Flooding Tolerance and Recovery Capacity of Carya illinoinensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
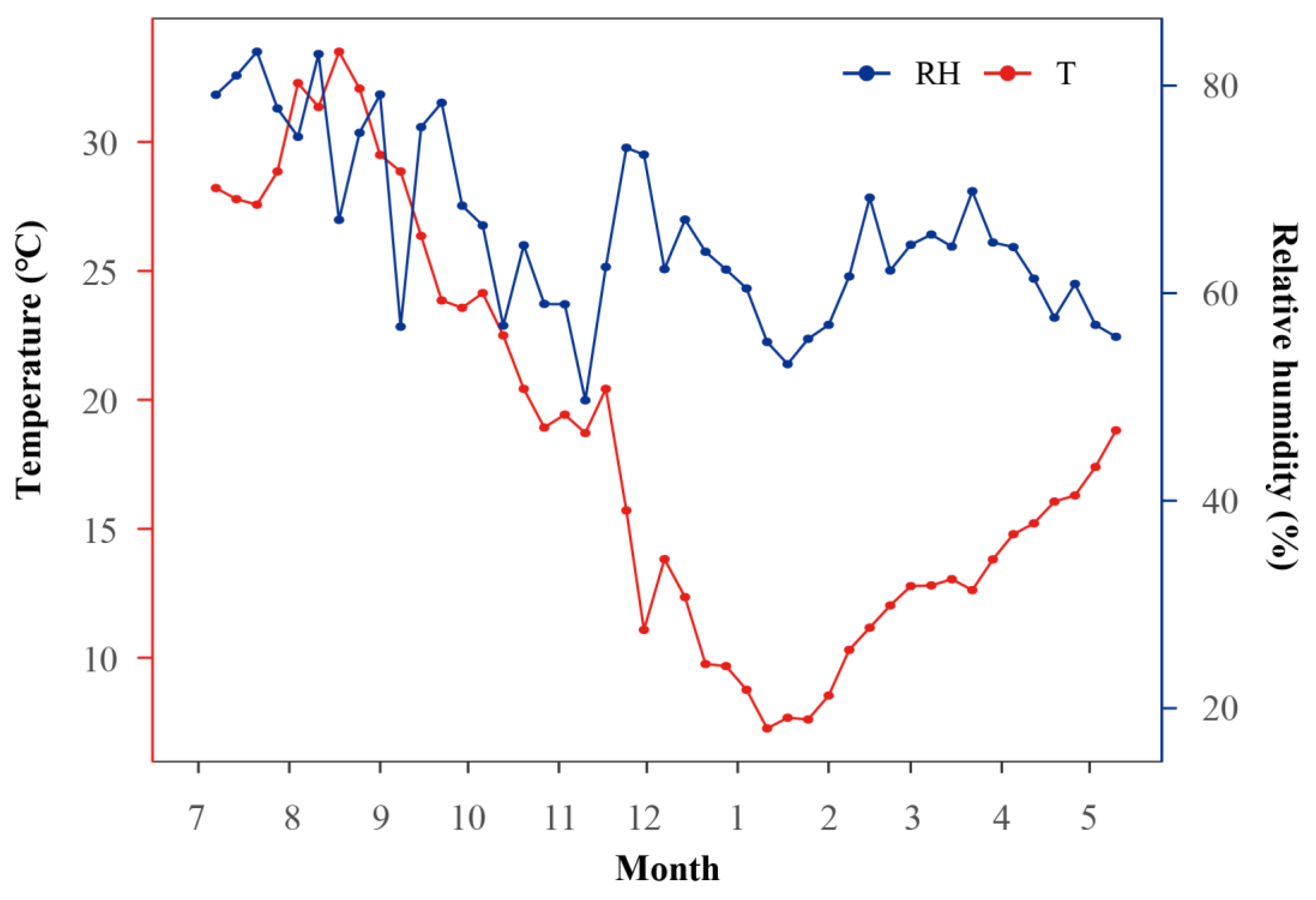
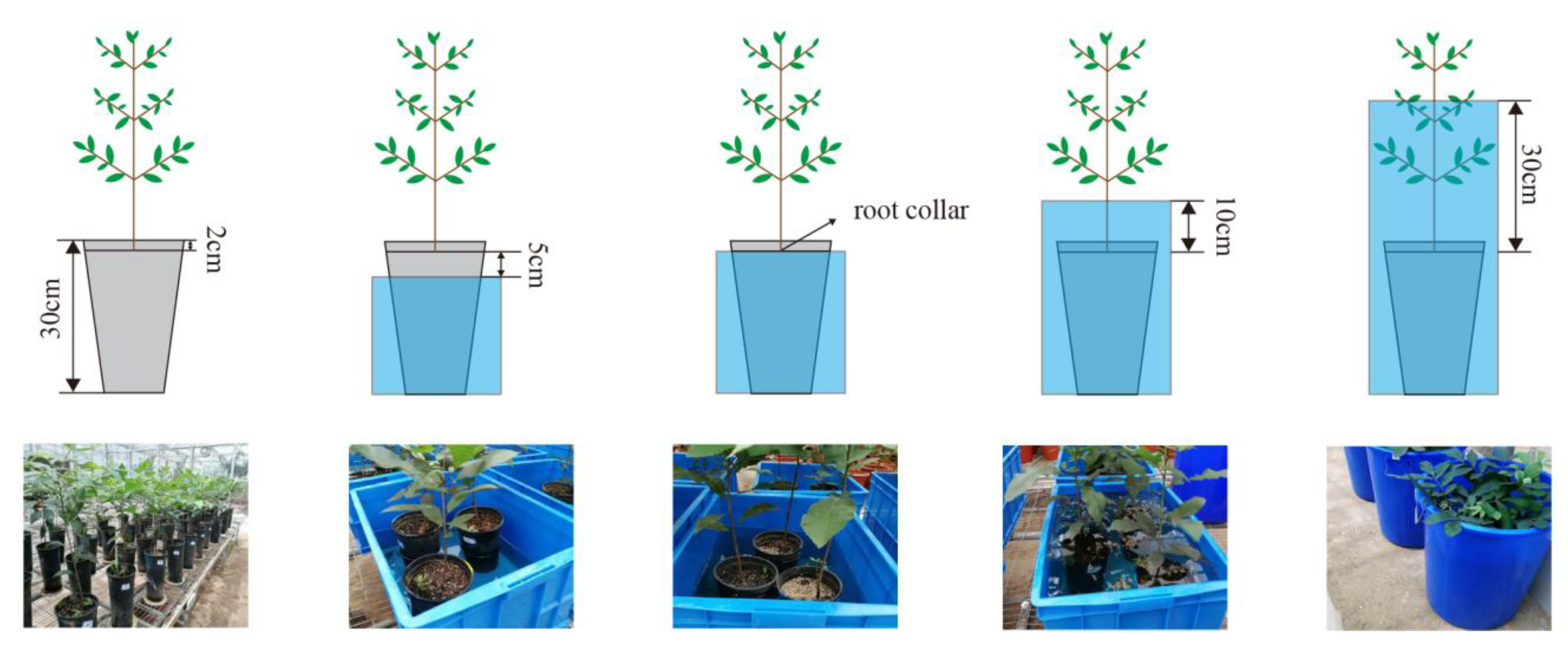
2.2. Experimental Design
2.3. Determination of Growth Indicators
2.4. Determination of Physiological and Biochemical Indicators
2.5. Statistical Analysis
3. Results
3.1. Growth Indicators of C. illinoinensis Under Different Flooding Treatments
3.1.1. The Growth Rate of the Height and Ground Diameter
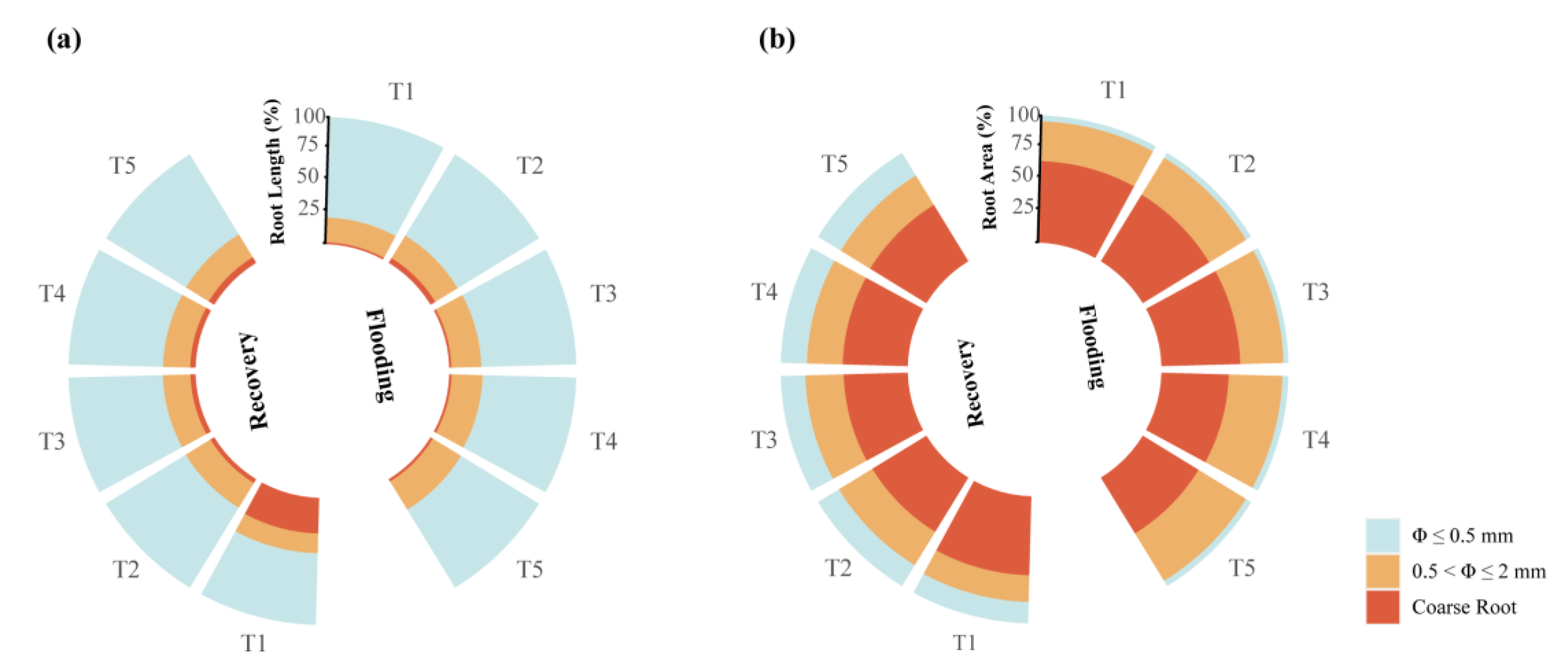
3.1.2. Root Length and Root Surface Area
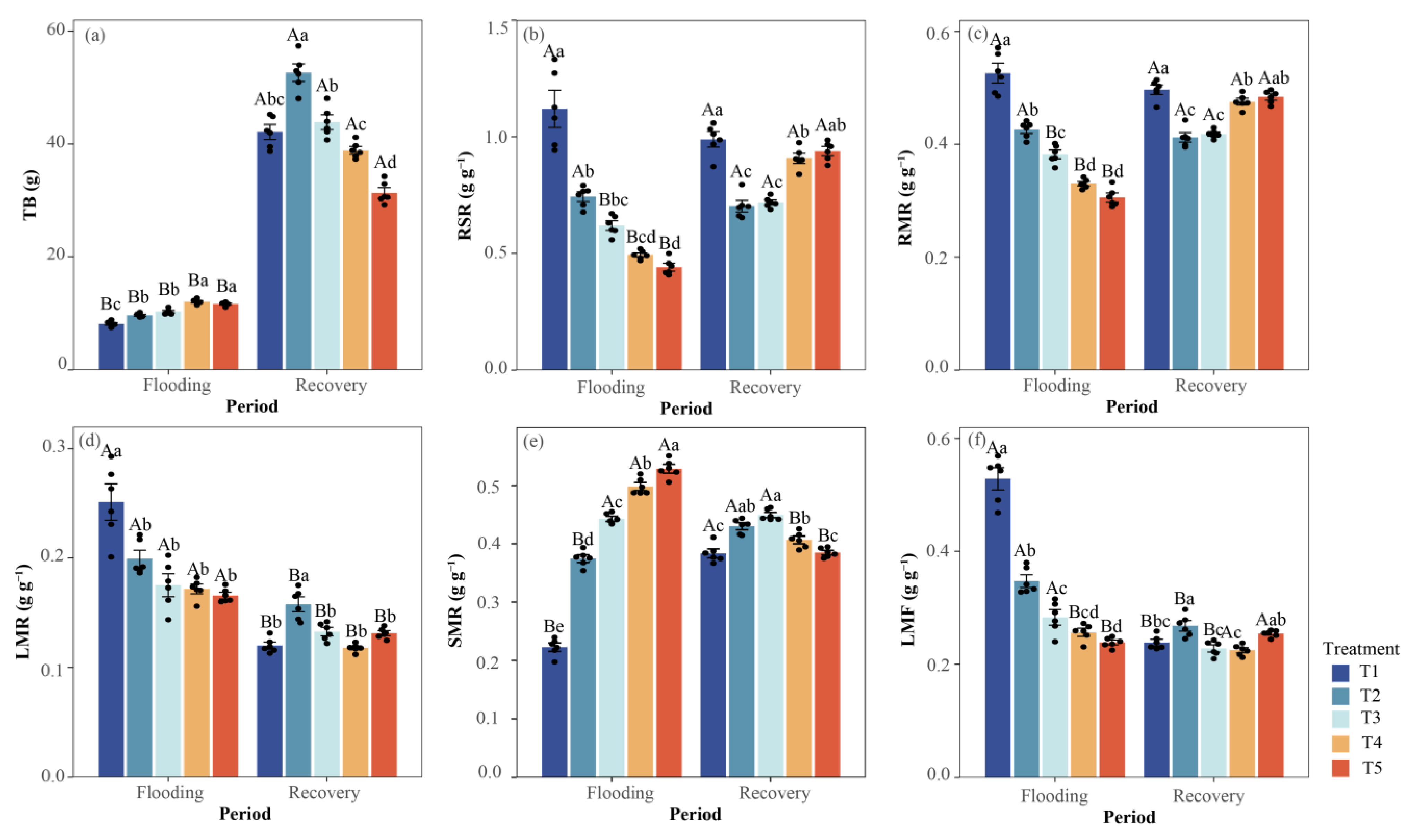
3.1.3. Dry Mass Accumulation
3.1.4. Survival Rates
3.2. Physiological Indicators of C. illinoinensis Under Different Flooding Treatments
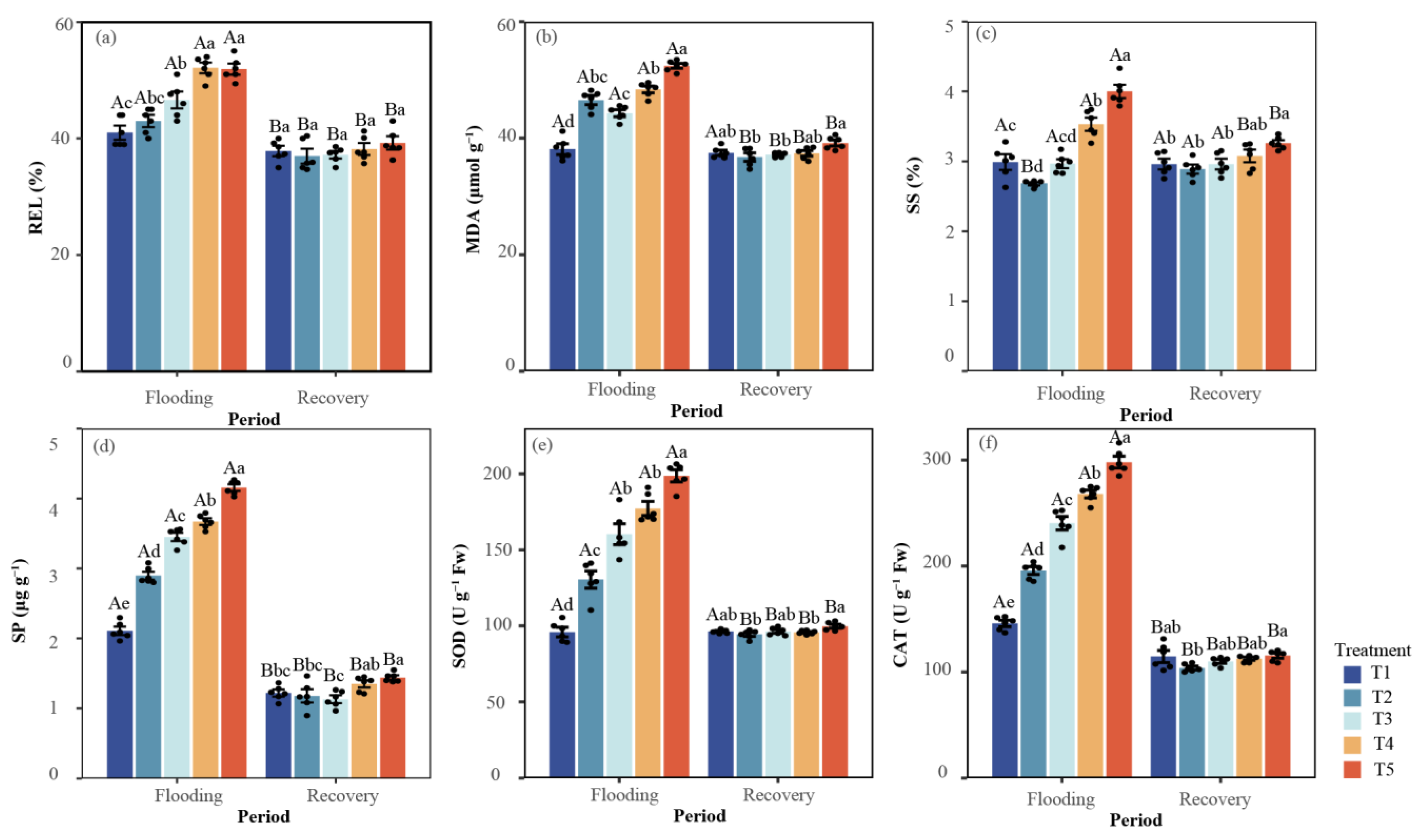
3.2.1. Relative Electrolytic Leakage and Malondialdehyde
3.2.2. Soluble Sugar and Soluble Protein
3.2.3. Superoxide Dismutase and Catalase
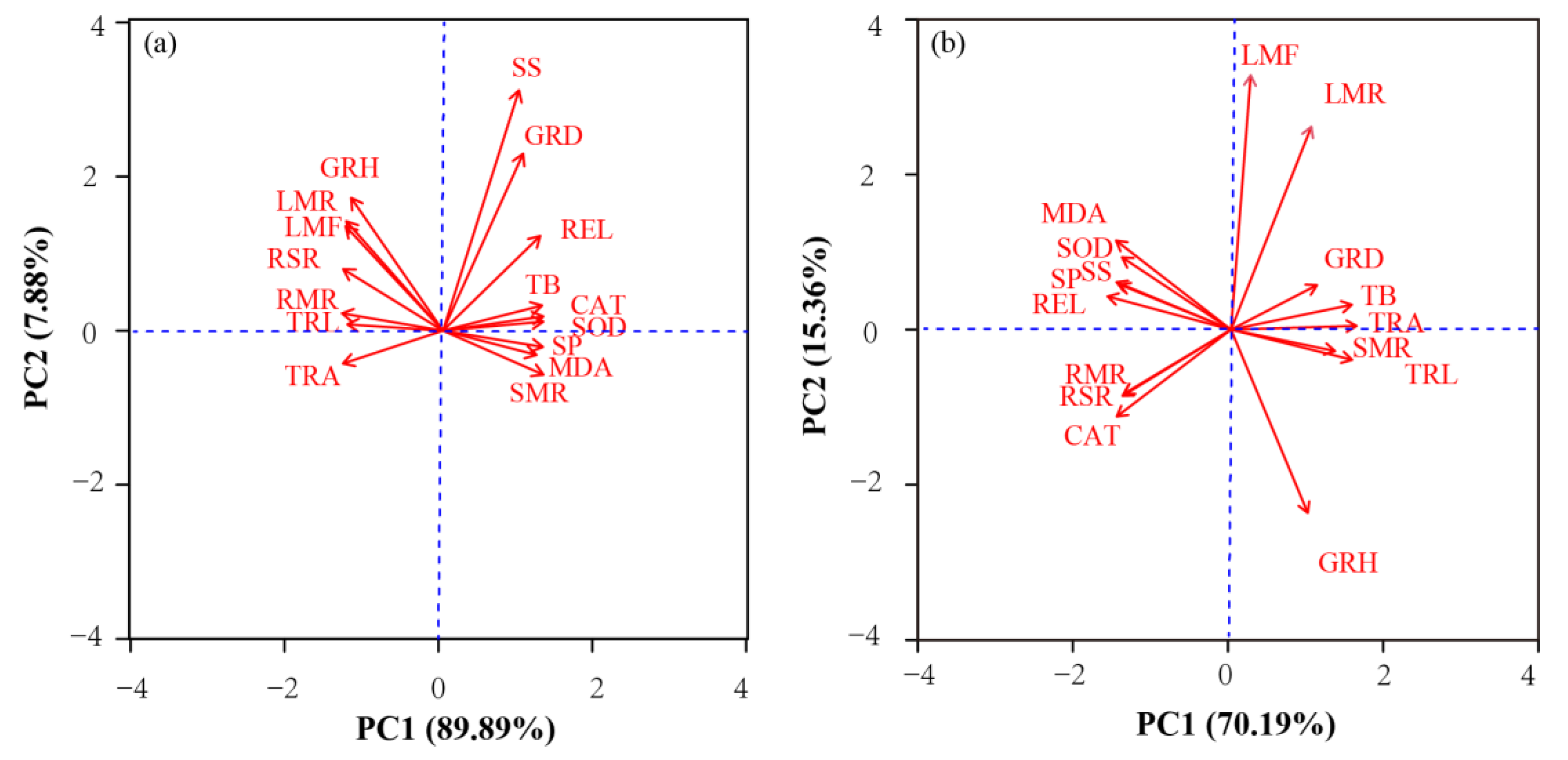
3.3. PCA Analysis
4. Discussion
4.1. Response of C. illinoinensis During Flooding Period
4.2. Response of C. illinoinensis During Recovery Period
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Knapp, A.K.; Beier, C.; Briske, D.D.; Classen, A.T.; Luo, Y.; Reichstein, M.; Smith, M.D.; Smith, S.D.; Bell, J.E.; Fay, P.A.; et al. Consequences of More Extreme Precipitation Regimes for Terrestrial Ecosystems. Bioscience 2008, 58, 811–821. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L. Flooding Stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Lopez, O.R.; Kursar, T.A. Does flood tolerance explain tree species distribution in tropical seasonally flooded habitats? Oecologia 2003, 136, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Renziehausen, T.; Frings, S.; Schmidt-Schippers, R. ‘Against all floods’: Plant adaptation to flooding stress and combined abiotic stresses. Plant J. 2024, 117, 1836–1855. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Z.; Shen, Z.J.; Luo, M.R.; Liu, Y.L.; Wei, M.Y.; Wang, W.H.; Qin, Y.Y.; Gao, C.H.; Li, K.K.; et al. Physiological and proteomic responses of mangrove plant seedlings to simulated periodical inundation. Plant Soil 2020, 450, 231–254. [Google Scholar] [CrossRef]
- Zeng, R.; Chen, T.; Wang, X.; Cao, J.; Li, X.; Xu, X.; Chen, L.; Xia, Q.; Dong, Y.; Huang, L.; et al. Physiological and Expressional Regulation on Photosynthesis, Starch and Sucrose Metabolism Response to Waterlogging Stress in Peanut. Front. Plant Sci. 2021, 12, 601771. [Google Scholar]
- Zhao, T.; Li, Q.; Pan, X.J.; Hua, X.F.; Zhang, W.E. Adaptation mechanism of terrestrial plants to waterlogging stress. J. Plant Physiol. 2021, 57, 2091–2103. (In Chinese) [Google Scholar]
