Comparative Study on Aquaponic and Hydroponic Systems for Sustainable Hemp Production in a Controlled Environment
Abstract
1. Introduction
2. Materials and Methods
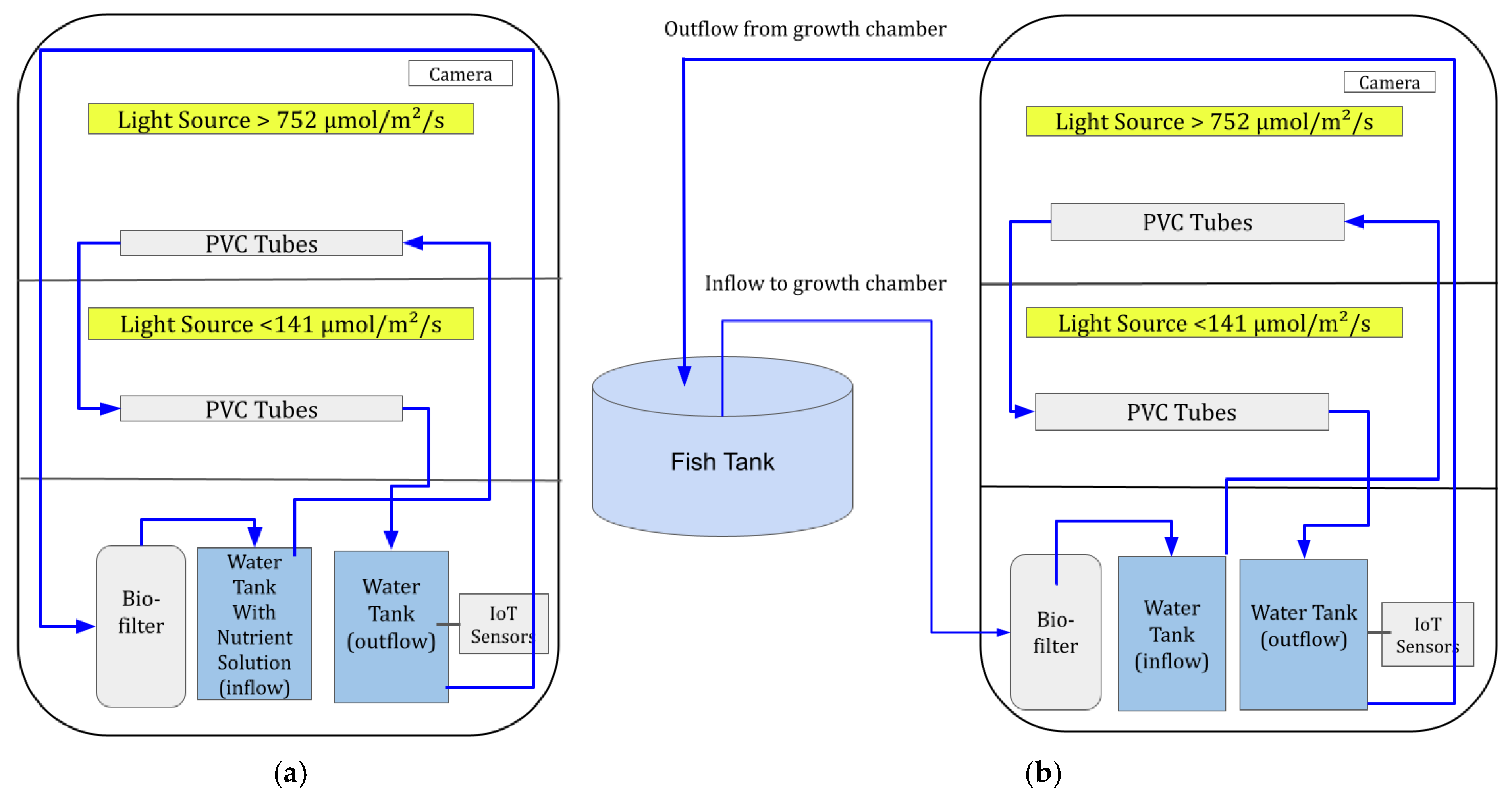
2.1. System Setup
2.2. Plant and Fish in the Systems
2.3. Light Intensity Treatments
2.4. Data Collection
2.5. Life Cycle Assessment
3. Results and Discussion
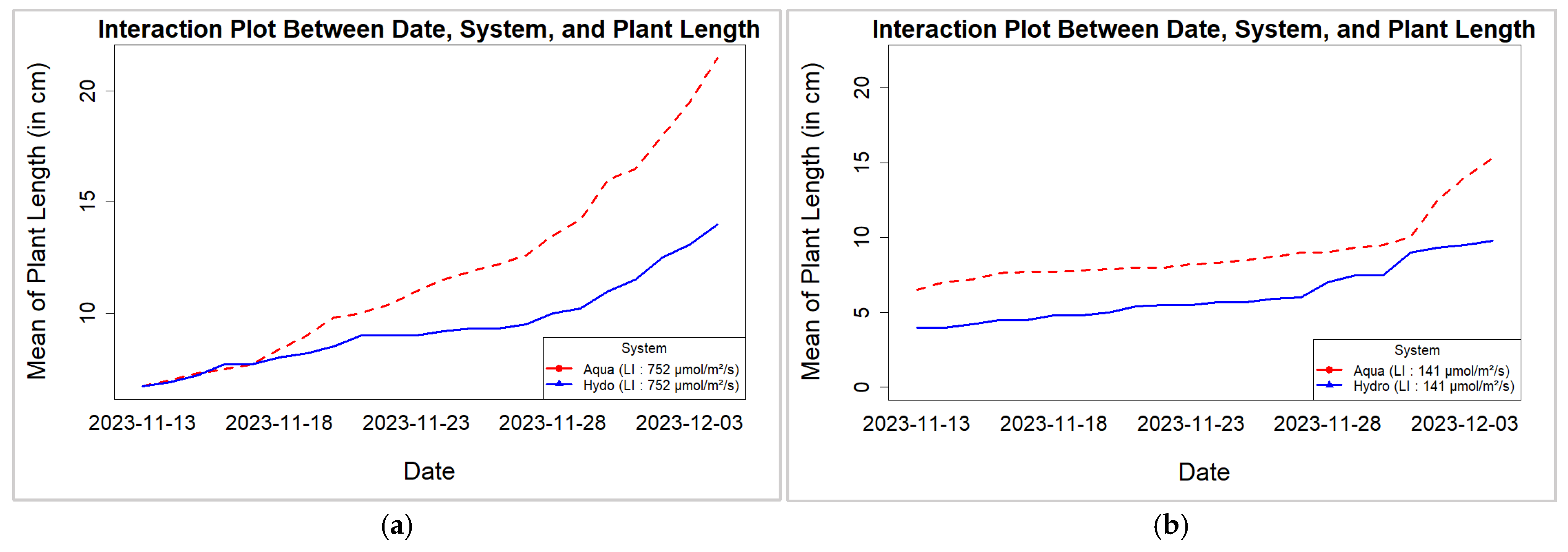
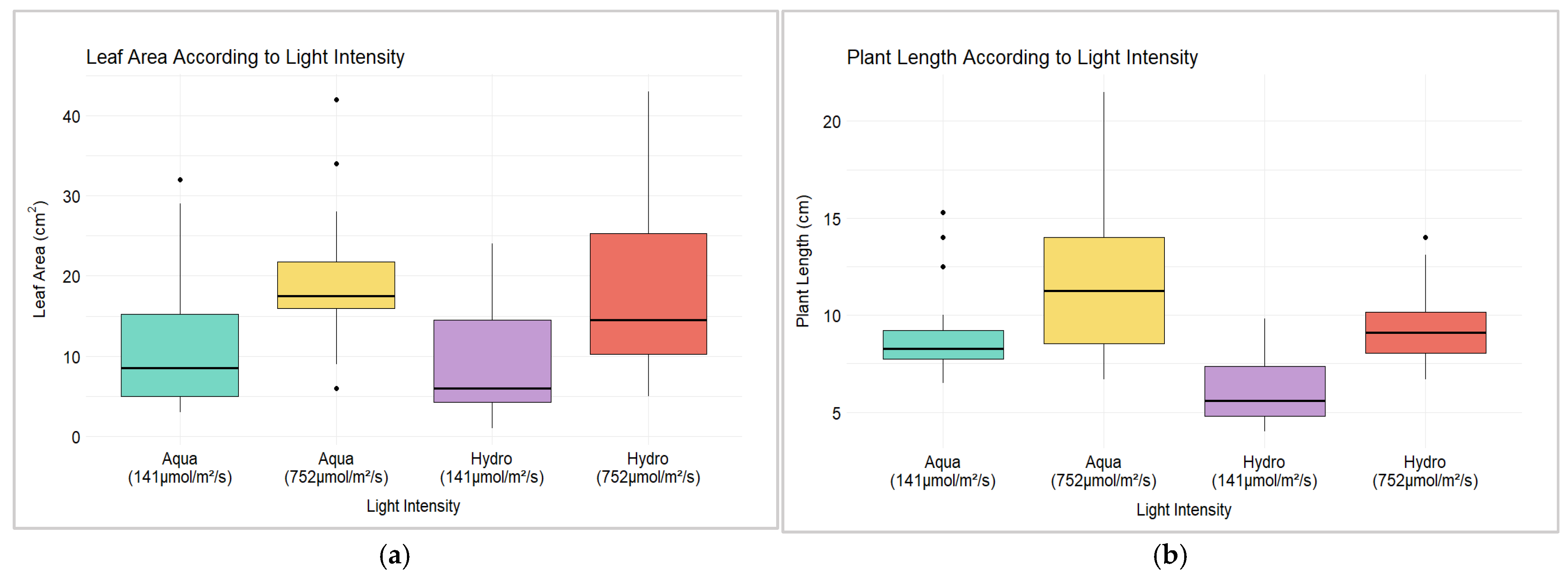
3.1. Hemp Leaf Area and Plant Length for Different Systems and LIs
3.2. Life Cycle Assessment Results
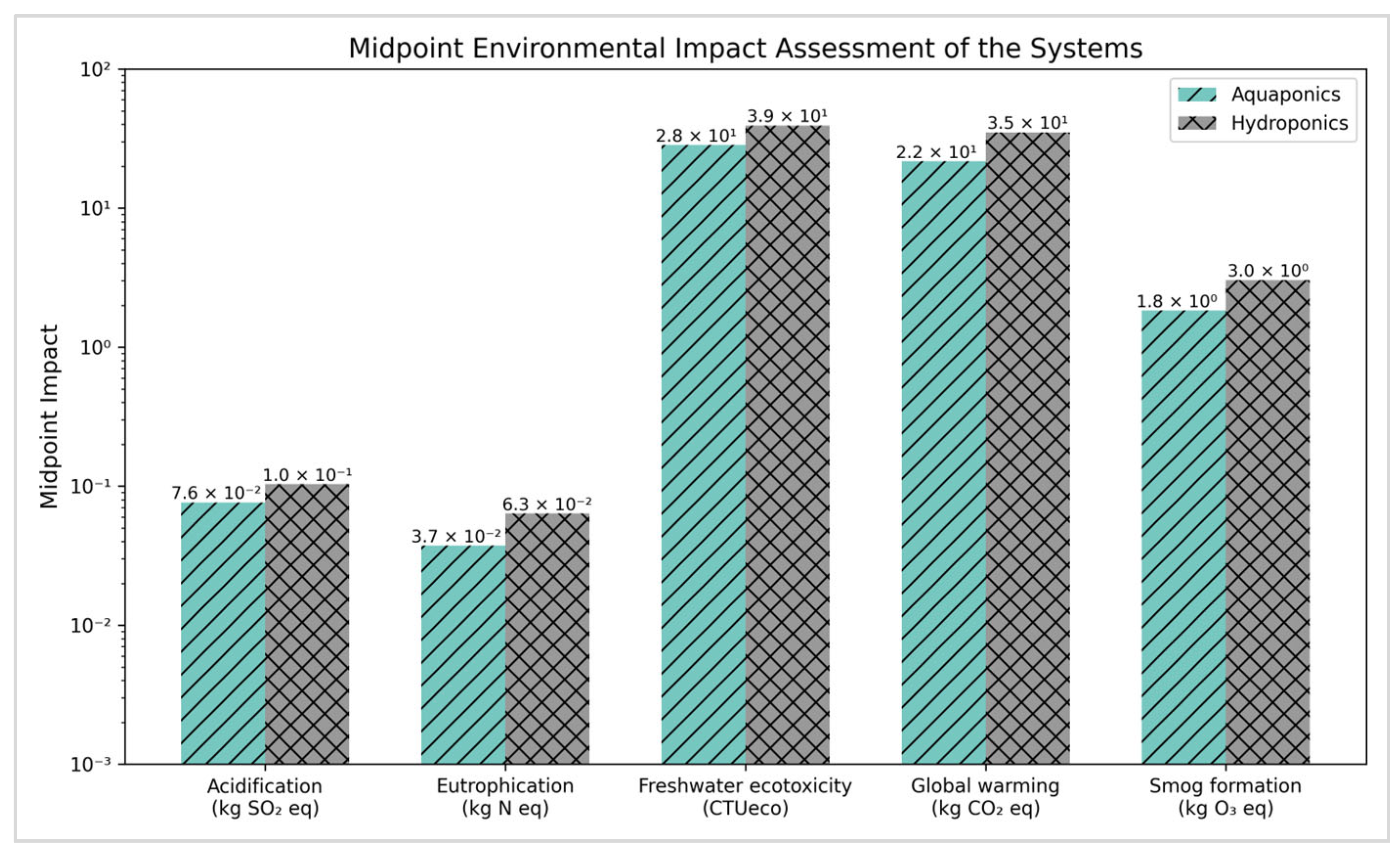
3.2.1. Midpoint Environmental Impacts
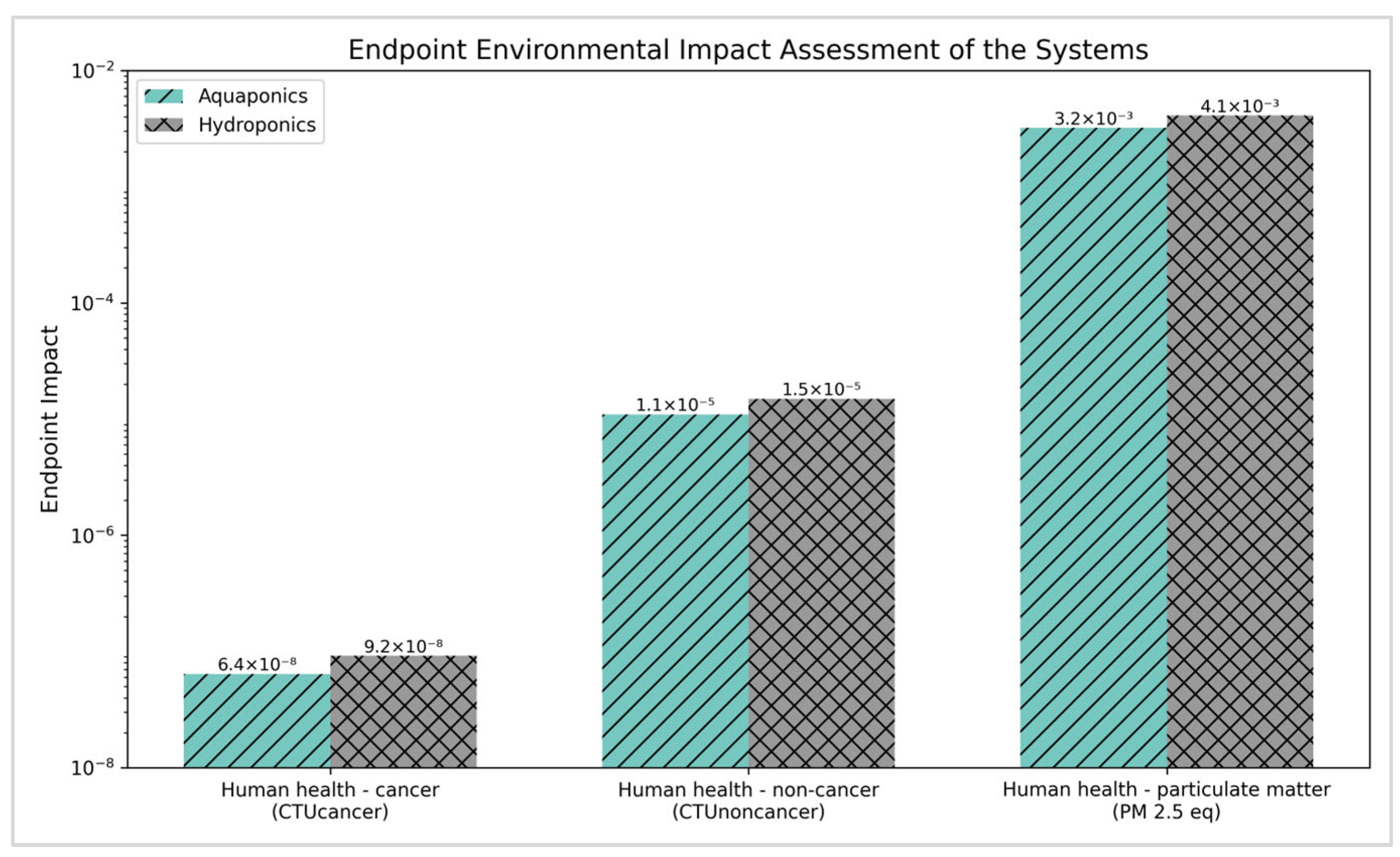
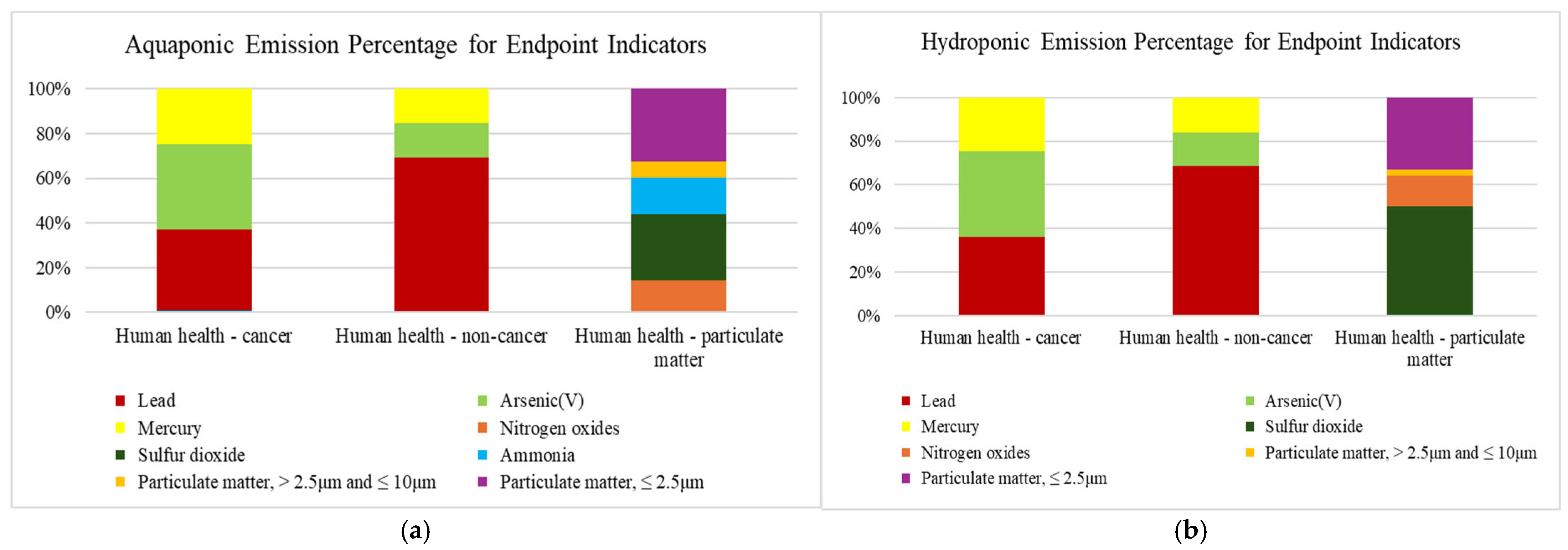
3.2.2. Endpoint Environmental Impacts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arsenault, T.L.; Prapayotin-Riveros, K.; Ammirata, M.A.; White, J.C.; Dimkpa, C.O. Compliance Testing of Hemp (Cannabis sativa L.) Cultivars for Total Delta-9 THC and Total CBD Using Gas Chromatography with Flame Ionization Detection. Plants 2024, 13, 519. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R. Defining hemp: A fact sheet. Congr. Res. Serv. 2019, 44742, 1–12. [Google Scholar]
- Small, E.; Marcus, D. Tetrahydrocannabinol levels in hemp (Cannabis sativa) germplasm resources. Econ. Bot. 2003, 57, 545–558. [Google Scholar] [CrossRef]
- Yang, R.; Berthold, E.C.; McCurdy, C.R.; da Silva Benevenute, S.; Brym, Z.T.; Freeman, J.H. Development of cannabinoids in flowers of industrial hemp (Cannabis sativa L.): A pilot study. J. Agric. Food Chem. 2020, 68, 6058–6064. [Google Scholar] [CrossRef]
