The Status of Esca Disease and the Disinfection of the Scion Prior to Grafting Affect the Phenolic Composition and Phenylpropanoid-Related Enzymes in the Callus of Vine Hetero-Grafts
Abstract
1. Introduction
2. Material and Methodes
2.1. Experiment Procedure
2.2. Individual Phenolic Compounds HPLC-MS Analysis
2.2.1. Phenolic Compounds Extraction
2.2.2. HPLC/MS Analysis
2.3. Analysis of Phenylpropanoid-Pathway-Related Enzymes
2.3.1. Crude Extract Preparation
2.3.2. Enzyme Activities
2.4. Statistic Analysis
3. Results
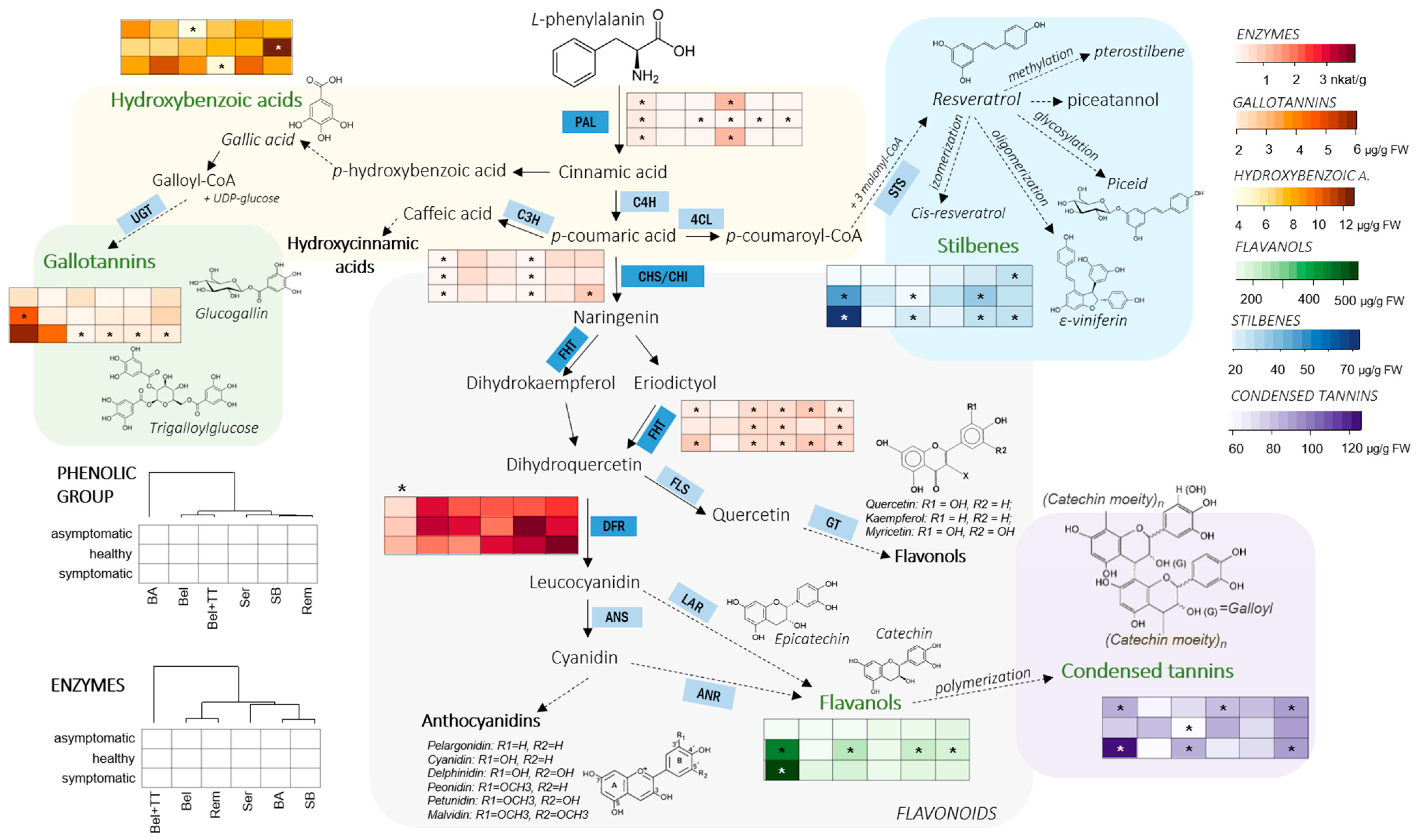
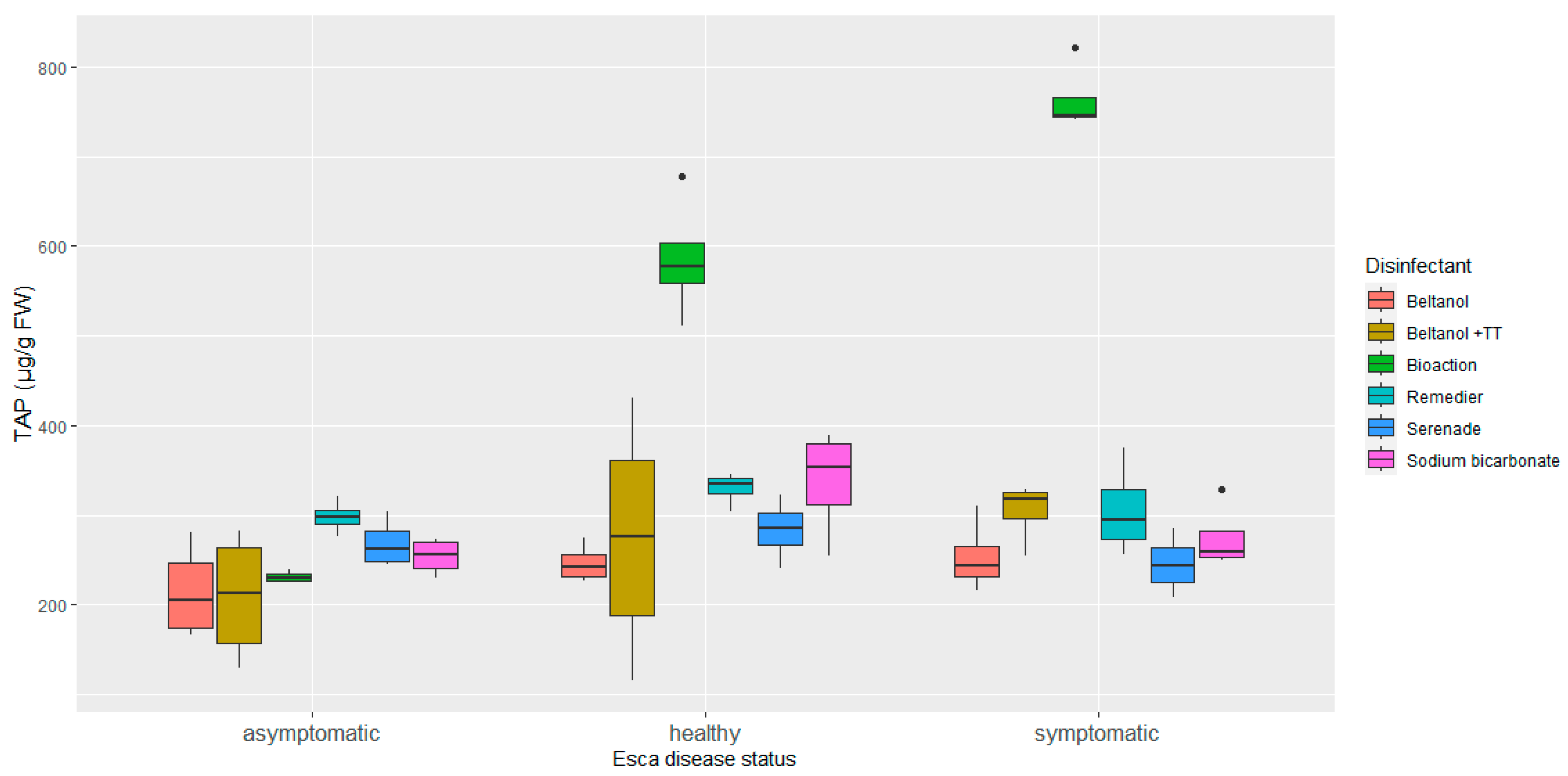
3.1. Phenolic Profile of Vine Graft Callus
3.2. Phenylpropanoid-Related Enzymes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tedesco, S.; Fevereiro, P.; Kragler, F.; Pina, A. Plant grafting and graft incompatibility: A review from the grapevine perspective. Sci. Hortic. 2022, 299, 111019. [Google Scholar] [CrossRef]
- Granett, J.; Walker, M.A.; Kocsis, L.; Omer, A.D. Biology and management of grape phylloxera. Annu. Rev. Entomol. 2001, 46, 387–412. [Google Scholar] [CrossRef]
- Marín, D.; Santesteban, L.G.; Dayer, S.; Villa-Llop, A.; Abad, F.J.; Gambetta, G.A.; Torres-Ruiz, J.M.; Torres, N. Connection matters: Exploring the implications of scion–rootstock alignment in grafted grapevines. Aust. J. Grape Wine Res. 2022, 28, 561–571. [Google Scholar] [CrossRef]
- Waite, H.; Whitelaw-Weckert, M.; Torley, P. Grapevine propagation: Principles and methods for the production of high-quality grapevine planting material. N. Z. J. Crop Hortic. Sci. 2015, 43, 144–161. [Google Scholar] [CrossRef]
- Gramaje, D.; Di Marco, S. Identifying practices likely to have impacts on grapevine trunk disease infections: A European nursery survey. Phytopathol. Mediterr. 2015, 54, 313–324. [Google Scholar]
- Bertsch, C.; Ramírez-Suero, M.; Magnin-Robert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clément, C.; Fontaine, F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2013, 62, 243–265. [Google Scholar] [CrossRef]