- Schindler, M.; Jungmann, L.; Donath, T.W.; Ludewig, K. Influence of flooding duration and aeration on saplings of ten hardwood floodplain forest species. PLoS ONE 2020, 15, e0234936. [Google Scholar] [CrossRef]
- Daniel, K.; Hartman, S. How plant roots respond to waterlogging. J. Exp. Bot. 2024, 75, 511–525. [Google Scholar] [CrossRef]
- Markus-Michalczyk, H.; Hanelt, D.; Jensen, K. Effects of Tidal Flooding on Juvenile Willows. Estuaries Coasts 2016, 39, 397–405. [Google Scholar] [CrossRef]
- Sun, L.; Li, X.; Wang, X.; Xiang, L.; Yang, J.; Min, Q.; Chen, G.; Chen, F.; Huang, C.; Wang, G. Growth and respiratory metabolic adaptation strategies of riparian plant Distylium chinense to submergence by the field study and controlled experiments. Plant Physiol. Biochem. 2020, 157, 1–12. [Google Scholar] [CrossRef]
- Zeng, R.; Cao, J.; Li, X.; Wang, X.; Wang, Y.; Yao, S.; Gao, Y.; Hu, J.; Luo, M.; Zhang, L.; et al. Waterlogging tolerance and recovery capability screening in peanut: A comparative analysis of waterlogging effects on physiological traits and yield. PeerJ 2022, 10, e12741. [Google Scholar] [CrossRef]
- Sanchez, F.W.; Crane, J.H.; Bayabil, H.; Sarkhosh, A.; Shahid, M.A.; Schaffer, B. Physiological and biochemical responses of the achachairu tree (Garcinia humilis) to prolonged flooding. Sci. Hortic. 2024, 337, 113573. [Google Scholar] [CrossRef]
- Voesenek, L.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Noguchi, K. Tolerant mechanisms to O2 deficiency under submergence conditions in plants. J. Plant Res. 2020, 133, 343–371. [Google Scholar] [CrossRef] [PubMed]
- Colmer, T.D.; Voesenek, L.A.C.J. Flooding tolerance: Suites of plant traits in variable environments. Funct. Plant Biol. 2009, 36, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Mozo, I.; Rodríguez, M.E.; Monteoliva, S.; Luquez, V.M.C. Floodwater Depth Causes Different Physiological Responses During Post-flooding in Willows. Front. Plant Sci. 2021, 12, 575090. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, G.; Chao, W.; Xu, F.; Sun, X.; Chen, Z. Physiological Responses and Tolerance Evaluation of Five Poplar Varieties to Waterlogging. Not. Bot. Horti Agrobot. 2019, 47, 658–667. [Google Scholar] [CrossRef]
- Samir, S.; Poliana, L.; Lucas, H.; Gonçalves, R.L.M.; Caroline, M.; Luis, C.R.; Luisa, S.T. Assessing the role of light in flooding tolerance for tree species recommendation in the restoration of riparian subtropical forests. Trees 2022, 37, 403–415. [Google Scholar] [CrossRef]
