Enhancing Tomato (Solanum lycopersicum L.) Resistance Against Bacterial Canker Disease (Clavibacter michiganensis ssp. michiganensis) via Seed Priming with β-Aminobutyric Acid (BABA)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Synthetic Elisitor Preparation: Preparation of β-aminobutyric Acid (BABA), BABA Seed Priming, and Spraying Applications
2.3. Bacterial Strain, Inoculation, and Disease Measurement
2.4. Gene Expression Analyses
2.5. Statistical Analyses
3. Results
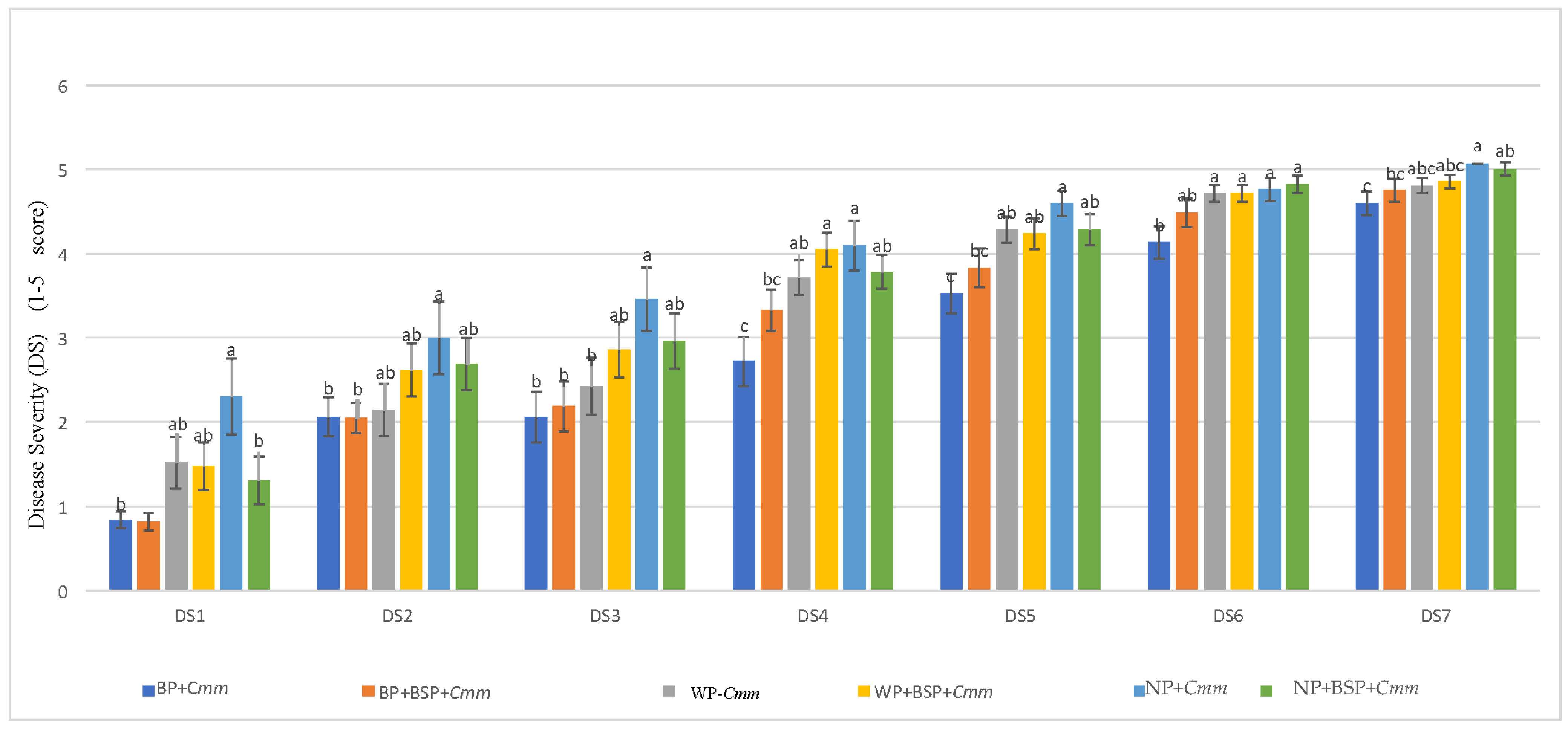
3.1. Effect of BABA Seed Priming on Cmm Disease Severity and Progression


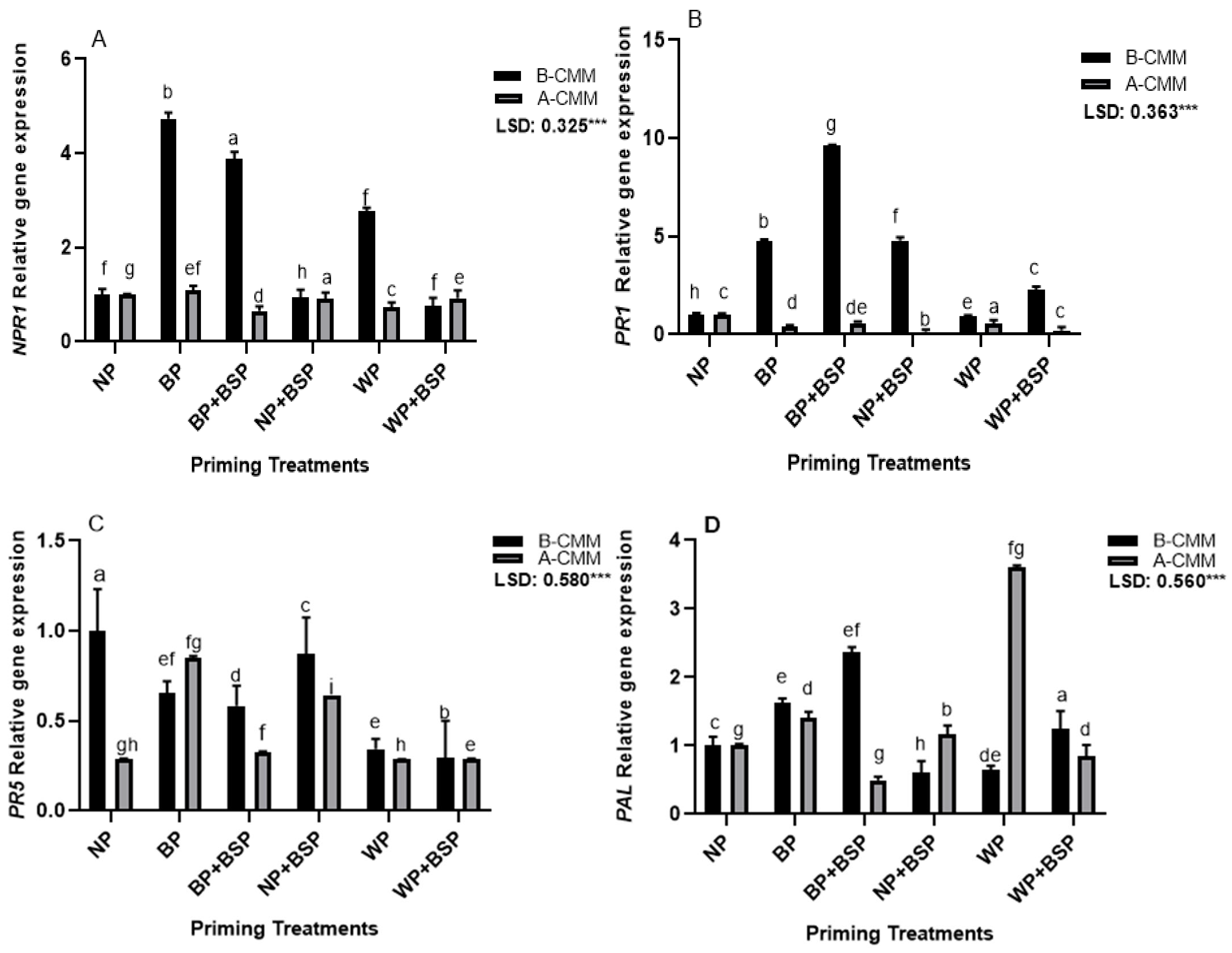
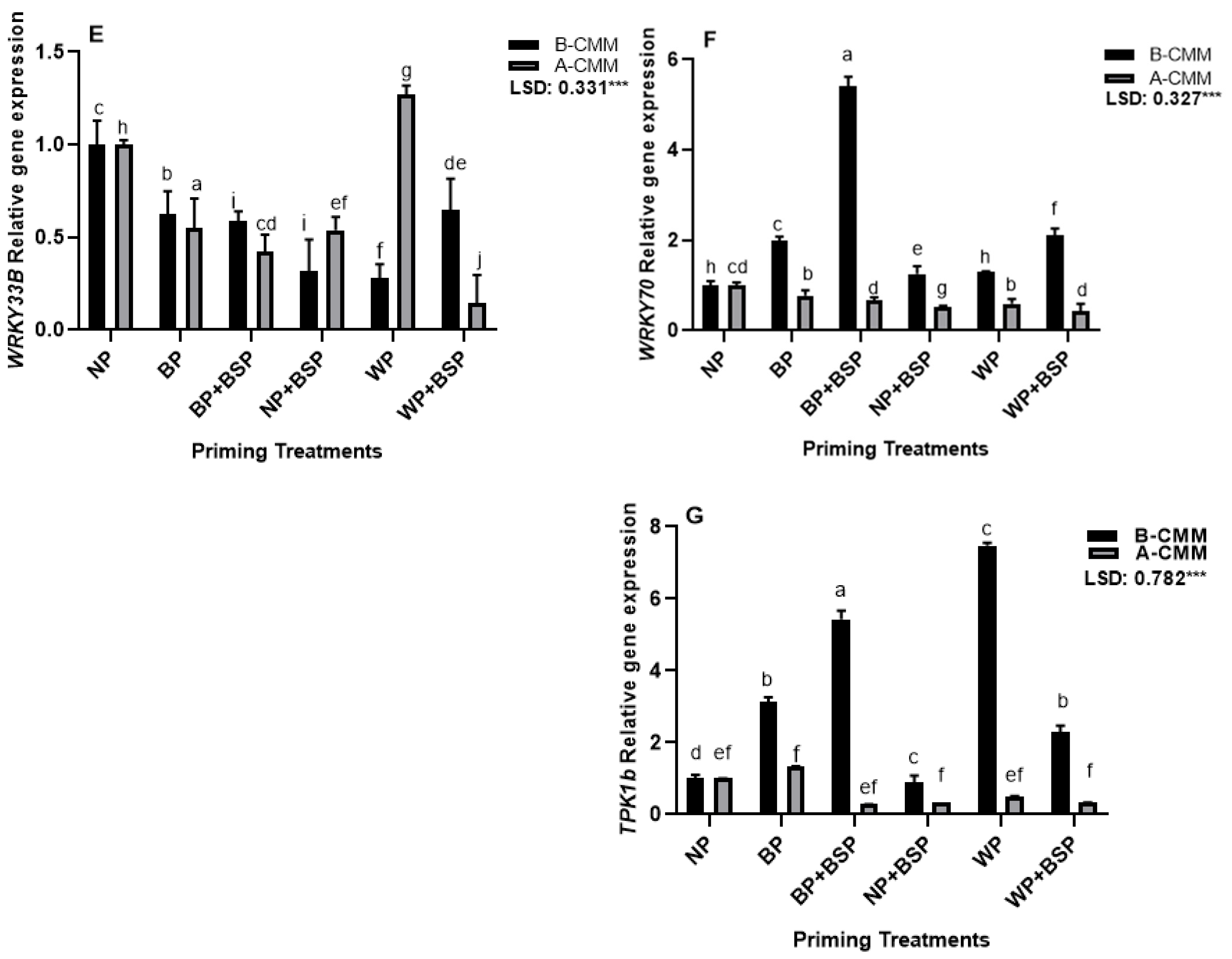
3.2. Gene Expression Analysis
4. Discussion
4.1. Effect of BABA Seed Priming on Cmm Disease Severity and Progression
4.2. Gene Expression Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PR1 | Pathogenesis-Related 1 gene |
| PR5 | Pathogenesis-Related 5 gene |
| NPR1 | Nonexpressor of the PR1 gene |
| WRKY70 | WRKY Transcription Factor 70 |
| WRKY33B | WRKY Transcription Factor 33b |
| TPK1b | Tomato Protein Kinase 1b |
| BABA | Beta-Aminobutyric Acid |
| LSD | Least Significant Difference |
| INA | 2,6-Dichloroisonicotinic acid |
| DPMP | 2,4-Dichloro-6-{(E)-[(3-Methoxyphenyl)imino]methyl}phenol |
| JA | Jasmonic Acid |
| SA | Salicylic Acid |
| NPK | Nitrogen, Phosphorus, and Potassium |
| DS | Disease Severity |
| WP | Water Priming |
| BP | BABA Priming |
| NP | Non-primed |
| BSP | BABA Spray Priming |
References
- Jisha, K.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant. 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Sher, A.; Sarwar, T.; Nawaz, A.; Ijaz, M.; Sattar, A.; Ahmad, S. Methods of Seed Priming. Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Springer: Singapore, 2019; pp. 1–10. [Google Scholar]
- Kaya, G. Tohum Uygulamaları (Priming)’nın Tohum Yağ Asitleri Kompozisyonuna Etkisi ve Tohum Kalitesi ile İliskisi. Tarla Bitk. Merk. Araştırma Enstitüsü Derg. 2008, 17, 53–62. [Google Scholar]
- McDonald, M. Seed deterioration: Physiology, repair and assessment. Seed Sci. Technol. 1999, 27, 177–237. [Google Scholar]
- Karakurt, H.; Aslantaş, R.; Eşitken, A. Tohum çimlenmesi ve bitki büyümesi üzerinde etkili olan çevresel faktörler ve bazı ön uygulamalar. Uludağ Üniversitesi Ziraat Fakültesi Derg. 2010, 24, 115–128. [Google Scholar]
- Gökçöl, A.; Duman, İ. Kapari tohumlarının çimlenmesinin iyileştirilmesinde farklı tohum uygulamalarının etkisinin belirlenmesi. J. Agric. Fac. Ege Univ. 2018, 55, 71–80. [Google Scholar]
