Comparative Assessment of Phytochemical Content and Antioxidant Activities in Different Parts of Pyrus ussuriensis Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Instruments, Chemicals, and Reagents
2.3. Crude Extraction
2.4. Total Polyphenol Content (TPC) Assay
2.5. Total Flavonoid Content (TFC) Assay
2.6. DPPH Radical Scavenging Assay
2.7. ABTS+ Radical Scavenging Assay
2.8. Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS)
2.9. High-Performance Liquid Chromatography (HPLC)
2.10. Statistical Analaysis
3. Results
3.1. TPC and TFC Assays
3.2. DPPH and ABTS+ Assays
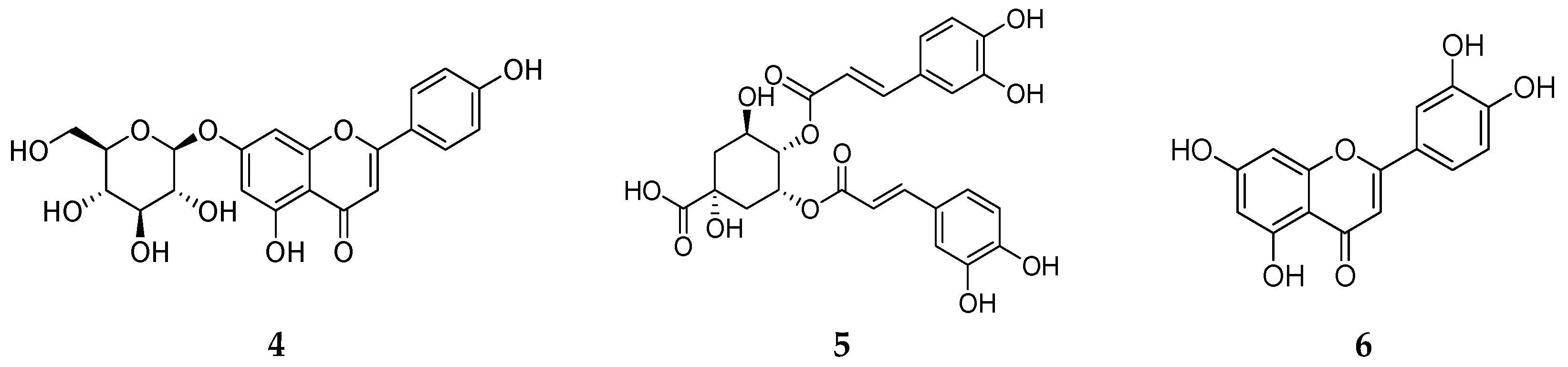
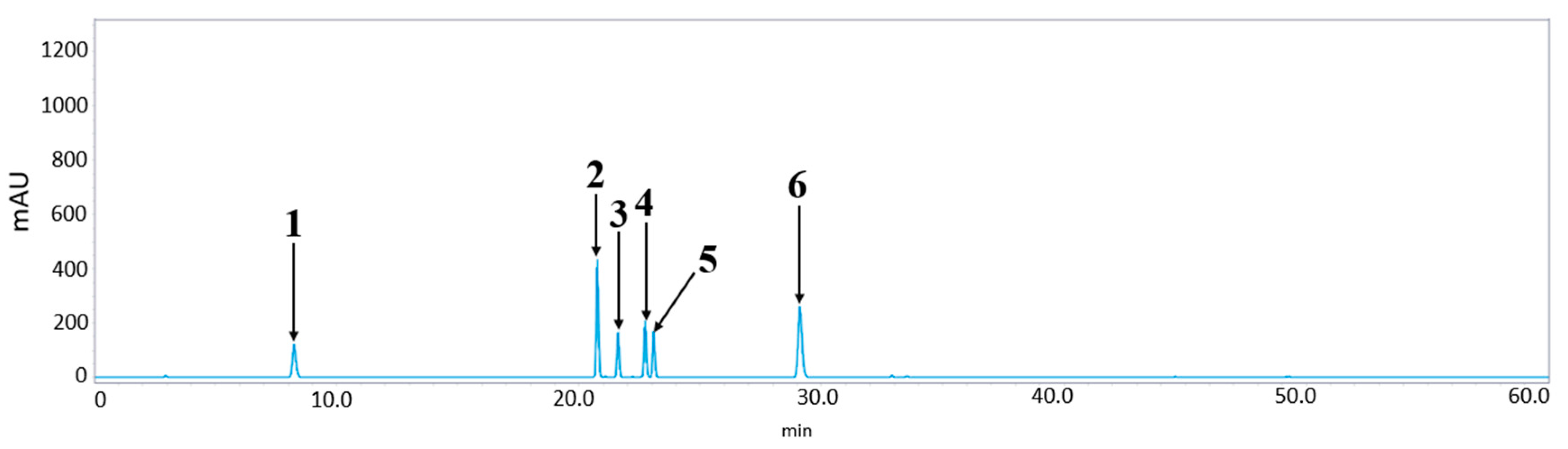
3.3. LC-MS/MS and HPLC/UV Analyses
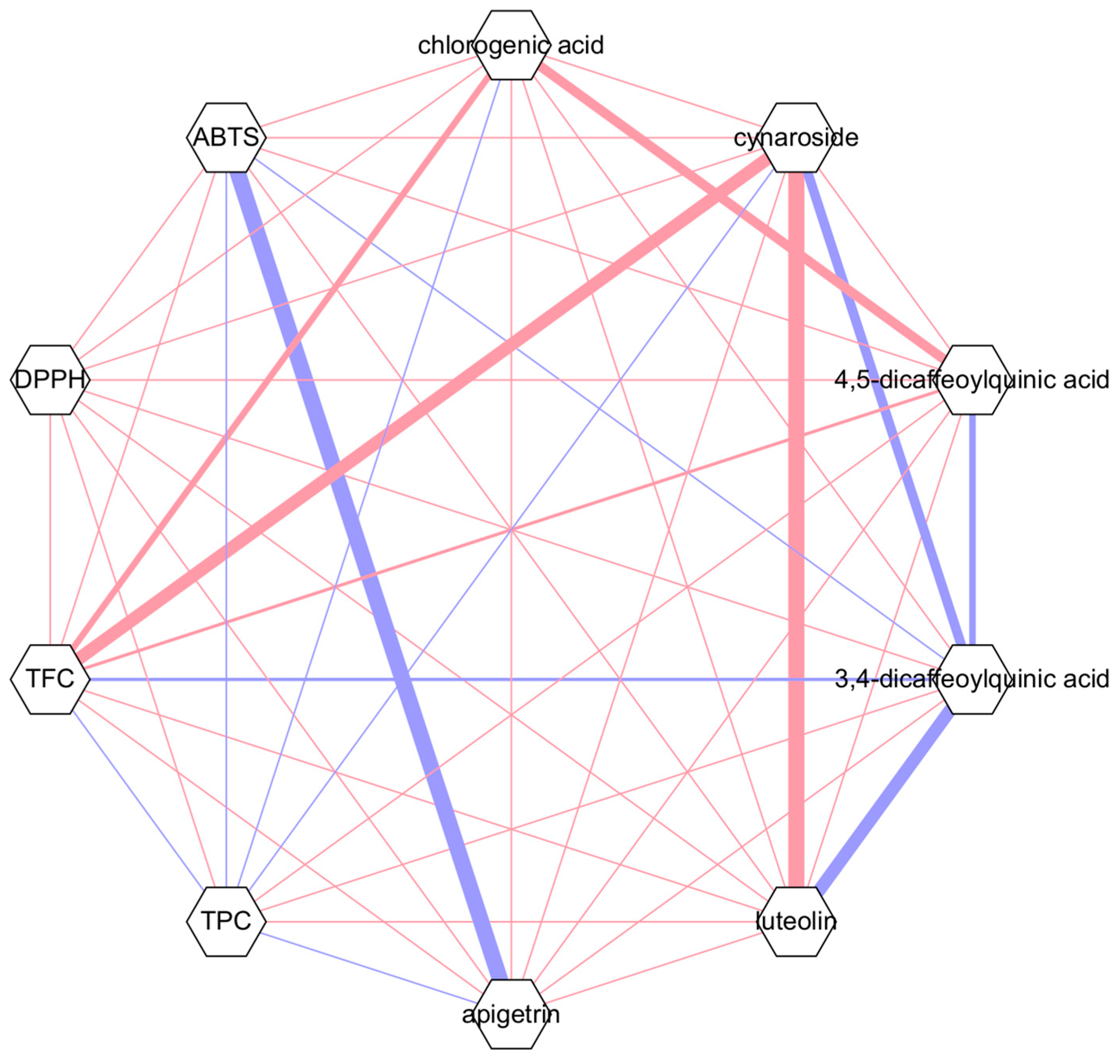
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, M.; Kim, Y.; Kim, S.; Park, J. The complete chloroplast genome of Korean Pyrus ussuriensis Maxim. (Rosaceae): Providing genetic background of two types of P. ussuriensis. Mitochondrial DNA Part B 2019, 4, 2424–2425. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Lee, S.; Lee, S.; Kang, K. Distribution and ecological characteristics of native Iris odesanensis in Mt. Naeyon. J. Environ. Sci. Int. 2006, 15, 1103–1107. [Google Scholar] [CrossRef]
- Wuyun, T.; Amo, H.; Xu, J.; Ma, T.; Uematsu, C.; Katayama, H. Population structure of and conservation strategies for wild Pyrus ussuriensis Maxim. in China. PLoS ONE 2015, 10, e0133686. [Google Scholar] [CrossRef]
- Bokszczanin, K.L.; Przybyla, A.A.; Schollenberger, M.; Gozdowski, D.; Madry, W.; Odziemkowski, S. Inheritance of fire blight resistance in Asian Pyrus species. Open J. Genet. 2012, 2, 109–120. [Google Scholar] [CrossRef]
- Katayama, H.; Amo, H.; Wuyun, T.; Uematsu, C.; Iketani, H. Genetic structure and diversity of the wild Ussurian pear in East Asia. Breed. Sci. 2016, 66, 90–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reighard, G.L. Evaluation of commercial potential of Asian pear cultivars in South Carolina. HortScience 1995, 30, 793. [Google Scholar] [CrossRef]