- Briceno-Contreras, E.A.; Moreno-Resendez, A.; Valenzuela-Nunez, L.M.; Garcia-De la Pena, C.; Esparza-Rivera, J.R.; Rodriguez-Martinez, R.; Molina-Ochoa, J. Influence of temperature and irradiation on starch concentration in Carya illinoinensis K. Koch varieties Wichita and Western. Rev. Chapingo Ser. Cie. 2019, 25, 305–314. [Google Scholar]
- Marco, R.; Goldschmidt, R.J.Z.; Herter, F.G.; Martins, C.R.; Mello-Farias, P.C.; Uberti, A. The irrigation effect on nuts’ growth and yield of Carya illinoinensis. An. Acad. Bras. Cienc. 2021, 93, e20181351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yong-Rong, L.I.; Peng, F.R. Cultivation adaptability and evaluation of Mahan in Carya illinoinensis. Non-Wood Forest Res. 2013, 31, 176–180. (In Chinese) [Google Scholar]
- Zhou, F.; Ma, J.; Hu, Y.J.; Yan, L.; Chen, B. Study on characteristics and causes of water-induced disaster in Yangtze Delta. J. China Inst. Water Resour. Hydropower Res. 2016, 14, 81–89. (In Chinese) [Google Scholar]
- Sun, F.; Zhao, J.M.; Yao, X.H.; Zhang, F.H.; Ma, S.L.; Zhang, L. Effects of water stress on physiological characteristics of Carya illinoensis—An economical plant for soil and water conservation. J. Southwest Univ. Nat. Sci. Ed. 2014, 36, 1–7. (In Chinese) [Google Scholar]
- Jiao, Z.Y.; Luo, Q.; Zhang, J.L.; Hao, R.M.; Xu, W.G.; Pan, W.M. The comparison of waterlogging resistance of 10 trees. J. Jiangsu For. Sci. Technol. 2007, 34, 15–18. (In Chinese) [Google Scholar]
- Miao, T.T.; Cao, Z.H.; Liu, J.L.; Sun, H.; Wu, Z.N. Effects of water stress on seedling growth and chlorophyll fluorescence characteristics of two Carya illinoensis varieties. Jiangsu Agric. Sci. 2022, 50, 138–146. (In Chinese) [Google Scholar]
- Huang, X.; Shi, H.; Hu, Z.; Liu, A.; Amombo, E.; Chen, L.; Fu, J. ABA Is Involved in Regulation of Cold Stress Response in Bermudagrass. Front. Plant Sci. 2017, 8, 1613. [Google Scholar] [CrossRef] [PubMed]
- Wassie, M.; Zhang, W.; Zhang, Q.; Ji, K.; Chen, L. Effect of Heat Stress on Growth and Physiological Traits of Alfalfa (Medicago sativa L.) and a Comprehensive Evaluation for Heat Tolerance. Agronomy 2019, 9, 597. [Google Scholar] [CrossRef]
- Moustakas, M.; Sperdouli, I.; Kouna, T.; Antonopoulou, C.I.; Therios, I. Exogenous proline induces soluble sugar accumulation and alleviates drought stress effects on photosystem II functioning of Arabidopsis thaliana leaves. Plant Growth Regul. 2011, 65, 315–325. [Google Scholar] [CrossRef]
- He, Y.X.; Wang, Q.; Xu, Y.P.; Li, Z.Y.; Yuan, J.; Lu, M.; Lin, Z.X. Climate change increased the compound extreme precipitation-flood events in a representative watershed of the Yangtze River Delta, China. Stoch. Env. Res. Risk A 2022, 36, 3803–3818. [Google Scholar] [CrossRef]