- Zulfiqar, F. Effect of seed priming on horticultural crops. Sci. Hortic. 2021, 286, 8. [Google Scholar] [CrossRef]
- Heydecker, W.; Gibbins, B. Attempts to synchronise seed germination. Acta Hortic. 1978, 72, 79–92. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Mostek, A.; Börner, A.; Weidner, S. Comparative proteomic analysis of β-aminobutyric acid-mediated alleviation of salt stress in barley. Plant Physiol. Biochem. 2016, 99, 150–161. [Google Scholar] [CrossRef]
- Uddin, S.; Ullah, S.; Nafees, M. Effect of seed priming on growth and performance of Vigna radiata L. under induced drought stress. J. Agric. Food Res. 2021, 4, 100140. [Google Scholar] [CrossRef]
- Yokotani, N.; Hasegawa, Y.; Sato, M.; Hirakawa, H.; Kouzai, Y.; Nishizawa, Y.; Yamamoto, E.; Naito, Y.; Isobe, S. Transcriptome Analysis of Clavibacter Michiganensis Subsp. Michiganensis-Infected Tomatoes: A Role of Salicylic Acid in the Host Response. BMC Plant Biol. 2021, 21, 476. [Google Scholar] [CrossRef] [PubMed]
- Lee SangHyeob, L.S.; Hong JongChan, H.J.; Jeon WoongBae, J.W.; Chung YoungSoo, C.Y.; Sung SoonKee, S.S.; Choi Doil, C.D.; Joung YoungHee, J.Y.; Oh BoungJun, O.B. The salicylic acid-induced protection of non-climacteric unripe pepper fruit against Colletotrichum gloeosporioides is similar to the resistance of ripe fruit. Plant Cell Rep. 2009, 28, 1573–1580. [Google Scholar]
- Özkaynak, E.; Orhan, Y.; Kargın, İ.; Tuncel, M. Biber ve domates tohumlarında organik priming uygulamaları. Black Sea J. Agric. 2020, 3, 301–307. [Google Scholar]
- Cohen, Y.; Vaknin, M.; Mauch-Mani, B. BABA-induced resistance: Milestones along a 55-year journey. Phytoparasitica 2016, 44, 513–538. [Google Scholar] [CrossRef]
- Jakab, G.; Manrique, A.; Zimmerli, L.; Métraux, J.-P.; Mauch-Mani, B. Molecular characterization of a novel lipase-like pathogen-inducible gene family of Arabidopsis. Plant Physiol. 2003, 132, 2230–2239. [Google Scholar] [CrossRef] [PubMed]
- Bektas, Y.; Eulgem, T. Synthetic Plant Defense Elicitors. Front. Plant Sci. 2015, 5, 804. [Google Scholar] [CrossRef]
- Weralupitiya, C.; Eccersall, S.; Meisrimler, C.-N. Shared signals, different fates: Calcium and ROS in plant PRR and NLR immunity. Cell Rep. 2024, 43, 114910. [Google Scholar] [CrossRef]
