Abstract
Hop (Humulus lupulus L.) is a crucial ingredient in beer, valued for its bitter acids and essential oils. Traditionally cultivated in temperate regions, hop production faces challenges from climate change, necessitating the exploration of new growing areas. This study evaluated the chemical quality of hop varieties grown in Brazil’s subtropical conditions to assess their suitability for brewing. Seven hop varieties (Cascade, Centennial, Columbus, Chinook, Comet, Fuggle, and Nugget) were analyzed for total α- and β-acids and essential oil composition. Bitter acids were quantified by spectrophotometric analysis, and volatile compounds were identified using gas chromatography coupled to mass spectrometry (GC-MS). Comet exhibited the highest a-acid level (10.54%), indicating strong bittering potential. Essential oil analysis revealed β-myrcene, (E)-caryophyllene, and α-humulene as predominant compounds. Centennial showed a distinct (E)-β-farnesene profile, a characteristic of noble hops. All varieties contained α- and β-selinene, suggesting a common metabolic pattern. The results indicate that Brazil has the potential to produce high-quality hops with suitable chemical profiles for brewing. As traditional hop-growing regions face climate-related challenges, hops cultivated in Brazil may offer a viable alternative for achieving the desired chemical composition required by the brewing industry.
1. Introduction
Humulus lupulus L. is an angiosperm belonging to the Cannabaceae family, originating from temperate climate countries [1]. It is a herbaceous, dioecious vine capable of reaching heights up to 7 m. The plant is notably sensitive to photoperiod, significantly affecting both its vegetative growth and the production of its inflorescences [2]. Known since antiquity, the species was initially described as a horticultural plant consumed in a manner similar to asparagus [3]. Today, global hop production is destined for the cosmetic, food, pharmaceutical, and animal industries, but approximately 97% of its use is allocated to the beer manufacturing industry [1].
Hops are one of the four key ingredients in beer production, along with water, malt, and yeast. Hops produce α- and β-acids, soft resins derived from specialized metabolism. These acids have antioxidant properties and antimicrobial actions, as well as contribute organoleptic characteristics such as bitterness and foam stability. Essential oils, on the other hand, are volatile chemical compounds related to aromatic components and impart aroma to beer [4,5,6].
The United States and Germany are the leading hop producers, followed by the Czech Republic, China, Poland, Slovenia, New Zealand, and Australia, where favorable climatic conditions support hop cultivation [7]. In Brazil, hop cultivation began in 2014, following the discovery of a variety that adapted well to the climatic conditions of the Mantiqueira mountain range. Since then, producers and researchers have started cultivating hops, still in an early stage, in various regions of Brazil [8]. In 2020, approximately 1400 new breweries were registered with the Ministry of Agriculture, Livestock, and Supply, with 80% located in the South and Southeast regions of the country, areas characterized as subtropical [1].
Climatic factors such as sunlight exposure, temperature, and water availability, along with genetic variability, influence the levels of active compounds in hops. This is one of the most important parameters for the brewing industry when assessing the quality of hop cones [9]. The growing focus on local production and the expansion of beer markets have increased interest in hops with unique phytochemical profiles and in new growing areas, producing local hops with characteristics specific to each region [10,11]. However, newly created and relocated varieties will bring changes in the sensory and other characteristics of hops [12]. Climate change threatens European hop production by accelerating ripening, reducing yields, and lowering α-acid content. By 2050, declines of 4–18% in yield and 20–31% in α-acid concentration are projected, underscoring the need for adaptation strategies in the brewing industry [13].
Given the increasing interest in expanding hop cultivation to new regions, this study aims to characterize the aroma and bitterness compounds of hop cones from varieties grown under subtropical conditions in Brazil to provide valuable insights into the potential of subtropical regions for hop production, despite the challenges posed by climate variability.
2. Materials and Methods
2.1. Experimental Area
The experiment was conducted in the Department of Crop Science, Division of Horticulture, School of Agricultural Sciences, São Paulo State University, located in the municipality of Botucatu, São Paulo (GPS coordinates: 22°50′ S, 48°26′ W, and altitude of 791 m). According to the Köppen climate classification, the region experiences a subtropical climate with hot summers (Cfa). During the cycle production period (November 2020 to March 2021), the average minimum temperature was 18.76 °C, and the average maximum temperature was 29.21 °C, with a total precipitation of 852.43 mm. The soil chemical analysis was conducted to characterize the edaphic conditions during the crop cultivation period (Table 1), including information on nutrient levels, organic matter content, and other relevant parameters for plant development.

Table 1.
Complete soil chemical analysis of experimental area in Botucatu, São Paulo, Brazil at depths of 0–20 cm and 20–40 cm.
2.2. Experimental Design
A randomized block design was adopted, consisting of seven treatments (different hop varieties), Cascade, Centennial, Columbus, Chinook, Comet, Fuggle, and Nugget, with four blocks and two plants per plot, totalizing 28 experimental plots. The seedlings were sourced from a licensed nursery Van den Bergen, located in the state of Minas Gerais, Brazil and certified for genetic authenticity.
2.3. Cultivation Characterization
Hop seedlings were planted in November 2020, at a spacing of 4.0 m × 1.0 m., and the plants were trained in a “V” shape, with the most vigorous branches selected, following the hexagonal form [14], to be guided along the sisal. Soil preparation involved scarification, followed by plowing, harrowing, furrowing, and hole opening. Soil correction and planting fertilization were carried out based on complete soil chemical analyses (Table 1), following recommendations established for the crop in the international literature [15]. Limestone and gypsum were applied, while the planting fertilization consisted of vegetable compost, Yoorin master®, calcitic lime, and potassium sulfate. Drip irrigation was used in the experimental area.