- Yang, P.; Xia, J.; Luo, X.G.; Meng, L.S.; Zhang, S.Q.; Cai, W.; Wang, W.Y. Impacts of climate change-related flood events in the Yangtze River Basin based on multi-source data. Atmos. Res. 2021, 263, 105819. [Google Scholar] [CrossRef]
- Kabrick, J.M.; Dey, D.C.; Van Sambeek, J.W.; Coggeshall, M.V.; Jacobs, D.F. Quantifying flooding effects on hardwood seedling survival and growth for bottomland restoration. New Forest. 2012, 43, 695–710. [Google Scholar] [CrossRef]
- Liu, Y.; Sheng, Z.P. Soil Moisture Status in an Irrigated Pecan Field. J. Irrig. Drain. Eng. 2013, 139, 26–40. (In Chinese) [Google Scholar] [CrossRef]
- Phukan, U.J.; Mishra, S.; Shukla, R.K. Waterlogging and submergence stress: Affects and acclimation. Crit. Rev. Biotechnol. 2016, 36, 956–966. [Google Scholar] [CrossRef]
- Argus, R.E.; Colmer, T.D.; Grierson, P.F. Early physiological flood tolerance is followed by slow post-flooding root recovery in the dryland riparian tree subsp. Plant Cell Environ. 2015, 38, 1189–1199. [Google Scholar] [CrossRef]
- Li, J.J.; Chen, Y.H.; Zhou, Z.H.; Wang, Y.J.; Yao, X.; Guo, L. Research progress on mechanisms of plant adaptation to flooding stress. Plant Sci. J. 2023, 41, 835–846. (In Chinese) [Google Scholar]
- Fukao, T.; Barrera-Figueroa, B.E.; Juntawong, P.; Pena-Castro, J.M. Submergence and Waterlogging Stress in Plants: A Review Highlighting Research Opportunities and Understudied Aspects. Front. Plant Sci. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Huang, S.R.; Yu, Y.P.; Huang, Q.W.; Zhang, J.; Tan, X.H. Growth and Physiological Response of Barringtonia acutangula to Freshwater Flooding Stress. J. Southwest Univ. Nat. Sci. Ed. 2019, 39, 18–25. (In Chinese) [Google Scholar]
- Han, L.W.; Shi, Q.; Xuan, L.; Yin, Y.L.; Hua, J.F. Growth traits of Taxodium ‘Zhongshanshan’ and T. distichum Under Different Waterlogging Stress. J. Zhejiang For. Sci. Technol. 2017, 37, 1–8. (In Chinese) [Google Scholar]
- Fan, F.F.; Yuan, W.G.; Li, T.T.; Wu, C.P.; Zhang, J.; Jiao, J.J. Effect of Water Logging and Drainage on Growth and Physiological Properties of Zelkova serrata. J. Zhejiang For. Sci. Technol. 2018, 38, 62–68. (In Chinese) [Google Scholar]
- Sun, H.; Wu, Z.N.; Liu, J.L.; Miao, T.T.; Cao, Z.H. Effects of Water Stress on Growth and Physiological Properties of Four Poplar Varieties. J. West. China For. Sci. 2020, 49, 62–67. (In Chinese) [Google Scholar]