- Gonçalves, J.; Rosado, T.; Soares, S.; Simão, A.Y.; Caramelo, D.; Luís, Â.; Fernández, N.; Barroso, M.; Gallardo, E.; Duarte, A.P. Cannabis and its secondary metabolites: Their use as therapeutic drugs, toxicological aspects, and analytical determination. Medicines 2019, 6, 31. [Google Scholar] [CrossRef]
- ElSohly, M.; Gul, W. Constituents of Cannabis sativa. Handb. Cannabis 2014, 3, 187–188. [Google Scholar]
- Gorelick, J.; Bernstein, N. Chemical and physical elicitation for enhanced cannabinoid production in cannabis. In Cannabis sativa L.-Botany and Biotechnology; Springer: Cham, Switzerland, 2017; pp. 439–456. [Google Scholar]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Bisson, J.; Singh, G.; Graham, J.G.; Chen, S.-N.; Friesen, J.B.; Dahlin, J.L.; Niemitz, M.; Walters, M.A.; Pauli, G.F. The essential medicinal chemistry of cannabidiol (CBD). J. Med. Chem. 2020, 63, 12137–12155. [Google Scholar] [CrossRef]
- Szaflarski, J.P.; Bebin, E.M.; Cutter, G.; DeWolfe, J.; Dure, L.S.; Gaston, T.E.; Kankirawatana, P.; Liu, Y.; Singh, R.; Standaert, D.G. Cannabidiol improves frequency and severity of seizures and reduces adverse events in an open-label add-on prospective study. Epilepsy Behav. 2018, 87, 131–136. [Google Scholar] [CrossRef]
- Rahn, E.J.; Hohmann, A.G. Cannabinoids as pharmacotherapies for neuropathic pain: From the bench to the bedside. Neurotherapeutics 2009, 6, 713–737. [Google Scholar] [CrossRef]
- Skrabek, R.Q.; Galimova, L.; Ethans, K.; Perry, D. Nabilone for the treatment of pain in fibromyalgia. J. Pain 2008, 9, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Farha, M.A.; El-Halfawy, O.M.; Gale, R.T.; MacNair, C.R.; Carfrae, L.A.; Zhang, X.; Jentsch, N.G.; Magolan, J.; Brown, E.D. Uncovering the hidden antibiotic potential of cannabis. ACS Infect. Dis. 2020, 6, 338–346. [Google Scholar] [CrossRef]