Green manure species were sown between the hop rows, including Crotalaria (Crotalaria breviflora), jack bean (Canavalia ensiformis), pearl millet (Pennisetum glaucum), and buckwheat (Fagopyrum esculentum). Phytosanitary control involved the application of Abamectin (Abamex®) for mites, Fipronil (Regent®) for ants, and Dipel® (Bacillus thuringiensis) in cases of caterpillar infestation. Green manure was mowed at the onset of flowering, and the biomass was distributed along the rows as mulch. Spontaneous vegetation was controlled mechanically, as needed, by mowing, weeding, and uprooting.
2.4. Harvest and Sample Processing
The harvest took place in March (2021), with the ideal timing determined based on qualitative sensory parameters—such as color, aroma, and cone texture [16]. Additionally, a quantitative parameter was considered by calculating the dry matter content, with the optimal harvest range set between 20% and 33% [17].
After harvesting the whole plants from the experimental area, the inflorescences were separated from the vegetative structure of the plant and placed in an oven to dry at 40 °C for 24 h until reaching a moisture content between 8% and 10%. The samples were obtained from two plants per plot following the experimental design. After that, the cones of each variety were placed in plastic bags, vacuum sealed, and stored in the freezer (−4 °C) until chemical analysis.
2.5. Essential Oil Extraction
Essential oil extraction was carried out via hydrodistillation using a Clevenger-type apparatus. For each extraction, 50 g of hop cones were utilized, and the distillation was conducted over a 90 min period [18]. Extractions were performed in triplicate for each pooled sample of the hop varieties. The yield (%) was calculated and expressed on a dry basis (Yield (%) = (Essential oil/Dry material mass) × 100).
2.6. Chemical Composition Analysis of Essential Oils by Gas Chromatography–Mass Spectrometry and Flame Ionization (GC-MS/FID)
The essential oil samples were diluted in ethyl acetate (Tedia, chromatographic grade, Fairfield, OH, USA, 1 mg.mL−1) and 1 μL of the solution was injected in triplicate. The analyses were performed using a Thermo Scientific gas chromatograph (model TRACE 1300 Series GC—Thermo Scientific—Waltham, MA, USA) equipped with a flame ionization detector (FID), a mass spectrometer (model ISQ 7000), and a Triplus RSH automatic injector (ThermoFisher Scientific). The injector was maintained at 220 °C, with a helium carrier gas flow (99.9999% purity) at a split ratio of 1:20. The mass spectrometer (MS) operated in full scan mode, using electron impact ionization (70 eV), with an acquisition range from 40 to 450 m/z. The interface temperature was maintained at 230 °C, and the detector temperature was set to 250 °C.
Separation of the substances was carried out using an Rtx-5MS capillary column (30 m × 0.25 mm, 0.25 μm, Restek Co., Bellefonte, PA, USA), with a carrier gas flow of 1.0 mL.min−1 under the following temperature program: 60 °C to 240 °C at 3 °C.min−1.
The Chromeleon software 7 (Thermo Scientific, Waltham, MA, USA) was used for data acquisition and processing. The identification of the substances was performed by comparing the mass spectra with the National Institute of Standards and Technology (NIST 14) library, the Flavour & Fragrance Natural & Synthetic Compounds (FFNSC 3) library, and the linear retention indices (LRI) of the substances with data from the literature (Adams, 2017 [19]). The linear retention indices were obtained by injecting a series of n-alkanes (C9–C24, Sigma-Aldrich, St. Louis, MI, USA, 99%) under the same chromatographic conditions as the samples, using the Van den Dool and Kratz (1963) equation [20]. The concentration of the metabolites was obtained by area normalization.
2.7. Total α- and β-Acid Content (%)
The determination of total α- and β-acid content was carried out by extracting the acids from the hop sample, followed by spectrophotometric analysis at three wavelengths [21]. Analyses were performed in triplicate for each pooled sample of the hop varieties.
Bitter acid extraction was performed using a 2.5 g sample of finely ground hop, which was added to 50.0 mL of methanol. The mixture was stirred for 30 min at room temperature, followed by a 10 min resting period. Next, membrane filtration (Millipore, 0.45 μm) was performed to remove particulate material. An aliquot of 50 μL of the filtrate was placed in a 25 mL volumetric flask, and the volume was completed with the extracting solution of methanolic NaOH (0.5 mL of 6M NaOH in 250 mL of methanol). The resulting solution was placed in a quartz cuvette with a 1 cm optical path length for UV–visible spectrum analysis, using 50 μL of methanol in 25 mL of methanolic NaOH as the blank. Absorbance values were recorded at wavelengths of 275, 325, and 355 nm. From these readings, the following were determined: α-acid content (%) = [(−51.26A355 nm) + (73.79A325 nm) − (19.07A275 nm)]; and β-acid content (%) = [(55.27A355 nm) − (47.59A325 nm) + (5.1A275 nm)]; where A = absorbance reading at each wavelength [13].
2.8. Statistical Analysis
The data for α- and β-acid were subjected to analysis of variance (ANOVA), followed by the Scott–Knott mean test at a 1% probability level, using Sisvar software 5.4 [22]. The chemical composition data of the essential oils were analyzed using multivariate analysis with the statistical software MetaboAnalyst 5.0 [23]. Sample data were normalized, and variable values were auto-scaled. The analyzed variables were subjected to multivariate data analysis using unsupervised methods—Principal Component Analysis (PCA) and Hierarchical Clustering Analysis (HCA).
3. Results and Discussion
3.1. Bitter Acids
The varieties presented different averages of α-acids (Table 2). Comet presented the highest concentration (10.54%), which was followed by the varieties Columbus (4.25%), Fuggle (2.86%), Chinook (1.41%), Centennial (1.35%), Nugget (1.15%), and Cascade (0.983%). The varieties do not show significant differences in β-acid averages at p < 0.01.