- Palta, J.A.; Ganjeali, A.; Turner, N.C.; Siddique, K.H.M. Effects of transient subsurface waterlogging on root growth, plant biomass and yield of chickpea. Agric. Water Manag. 2010, 97, 1469–1476. [Google Scholar] [CrossRef]
- Patel, M.K.; Pandey, S.; Burritt, D.J.; Tran, L.S.P. Plant responses to low-oxygen stress: Interplay between ROS and NO signaling pathways. Environ. Exp. Bot. 2019, 161, 134–142. [Google Scholar] [CrossRef]
- Yu, B.; Zhao, C.Y.; Li, J.; Li, J.Y.; Peng, G. Morphological, physiological, and biochemical responses of Populus euphratica to soil flooding. Photosynthetica 2015, 53, 110–117. [Google Scholar] [CrossRef]
- Fan, L.H.; Li, P.J.; Wang, J.; Gao, X.Q.; Zhen, Z.Y.; Wang, H.R.; Xing, W.X.; Zheng, C.W.; Liu, H. Study on waterlogging tolerance of Juglans sigillata. J. Anhui Agric. Univ. 2024, 51, 563–568. [Google Scholar]
- Wang, B.M.; Chen, J.J.; Chen, L.S.; Wang, X.N.; Wang, R.; Ma, L.; Peng, S.F.; Luo, J.; Chen, Y.Z. Combined drought and heat stress in Camellia oleifera cultivars: Leaf characteristics, soluble sugar and protein contents, and Rubisco gene expression. Trees-Struct. Funct. 2015, 29, 1483–1492. [Google Scholar] [CrossRef]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.N.; Wang, L.C. Chilling and Drought Stresses in Crop Plants: Implications, Cross Talk, and Potential Management Opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Azarabadi, S.; Abdollahi, H.; Torabi, M.; Salehi, Z.; Nasiri, J. ROS generation, oxidative burst and dynamic expression profiles of ROS-scavenging enzymes of superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) in response to Erwinia amylovora in pear (Pyrus communis L). Eur. J. Plant Pathol. 2016, 147, 279–294. [Google Scholar] [CrossRef]
- Lan, Z.Q.; Wen, J.H.; Chen, C.Y.; Xie, D.Z.; Zhao, Z.H.; Huang, X.L.; Liao, J.M. Physiological Responses and Tolerance Evaluation of Different Varieties of Carya illinoinensis Seedlings Under Water Logging Stress. Guangxi For. Sci. 2024, 53, 144–151. (In Chinese) [Google Scholar]
- Yan, Z.; Zhang, J.T.; Zhao, P.H.; Yang, S.H.; Ma, Y.T.; Zhu, D. Effects of continuous waterlogging stress on growth, physiology and biochemistry of Populus deltoides seedlings. J. Cent. South. Univ. For. Technol. 2019, 39, 16–23. (In Chinese) [Google Scholar]
- Liu, C.C.; Lan, C.J.; Li, H.; Li, C.J.; Qiu, Y.; Wang, X.L. Effects of Flooding Stress and Recovery on Root and Leaf Cell Membrane Stability at Cherry Tomato Seedlings. J. Irrig. Drain. 2022, 41, 61–70. (In Chinese) [Google Scholar]