- Yep, B.; Gale, N.V.; Zheng, Y. Comparing hydroponic and aquaponic rootzones on the growth of two drug-type Cannabis sativa L. cultivars during the flowering stage. Ind. Crops Prod. 2020, 157, 112881. [Google Scholar] [CrossRef]
- Perez, J.; Ribera, M.V. Managing neuropathic pain with Sativex®: A review of its pros and cons. Expert Opin. Pharmacother. 2008, 9, 1189–1195. [Google Scholar] [CrossRef]
- Vernon, M.; Kouzani, A.Z.; Webb, L.D.; Adams, S.D. A Survey of Modern Greenhouse Technologies and Practices for Commercial Cannabis Cultivation. IEEE Access 2023, 11, 62077–62090. [Google Scholar] [CrossRef]
- U.S. Congress. Agricultural Act of 2014, H.R. 2642, 113th Cong. Enacted. 7 February 2014. Available online: https://www.congress.gov/bill/113th-congress/house-bill/2642 (accessed on 22 June 2024).
- Abernethy, A. Hemp Production and the 2018 Farm Bill; US Food and Drug Administration: Silver Spring, MD, USA, 2019. [Google Scholar]
- Idaho State Legislature. Industrial Hemp Research and Development Act, H.B. 126, 66th Legislature, 1st Regular Session. 2021. Available online: https://legislature.idaho.gov/wp-content/uploads/sessioninfo/2021/legislation/H0126.pdf (accessed on 5 June 2024).
- Farag, S.; Kayser, O. Cultivation and breeding of Cannabis sativa L. for preparation of standardized extracts for medicinal purposes. In Medicinal and Aromatic Plants of the World: Scientific, Production, Commercial and Utilization Aspects; Springer: Dordrecht, The Netherlands, 2015; pp. 165–186. [Google Scholar]