Table 2.
Total α- and β-acids (%) of cones of Humulus lupulus L. varieties grown in Botucatu, São Paulo, Brazil.
Hop-derived α-acids are among the most significant components of hops. Hops have a complex composition, with α-acids comprising 2 to 12% of their weight and β-acids accounting for 1 to 10%. Both substances are precursors of bittering agents in beer. Furthermore, α-acids play a crucial role in enhancing foam stability, preventing gushing, and contributing to beer preservation [24]. Their primary function occurs during the boiling process of wort with hops, where they undergo isomerization, leading to the formation of iso-α-acids, which are responsible for the intense bitterness of beer. The bitter potential of hops is a key quality criterion, which is why varieties with a high percentage of α-acids are highly valued. However, in terms of bitterness, iso-α-acids are far more important than α-acids themselves in beer. Since iso-α-acids result from the isomerization of α-acids, high α-acids hop varieties have been extensively used to develop improved cultivars [25].
According to Barth Haas (the major global company specializing in hops and hop products), the Comet variety exhibits an α-acid content of 11.9%, closely aligning with its highest observed concentration in this study (10.54%), demonstrating its potential adaptability to tropical climates, highlighting its viability for cultivation in such conditions. In contrast, Columbus, reported to have the highest α-acid content (15.0%) by Barth Haas, presented a significantly lower concentration in this study (4.25%), indicating the influence of additional factors beyond α-acid content alone [26]. Fuggle, with a moderate α-acid content of 5.1%, demonstrated a reasonable alignment with its measured concentration (2.86%). Meanwhile, Chinook and Centennial, despite their relatively high α-acid contents of 11.4% and 9.2% [26], respectively, exhibited substantially lower concentrations (1.41% and 1.35%), suggesting the interplay of other environmental or physiological variables. Similarly, Nugget, which possesses one of the highest α-acid levels (15.2%), displayed a low concentration (1.15%), further emphasizing indications of the complex interaction with the environment and the synthesis of these compounds [27]. Cascade showed the lowest concentration in this study (0.98%). Interestingly, β-acid concentrations did not present statistically significant differences across the studied varieties (p < 0.01), suggesting a more uniform distribution of these compounds in contrast to the variability observed in α-acids.
Cascade and Chinook hop varieties were evaluated under tropical conditions in Brazil during the years 2020 and 2021 [28]. The average concentration of bitter acids in both varieties was lower than the expected reference values from Barth Haas (4.22% and 9.9%, respectively). Specifically, the α-acid concentration in the Cascade variety should range between 4.5% and 9.0%, while for Chinook, it is expected to fall between 12.0% and 15.3%. The β-acid concentration in Cascade and Chinook should be within 3.6–9.0% and 3.0–4.9%, respectively. This difference might be attributed to the age of the plant, which may present a higher content and stability of compounds at maturity [16,29,30]. The quality of Cascade variety hops was also evaluated under subtropical conditions in Florida, USA, over two consecutive years and a double-season hop production system. They found that from spring to fall, the total α-acid concentration decreased by 10% in year 1 (from 5.94% to 5.35%) and by 71% in year 2 (from 8.25% to 2.39%). A similar trend was observed for total β-acid concentration, which decreased by 25% in year 1 (from 3.55% to 2.66%) and by 70% in year 2 (from 5.15% to 1.55%) [31].
The lower α-acid levels observed in this study compared to expected values from Barth Haas may be attributed to environmental factors, plant maturity, and post-harvest handling. Genetics plays a crucial role in the expression of α-acid content, and it is possible that some cultivars have already reached satisfactory levels of these compounds. Overall, the improvement and selection of hop cultivars in Brazil should focus on genotypes that either enhance or fully exploit taste and aroma, with α-acid levels below 7% [32]. On the other hand, climate conditions such as higher temperatures, increased UV exposure, and soil nutrient availability can influence α-acid biosynthesis, potentially leading to lower accumulation [33,34]. Monitoring the maturity of hop plants also becomes an important point to understand the increase in the content of compounds according to maturation, especially focused on tropical conditions. Additionally, it is important to keep the hops, pellets, or cones vacuum packed, if possible, with a modified atmosphere for N2 and refrigerated. Anaerobic conditions at 4 °C were the best to keep bitter acids stable over the time period [35].
3.2. Essential Oil Yield and Composition
The essential oil yields measured in this study ranged from 0.70% in Cascade to 2.36% in Comet. Table 3 shows the essential oil yield for each variety.

Table 3.
Essential oil yield (%) of hop varieties cultivated in Botucatu, São Paulo, Brazil.
The values are within the broader interval of 0.5–3.0% (dry-cone basis) documented by Almaguer et al. (2014) for hop cultivars grown under temperate conditions [36]. This alignment suggests that the Brazilian subtropical environment does not inherently suppress overall oil production relative to standards established in temperate regions. A significant increase was observed in volatile oil content between the first and second years of hop cultivation, emphasizing the role of plant maturity in essential oil accumulation. In 2021, Cascade (0.75 mL/100 g) and Chinook (1.43 mL/100 g) fell within their expected reference ranges, indicating that oil composition stabilizes as plants mature. This supports the notion that younger hop plants yield lower essential oil amounts, with environmental factors such as temperature and soil conditions also contributing to variability [28].
In the seven analyzed varieties, 49 substances were identified in the essential oil, with variations in their relative proportions among the studied varieties. Five major compounds were consistently identified across all hop varieties analyzed. β-Myrcene content ranged from 7.98% in Centennial to 62.05% in Fuggle. Caryophyllene was found in concentrations ranging from 2.38% in Centennial to 6.65% in Chinook. α-Humulene varied from 0.12% in Fuggle to 11.34% in Cascade. β-Selinene ranged from 0.28% in Columbus to 7.48% in Centennial, while α-selinene ranged from 0.68% in Cascade to 11.18% in Centennial. The following table presents all compounds identified, highlighting their respective relative contents (%) (Table 4).