- Feng, Y.; Cheng, H.; Cheng, Y.; Zhao, J.; He, J.; Li, N.; Wang, J.; Guan, J. Chinese traditional pear paste: Physicochemical properties, antioxidant activities, and quality evaluation. Foods 2023, 12, 187. [Google Scholar] [CrossRef]
- Kar, A.; Bhattacharjee, S. Bioactive polyphenolic compounds, water-soluble vitamins, in vitro anti-inflammatory, anti-diabetic and free radical scavenging properties of underutilized alternate crop Amaranthus spinosus L. from Gangetic plain of West Bengal. Food Biosci. 2022, 50, 102072. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef]
- Moar, K.; Yadav, S.; Pant, A.; Deepika; Maurya, P.K. Anti-tumor effects of polyphenols via targeting cancer-driving signaling pathways: A review. Indian J. Clin. Biochem. 2024, 39, 470–488. [Google Scholar] [CrossRef] [PubMed]
- Manso, T.; Lores, M.; De Miguel, T. Antimicrobial activity of polyphenols and natural polyphenolic extracts on clinical isolates. Antibiotics 2021, 11, 46. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Izydorczyk, G.; Mikula, K.; Szopa, D.; Witek-Krowiak, A. Antiviral properties of polyphenols from plants. Foods 2021, 10, 2277. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Prabakaran, M.; Kim, S.; Sasireka, A.; Chandrasekaran, M.; Chung, I.-M. Polyphenol composition and antimicrobial activity of various solvent extracts from different plant parts of Moringa oleifera. Food Biosci. 2018, 26, 23–29. [Google Scholar] [CrossRef]
- Hancock, J.; Lobos, G. Pears. In Temperate Fruit Crop Breeding: Germplasm to Genomics, 1st ed.; Hancock, J.F., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 265–298. [Google Scholar]