- Wang, D.; Wei, L.; Liu, T.; Ma, J.; Huang, K.; Guo, H.; Huang, Y.; Zhang, L.; Zhao, J.; Tsuda, K. Suppression of ETI by PTI priming to balance plant growth and defense through an MPK3/MPK6-WRKYs-PP2Cs module. Mol. Plant 2023, 16, 903–918. [Google Scholar] [CrossRef]
- Zimmerli, L.; Hou, B.H.; Tsai, C.H.; Jakab, G.; Mauch-Mani, B.; Somerville, S. The xenobiotic β-aminobutyric acid enhances Arabidopsis thermotolerance. Plant J. 2008, 53, 144–156. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kim, Y.H.; Lee, Y.H.; Lee, S.W.; Chae, Y.-S.; Kang, H.-K.; Yun, B.-W.; Hong, J.K. β-Amino-n-butyric acid regulates seedling growth and disease resistance of kimchi cabbage. Plant Pathol. J. 2013, 29, 305. [Google Scholar] [CrossRef]
- Baccelli, I.; Mauch-Mani, B. Beta-aminobutyric acid priming of plant defense: The role of ABA and other hormones. Plant Mol. Biol. 2016, 91, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.R. β-aminobutyric acid-induced resistance against plant pathogens. Plant Dis. 2002, 86, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Luna, E.; Beardon, E.; Ravnskov, S.; Scholes, J.; Ton, J. Optimizing chemically induced resistance in tomato against Botrytis cinerea. Plant Dis. 2016, 100, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Luo, H.; He, L.; Zhang, L.; Liu, Y.; Mo, Z.; Pan, S.; Tian, H.; Duan, M.; Tang, X. Rice seed priming with sodium selenate: Effects on germination, seedling growth, and biochemical attributes. Sci. Rep. 2019, 9, 4311. [Google Scholar] [CrossRef]
- Jisha, K.; Puthur, J.T. Seed priming with BABA (β-amino butyric acid): A cost-effective method of abiotic stress tolerance in Vigna radiata (L.) Wilczek. Protoplasma 2016, 253, 277–289. [Google Scholar] [CrossRef]
- Kulak, M.; Jorrín-Novo, J.V.; Romero-Rodriguez, M.C.; Yildirim, E.D.; Gul, F.; Karaman, S. Seed priming with salicylic acid on plant growth and essential oil composition in basil (Ocimum basilicum L.) plants grown under water stress conditions. Ind. Crop. Prod. 2021, 161, 113235. [Google Scholar] [CrossRef]
- Martínez-Aguilar, K.; Hernández-Chávez, J.L.; Alvarez-Venegas, R. Priming of seeds with INA and its transgenerational effect in common bean (Phaseolus vulgaris L.) plants. Plant Sci. 2021, 305, 110834. [Google Scholar] [CrossRef]
- Chang, R.; Ries, S.; Pataky, J. Reductions in yield of processing tomatoes and incidence of bacterial canker. Plant Dis. 1992, 76, 85–89. [Google Scholar] [CrossRef]
- Nandi, M.; Macdonald, J.; Liu, P.; Weselowski, B.; Yuan, Z.C. Clavibacter michiganensis ssp. michiganensis: Bacterial canker of tomato, molecular interactions and disease management. Mol. Plant Pathol. 2018, 19, 2036–2050. [Google Scholar] [CrossRef]