- Kenfaoui, J.; Radouane, N.; Mennani, M.; Tahiri, A.; El Ghadraoui, L.; Belabess, Z.; Fontaine, F.; El Hamss, H.; Amiri, S.; Lahlali, R.; et al. A Panoramic View on Grapevine Trunk Diseases Threats: Case of Eutypa Dieback, Botryosphaeria Dieback, and Esca Disease. J. Fungi 2022, 8, 595. [Google Scholar] [CrossRef]
- Battiston, E.; Compant, S.; Antonielli, L.; Mondello, V.; Clément, C.; Simoni, A.; Di Marco, S.; Mugnai, L.; Fontaine, F. In planta Activity of Novel Copper(II)-Based Formulations to Inhibit the Esca-Associated Fungus Phaeoacremonium minimum in Grapevine Propagation Material. Front. Plant Sci. 2021, 12, 649694. [Google Scholar] [CrossRef]
- Bruez, E.; Vallance, J.; Gerbore, J.; Lecomte, P.; Costa, J.-P.D.; Guerin-Dubrana, L.; Rey, P. Analyses of the Temporal Dynamics of Fungal Communities Colonizing the Healthy Wood Tissues of Esca Leaf-Symptomatic and Asymptomatic Vines. PLoS ONE 2014, 9, e95928. [Google Scholar] [CrossRef]
- Surico, G. Towards a redefinition of the diseases within the esca complex of grapevine. Phytopathol. Mediterr. 2009, 48, 5–10. [Google Scholar]
- Surico, G.; Mugnai, L.; Marchi, G. The esca disease complex. In Integrated Management of Diseases Caused by Fungi, Phytoplasma and Bacteria; Springer: Dordrecht, The Netherlands, 2008; pp. 119–136. [Google Scholar]
- Fourie, P.H.; Halleen, F. Chemical and biological protection of grapevine propagation material from trunk disease pathogens. Eur. J. Plant Pathol. 2006, 116, 255–265. [Google Scholar] [CrossRef]
- Gramaje, D.; Úrbez-Torres, J.R.; Sosnowski, M.R. Managing Grapevine Trunk Diseases With Respect to Etiology and Epidemiology: Current Strategies and Future Prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine Trunk Diseases: A Review of Fifteen Years of Trials for Their Control with Chemicals and Biocontrol Agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.; Pierron, R.; Larignon, P.; Lecomte, P.; Abou-Mansour, E.; Farine, S.; Bertsch, C.; Jacques, A.; Trotel-Aziz, P.; Rego, C.; et al. Vitis Methods to Understand and Develop Strategies for Diagnosis and Sustainable Control of Grapevine Trunk Diseases. Phytopathology® 2019, 109, 916–931. [Google Scholar] [CrossRef]
