | 48.10 ± 1.68 aA | ||
| Chl b (mg 100 g−1 leaves) | Low | 21.94 ± 1.38 aA | 16.62 ± 1.02 bB | 6.80 |
| High | 17.37 ± 1.50 bB | 21.92 ± 1.58 aA | ||
| Chl total (mg 100 g−1 leaves) | Low | 68.16 ± 4.96 aA | 50.18 ± 1.83 bB | 4.63 |
| High | 58.54 ± 3.40 bB | 70.02 ± 2.05 aA | ||
| POD (µmol mg−1 min−1 protein) | Low | 33.38 ± 4.78 aA | 16.44 ± 1.19 bB | 8.23 |
| High | 27.69 ± 1.48 bA | 24.33 ± 0.75 aB | ||
| SOD (mg U−1 protein) | Low | 5040.23 ± 57.56 bA | 4824.10 ± 57.27 aA | 11.62 |
| High | 7193.32 ± 38.84 aA | 4769.08 ± 49.44 aB | ||
| CAT (µg mKat−1 protein) | Low | 129.68 ± 1.93 aA | 47.04 ± 3.33 aB | 5.21 |
| High | 52.01 ± 5.11 bA | 46.06 ± 1.92 aB | ||
| Berry Ripening | ||||
| Variables | Trellis System | Rootstocks | CV (%) | |
| ‘IAC 766 Campinas’ | ‘106-8 Mgt’ | |||
| SOD (mg U−1 protein) | Low | 3533.86 ± 55.18 aA | 2900.90 ± 60.04 aB | 4.69 |
| High | 2741.31 ± 57.92 bA | 2698.74 ± 60.09 aA | ||
| CAT (µg mKat−1 protein) | Low | 36.80 ± 2.91 aA | 12.95 ± 0.63 bB | 4.75 |
| High | 10.98 ± 1.15 bB | 43.29 ± 1.50 aA | ||
| Variables | Trellis System | Rootstocks | CV (%) | ||
|---|---|---|---|---|---|
| Low | High | ‘IAC 766 Campinas’ | ‘106-8 Mgt’ | ||
| Number of clusters per plant | 15.15 ± 4.88 b | 21.26 ± 5.20 a | 15.05 ± 5.05 b | 21.37 ± 4.89 a | 19.14 |
| Yield (kg per plant) | 2.21 ± 0.97 b | 2.80 ± 1.00 a | 2.00 ± 0.99 b | 3.01 ± 0.78 a | 24.57 |
| Productivity (t ha⁻1) | 8.86 ± 3.90 b | 11.21 ± 3.99 a | 8.01 ± 3.94 b | 12.06 ± 3.13 a | 24.57 |
| Soluble solids (°Brix) | 16.83 ± 1.18 b | 17.34 ± 1.14 a | 17.04 ± 1.01 a | 16.93 ± 1.24 a | 3.94 |
| pH | 3.52 ± 0.08 a | 3.65 ± 0.14 a | 3.55 ± 0.06 a | 3.53 ± 0.09 a | 2.01 |
| Titratable acidity (g/L tartaric acid) | 8.6 ± 0.15 a | 8.7 ± 0.11 a | 8.9 ± 0.15 a | 8.4 ± 0.10 a | 4.89 |
| Reducing sugars (%) | 10.40 ± 1.88 b | 13.17 ± 2.84 a | 12.44 ± 3.07 a | 11.93 ± 2.23 a | 3.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domingues Neto, F.J.; Tecchio, M.A.; Pimentel Junior, A.; Monteiro, H.S.A.; Moura-Furlan, M.F.; Hernandes, J.L.; Ono, E.O.; Lima, G.P.P.; Rodrigues, J.D. Interactive Effects of Rootstock and Training System on Photosynthesis, Biochemical Responses, and Yield in Vitis labrusca Under Subtropical Climate Conditions. Horticulturae 2025, 11, 589. https://doi.org/10.3390/horticulturae11060589
Domingues Neto FJ, Tecchio MA, Pimentel Junior A, Monteiro HSA, Moura-Furlan MF, Hernandes JL, Ono EO, Lima GPP, Rodrigues JD. Interactive Effects of Rootstock and Training System on Photosynthesis, Biochemical Responses, and Yield in Vitis labrusca Under Subtropical Climate Conditions. Horticulturae. 2025; 11(6):589. https://doi.org/10.3390/horticulturae11060589
Chicago/Turabian StyleDomingues Neto, Francisco José, Marco Antonio Tecchio, Adilson Pimentel Junior, Harleson Sidney Almeida Monteiro, Mara Fernandes Moura-Furlan, José Luiz Hernandes, Elizabeth Orika Ono, Giuseppina Pace Pereira Lima, and João Domingos Rodrigues. 2025. "Interactive Effects of Rootstock and Training System on Photosynthesis, Biochemical Responses, and Yield in Vitis labrusca Under Subtropical Climate Conditions" Horticulturae 11, no. 6: 589. https://doi.org/10.3390/horticulturae11060589
APA StyleDomingues Neto, F. J., Tecchio, M. A., Pimentel Junior, A., Monteiro, H. S. A., Moura-Furlan, M. F., Hernandes, J. L., Ono, E. O., Lima, G. P. P., & Rodrigues, J. D. (2025). Interactive Effects of Rootstock and Training System on Photosynthesis, Biochemical Responses, and Yield in Vitis labrusca Under Subtropical Climate Conditions. Horticulturae, 11(6), 589. https://doi.org/10.3390/horticulturae11060589