- Poysa, V. Evaluation of tomato breeding lines resistant to bacterial canker. Can. J. Plant Pathol. 1993, 15, 301–304. [Google Scholar] [CrossRef]
- Chalupowicz, L.; Barash, I.; Reuven, M.; Dror, O.; Sharabani, G.; Gartemann, K.H.; Eichenlaub, R.; Sessa, G.; Manulis-Sasson, S. Differential contribution of Clavibacter michiganensis ssp. michiganensis virulence factors to systemic and local infection in tomato. Mol. Plant Pathol. 2017, 18, 336–346. [Google Scholar] [CrossRef]
- Holtsmark, I.; Mantzilas, D.; Eijsink, V.; Brurberg, M. The tomato pathogen Clavibacter michiganensis ssp. michiganensis: Producer of several antimicrobial substances. J. Appl. Microbiol. 2007, 102, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Eichenlaub, R.; Gartemann, K.-H. The Clavibacter michiganensis subspecies: Molecular investigation of gram-positive bacterial plant pathogens. Annu. Rev. Phytopathol. 2011, 49, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Sharabani, G.; Manulis-Sasson, S.; Borenstein, M.; Shulhani, R.; Lofthouse, M.; Chalupowicz, L.; Shtienberg, D. The significance of guttation in the secondary spread of Clavibacter michiganensis subsp. michiganensis in tomato greenhouses. Plant Pathol. 2013, 62, 578–586. [Google Scholar] [CrossRef]
- Vega, D.; Romero, A.M. Survival of Clavibacter michiganensis subsp. michiganensis in tomato debris under greenhouse conditions. Plant Pathol. 2016, 65, 545–550. [Google Scholar] [CrossRef]
- Yuqing, W.; Zhang, Y.; Zhipeng, G.; Wencai, Y. Breeding for resistance to tomato bacterial diseases in China: Challenges and prospects. Hortic. Plant J. 2018, 4, 193–207. [Google Scholar]
- Mohammad, H.B.; Lee, S.E.; Kim, M.S. Refining a Pathogen’s Genome to Know the Enemy Better for the Winning Battles. Proteomics 2019, 19, 1900005. [Google Scholar] [CrossRef]
- Mohd Nadzir, M.; Vieira Lelis, F.; Thapa, B.; Ali, A.; Visser, R.; van Heusden, A.; Van der Wolf, J. Development of an in vitro protocol to screen Clavibacter michiganensis subsp. michiganensis pathogenicity in different Solanum species. Plant Pathol. 2019, 68, 42–48. [Google Scholar] [CrossRef]
- Bektas, Y. The synthetic elicitors 2, 6-dichloro-isonicotinic acid (INA) and 2, 4-dichloro-6-{(E)-[(3-methoxyphenyl) imino] methyl} phenol (DPMP) enhances tomato resistance against bacterial canker disease with different molecular mechanisms. Physiol. Mol. Plant Pathol. 2021, 116, 101740. [Google Scholar] [CrossRef]