Table 4.
Chemical composition (relative content%) of hops varieties grown in Botucatu, São Paulo, Brazil.
Essential oils are chemical compounds responsible for the aromatic characteristics of hop varieties and play a key role in imparting aroma to beer. These compounds are primarily classified into three main groups: monoterpenes (β-myrcene, limonene, and α-pinene), sesquiterpenes (farnesene, α-humulene, and β-caryophyllene) and terpene alcohols (linalool). Hydrocarbons generally account for 50–80% of the total oil and include monoterpenes (such as α- and β-pinene, β-myrcene, and limonene) and sesquiterpenes (like α-humulene, β-farnesene, β-caryophyllene, α- and β-selinene, and γ-muurolene) [4]. The hop varieties exhibited a high content of monoterpene and sesquiterpene hydrocarbons. Except for Centennial, the monoterpene hydrocarbon class was the most predominant in all varieties, mainly due to the high concentration of β-myrcene, which was the most abundant compound across all samples. Notably, the Fuggle variety displayed the highest β-myrcene content (62.05%), while Centennial had the lowest (7.98%). β-Myrcene is considered the main precursor to the aroma of green hops, with a scent described as herbaceous, resinous, green, balsamic, and reminiscent of fresh hops [37].
Sesquiterpene hydrocarbons are also the class of substances most present in hops, such as (E)-caryophyllene and α-humulene. However, the hop varieties analyzed exhibited distinct essential oil compositions, with β-myrcene, (E)-caryophyllene, and α-humulene being the only substances common to all varieties. While major components, such as α-acids, tend to remain relatively stable, minor components like essential oils are more susceptible to environmental variation. However, the changes induced by edaphoclimatic and biotic conditions result in region-specific traits, collectively referred to as terroir [38,39]. Therefore, more abundant and less abundant substances are both important for characterizing hop varieties by locality. According to the HCA (Figure 1), five groups are formed based on the chemical composition of essential oil and bitter acid content, with a cutoff at 75% of the Euclidean distance for classification.
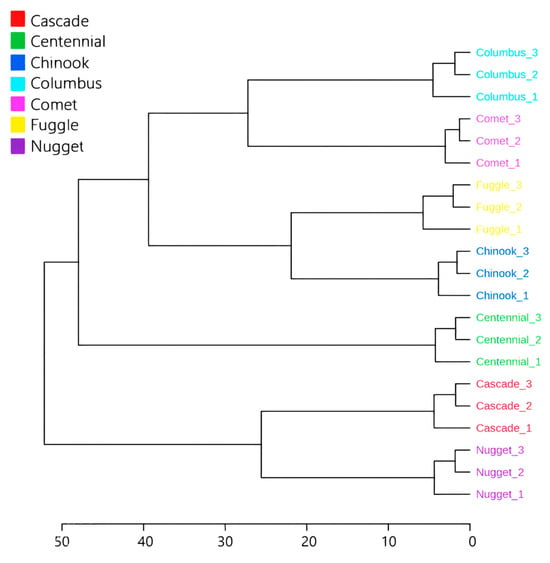
Figure 1.
Hierarchical Cluster Analysis (HCA) of Humulus lupulus L. essential oil from different varieties grown in Brazil.
The first group consists of the Cascade and Nugget variety, which differed most among the samples. This variation could be attributed to their similar levels of α-humulene, which are notably higher than those observed in the other varieties. The second group includes just the Centennial variety, displaying a markedly different profile, with only 7.98% β-myrcene but a substantial 31.09% of (E)-β-farnesene, making it the predominant compound in this variety. This hop is commonly used for dual purposes, both aroma and bittering, though farnesene is listed as one of its minor constituents on commercial pellet packaging. The third group consists of Chinook and Fuggle, the fourth group is formed by Comet variety, and the fifth is formed by Columbus. These results can be explained by PCA, where the weights of each variable for the formed groups are shown in Figure 2. The first two principal components explained 60.15% of the total variance, with PC1 accounting for 37.61% and PC2 for 22.54%.
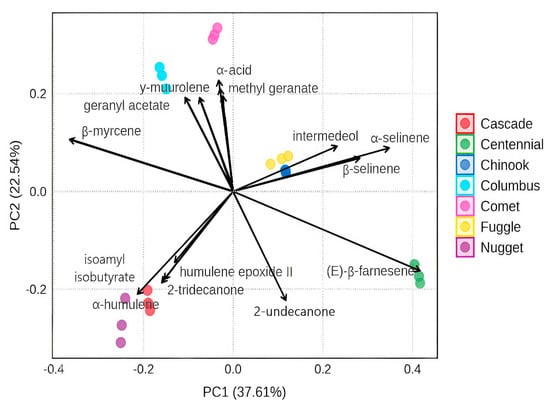
Figure 2.
A biplot of the Principal Component Analysis (PCA). The vectors represent key chemical compounds contributing to the differentiation of the hop samples.
The analysis reveals the formation of five distinct groups of hop varieties, each characterized by correlations with specific chemical compounds: the first group, located towards the negative side of PC1, includes Cascade and Nugget. These varieties exhibit a strong correlation with α-humulene and humulene epoxide II in addition to isoamyl isobutyrate and 2-tridecanone, suggesting a chemical profile differentiated by these compounds. The second group, positioned on the positive side of PC1, is represented by Centennial, strongly associated with (E)-β-farnesene, indicating a distinct compositional pattern compared to the first group. The third group, located near the center of the biplot, consists of Fuggle and Chinook. These samples are associated with a broader distribution of compounds, including α-selinene and β-selinene, without a strong correlation to a specific subset of chemical markers.