- Vanhove, W.; Van Damme, P.; Meert, N. Factors determining yield and quality of illicit indoor cannabis (Cannabis spp.) production. Forensic Sci. Int. 2011, 212, 158–163. [Google Scholar] [CrossRef]
- Heo, J.; Baek, J.; Subah, Z.; Ryu, J.H. Evaluating crop growth between hydroponics and aquaponics with different light inputs. Front. Hortic. 2024, 3, 1413224. [Google Scholar] [CrossRef]
- Bouchard, M.; Dion, C.B. Growers and facilitators: Probing the role of entrepreneurs in the development of the cannabis cultivation industry. J. Small Bus. Entrep. 2009, 22, 25–37. [Google Scholar] [CrossRef]
- Ali, A.; Niu, G.; Masabni, J.; Ferrante, A.; Cocetta, G. Integrated nutrient management of fruits, vegetables, and crops through the use of biostimulants, soilless cultivation, and traditional and modern approaches—A mini review. Agriculture 2024, 14, 1330. [Google Scholar] [CrossRef]
- Bevan, L.; Jones, M.; Zheng, Y. Optimisation of nitrogen, phosphorus, and potassium for soilless production of Cannabis sativa in the flowering stage using response surface analysis. Front. Plant Sci. 2021, 12, 764103. [Google Scholar] [CrossRef]
- Jin, D.; Jin, S.; Chen, J. Cannabis indoor growing conditions, management practices, and post-harvest treatment: A review. Am. J. Plant Sci. 2019, 10, 925. [Google Scholar] [CrossRef]
- Ryu, J.H.; Subah, Z.; Baek, J. An Application of System Dynamics to Characterize Crop Production for Autonomous Indoor Farming Platforms (AIFP). Horticulturae 2023, 9, 1318. [Google Scholar] [CrossRef]
- Ryu, J.H.; Baek, J.; Subah, Z. A low-cost autonomous and scalable hydroponics system for space farming. HardwareX 2025, 21, e00625. [Google Scholar] [CrossRef]