- Abbasi, S.; Sadeghi, A.; Omidvari, M.; Tahan, V. The stimulators and responsive genes to induce systemic resistance against pathogens: An exclusive focus on tomato as a model plant. Biocatal. Agric. Biotechnol. 2021, 33, 101993. [Google Scholar] [CrossRef]
- Bektas, H.; Hohn, C.E.; Waines, J.G. Root and shoot traits of bread wheat (Triticum aestivum L.) landraces and cultivars. Euphytica 2016, 212, 297–311. [Google Scholar] [CrossRef]
- Baysal, Ö.; Soylu, E.M.; Soylu, S. Induction of defence-related enzymes and resistance by the plant activator acibenzolar-S-methyl in tomato seedlings against bacterial canker caused by Clavibacter michiganensis ssp. michiganensis. Plant Pathol. 2003, 52, 747–753. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Molinari, S.; Fanelli, E.; Leonetti, P. Expression of tomato salicylic acid (SA)-responsive pathogenesis-related genes in Mi-1-mediated and SA-induced resistance to root-knot nematodes. Mol. Plant Pathol. 2014, 15, 255–264. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, J.; Wang, N. Control of citrus Huanglongbing via trunk injection of plant defense activators and antibiotics. Phytopathology 2018, 108, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Atamian, H.S.; Eulgem, T.; Kaloshian, I. SlWRKY70 is required for Mi-1-mediated resistance to aphids and nematodes in tomato. Planta 2012, 235, 299–309. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Chun, S.C. Expression of PR-protein genes and induction of defense-related enzymes by Bacillus subtilis CBR05 in tomato (Solanum lycopersicum) plants challenged with Erwinia carotovora subsp. carotovora. Biosci. Biotechnol. Biochem. 2016, 80, 2277–2283. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Zheng, Z.; Fan, B.; Yu, J.-Q.; Chen, Z. Characterization of the promoter and extended C-terminal domain of Arabidopsis WRKY33 and functional analysis of tomato WRKY33 homologues in plant stress responses. J. Exp. Bot. 2015, 66, 4567–4583. [Google Scholar] [CrossRef]
- Ray, S.; Mondal, S.; Chowdhury, S.; Kundu, S. Differential responses of resistant and susceptible tomato varieties to inoculation with Alternaria solani. Physiol. Mol. Plant Pathol. 2015, 90, 78–88. [Google Scholar] [CrossRef]
- Worrall, D.; Holroyd, G.H.; Moore, J.P.; Glowacz, M.; Croft, P.; Taylor, J.E.; Paul, N.D.; Roberts, M.R. Treating seeds with activators of plant defence generates long-lasting priming of resistance to pests and pathogens. New Phytol. 2012, 193, 770–778. [Google Scholar] [CrossRef]