- Lee, C.-D.; Ku, J.; Lee, S.; Lee, S. Quantification of phytochemicals in Cephalotaxus harringtonia: Insights into plant tissue-specific allocation. Horticulturae 2024, 10, 1286. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; A Haddad, M.; Al-Hiary, M. Natural polyphenols: Chemical classification, definition of classes, subcategories, and structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- da Silva, P.R.; de Lima, M.d.C.A.; Souza, T.P.; Sandes, J.M.; Lima, A.d.C.A.d.; Neto, P.J.R.; dos Santos, F.A.B.; Alves, L.C.; da Silva, R.M.F.; Rocha, G.J.d.M.; et al. Lignin from Morinda citrifolia leaves: Physical and chemical characterization, in vitro evaluation of antioxidant, cytotoxic, antiparasitic and ultrastructural activities. Int. J. Biol. Macromol. 2021, 193, 1799–1812. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, M.L.F.; Serra, P.; Casati, P. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. Physiol. Plant. 2021, 173, 736–749. [Google Scholar] [CrossRef]
- Hasan, Z.Y.M. Total phenolic, flavonoid contents, and antioxidant activities of different parts of Malus domestica L. in Iraq. Iraqi J. Pharm. Sci. 2024, 33, 174–189. [Google Scholar] [CrossRef]
- Dong, D.; Shi, Y.; Mou, Z.; Chen, S.-Y.; Zhao, D.-K. Grafting: A potential method to reveal the differential accumulation mechanism of secondary metabolites. Hortic. Res. 2022, 9, uhac050. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Oh, Y.C.; Cho, W.K.; Ma, J.Y. Antioxidant and anti-inflammatory activity determination of one hundred kinds of pure chemical compounds using offline and online screening HPLC assay. Evid.-Based Complement. Altern. Med. 2015, 2015, 165457. [Google Scholar] [CrossRef] [PubMed]