PCA placed Comet and Columbus in distinct clusters, showing that, despite some compositional overlap, their volatile oil profiles differ enough to separate them statistically. Both cultivars exhibit very high β-myrcene levels, Comet (55.8%) and Columbus (56.5%), and a complete absence of (E)-β-farnesene; however, their sesquiterpene profiles differ significantly. Comet contains extremely low α-humulene (0.51%) and moderate (E)-caryophyllene (9.7%), whereas Columbus shows a higher α-humulene level (6.14%) but a lower (E)-caryophyllene fraction (5.18%). These quantitative shifts strongly influence the loadings of PC1, which is weighted toward α-humulene and (E)-caryophyllene, thereby positioning the two varieties in separate regions of the score plot.
Notably, the lack of detectable farnesene in Columbus matches the reference range reported for its essential oil, and its β-myrcene content (25–55%) falls within the values documented in the literature [40]. Consequently, although Comet and Columbus share a myrcene-dominant profile, their distinct sesquiterpene balances create clearly differentiated chemometric signatures. Regarding Comet, previous studies have reported that (E)-β-ocimene accounts for more than 2% of its chemical composition, which aligns with our findings (2.65%), as it was the only variety to present this compound. Additionally, while Comet is not a variety rich in α-humulene, it does contain a considerable percentage of α- and β-selinene, as well as a notable amount of geranyl isobutanoate (4.89%). The variety had 56.8% of β-myrcene and the highest value of α-acids [41].
Monoterpene hydrocarbons, particularly β-myrcene, also dominate the essential oil profiles of Cascade, Chinook, and Comet varieties cultivated in Southern Italy, with β-myrcene comprising 42–62% of the total composition [41]. Similarly, significant variability was found in β-myrcene levels among Nugget, Columbus, and Chinook varieties, ranging from 15% to 52.06%, alongside consistent α- and β-acid content, which are critical for bitterness in brewing [42]. In contrast, environmental factors, such as seasonal changes in subtropical Florida, significantly impact the total essential oil content of the Cascade variety, with an 85% decrease observed over two consecutive years [31]. A study further corroborated these findings, showing that the Cascade variety cultivated in Florida exhibited seasonal and yearly variations in essential oil content and bitter acid profiles. Myrcene remained the most abundant terpene (over 31%), while α-acid content varied significantly between seasons and years, ranging from 2.7% to 6.2% [43].
Studies analyzing Brazilian hops identified α-selinene and β-selinene as potential discriminants of Brazilian varieties, along with 2-dodecanone and 2-decanone, which contribute floral aromas. The authors also found that (E)-α-bergamotene played a significant role in distinguishing Brazilian hops [44]. Our results showed that Centennial contained 1.39% of this compound, which is associated with floral, sweet, and citrus aromas, reinforcing its classification as a highly valued aroma variety. In another study evaluating four varieties, Centennial exhibited (E)-β-farnesene as the major compound, along with a high content of selinene isomers, which were also present in Fuggle varieties [45]. All varieties contained high levels of these compounds, ranging from 0.28% of α-selinene and 0.95% of β-selinene in Columbus to 7.79% of α-selinene and 11.18% of β-selinene in Centennial. Cascade and Nugget exhibited less content of these substances. Consequently, although these varieties showed a lower relative percentage of these compounds, all varieties contained α- and β-selinenes, showing that this pattern repeats for the varieties.
3.3. Climate Adaptation Implications
The difference between the values presented in this study and the values disseminated for each variety can be explained because, as these are compounds of specialized metabolism, their production is influenced by climatic factors, type of cultivation soil, hours of sunlight, and temperature [27]. Studies have demonstrated that growing conditions (such as soil composition, moisture, microclimate, etc.) significantly influence the aroma, particularly in certain cultivars. Climate change, especially adverse temperatures and more frequent droughts have already led to stagnation in crop yields and a decline in α-acid content in certain farming regions. This makes it difficult to maintain stable hop characteristics, which are essential for consistent quality in the brewing market [46]. Additionally, hops are plants more sensitive to climate warming because their adaptation is slower [47].
Climate change has significantly impacted hop cultivation in the Czech Republic, with rising temperatures, increased sunshine, and reduced humidity observed between 1951 and 2006. The Saaz yield has stagnated in recent years and so α-acid content in Saaz hops has declined at a statistically significant rate, which could affect the quality of the hops used in brewing [48]. Given that hop cultivation is concentrated in a small region, these climatic shifts may necessitate a regional redistribution of production to mitigate risks and ensure long-term sustainability. Despite global warming, hop cultivation has expanded in regions with higher temperatures and lower rainfall, supported by well-developed hop industries [12].
Climate sensitivity of European hop yield, α-acid content, and cone development was assessed from 1970 to 2050 using meteorological measurements and model projections, with a 1.4 °C temperature rise and a 24 mm decrease in precipitation. Data from Germany, the Czech Republic, and Slovenia, which represent nearly 90% of hop-growing regions, show that hop ripening started 20 days earlier, production dropped by 0.2 t/ha/year, and α-acid content fell by 0.6% after 1994. By 2050, hop yield and α-acid content are predicted to decline by 4–18% and 20–31%, respectively, highlighting the need for immediate adaptation measures [13]. Central Europe is undergoing significant climatic shifts that are expected to intensify during the 21st century, with major implications for agricultural productivity [49].
By leveraging advanced analytical methods, such as GC-MS and spectrophotometry, they have been able to monitor and maintain the chemical profiles of hops, particularly essential oils and bitter acids, to meet the stringent quality standards required for brewing. This commitment to quality, combined with innovative adaptation strategies, has not only strengthened the domestic hop supply chain but also positioned Brazil as a promising region for hop cultivation in the face of global climate challenges [8,37,50,51]. These advancements demonstrate that Brazil, with its unique climate and technological adaptations, can serve as a promising alternative for hop production, offering resilience against the climate-induced declines in quality and quantity faced by traditional hop-growing regions in Europe and North America.