- Anup, C.P.; Melvin, P.; Shilpa, N.; Gandhi, M.N.; Jadhav, M.; Ali, H.; Kini, K.R. Proteomic analysis of elicitation of downy mildew disease resistance in pearl millet by seed priming with β-aminobutyric acid and Pseudomonas fluorescens. J. Proteom. 2015, 120, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Baysal, Ö.; Gürsoy, Y.Z.; Örnek, H.; Duru, A. Induction of oxidants in tomato leaves treated with DL-β-Amino butyric acid (BABA) and infected with Clavibacter michiganensis ssp. michiganensis. Eur. J. Plant Pathol. 2005, 112, 361–369. [Google Scholar] [CrossRef]
- Dong, X. NPR1, all things considered. Curr. Opin. Plant Biol. 2004, 7, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, L.; Métraux, J.-P.; Mauch-Mani, B. β-Aminobutyric acid-induced protection of Arabidopsis against the necrotrophic fungus Botrytis cinerea. Plant Physiol. 2001, 126, 517–523. [Google Scholar] [CrossRef]
- Uehara, T.; Sugiyama, S.; Matsuura, H.; Arie, T.; Masuta, C. Resistant and susceptible responses in tomato to cyst nematode are differentially regulated by salicylic acid. Plant Cell Physiol. 2010, 51, 1524–1536. [Google Scholar] [CrossRef]
- Tsai, C.H.; Singh, P.; Chen, C.W.; Thomas, J.; Weber, J.; Mauch-Mani, B.; Zimmerli, L. Priming for enhanced defence responses by specific inhibition of the Arabidopsis response to coronatine. Plant J. 2011, 65, 469–479. [Google Scholar] [CrossRef]
- Martínez-Aguilar, K.; Ramírez-Carrasco, G.; Hernández-Chávez, J.L.; Barraza, A.; Alvarez-Venegas, R. Use of BABA and INA as activators of a primed state in the common bean (Phaseolus vulgaris L.). Front. Plant Sci. 2016, 7, 653. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Cumplido-Nájera, C.F.; González-Morales, S.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; Benavides-Mendoza, A.; Juárez-Maldonado, A. The application of copper nanoparticles and potassium silicate stimulate the tolerance to Clavibacter michiganensis in tomato plants. Sci. Hortic. 2019, 245, 82–89. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Mao, G.; Meng, X.; Liu, Y.; Zheng, Z.; Chen, Z.; Zhang, S. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 2011, 23, 1639–1653. [Google Scholar] [CrossRef]
- Liu, S.; Ziegler, J.; Zeier, J.; Birkenbihl, R.P.; Somssich, I.E. Botrytis cinerea B05. 10 promotes disease development in Arabidopsis by suppressing WRKY33-mediated host immunity. Plant Cell Environ. 2017, 40, 2189–2206. [Google Scholar] [CrossRef] [PubMed]