- Yep, B.; Zheng, Y. Aquaponic trends and challenges—A review. J. Clean. Prod. 2019, 228, 1586–1599. [Google Scholar] [CrossRef]
- König, B.; Janker, J.; Reinhardt, T.; Villarroel, M.; Junge, R. Analysis of aquaponics as an emerging technological innovation system. J. Clean. Prod. 2018, 180, 232–243. [Google Scholar] [CrossRef]
- Palm, H.W.; Knaus, U.; Appelbaum, S.; Goddek, S.; Strauch, S.M.; Vermeulen, T.; Haїssam Jijakli, M.; Kotzen, B. Towards commercial aquaponics: A review of systems, designs, scales and nomenclature. Aquac. Int. 2018, 26, 813–842. [Google Scholar] [CrossRef]
- Punja, Z.K.; Rodriguez, G. Fusarium and Pythium species infecting roots of hydroponically grown marijuana (Cannabis sativa L.) plants. Can. J. Plant Pathol. 2018, 40, 498–513. [Google Scholar] [CrossRef]
- Arru, L.; Rognoni, S.; Baroncini, M.; Bonatti, P.M.; Perata, P. Copper localization in Cannabis sativa L. grown in a copper-rich solution. Euphytica 2004, 140, 33–38. [Google Scholar] [CrossRef]
- Pannico, A.; Kyriacou, M.C.; El-Nakhel, C.; Graziani, G.; Carillo, P.; Corrado, G.; Ritieni, A.; Rouphael, Y.; De Pascale, S. Hemp microgreens as an innovative functional food: Variation in the organic acids, amino acids, polyphenols, and cannabinoids composition of six hemp cultivars. Food Res. Int. 2022, 161, 111863. [Google Scholar] [CrossRef]
- Mi, R. Cultural Management, Production, and Consumer Sensory Evaluation of Baby Leaf Hemp (Cannabis sativa L.) as an Edible Salad Green. 2020. Available online: https://ecommons.cornell.edu/bitstream/1813/103091/1/Mi_cornell_0058O_10970.pdf (accessed on 5 June 2024).