- Saha, J.C.; Dikshit, A.K.; Bandyopadhyay, M.; Saha, K.C. A Review of Arsenic Poisoning and its Effects on Human Health. Crit. Rev. Environ. Sci. Technol. 1999, 29, 281–313. [Google Scholar] [CrossRef]
- Trouvelot, S.; Lemaitre-Guillier, C.; Vallet, J.; Jacquens, L.; Douillet, A.; Harir, M.; Larignon, P.; Roullier-Gall, C.; Schmitt-Kopplin, P.; Adrian, M.; et al. Sodium arsenite-induced changes in the wood of esca-diseased grapevine at cytological and metabolomic levels. Front. Plant Sci. 2023, 14, 1141700. [Google Scholar] [CrossRef]
- Assunção, M.; Canas, S.; Cruz, S.; Brazão, J.; Zanol, G.C.; Eiras-Dias, J.E. Graft compatibility of Vitis spp.: The role of phenolic acids and flavanols. Sci. Hortic. 2016, 207, 140–145. [Google Scholar] [CrossRef]
- Bester, A.J. Factors Influencing the Success or Failure of Graft Unions in Grapevine. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2020. [Google Scholar]
- Rasool, A.; Mansoor, S.; Bhat, K.M.; Hassan, G.I.; Baba, T.R.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; Paray, B.A.; Ahmad, P. Mechanisms Underlying Graft Union Formation and Rootstock Scion Interaction in Horticultural Plants. Front. Plant Sci. 2020, 11, 590847. [Google Scholar] [CrossRef]
- Pina, A.; Cookson, S.J.; Calatayud, A.; Trinchera, A.; Errea, P. Physiological and molecular mechanisms underlying graft compatibility. In Vegetable Grafting: Principles and Practices; Colla, G., Pérez-Alfocea, F., Schwarz, D., Eds.; CABI: Wallingford, UK, 2017; pp. 132–154. ISBN 978-1-78064-897-2. [Google Scholar]
- Cookson, S.J.; Clemente Moreno, M.J.; Hevin, C.; Nyamba Mendome, L.Z.; Delrot, S.; Trossat-Magnin, C.; Ollat, N. Graft union formation in grapevine induces transcriptional changes related to cell wall modification, wounding, hormone signalling, and secondary metabolism. J. Exp. Bot. 2013, 64, 2997–3008. [Google Scholar] [CrossRef]