- Li, Y.; Shi, L.C.; Yang, J.; Qian, Z.H.; He, Y.X.; Li, M.W. Physiological and transcriptional changes provide insights into the effect of root waterlogging on the aboveground part of Pterocarya stenoptera. Genomics 2021, 113, 2583–2590. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Xuanyuan, X.T.; Zheng, B.T.; Wang, G.P.; Zhai, J.T.; Yu, P.F. Effects of waterlogging stress and restoration on morphological and physiological characteristics of Xanthoceras sorbifolia Bunge seedlings. Non-Wood For. Res. 2024, 42, 77–85. (In Chinese) [Google Scholar]
- Zhao, Y.Q.; Wang, C.Y.; Yang, J.F.; Wang, X.X.; Zhang, B.G.; Li, Z.G.; Zu, C.; Chen, X.A.; Zhang, Y.J.; Tan, M.Y.; et al. Effects of short-term waterlogging stress and stress relief on physiology and yield of Noni. Chin. J. Trop. Crops 2024, 45, 2117–2128. (In Chinese) [Google Scholar]
- Li, X.; Cao, Y.; Xie, Q.Z.; Yao, X.C.; Tang, S.W.; Feng, K.P. Effects of water level change and normal moisture after waterlogging on the growth and physiology of Artemisia selengensis. Chin. J. Ecol. 2022, 41, 1109–1120. (In Chinese) [Google Scholar] [CrossRef]
- Li, M.; López, R.; Venturas, M.; Pita, P.; Gordaliza, G.G.; Gil, L.; Rodríguez-Calcerrada, J. Greater resistance to flooding of seedlings of Ulmus laevis than Ulmus minor is related to the maintenance of a more positive carbon balance. Trees 2015, 29, 835–848. [Google Scholar] [CrossRef]
- Agayev, Y.M.; Zarifi, E. Peculiar Evolution of Saffron (Crocus sativus L.): Prosperity and Decline. Acta Hortic. 2010, 850, 29–33. [Google Scholar] [CrossRef]
- Acevedo, E.; Hsiao, T.C.; Henderson, D.W. Immediate and subsequent growth responses of maize leaves to changes in water status. Plant Physiol. 1971, 48, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.-L.; Nagel, K.A.; Scharr, H.; Zeng, B.; Schurr, U.; Matsubara, S. Recovery dynamics of growth, photosynthesis and carbohydrate accumulation after de-submergence: A comparison between two wetland plants showing escape and quiescence strategies. Ann. Bot. 2011, 107, 49–63. [Google Scholar] [CrossRef]
- Shao, C.C.; Duan, H.L.; Ding, G.J.; Luo, X.Y.; Fu, Y.H.; Lou, Q. Physiological and Biochemical Dynamics of Pinus massoniana Lamb. Seedlings under Extreme Drought Stress and during Recovery. Forests 2022, 13, 65. [Google Scholar] [CrossRef]
- Crawford, R.M.M.; Braendle, R. Oxygen deprivation stress in a changing environment. J. Exp. Bot. 1996, 47, 145–159. [Google Scholar] [CrossRef]
- Striker, G.G. Time is on our side: The importance of considering a recovery period when assessing flooding tolerance in plants. Ecol. Res. 2012, 27, 983–987. [Google Scholar] [CrossRef]
- Diamond, J.M. Ecology: Laboratory, field and natural experiments. Nature 1983, 304, 586–587. [Google Scholar] [CrossRef]
| Growth Rate in Ground Diameter (mm) | Growth Rate in Height (cm) | |||