- Mi, R.; Taylor, A.G.; Smart, L.B.; Mattson, N.S. Developing production guidelines for baby leaf hemp (Cannabis sativa L.) as an edible salad green: Cultivar, sowing density and seed size. Agriculture 2020, 10, 617. [Google Scholar] [CrossRef]
- Roman, M.; Li, M.; Yuan, J.; Rehman, M.; Liu, L. Dynamics of industrial hemp vegetative growth and metabolite accumulation in response to light intensity. S. Afr. J. Bot. 2024, 169, 82–94. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, X.; Long, S.; Xiao, Q.; Guo, Y.; Qiu, C.; Qiu, H.; Wang, Y. Wavelengths of LED light affect the growth and cannabidiol content in Cannabis sativa L. Ind. Crops Prod. 2021, 165, 113433. [Google Scholar] [CrossRef]
- Moher, M.; Llewellyn, D.; Jones, M.; Zheng, Y. Light intensity can be used to modify the growth and morphological characteristics of cannabis during the vegetative stage of indoor production. Ind. Crops Prod. 2022, 183, 114909. [Google Scholar] [CrossRef]
- Eaves, J.; Eaves, S.; Morphy, C.; Murray, C. The relationship between light intensity, cannabis yields, and profitability. Agron. J. 2020, 112, 1466–1470. [Google Scholar] [CrossRef]
- Xie, K.; Rosentrater, K. Life cycle assessment (LCA) and Techno-economic analysis (TEA) of tilapia-basil aquaponics. In Proceedings of the 2015 ASABE Annual International Meeting, New Orleans, LA, USA, 26–29 July 2015; p. 1. [Google Scholar]
- Chen, P.; Zhu, G.; Kim, H.-J.; Brown, P.B.; Huang, J.-Y. Comparative life cycle assessment of aquaponics and hydroponics in the Midwestern United States. J. Clean. Prod. 2020, 275, 122888. [Google Scholar] [CrossRef]
- Boxman, S.E.; Zhang, Q.; Bailey, D.; Trotz, M.A. Life cycle assessment of a commercial-scale freshwater aquaponic system. Environ. Eng. Sci. 2017, 34, 299–311. [Google Scholar] [CrossRef]
- Lobillo-Eguíbar, J.; Fernández-Cabanás, V.M.; Bermejo, L.A.; Pérez-Urrestarazu, L. Economic sustainability of small-scale aquaponic systems for food self-production. Agronomy 2020, 10, 1468. [Google Scholar] [CrossRef]
- Love, D.C.; Fry, J.P.; Genello, L.; Hill, E.S.; Frederick, J.A.; Li, X.; Semmens, K. An international survey of aquaponics practitioners. PLoS ONE 2014, 9, e102662. [Google Scholar] [CrossRef]
- Lages Barbosa, G.; Almeida Gadelha, F.D.; Kublik, N.; Proctor, A.; Reichelm, L.; Weissinger, E.; Wohlleb, G.M.; Halden, R.U. Comparison of land, water, and energy requirements of lettuce grown using hydroponic vs. conventional agricultural methods. Int. J. Environ. Res. Public Health 2015, 12, 6879–6891. [Google Scholar] [CrossRef]
- Goddek, S.; Delaide, B.; Mankasingh, U.; Ragnarsdottir, K.V.; Jijakli, H.; Thorarinsdottir, R. Challenges of sustainable and commercial aquaponics. Sustainability 2015, 7, 4199–4224. [Google Scholar] [CrossRef]
- Cohen, A.; Malone, S.; Morris, Z.; Weissburg, M.; Bras, B. Combined fish and lettuce cultivation: An aquaponics life cycle assessment. Procedia Cirp 2018, 69, 551–556. [Google Scholar] [CrossRef]
- Forchino, A.A.; Lourguioui, H.; Brigolin, D.; Pastres, R. Aquaponics and sustainability: The comparison of two different aquaponic techniques using the Life Cycle Assessment (LCA). Aquac. Eng. 2017, 77, 80–88. [Google Scholar] [CrossRef]
- Nuwansi, K.; Verma, A.; Chandrakant, M.; Prabhath, G.; Peter, R. Optimization of stocking density of koi carp (Cyprinus carpio var. koi) with gotukola (Centella asiatica) in an aquaponic system using phytoremediated aquaculture wastewater. Aquaculture 2021, 532, 735993. [Google Scholar] [CrossRef]
- Hussain, T.; Verma, A.; Tiwari, V.; Prakash, C.; Rathore, G.; Shete, A.; Nuwansi, K. Optimizing koi carp, Cyprinus carpio var. koi (Linnaeus, 1758), stocking density and nutrient recycling with spinach in an aquaponic system. J. World Aquac. Soc. 2014, 45, 652–661. [Google Scholar] [CrossRef]
- Jones, B.; Nachtsheim, C.J. Split-plot designs: What, why, and how. J. Qual. Technol. 2009, 41, 340–361. [Google Scholar] [CrossRef]
- Altman, N.; Krzywinski, M. Points of significance: Split plot design. Nat. Methods 2015, 12, 165. [Google Scholar] [CrossRef]
- Ferrarezi, R.S.; Bailey, D.S. Basil performance evaluation in aquaponics. HortTechnology 2019, 29, 85–93. [Google Scholar] [CrossRef]
- Bare, J.C. TRACI: The tool for the reduction and assessment of chemical and other environmental impacts. J. Ind. Ecol. 2002, 6, 49–78. [Google Scholar] [CrossRef]
- Pacheco, R.; Silva, C. Global warming potential of biomass-to-ethanol: Review and sensitivity analysis through a case study. Energies 2019, 12, 2535. [Google Scholar] [CrossRef]