- Gao, F.; Wang, R.; Shi, Y.; Shen, H.; Yang, L. Reactive oxygen metabolism in the proliferation of Korean pine embryogenic callus cells promoted by exogenous GSH. Sci. Rep. 2023, 13, 2218. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Iwase, A.; Rymen, B.; Lambolez, A.; Kojima, M.; Takebayashi, Y.; Heyman, J.; Watanabe, S.; Seo, M.; De Veylder, L.; et al. Wounding Triggers Callus Formation via Dynamic Hormonal and Transcriptional Changes. Plant Physiol. 2017, 175, 1158–1174. [Google Scholar] [CrossRef]
- Prodhomme, D.; Fonayet, J.; Hévin, C.; Franc, C.; Hilbert, G.; Revel, G.; Richard, T.; Ollat, N.; Cookson, S. Metabolite profiling during graft union formation reveals the reprogramming of primary metabolism and the induction of stilbene synthesis at the graft interface in grapevine. BMC Plant Biol. 2019, 19, 599. [Google Scholar] [CrossRef] [PubMed]
- Canas, S.; Assunção, M.; Brazão, J.; Zanol, G.; Eiras-Dias, J.E. Phenolic Compounds Involved in Grafting Incompatibility of Vitis spp: Development and Validation of an Analytical Method for their Quantification. Phytochem. Anal. 2015, 26, 1–7. [Google Scholar] [CrossRef]
- Loupit, G.; Valls Fonayet, J.; Prigent, S.; Prodhomme, D.; Spilmont, A.-S.; Hilbert, G.; Franc, C.; De Revel, G.; Richard, T.; Ollat, N.; et al. Identifying early metabolite markers of successful graft union formation in grapevine. Hortic. Res. 2022, 9, uhab070. [Google Scholar] [CrossRef] [PubMed]
- Rusjan, D.; Persic, M.; Likar, M.; Biniari, K.; Mikulic-Petkovsek, M. Phenolic Responses to Esca-Associated Fungi in Differently Decayed Grapevine Woods from Different Trunk Parts of ‘Cabernet Sauvignon’. J. Agric. Food Chem. 2017, 65, 6615–6624. [Google Scholar] [CrossRef] [PubMed]
- List of Registered Plant Protection Products. Available online: https://spletni2.furs.gov.si/FFS/REGSR/index.htm (accessed on 16 March 2025).