|---|---|---|---|---|
| Flooding | Recovery | Flooding | Recovery | |
| T1 | 0.03 ± 0.03 Ac | 0.03 ± 0.04 Aa | 0.87 ± 0.27 Ba | 21.86 ± 0.82 Aab |
| T2 | 0.03 ± 0.13 Ac | 0.03 ± 0.13 Aa | 0.06 ± 0.67 Ba | 23.50 ± 5.14 Aab |
| T3 | 0.37 ± 0.65 Ac | −0.03 ± 0.04 Ba | −0.06 ± 0.85 Ba | 26.26 ± 4.47 Aa |
| T4 | 2.87 ± 0.22 Aa | −1.54 ± 0.27 Bc | −0.30 ± 1.27 Ba | 26.28 ± 4.59 Aa |
| T5 | 1.75 ± 0.09 Ab | −1.20 ± 0.08 Bb | −0.10 ± 0.31 Ba | 19.27 ± 2.50 Ab |
| F | 97.006 | 179.435 | 2.082 | 3.615 |
| P | <0.001 *** | < 0.001 *** | 0.113 | 0.119 |
| Treatment | Df = 4, F = 17.963, p < 0.001 *** | Df = 4, F = 2.946, p = 0.040 * | ||
| Moment | Df = 1, F = 501.165, p < 0.001 *** | Df = 1, F = 1143.563, p < 0.001 *** | ||
| Treatment × Moment | Df = 4, F = 169.792, p < 0.001 *** | Df = 4, F = 4.269, p = 0.009 ** | ||
| Total Root Length (m) | Total Root Area (cm2) | |||
|---|---|---|---|---|
| Flooding | Recovery | Flooding | Recovery | |
| T1 | 16.28 ± 2.76 Ba | 33.22 ± 5.15 Aab | 634.53 ± 75.08 Aa | 649.97 ± 87.75 Abc |
| T2 | 15.53 ± 2.12 Ba | 39.55 ± 6.39 Aa | 509.61 ± 105.46 Bab | 855.49 ± 110.84 Aa |
| T3 | 14.44 ± 2.24 Ba | 35.49 ± 6.04 Aab | 482.13 ± 167.03 Babc | 788.82 ± 70.06 Aab |
| T4 | 14.55 ± 1.01 Ba | 34.22 ± 7.09 Aab | 356.15 ± 47.99 Bbc | 672.81 ± 102.45 Abc |
| T5 | 13.52 ± 2.56 Ba | 26.86 ± 2.29 Ab | 336.99 ± 30.08 Bc | 538.33 ± 72.61 Ac |
| F | 1.373 | 3.978 | 9.284 | 11.332 |
| P | 0.272 | 0.012 ** | < 0.001 *** | < 0.001 *** |
| Treatment | Df = 4, F = 4.419, p = 0.008 ** | Df = 4, F = 17.279, p < 0.001 *** | ||
| Moment | Df = 1, F = 304.251, p < 0.001 *** | Df = 1, F = 81.032, p < 0.001 *** | ||
| Treatment × Moment | Df = 4, F = 2.784, p = 0.049 * | Df = 4, F = 5.287, p = 0.003 ** | ||
| Indicators | Treatment | Moment | Treatment × Moment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Df | F | p | Df | F | p | Df | F | p | |
| TB | 4 | 39.557 | 0.040 * | 1 | 6524.849 | <0.001 *** | 4 | 100.314 | <0.001 *** |
| RSR | 4 | 55.408 | <0.001 *** | 1 | 173.509 | <0.001 *** | 4 | 95.963 | <0.001 *** |
| RMR | 4 | 83.196 | <0.001 *** | 1 | 321.174 | <0.001 *** | 4 | 140.725 | <0.001 *** |
| LMR | 4 | 15.678 | <0.001 *** | 1 | 249.323 | <0.001 *** | 4 | 21.317 | <0.001 *** |
| SMR | 4 | 388.891 | <0.001 *** | 1 | 0.859 | 0.363 | 4 | 223.690 | <0.001 *** |
| LMF | 4 | 112.960 | <0.001 *** | 1 | 267.830 | <0.001 *** | 4 | 95.528 | <0.001 *** |
| Treatment | Moment | Treatment × Moment |
|---|---|---|
| T1 | 100% | 100% |
| T2 | 100% | 100% |
| T3 | 100% | 100% |
| T4 | 100% | 100% |
| T5 | 83.3% | 100% |