- Knoth, C.; Ringler, J.; Dangl, J.L.; Eulgem, T. Arabidopsis WRKY70 is required for full RPP4-mediated disease resistance and basal defense against Hyaloperonospora parasitica. Mol. Plant-Microbe Interact. 2007, 20, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhong, R.; Palva, E.T. WRKY70 and its homolog WRKY54 negatively modulate the cell wall-associated defenses to necrotrophic pathogens in Arabidopsis. PLoS ONE 2017, 12, e0183731. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.E.; Mengesha, B.; Tang, H.; Mengiste, T.; Bluhm, B.H. Resistance to Botrytis cinerea in Solanum lycopersicoides involves widespread transcriptional reprogramming. BMC Genom. 2014, 15, 334. [Google Scholar] [CrossRef]
- AbuQamar, S.; Chai, M.-F.; Luo, H.; Song, F.; Mengiste, T. Tomato protein kinase 1b mediates signaling of plant responses to necrotrophic fungi and insect herbivory. Plant Cell 2008, 20, 1964–1983. [Google Scholar] [CrossRef]
| Seed Priming Application | YES | YES | NO | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Seed priming duration | 72 h | 72 h | 0 h | ||||||
| Application | 12 mM BABA priming | Water priming | Normal germination (positive control) | ||||||
| Application when the seedlings reach the 3–4 leaf stage (7 days and 1 day before Cmm application) | Distilled water spray | 2 mM BABA spray | Distilled water spray | 2 mM BABA spray | Distilled water spray | 2 mM BABA spray | |||
| Cmm applications (100 μL OD600:0.02) | - Cmm | + Cmm | + Cmm | - Cmm | + Cmm | + Cmm | - Cmm | + Cmm | + Cmm |
| Label | BABA priming- Cmm (BP-Cmm) | BABA priming+Cmm (BP+Cmm) | BABA priming+BABA-Sp+Cmm (BP+BSP+Cmm) | Water priming- Cmm (WP-Cmm) | Water priming+Cmm (WP+Cmm) | Water priming+BABA- Sp+Cmm (WP+BSP+Cmm) | Non-primed- Cmm (NP-Cmm) | Non-primed+Cmm (NP+Cmm) | Non-primed+BABA- Sp+Cmm (NP+BSP+Cmm) |
| Genes | Functions of Genes | Primer Sequences (5′-3′) | Temperature (°C) | References |
|---|---|---|---|---|
| PR1 | Pathogenesis 1 gene | F: GGATCGGACAACGTCCTTAC R:GCAACATCAAAAGGGAAATAAT | 52 | Molinari et al. [45] |
| PR5 | Pathogenesis 5 gene | F: GCAACAACTGTCCATACACC R: AGACTCCACCACAATCACC | 52 | |
| NPR1 | Non-expression of PR1 gene | F: GGGAAAGATAGCAGCACG R: GTCCACACAAACACACACATC | 52 | Hu et al. [46] |
| WRKY70 | WRKY transcription factor 70 | F: CATGGATGAGAGAATCTGCA R: GATTTTCTTGGATTATTTGAAC | 52 | Atamian et al. [47] |