- Cesari, L.; Mutelet, F.; Canabady-Rochelle, L. Antioxidant properties of phenolic surrogates of lignin depolymerisation. Ind. Crops Prod. 2019, 129, 480–487. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.-M. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The biological activity mechanism of chlorogenic acid and its applications in food industry: A review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, M.; He, L.; Song, X.; Cao, T. Chlorogenic acid: A review on its mechanisms of anti-inflammation, disease treatment, and related delivery systems. Front. Pharmacol. 2023, 14, 1218015. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.N.T.; Vu, T.T.T.; Do, T.H.T.; Nguyen, T.H.; Van Le, H.; Pham, H.K.T.; Truong, P.C.H.; Pham, D.P.; Tran, M.H. Identification of phenolic compounds from Vietnamese artichoke (Cynara scolymus L.) leaf and their antioxidant activities. Nat. Prod. Sci. 2024, 30, 39–112. [Google Scholar] [CrossRef]
- Kim, J.S.; Lim, J.H.; Cho, S.K. Effect of antioxidant and anti-inflammatory on bioactive components of carrot (Daucus carota L.) leaves from Jeju Island. Appl. Biol. Chem. 2023, 66, 34. [Google Scholar] [CrossRef]
- Liang, N.; Dupuis, J.H.; Yada, R.Y.; Kitts, D.D. Chlorogenic acid isomers directly interact with Keap 1-Nrf2 signaling in Caco-2 cells. Mol. Cell. Biochem. 2019, 457, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.; Veiga, F.; Cardoso, C.; Dias, F.; Cerqueira, F.; Medeiros, R.; Paiva-Santos, A.C. A rapid and simplified DPPH assay for analysis of antioxidant interactions in binary combinations. Microchem. J. 2024, 202, 110801. [Google Scholar] [CrossRef]
- Kim, H.; Kim, T.H.; Jeon, B.; Bang, M.H.; Kim, W.J.; Park, J.W.; Chung, D.-K. Evaluation of the physiological activity on the skin and identification of the active ingredient of leaf extract from Sanhyang Sandolbae (Pyrus ussuriensis) as a new variety. J. Korean Soc. Food Sci. Nutr. 2020, 49, 35–45. [Google Scholar] [CrossRef]