- Salam, M.; Prodhan, M.; Sayem, S.; Islam, M. Comparative growth performances of taro plant in aquaponics vs other systems. Int. J. Innov. Appl. Stud. 2014, 7, 941. [Google Scholar]
- Gebhart, B.; White, M.; Montouri, C.; Karl, V. Comparing Multiple Forms of Light on Different Cultivars of Swiss Chard in an Ebb and Flow Bench Aquaponics System; University of Minnesota Aquaponics: St. Paul, MN, USA, 2015. [Google Scholar]
- Delaide, B.; Goddek, S.; Gott, J.; Soyeurt, H.; Jijakli, M.H. Lettuce (Lactuca sativa L. var. Sucrine) growth performance in complemented aquaponic solution outperforms hydroponics. Water 2016, 8, 467. [Google Scholar] [CrossRef]
- Suhl, J.; Dannehl, D.; Kloas, W.; Baganz, D.; Jobs, S.; Scheibe, G.; Schmidt, U. Advanced aquaponics: Evaluation of intensive tomato production in aquaponics vs. conventional hydroponics. Agric. Water Manag. 2016, 178, 335–344. [Google Scholar] [CrossRef]
- Jordan, R.A.; Ribeiro, E.F.; Oliveira, F.C.d.; Geisenhoff, L.O.; Martins, E.A. Yield of lettuce grown in hydroponic and aquaponic systems using different substrates. Rev. Bras. de Eng. Agrícola e Ambient. 2018, 22, 525–529. [Google Scholar] [CrossRef]
- Jaeger, C.; Foucard, P.; Tocqueville, A.; Nahon, S.; Aubin, J. Mass balanced based LCA of a common carp-lettuce aquaponics system. Aquac. Eng. 2019, 84, 29–41. [Google Scholar] [CrossRef]
- Bugbee, B. Nutrient management in recirculating hydroponic culture. In Proceedings of the South Pacific Soilless Culture Conference-SPSCC 648, Palmerston North, New Zealand, 10–13 February 2003; pp. 99–112. [Google Scholar]
- Colt, J.; Schuur, A.M. Comparison of nutrient costs from fish feeds and inorganic fertilizers for aquaponics systems. Aquac. Eng. 2021, 95, 102205. [Google Scholar] [CrossRef]
- Cerozi, B.d.S.; Fitzsimmons, K. Phosphorus dynamics modeling and mass balance in an aquaponics system. Agric. Syst. 2017, 153, 94–100. [Google Scholar] [CrossRef]
- Dell, M.v.L.-M. Analysis of the Environmental Impacts of a Hydroponic System with Combined Wastewater Treatment Using Life Cycle Assessment. 2023. Available online: https://www.hypowave.de/fileadmin/user_upload/Ergebnisse/Publikationen/Masterarbeit_Laura-Maria_Dell_HyW_Website.pdf (accessed on 22 June 2024).
- Dessus, B.; Le Treut, H.; Laponche, B. Global Warming: The Significance of Methane. 2008. Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:42007554 (accessed on 22 June 2024).
- Hardaker, A.; Styles, D.; Williams, P.; Chadwick, D.; Dandy, N. A framework for integrating ecosystem services as endpoint impacts in life cycle assessment. J. Clean. Prod. 2022, 370, 133450. [Google Scholar] [CrossRef]
- Ghamkhar, R.; Hartleb, C.; Wu, F.; Hicks, A. Life cycle assessment of a cold weather aquaponic food production system. J. Clean. Prod. 2020, 244, 118767. [Google Scholar] [CrossRef]
- Barone, G.; Storelli, A.; Garofalo, R.; Mallamaci, R.; Storelli, M.M. Residual levels of mercury, cadmium, lead and arsenic in some commercially key species from Italian coasts (Adriatic Sea): Focus on human health. Toxics 2022, 10, 223. [Google Scholar] [CrossRef]
- Parker, G.H.; Gillie, C.E.; Miller, J.V.; Badger, D.E.; Kreider, M.L. Human health risk assessment of arsenic, cadmium, lead, and mercury ingestion from baby foods. Toxicol. Rep. 2022, 9, 238–249. [Google Scholar] [CrossRef]
- Pietrzak, S.; Wójcik, J.; Baszuk, P.; Marciniak, W.; Wojtyś, M.; Dębniak, T.; Cybulski, C.; Gronwald, J.; Alchimowicz, J.; Masojć, B. Influence of the levels of arsenic, cadmium, mercury and lead on overall survival in lung cancer. Biomolecules 2021, 11, 1160. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.K.; Baki, M.A.; Islam, M.S.; Kundu, G.K.; Habibullah-Al-Mamun, M.; Sarkar, S.K.; Hossain, M.M. Human health risk assessment of heavy metals in tropical fish and shellfish collected from the river Buriganga, Bangladesh. Environ. Sci. Pollut. Res. 2015, 22, 15880–15890. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, M.; Asaduzzaman, K.; Nawi, S.; Usman, A.; Amin, Y.; Daar, E.; Bradley, D.; Ahmed, H.; Okhunov, A. Assessment of radiation and heavy metals risk due to the dietary intake of marine fishes (Rastrelliger kanagurta) from the Straits of Malacca. PLoS ONE 2015, 10, e0128790. [Google Scholar] [CrossRef] [PubMed]
- US-EPA. Non-Cancer Screening Approaches for Health Effects. Available online: https://www.epa.gov/tsca-screening-tools/non-cancer-screening-approaches-health-effects (accessed on 21 June 2024).