In recent years, Brazilian producers and researchers have made significant efforts to establish a sustainable and high-quality hop production chain in Brazil, despite the challenges posed by the country’s tropical and subtropical climates. Recognizing the growing demand for locally sourced ingredients in the craft beer industry, these stakeholders have invested in agronomic research, varietal selection, and cultivation techniques tailored to Brazil’s unique environmental conditions [50]. Nevertheless, Brazil lacks rigorously validated, region-specific hop quality standards, obliging researchers to compare local material against international benchmarks that may not accurately reflect domestic agronomic and chemical realities. In this investigation, the data set was generated on a small scale at a single experimental site; future investigations conducted across multiple locations and seasons will be essential to strengthen statistical power and broaden the applicability of these findings.
4. Conclusions
Our results show that hops grown in Brazil’s subtropical climate can meet key brewing requirements, especially when the right varieties are chosen. Comet delivered the highest α-acid levels alongside a favorable mix of aroma compounds, indicating that the careful matching of cultivar and environment can yield cones comparable to those from traditional temperate regions that are facing climate changes. Other varieties, although lower in α-acids, exhibited rich terpene profiles that make them attractive for aroma purposes. For local producers, this reinforces the importance of selecting varieties with desirable phytochemical profiles, particularly those adapted to tropical conditions and suited to aroma or bitterness goals. For brewers, it opens opportunities to work with cultivars that either mimic traditional profiles or offer unique characteristics, fostering the creation of beer with a distinctive Brazilian terroir.
Future studies should prioritize understanding how environmental and management factors, such as nutrient supply, irrigation, and optimal harvest maturity, affect α-acid synthesis and overall productivity. Integrating chemical and yield data will be essential to identify hop varieties that meet both agronomic and industry standards, reducing dependence on imports and supporting the development of a competitive national hop market.
Author Contributions
Conceptualization, B.C.C.S.; methodology, B.C.C.S., O.P.C., G.d.C.F., C.S.N. and J.A.O.G.; validation, R.F. and M.N.F.C.; formal analysis, B.C.C.S. and F.P.G.B.; investigation, B.C.C.S.; data curation, F.P.G.B., V.V. and M.N.F.C.; writing—original draft preparation, B.C.C.S.; writing—review and editing, M.N.F.C. and V.V.; visualization, F.P.G.B.; supervision, F.P.G.B. and M.O.M.M.; funding acquisition, F.P.G.B. and M.O.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financed, in part, by the São Paulo Research Foundation (FAPESP)—Research grants: 2017/50338-9; 2018/25812-1; 2019/27066-8; 2023/12485-0 and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), grant number 001.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors would like to thank the funding agencies São Paulo Research Foundation (FAPESP) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for funding the research and scholarships.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brasil. Ministério da Agricultura, Pecuária e Abastecimento. Lúpulo no Brasil: Perspectivas e Realidades; Ministério da Agricultura, Pecuária e Abastecimento; Secretaria de Agricultura Familiar e Cooperativismo: Brasília, Brazil, 2022; 175p, ISBN 978-65-86803-89-1.
- Gutiérrez, R.M.; de Oliveira, R.R.; Ribeiro, T.H.; de Oliveira, K.K.; Silva, J.V.; Alves, T.C.; do Amaral, L.R.; de Souza Gomes, M.; de Souza Gomes, M.; Chalfun-Junior, A. Unveiling the phenology and associated floral regulatory pathways of Humulus lupulus L. in subtropical conditions. Planta 2024, 259, 150. [Google Scholar] [CrossRef] [PubMed]
- Spósito, M.B.; Ismael, R.V.; Barbosa, C.D.A.; Tagliaferro, A.L. A cultura do lúpulo. In Série Produtor Rural, 68; ESALQ-Divisão de Biblioteca: Piracicaba, Brazil, 2019. [Google Scholar]
- Durello, R.S.; Silva, L.M.; Bogusz, S. Química do lúpulo. Quim. Nova 2019, 42, 900–919. [Google Scholar] [CrossRef]
- Kowalska, G.; Bouchentouf, S.; Kowalski, R.; Wyrostek, J.; Pankiewicz, U.; Mazurek, A.; Włodarczyk-Stasiak, M. The hop cones (Humulus lupulus L.): Chemical composition, antioxidant properties and molecular docking simulations. J. Herb. Med. 2022, 33, 100566. [Google Scholar] [CrossRef]
- Astray, G.; Gullón, P.; Gullón, B.; Munekata, P.E.S.; Lorenzo, J.M. Humulus lupulus L. as a Natural Source of Functional Biomolecules. Appl. Sci. 2020, 10, 5074. [Google Scholar] [CrossRef]
- IHGC—International Hop Growers Convention. 2023. Available online: https://www.ihgc.org/wp-content/uploads/IHGC_CountryReportsSummary.pdf (accessed on 12 January 2025).