- Boby, N.; Abbas, M.A.; Lee, E.; Im, Z.-E.; Hsu, W.H.; Park, S.-C. Protective effect of Pyrus ussuriensis Maxim. extract against ethanol-induced gastritis in rats. Antioxidants 2021, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Brahem, M.; Renard, C.M.; Eder, S.; Loonis, M.; Ouni, R.; Mars, M.; Le Bourvellec, C. Characterization and quantification of fruit phenolic compounds of European and Tunisian pear cultivars. Food Res. Int. 2017, 95, 125–133. [Google Scholar] [CrossRef]
- Dibacto, R.E.K.; Tchuente, B.R.T.; Nguedjo, M.W.; Tientcheu, Y.M.T.; Nyobe, E.C.; Edoun, F.L.E.; Kamini, M.F.G.; Dibanda, R.F.; Medoua, G.N. Total polyphenol and flavonoid content and antioxidant capacity of some varieties of Persea americana peels consumed in Cameroon. Sci. World J. 2021, 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Choi, S.; Park, J.; Kim, K.; Oh, J.-E. Phenolic compounds in the freshwater environment in South Korea: Occurrence and tissue-specific distribution. Sci. Total Environ. 2023, 905, 166914. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Song, Z.; Cai, N.; Wang, Q.; Xiao, X.; Yang, X.; He, Y.; Zou, S. Pharmacokinetics, tissue distribution, and excretion of six bioactive components from total glucosides picrorhizae rhizoma, as simultaneously determined by a UHPLC-MS/MS method. J. Chromatogr. B 2023, 1227, 123830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tian, J.; Gao, N.; Gong, E.S.; Xin, G.; Liu, C.; Si, X.; Sun, X.; Li, B. Assessment of the phytochemical profile and antioxidant activities of eight kiwi berry (Actinidia arguta (Siebold & Zuccarini) Miquel) varieties in China. Food Sci. Nutr. 2021, 9, 5616–5625. [Google Scholar] [CrossRef]
- Akullo, J.O.; Kiage-Mokua, B.N.; Nakimbugwe, D.; Ng’ang’a, J.; Kinyuru, J. Phytochemical profile and antioxidant activity of various solvent extracts of two varieties of ginger and garlic. Heliyon 2023, 9, e18806. [Google Scholar] [CrossRef] [PubMed]