| Inputs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ingredients | Unit | Amount | Per FU (Aqua) | Per FU (Hydro) | ||||
| Aqua Fish Feed | Total | kg | 0.90 | 0.30 | - | |||
| Soybean meal | kg | 0.27 | 0.09 | - | ||||
| Ground corn | kg | 0.13 | 0.04 | - | ||||
| Fishmeal (including meat meal) | kg | 0.36 | 0.12 | - | ||||
| Wheat | kg | 0.05 | 0.02 | - | ||||
| Nutrient | Total N | L | 0.08 | - | 0.04 | |||
| P2O5 | L | 0.04 | - | 0.02 | ||||
| K2O | L | 0.23 | - | 0.13 | ||||
| MgSO4 | L | 0.02 | - | 0.01 | ||||
| Biofilter | Filter material | kg | 0.15 | 0.05 | 0.03 | |||
| Gravel | kg | 3.85 | 1.28 | 2.14 | ||||
| Fish Tank | Glass Aquarium | kg | 136.08 | 45.36 | - | |||
| Hemp Seeds | - | kg | 0.14 | 0.05 | 0.08 | |||
| Electronic Equipment | IoT devices | kg | 1.22 | 0.41 | 0.68 | |||
| Light bulbs | kg | 1.86 | 0.62 | 1.03 | ||||
| Koi Fish | - | kg | 0.85 | 0.28 | - | |||
| Polyethylene | - | kg | 9.49 | 3.16 | 5.27 | |||
| PVC | - | kg | 8.85 | 2.95 | 4.92 | |||
| Water Usage (aqua) | - | L | 151.42 | 50.47 | - | |||
| Water Usage (hydro) | - | L | 64.00 | - | 35.55 | |||
| Electricity (aqua) | Total | Wh | 172.00 | 57.33 | - | |||
| Light | Wh | 43.00 | 14.33 | - | ||||
| IoT devices | Wh | 8.58 | 2.86 | - | ||||
| Water pump motor | Wh | 120.42 | 40.14 | - | ||||
| Electricity (hydro) | Total | Wh | 144.00 | - | 80.00 | |||
| Light | Wh | 36.00 | - | 20.00 | ||||
| IoT devices | Wh | 7.20 | - | 4.00 | ||||
| Water pump motor | Wh | 100.80 | - | 56.00 | ||||
| Outputs | ||||||||
| Unit | Aquaponics | Hydroponics | ||||||
| Mass Produced | kg | 3.0 | 1.8 | |||||
| Model: ‘Leaf Area’ ~ LI + (1 | LI:Day) + Systems + LI:Systems | ||||
|---|---|---|---|---|
| Effect | df | F Value | p-Value | |
| 1 | Light Intensity (LI) | 1, 30 | 3.57 + | 0.069 |
| 2 | Systems (Hydroponics and Aquaponics) | 5, 150 | 34.33 *** | <0.001 |
| 3 | LI:Systems | 5, 150 | 15.25 *** | <0.001 |
| Model: ‘Plant Length’ ~ LI + (1 | LI:Day) + Systems + LI:Systems | ||||
|---|---|---|---|---|
| Effect | df | F Value | p-Value | |
| 1 | Light Intensity (LI) | 1, 42 | 7.26 * | 0.010 |
| 2 | Systems (Hydroponics and Aquaponics) | 5, 210 | 62.60 *** | <0.001 |
| 3 | LI:Systems | 5, 210 | 7.98 *** | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subah, Z.; Ryu, J.H.; Mirkouei, A. Comparative Study on Aquaponic and Hydroponic Systems for Sustainable Hemp Production in a Controlled Environment. Horticulturae 2025, 11, 588. https://doi.org/10.3390/horticulturae11060588
Subah Z, Ryu JH, Mirkouei A. Comparative Study on Aquaponic and Hydroponic Systems for Sustainable Hemp Production in a Controlled Environment. Horticulturae. 2025; 11(6):588. https://doi.org/10.3390/horticulturae11060588
Chicago/Turabian StyleSubah, Zarin, Jae Hyeon Ryu, and Amin Mirkouei. 2025. "Comparative Study on Aquaponic and Hydroponic Systems for Sustainable Hemp Production in a Controlled Environment" Horticulturae 11, no. 6: 588. https://doi.org/10.3390/horticulturae11060588
APA StyleSubah, Z., Ryu, J. H., & Mirkouei, A. (2025). Comparative Study on Aquaponic and Hydroponic Systems for Sustainable Hemp Production in a Controlled Environment. Horticulturae, 11(6), 588. https://doi.org/10.3390/horticulturae11060588