- EPPO PM 10/16: Hot Water Treatment of Grapevine to Control Viteus Vitifoliae. EPPO Bull. 2009, 39, 484–485. [CrossRef]
- Gačnik, S. Vpliv Zdravstvenega Stanja Cepičev ter Sredstev za Razkuževanje na Vsebnost Fenolnih Spojin v Kalusu Cepljenk Žlahtne Vinske Trte (Vitis vinifera L.) Sorte “Cabernet sauvignon”. Master’s Thesis, Univerza v Ljubljani, Biotehniška Fakulteta, Ljubljana, Slovenia, 2018. [Google Scholar]
- Halbwirth, H.; Waldner, I.; Miosic, S.; Ibanez, M.; Costa, G.; Stich, K. Measuring Flavonoid Enzyme Activities in Tissues of Fruit Species. J. Agric. Food Chem. 2009, 57, 4983–4987. [Google Scholar] [CrossRef]
- Sandermann, H.; Strominger, J.L. Purification and Properties of C55-Isoprenoid Alcohol Phosphokinase from Staphylococcus aureus. J. Biol. Chem. 1972, 247, 5123–5131. [Google Scholar] [CrossRef]
- Choi, M.; Sathasivam, R.; Nguyen, B.V.; Park, N.I.; Woo, S.-H.; Park, S.U. Expression Analysis of Phenylpropanoid Pathway Genes and Metabolomic Analysis of Phenylpropanoid Compounds in Adventitious, Hairy, and Seedling Roots of Tartary Buckwheat. Plants 2022, 11, 90. [Google Scholar] [CrossRef]
- Gacnik, S.; Veberič, R.; Hudina, M.; Marinovic, S.; Halbwirth, H.; Mikulič-Petkovšek, M. Salicylic and Methyl Salicylic Acid Affect Quality and Phenolic Profile of Apple Fruits Three Weeks before the Harvest. Plants 2021, 10, 1807. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Milke, L.; Aschenbrenner, J.; Marienhagen, J.; Kallscheuer, N. Production of plant-derived polyphenols in microorganisms: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 1575–1585. [Google Scholar] [CrossRef]
- Wei, Y.-J.; Zhao, S.-R.; Li, J.-M.; Xue, B. Stilbene profiles in different tissues of Vitis vinifera L. cv. Cabernet Sauvignon and a comparison of their antioxidant activity. Aust. J. Grape Wine Res. 2016, 22, 226–231. [Google Scholar] [CrossRef]
- Bruno, G.; Sparapano, L. Effects of three esca-associated fungi on Vitis vinifera L.: I. Characterization of secondary metabolites in culture media and host responses to the pathogens in calli. Physiol. Mol. Plant Pathol. 2006, 69, 209–223. [Google Scholar] [CrossRef]
- BioAction, E.S. Instructions for Use of the Preparation; Izimed: Dobrovo, Slovenia, 2018; p. 2. [Google Scholar]
- Dias, M.I.; Sousa, M.J.; Alves, R.C.; Ferreira, I.C.F.R. Exploring plant tissue culture to improve the production of phenolic compounds: A review. Ind. Crops Prod. 2016, 82, 9–22. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Gil-Muñoz, R.; López-Roca, J.M.; Martínez-Cutillas, A.; Romero-Cascales, I.; Gómez-Plaza, E. Increasing the Phenolic Compound Content of Grapes by Preharvest Application of Abcisic Acid and a Combination of Methyl Jasmonate and Benzothiadiazole. J. Agric. Food Chem. 2013, 61, 3978–3983. [Google Scholar] [CrossRef]
- Li, H.; Qiu, J.; Chen, F.; Lv, X.; Fu, C.; Zhao, D.; Hua, X.; Zhao, Q. Molecular characterization and expression analysis of dihydroflavonol 4-reductase (DFR) gene in Saussurea medusa. Mol. Biol. Rep. 2012, 39, 2991–2999. [Google Scholar] [CrossRef]