- Jastrombek, J.M.; Fagherazzi, M.M.; de Cássio Pierezan, H.; Rufato, L.; Sato, A.J.; da Silva Ricce, W.; Marques, V.V.; Leles, N.R.; Roberto, S.R. Hop: An emerging crop in subtropical areas in Brazil. Horticulturae 2022, 8, 393. [Google Scholar] [CrossRef]
- Pavolovic, V.; Cerenak, A.; Pavolovic, M.; Kosir, I.J.; Rozman, C.; Bohanec, M.; Ceh, B.; Naglic, B. Modelling of quality prediction for hops (Humulus lupulus L.) in relation to meteorological variables. In Proceedings of the Balwois Conference Proceedings, Ohrid, North Macedonia, 25–29 May 2010; pp. 1–10. [Google Scholar]
- Mongelli, A.; Rodolfi, M.; Ganino, T.; Marieschi, M.; Caligiani, A.; Dall’Asta, C.; Bruni, R. Are Humulus lupulus L. ecotypes and cultivars suitable for the cultivation of aromatic hop in Italy? A phytochemical approach. Ind. Crops Prod. 2016, 83, 693–700. [Google Scholar] [CrossRef]
- Ruggeri, R.; Rossini, F.; Roberto, S.R.; Sato, A.J.; Perrine, L.; Laban, K.R.; Agehara, S. Development of hop cultivation in new growing areas: The state of the art and the way forward. Eur. J. Agron. 2024, 161, 127335. [Google Scholar] [CrossRef]
- Kubes, J. Changing Geography of Hop Regions in the World 1990–2022. J. Am. Soc. Brew. Chem. 2024, 83, 238–247. [Google Scholar] [CrossRef]
- Mozny, M.; Trnka, M.; Vlach, V.; Zalud, Z.; Cejka, T.; Hajkova, L.; Potopova, V.; Semenov, M.A.; Semeradova, D.; Büntgen, U. Climate-induced decline in the quality and quantity of European hops calls for immediate adaptation measures. Nat. Commun. 2023, 14, 6028. [Google Scholar] [CrossRef]
- Peragine, J.N. The Complete Guide to Growing Your Own Hops, Malts, and Brewing Herbs: Everything You Need to Know Explained Simply; Atlantic Publishing Company: Ocala, FL, USA, 2011. [Google Scholar]
- Gingrich, C.; Hart, J.; Christensen, N. Hops Fertilizer Guide; OSU Extension Catalog, Oregon State University: Corvallis, OR, USA, 2018. [Google Scholar]
- Dodds, K. Hops—A Guide for New Growers; NSW Department of Primary Industries: Orange, NSW, Australia, 2017; ISBN 978-1-76058-007-0. [Google Scholar]
- Darby, H.; Madden, R. The UVM Mobile Hop Harvester; University of Vermont: Burlington, VT, USA, 2012. [Google Scholar]
- Lagos, F.S.; Deschamps, C.; Zuffellato-Ribas, K.C.; Antoniazzi, N. Biomass and essential oil production of hops cv Chinook in response to nitrogen fertilization. Rev. Ceres. 2023, 70, e70509. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5th ed.; Texensis Publishing: Gruver, TX, USA, 2017; pp. 46–52. [Google Scholar]
- van Den Dool, H.A.N.D.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Egts, H.; Durben, D.J.; Dixson, J.A.; Zehfus, M.H.A. Multicomponent UV Analysis of α- and β-Acids in Hops. J. Chem. Educ. 2012, 89, 117–120. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: Um sistema computacional de análise estatística. Ciência Agrotecnol. 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Pang, Z.; Xu, L.; Viau, C.; Lu, Y.; Salavati, R.; Basu, N.; Xia, J. MetaboAnalystR 4.0: A unified LC-MS workflow for global metabolomics. Nat. Commun. 2024, 15, 3675. [Google Scholar] [CrossRef]
- Van Cleemput, M.; Cattoor, K.; De Bosscher, K.; Haegeman, G.; De Keukeleire, D.; Heyerick, A. Hop (Humulus lupulus)-derived bitter acids as multipotent bioactive compounds. J. Nat. Prod. 2009, 72, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Verzele, M.; De Keukeleire, D. Chemistry and Analysis of Hop and Beer Bitter Acids; Elsevier: Amsterdam, The Netherlands, 1991; Volume 27, pp. 1–411. [Google Scholar]
- Haas, J.I. Barth-Haas Hops Companion: A Guide to the Varieties of Hops and Hop Products. Barth-Haas Group. 2009. Available online: https://www.johnihaas.com/wp-content/uploads/2021/11/HAAS_HopsCompanion-Final-ForWeb.pdf (accessed on 12 January 2025).
- Huang, X.Q.; Dudareva, N. Plant specialized metabolism. Curr. Biol. 2023, 33, R473–R478. [Google Scholar] [CrossRef]
- Contin, D.R.; Habermann, E.; de Souza, B.C.; de Oliveira, E.A.; Martinez, C.A.; Vieira, P.C.; Da Costa, F.B. Exploring the tropical acclimation of European and American hop cultivars (Humulus lupulus L.): Focus on physiology, productivity, and chemical composition. Eur. J. Agron. 2023, 151, 126990. [Google Scholar] [CrossRef]
- Fortuna, G.C.; Neves, C.S.; Campos, O.P.; Gomes, J.A.O.; Silva, J.C.R.L.; Souza, A.A.; Funari, C.S.; Marques, M.O.M.; Bonfim, F.P.G. Hop Tropicalization: Chemical Compositions of Varieties Grown under Organic and Conventional Systems in Subtropical Conditions. Horticulturae 2023, 9, 855. [Google Scholar] [CrossRef]
- McAdam, E.L.; Vaillancourt, R.E.; Koutoulis, A.; Whittock, S.P. Quantitative genetic parameters for yield, plant growth and cone chemical traits in hop (Humulus lupulus L.). BMC Genet. 2014, 15, 22. [Google Scholar] [CrossRef]
- Gallardo, M.; Agehara, S.; Rechcigl, J. Optimization of trellis design and height for double-season hop (Humulus lupulus L.) production in a subtropical climate: Growth, morphology, yield, and cone quality during establishment years. Eur. J. Agron. 2025, 162, 127415. [Google Scholar] [CrossRef]