- Lekshmi, S.G.; Sethi, S.; Pooja, B.K.; Nayak, S.L.; Menaka, M. Ornamental plant extracts: Application in food colouration and packaging, antioxidant, antimicrobial and pharmacological potential—A concise review. Food Chem. Adv. 2023, 3, 100529. [Google Scholar] [CrossRef]
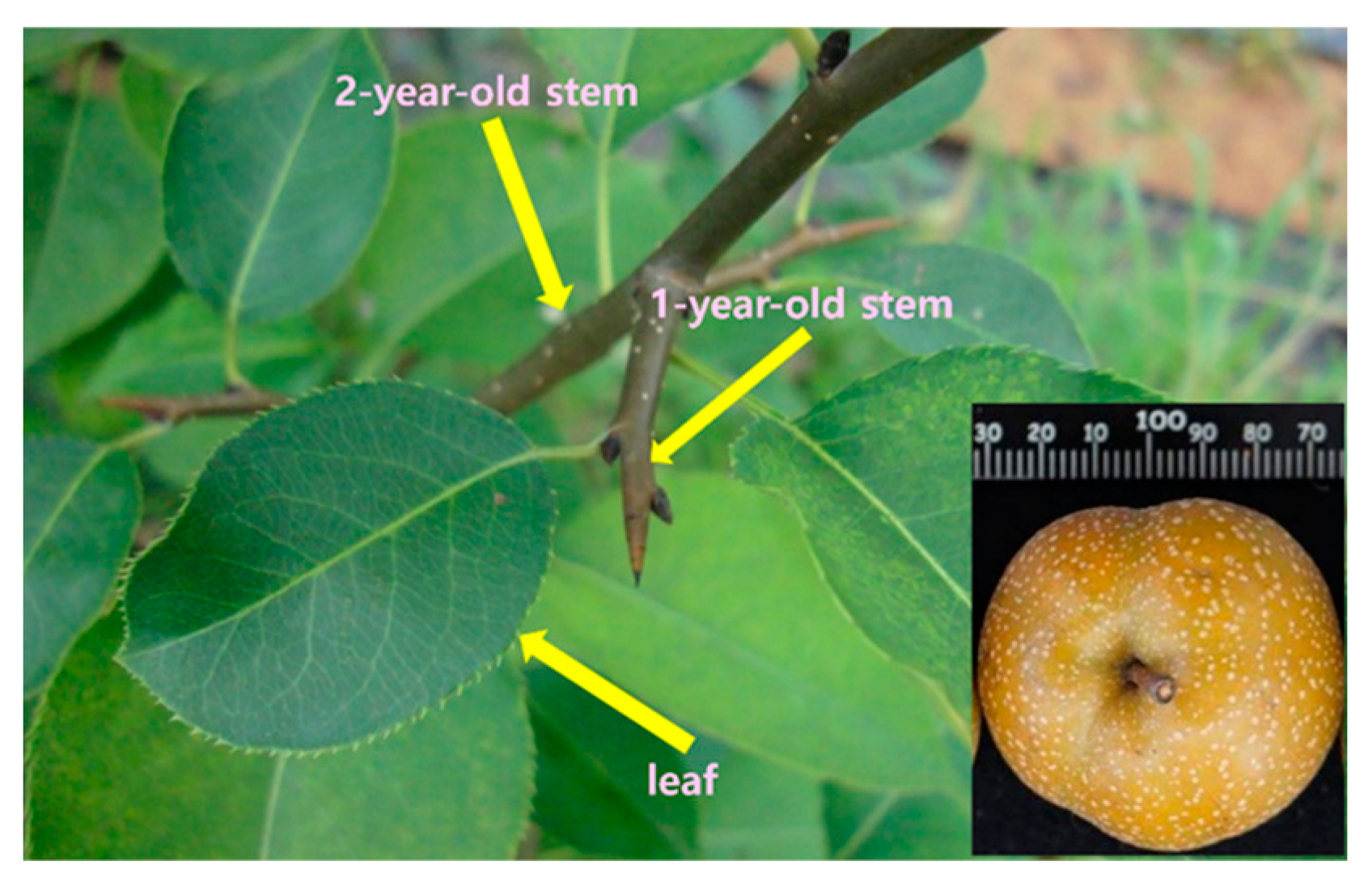




| Sample | TPC (mg TAE/g) | TFC (mg QE/g) |
|---|---|---|
| 1PUR | 60.7 ± 3.29 b | 0.45 ± 0.22 c |
| 2PUR | 82.9 ± 11.3 ab | 0.78 ± 0.06 c |
| LPUR | 79.6 ± 9.08 ab | 13.5 ± 0.82 a |
| FPUR | 41.5 ± 2.14 c | 0.30 ± 0.12 d |
| 1PUM | 81.1 ± 6.77 ab | 0.52 ± 0.09 c |
| 2PUM | 94.7 ± 20.9 a | 1.05 ± 0.04 c |
| LPUM | 76.5 ± 13.3 ab | 6.72 ± 0.73 b |
| tR (min) | Molecular Formula | Molecular Weight | Tentative Identification |
|---|---|---|---|
| 7.55 | C16H18O9 | 354.1 | Neochlorogenic acid |
| 10.82 | C16H18O9 | 354.1 | Chlorogenic acid |
| 13.41 | C16H18O9 | 354.1 | 1-Caffeoylquinic acid |
| 13.88 | C16H18O8 | 338.1 | 4-Coumaroylquinic acid |
| 15.80 | C17H20O9 | 368.1 | 3-Feruloylquinic acid |
| 16.30 | C16H18O8 | 338.1 | 1-Coumaroylquinic acid |
| 17.03 | C21H22O10 | 434.1 | 6-Caffeoylarbutin |
| 18.17 | C17H20O9 | 368.1 | Chlorogenic acid methyl ester |
| 19.94 | C21H22O10 | 464.1 | Hirsutrin |
| 20.21 | C21H20O11 | 448.1 | Cynaroside |
| 21.17 | C21H20O12 | 516.1 | 3,4-Dicaffeoylquinic acid |
| 21.67 | C21H20O10 | 432.1 | Apigetrin |
| 21.94 | C25H24O12 | 516.1 | 4,5-Dicaffeoylquinic acid |
| 22.24 | C22H22O11 | 462.1 | Isoscoparin |
| 23.41 | C25H24O12 | 516.1 | Phellopterin |
| 24.51 | C15H10O6 | 516.1 | Luteolin |
| 26.37 | C15H10O5 | 286.1 | Apigenin |
| 26.98 | C16H12O6 | 300.1 | Disometin |
| Compound | tR (min) | Regression Equation | Coefficient of Determination (r2) |
|---|---|---|---|
| 1 | 8.19 | y = 7.9045x + 100.64 | 0.9979 |
| 2 | 20.70 | y = 10.729x + 328.35 | 0.9916 |
| 3 | 21.56 | y = 5.4865x + 154.18 | 0.9836 |
| 4 | 22.67 | y = 7.4078x + 251.79 | 0.9864 |