| PAL | Phenylalanine ammonialyase like gene | F: CGCTATGCTCTCCGAACATCTC R: ATTCACCGAGTTAATCTCCCTCTC | 55 | Chandrasekaran and Chun [48] |
| WRKY33B | WRKY transcription factor 33b | F: CCACAACAGTCTGAAATGGG R: CAGCAAAGCAATGACTCCAT | 52 | Zhou et al. [49] |
| ACTIN | Actin gene | F: TGTCCCTATTTACGAGGGTTATGC R: CAGTTAAATCACGACCAGCAAGAT | 52 | |
| TPK1b | Tomato protein kinase 1b | F: ATGGGGATATGTTTGAGTGCTAGAA R: GAACGTGTTCTCGTCGATCCACCCT | 55 | Ray et al. [50] |
| Application | Plant Fresh Weight (g) | Plant Dry Weight (g) |
|---|---|---|
| Water priming-Cmm (WP-Cmm) | 31.14 ± 1.18 a | 2.79 ± 0.18 a |
| Non-primed-Cmm (NP-Cmm) | 26.34 ± 2.45 b | 2.39 ± 0.28 a |
| BABA priming-Cmm (BP-Cmm) | 16.89 ± 0.94 c | 1.61 ± 0.12 b |
| Water priming+Cmm (WP+Cmm) | 6.82 ± 0.95 d | 0.93 ± 0.12 c |
| BABA priming+BABA-SP+Cmm (BP+BSP+Cmm) | 6.79 ± 0.96 d | 0.92 ± 0.11 c |
| Water priming+BABA-SP+Cmm (WP+BSP+Cmm) | 6.40 ± 1.04 de | 0.81 ± 0.12 cd |
| BABA priming+Cmm (BP+Cmm) | 6.37 ± 0.90 de | 0.75 ± 0.08 cd |
| Non-primed+BABA-SP+Cmm (NP+BSP+Cmm) | 4.39 ± 1.02 de | 0.63 ± 0.12 cd |
| Non-primed+Cmm (NP+Cmm) | 3.72 ± 0.94 e | 0.58 ± 0.11 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özkurt, N.; Bektas, H.; Bektas, Y. Enhancing Tomato (Solanum lycopersicum L.) Resistance Against Bacterial Canker Disease (Clavibacter michiganensis ssp. michiganensis) via Seed Priming with β-Aminobutyric Acid (BABA). Horticulturae 2025, 11, 587. https://doi.org/10.3390/horticulturae11060587
Özkurt N, Bektas H, Bektas Y. Enhancing Tomato (Solanum lycopersicum L.) Resistance Against Bacterial Canker Disease (Clavibacter michiganensis ssp. michiganensis) via Seed Priming with β-Aminobutyric Acid (BABA). Horticulturae. 2025; 11(6):587. https://doi.org/10.3390/horticulturae11060587
Chicago/Turabian StyleÖzkurt, Nazlı, Harun Bektas, and Yasemin Bektas. 2025. "Enhancing Tomato (Solanum lycopersicum L.) Resistance Against Bacterial Canker Disease (Clavibacter michiganensis ssp. michiganensis) via Seed Priming with β-Aminobutyric Acid (BABA)" Horticulturae 11, no. 6: 587. https://doi.org/10.3390/horticulturae11060587
APA StyleÖzkurt, N., Bektas, H., & Bektas, Y. (2025). Enhancing Tomato (Solanum lycopersicum L.) Resistance Against Bacterial Canker Disease (Clavibacter michiganensis ssp. michiganensis) via Seed Priming with β-Aminobutyric Acid (BABA). Horticulturae, 11(6), 587. https://doi.org/10.3390/horticulturae11060587