- Dos Santos, F.C.; Dos Santos, M.; Huezsmann, R.D.; Ceola, D.; De Souza, E.M.; Junior, C.F.; Guidolin, A.F.; Coimbra, J.L. Phenotypic variability in the induction of alpha acids in hops (Humulus lupulus L.) in Brazil. J. Agric. Sci. 2022, 14, 198–205. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, N.; Yang, A.; Huang, J.; Ren, X.; Xian, M.; Zou, H. Hop bitter acids: Resources, biosynthesis, and applications. Appl. Microbiol. Biotechnol. 2021, 105, 4343–4356. [Google Scholar] [CrossRef] [PubMed]
- Czubacka, A.; Skomra, U.; Agacka-Mołdoch, M.; Koziara-Ciupa, M. The Expression of Genes Involved in Synthesis of Bitter Acids and Xanthohumol and the Content of These Compounds in Aroma and Bitter Hop under Reduced Nitrogen Fertilisation. Agronomy 2024, 14, 1680. [Google Scholar] [CrossRef]
- Rutnik, K.; Ocvirk, M.; Košir, I.J. The stability of hop (Humulus lupulus L.) resins during long-period storage. Plants 2023, 12, 936. [Google Scholar] [CrossRef]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Duarte, P.F.; do Nascimento, L.H.; Bandiera, V.J.; Fischer, B.; Fernandes, I.A.; Paroul, N.; Alexander, J. Exploring the versatility of hop essential oil (Humulus lupulus L.): Bridging brewing traditions with modern industry applications. Ind. Crops Prod. 2024, 218, 118974. [Google Scholar] [CrossRef]
- Morcol, T.B.; Negrin, A.; Matthews, P.D.; Kennelly, E.J. Hop (Humulus lupulus L.) terroir has large effect on a glycosylated green leaf volatile but not on other aroma glycosides. Food Chem. 2020, 321, 126644. [Google Scholar] [CrossRef]
- Guimarães, B.P.; Nascimento, P.G.B.D.; Ghesti, G.F. Intellectual property and plant variety protection: Prospective study on Hop (Humulus lupulus L.) cultivars. World Pat. Inf. 2021, 65, 102041. [Google Scholar] [CrossRef]
- Duarte, L.M.; Amorim, T.L.; Grazul, R.M.; de Oliveira, M.A.L. Differentiation of aromatic, bittering and dual-purpose commercial hops from their terpenic profiles: An approach involving batch extraction, GC–MS and multivariate analysis. Food Res. Int. 2020, 138, 109768. [Google Scholar] [CrossRef]
- Gresta, F.; Calvi, A.; Santonoceto, C.; Strano, T.; Ruberto, G. Agronomic traits and essential oil profiles of Humulus lupulus L. cultivated in southern Italy. J. Esssent. Oil Res. 2022, 35, 60–70. [Google Scholar] [CrossRef]
- Alfaro-Saiz, E.; Cámara-Leret, S.; González-González, M.; Fernández-Álvarez, O.; Rodríguez-Fernández, S.; López-López, D.; Paniagua-García, A.I.; Acedo, C.; Díez-Antolínez, R. The Memory of Hops: Rural Bioculture as a Collective Means of Reimagining the Future. Sustainability 2024, 16, 2470. [Google Scholar] [CrossRef]
- Acosta-Rangel, A.; Agehara, S.; Rechcigl, J. Double-season production of hops (Humulus lupulus L.) with photoperiod manipulation in a subtropical climate. Sci. Hortic. 2024, 332, 113177. [Google Scholar] [CrossRef]
- Dias, G.S.; Gallon, M.E.; Gobbo-Neto, L. Comparative analysis of four hop cultivars grown in Brazil and the USA by GC-MS-based metabolomics. J. Inst. Brew. 2024, 130, 238–249. [Google Scholar] [CrossRef]
- Cabral, M.N.F. Óleos Essenciais e Hidrolatos de Humulus lupulus L.: Caracterização Química de Variedades Cultivadas No Estado de São Paulo. Master’s Thesis, Faculdade de Ciências Agronômicas UNESP, Botucatu, Brazil, 2023. [Google Scholar]
- Korpelainen, H.; Pietiläinen, M. Hop (Humulus lupulus L.): Traditional and present use, and future potential. Econ. Bot. 2021, 75, 302–322. [Google Scholar] [CrossRef]
- Mozny, M.; Tolasz, R.; Nekovar, J.; Sparks, T.; Trnka, M.; Zalud, Z. The impact of climate change on the yield and quality of Saaz hops in the Czech Republic. Agric. For. Meteorol. 2009, 149, 913–919. [Google Scholar] [CrossRef]
- Donner, P.; Pokorný, J.; Ježek, J.; Krofta, K.; Patzak, J.; Pulkrábek, J. Influence of weather conditions, irrigation and plant age on yield and alpha-acids content of Czech hop (Humulus lupulus L.) cultivars. Plant Soil Environ. 2020, 66, 41–46. [Google Scholar] [CrossRef]
- Torbenson, M.C.A.; Esper, J.; Brázdil, R.; Büntgen, U.; Olesen, J.E.; Semarádová, D.; Vlach, M.; Urban, O.; Balek, J.; Kolar, T.; et al. Past and future climate-driven changes of agricultural land in central Europe. Geophys. Res. Lett. 2024, 51, e2024GL112363. [Google Scholar] [CrossRef]
- Almeida, A.d.R.; de Conto, L.C. Lúpulo no Brasil: Uma cultura promissora em ascensão. Food Sci. Technol. 2024, 3, 1–7. [Google Scholar] [CrossRef]
- Neves, C.S.; Aires, E.S.; Campos, O.P.; Fortuna, G.C.; de Oliveira Gomes, J.A.; Callili, D.; Ono, E.O.; Rodrigues, J.D.; Bonfim, F.P.G. Physiological and productive performance of hop (‘Humulus lupulus L.’) varieties grown under subtropical conditions in Brazil. Aust. J. Crop Sci. 2020, 18, 280–287. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).