- Saltveit, M.E. Synthesis and Metabolism of Phenolic Compounds. In Fruit and Vegetable Phytochemicals; Yahia, E.M., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 115–124. ISBN 978-1-119-15804-2. [Google Scholar]
- Garner, R.J. The Grafter’s Handbook; Chelsea Green Publishing: Chelsea, VT, USA, 2013; ISBN 978-1-60358-482-1. [Google Scholar]
- Melnyk, C.W. Plant grafting: Insights into tissue regeneration. Regeneration 2016, 4, 3–14. [Google Scholar] [CrossRef]
- Mahunu, G.K.; Osei-Kwarteng, M.; Quainoo, A.K. Dynamics of graft formation in fruit trees: A review. Albanian J. Agric. Sci. 2013, 12, 177–180. [Google Scholar]
- Kovačec, E.; Regvar, M. Effects of Copper Compounds on Phenolic Composition of the Common and Tartary Buckwheat Seedlings. Agriculture 2024, 14, 269. [Google Scholar] [CrossRef]
- Sun, X.; Ma, T.; Han, L.; Huang, W.; Zhan, J. Effects of Copper Pollution on the Phenolic Compound Content, Color, and Antioxidant Activity of Wine. Molecules 2017, 22, 726. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, F.; Pinto, C.; Vallet, J.; Clément, C.; Gomes, A.C.; Spagnolo, A. The effects of grapevine trunk diseases (GTDs) on vine physiology. Eur. J. Plant Pathol. 2016, 144, 707–721. [Google Scholar] [CrossRef]
- Goufo, P.; Marques, A.C.; Cortez, I. Exhibition of Local but Not Systemic Induced Phenolic Defenses in Vitis vinifera L. Affected by Brown Wood Streaking, Grapevine Leaf Stripe, and Apoplexy (Esca Complex). Plants 2019, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Cooman, L.D.; Everaert, E.; Curir, P.; Dolci, M. The Possible Role of Phenolics in Incompatibility Expression in Eucalyptus gunnii Micrografts. Phytochem. Anal. 1996, 7, 92–96. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krošelj, S.; Mikulic-Petkovsek, M.; Likar, M.; Škvarč, A.; Halbwirth, H.; Biniari, K.; Rusjan, D. The Status of Esca Disease and the Disinfection of the Scion Prior to Grafting Affect the Phenolic Composition and Phenylpropanoid-Related Enzymes in the Callus of Vine Hetero-Grafts. Horticulturae 2025, 11, 371. https://doi.org/10.3390/horticulturae11040371
Krošelj S, Mikulic-Petkovsek M, Likar M, Škvarč A, Halbwirth H, Biniari K, Rusjan D. The Status of Esca Disease and the Disinfection of the Scion Prior to Grafting Affect the Phenolic Composition and Phenylpropanoid-Related Enzymes in the Callus of Vine Hetero-Grafts. Horticulturae. 2025; 11(4):371. https://doi.org/10.3390/horticulturae11040371
Chicago/Turabian StyleKrošelj, Saša, Maja Mikulic-Petkovsek, Matevž Likar, Andreja Škvarč, Heidi Halbwirth, Katerina Biniari, and Denis Rusjan. 2025. "The Status of Esca Disease and the Disinfection of the Scion Prior to Grafting Affect the Phenolic Composition and Phenylpropanoid-Related Enzymes in the Callus of Vine Hetero-Grafts" Horticulturae 11, no. 4: 371. https://doi.org/10.3390/horticulturae11040371
APA StyleKrošelj, S., Mikulic-Petkovsek, M., Likar, M., Škvarč, A., Halbwirth, H., Biniari, K., & Rusjan, D. (2025). The Status of Esca Disease and the Disinfection of the Scion Prior to Grafting Affect the Phenolic Composition and Phenylpropanoid-Related Enzymes in the Callus of Vine Hetero-Grafts. Horticulturae, 11(4), 371. https://doi.org/10.3390/horticulturae11040371