| Indicators | Treatment | Moment | Treatment × Moment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Df | F | p | Df | F | p | Df | F | p | |
| REL | 4 | 97.467 | <0.001 *** | 1 | 261.824 | <0.001 *** | 4 | 12.960 | <0.001 *** |
| MDA | 4 | 48.074 | <0.001 *** | 1 | 1325.803 | <0.001 *** | 4 | 89.298 | <0.001 *** |
| SS | 4 | 56.810 | <0.001 *** | 1 | 24.675 | <0.001 *** | 4 | 17.526 | <0.001 *** |
| SP | 4 | 122.814 | <0.001 *** | 1 | 7203.544 | <0.001 *** | 4 | 183.909 | <0.001 *** |
| SOD | 4 | 86.727 | <0.001 *** | 1 | 1026.6 | <0.001 *** | 4 | 100.484 | <0.001 *** |
| CAT | 4 | 151.024 | <0.001 *** | 1 | 4037.111 | <0.001 *** | 4 | 200.944 | <0.001 *** |
| Indicators | Flooding | Recovery | ||
|---|---|---|---|---|
| PC1 | PC2 | PC1 | PC2 | |
| GRD | 0.794 | 0.440 | −0.684 | 0.203 |
| GRH | −0.908 | 0.353 | −0.598 | −0.724 |
| TRL | −0.944 | 0.006 | −0.963 | −0.122 |
| TRA | −0.990 | −0.080 | −0.997 | −0.007 |
| TB | 0.981 | 0.057 | −0.963 | 0.096 |
| RSR | −0.987 | 0.161 | 0.852 | −0.200 |
| RMR | −0.998 | 0.057 | 0.828 | −0.282 |
| LMR | −0.962 | 0.259 | −0.657 | 0.714 |
| SMR | 0.995 | −0.098 | −0.816 | −0.189 |
| LMF | −0.960 | 0.277 | −0.302 | 0.930 |
| REL | 0.963 | 0.239 | 0.977 | 0.127 |
| MDA | 0.924 | −0.062 | 0.910 | 0.334 |
| SS | 0.751 | 0.623 | 0.907 | 0.155 |
| SP | 0.987 | −0.038 | 0.875 | 0.161 |
| SOD | 0.991 | 0.026 | 0.866 | 0.271 |
| CAT | 0.992 | 0.039 | 0.913 | −0.296 |
| Eigenvalue | 14.382 | 1.260 | 11.231 | 2.457 |
| Proportion (%) | 89.889 | 7.880 | 70.193 | 15.359 |
| Cumulative (%) | 89.889 | 97.750 | 70.193 | 85.552 |
| Treatments | Flooding | Recovery | ||
|---|---|---|---|---|
| Synthesis Score | Ranking | Synthesis Score | Ranking | |
| T1 | −4.91 | 5 | 0.56 | 2 |
| T2 | −1.54 | 4 | −2.68 | 5 |
| T3 | 0.27 | 3 | −1.73 | 4 |
| T4 | 2.67 | 2 | 0.43 | 3 |
| T5 | 3.51 | 1 | 3.41 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Hu, H.; Wu, C.; Zhu, L. Flooding Tolerance and Recovery Capacity of Carya illinoinensis. Horticulturae 2025, 11, 590. https://doi.org/10.3390/horticulturae11060590
Chen X, Hu H, Wu C, Zhu L. Flooding Tolerance and Recovery Capacity of Carya illinoinensis. Horticulturae. 2025; 11(6):590. https://doi.org/10.3390/horticulturae11060590
Chicago/Turabian StyleChen, Xue, Haibo Hu, Chaoming Wu, and Li Zhu. 2025. "Flooding Tolerance and Recovery Capacity of Carya illinoinensis" Horticulturae 11, no. 6: 590. https://doi.org/10.3390/horticulturae11060590
APA StyleChen, X., Hu, H., Wu, C., & Zhu, L. (2025). Flooding Tolerance and Recovery Capacity of Carya illinoinensis. Horticulturae, 11(6), 590. https://doi.org/10.3390/horticulturae11060590