| 5 | 23.04 | y = 5.3829x + 91.65 | 0.9981 |
| 6 | 29.01 | y = 16.394x + 513.67 | 0.9893 |
| Sample | Content (mg/g) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | Total | |
| 1PUR | 1.23 ± 0.01 e | a tr | 0.16 ± 0.00 e | b ND | tr | ND | 1.39 |
| 2PUR | 0.69 ± 0.04 f | tr | 0.19 ± 0.02 e | tr | tr | tr | 0.88 |
| LPUR | 28.53 ± 0.03 a | 20.21 ± 0.02 a | 1.22 ± 0.04 d | 8.06 ± 0.00 b | 20.21± 0.03 a | 0.27 ± 0.00 | 79.5 |
| FPUR | 9.41 ± 0.11 c | tr | 0.05 ± 0.00 f | tr | tr | tr | 9.46 |
| 1PUM | 2.96 ± 0.00 d | tr | 9.07 ± 0.01 a | 0.67 ± 0.01 c | tr | ND | 12.70 |
| 2PUM | 2.09 ± 0.02 d | tr | 7.57 ± 0.09 b | 0.74 ± 0.02 c | tr | ND | 10.40 |
| LPUM | 25.55 ± 0.06 b | 3.31 ± 0.01 b | 3.69 ± 0.01 c | 18.39 ± 0.04 a | 16.90 ± 0.04 b | tr | 67.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uy, N.P.; Ku, J.; Lee, D.-H.; Nam, S.J.; Lee, S. Comparative Assessment of Phytochemical Content and Antioxidant Activities in Different Parts of Pyrus ussuriensis Cultivars. Horticulturae 2025, 11, 184. https://doi.org/10.3390/horticulturae11020184
Uy NP, Ku J, Lee D-H, Nam SJ, Lee S. Comparative Assessment of Phytochemical Content and Antioxidant Activities in Different Parts of Pyrus ussuriensis Cultivars. Horticulturae. 2025; 11(2):184. https://doi.org/10.3390/horticulturae11020184
Chicago/Turabian StyleUy, Neil Patrick, Jajung Ku, Doo-Hee Lee, Sang June Nam, and Sanghyun Lee. 2025. "Comparative Assessment of Phytochemical Content and Antioxidant Activities in Different Parts of Pyrus ussuriensis Cultivars" Horticulturae 11, no. 2: 184. https://doi.org/10.3390/horticulturae11020184
APA StyleUy, N. P., Ku, J., Lee, D.-H., Nam, S. J., & Lee, S. (2025). Comparative Assessment of Phytochemical Content and Antioxidant Activities in Different Parts of Pyrus ussuriensis Cultivars. Horticulturae, 11(2), 184. https://doi.org/10.3390/horticulturae11020184









