Effects of Macronutrients on the Growth, Essential Oil Production, and Quality of Echinophora platyloba (DC.) in Natural Ecosystems
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Experimental Site
2.2. Specifications of the Experimental Design
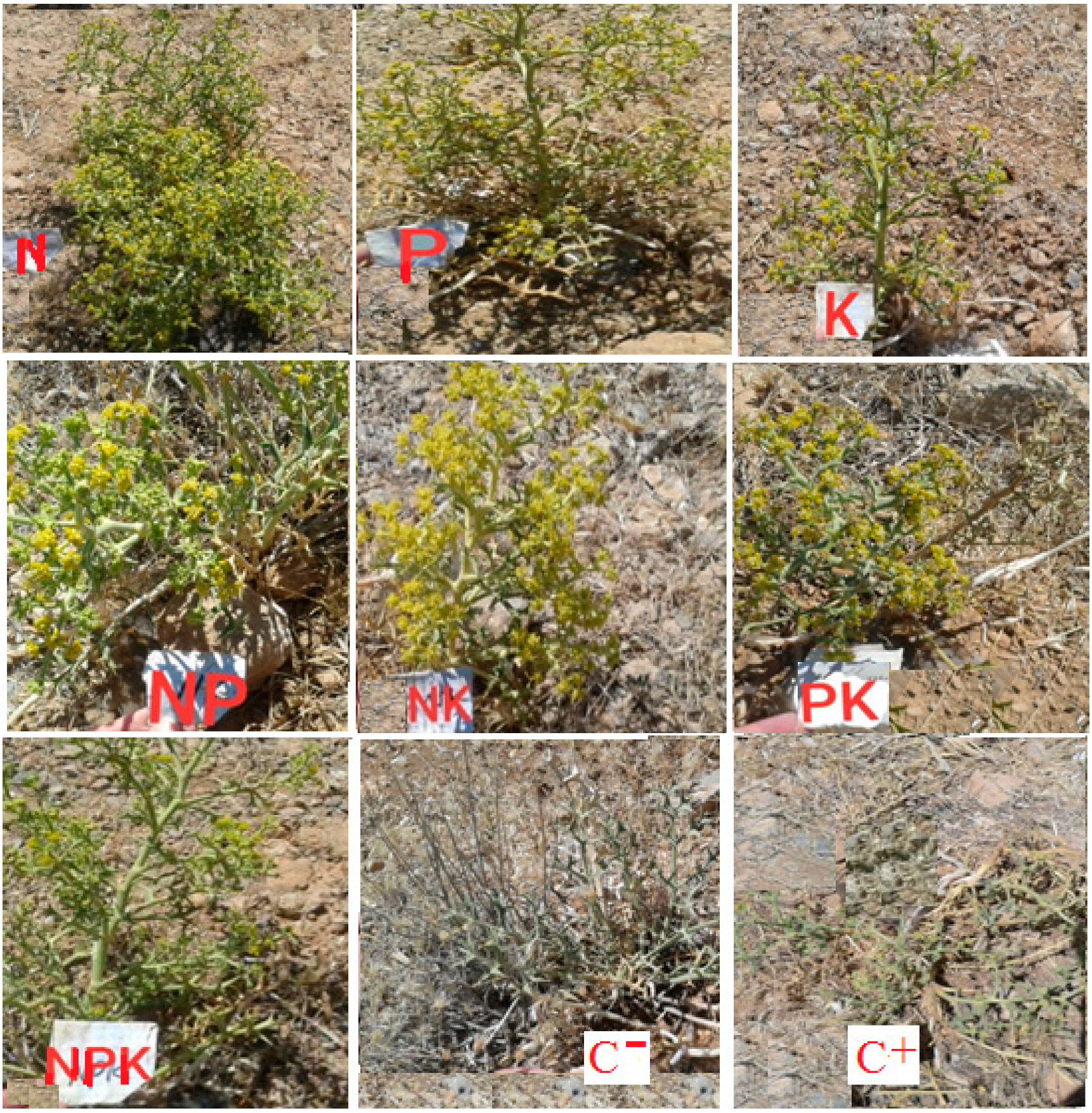
2.3. Experimental Operation
2.4. Measurement of Attributes
2.5. Qualitative and Semi-Quantitative Analysis of Essential Oils Samples
2.6. Statistical Analysis
3. Results
3.1. Plant Height
3.2. Branch Number
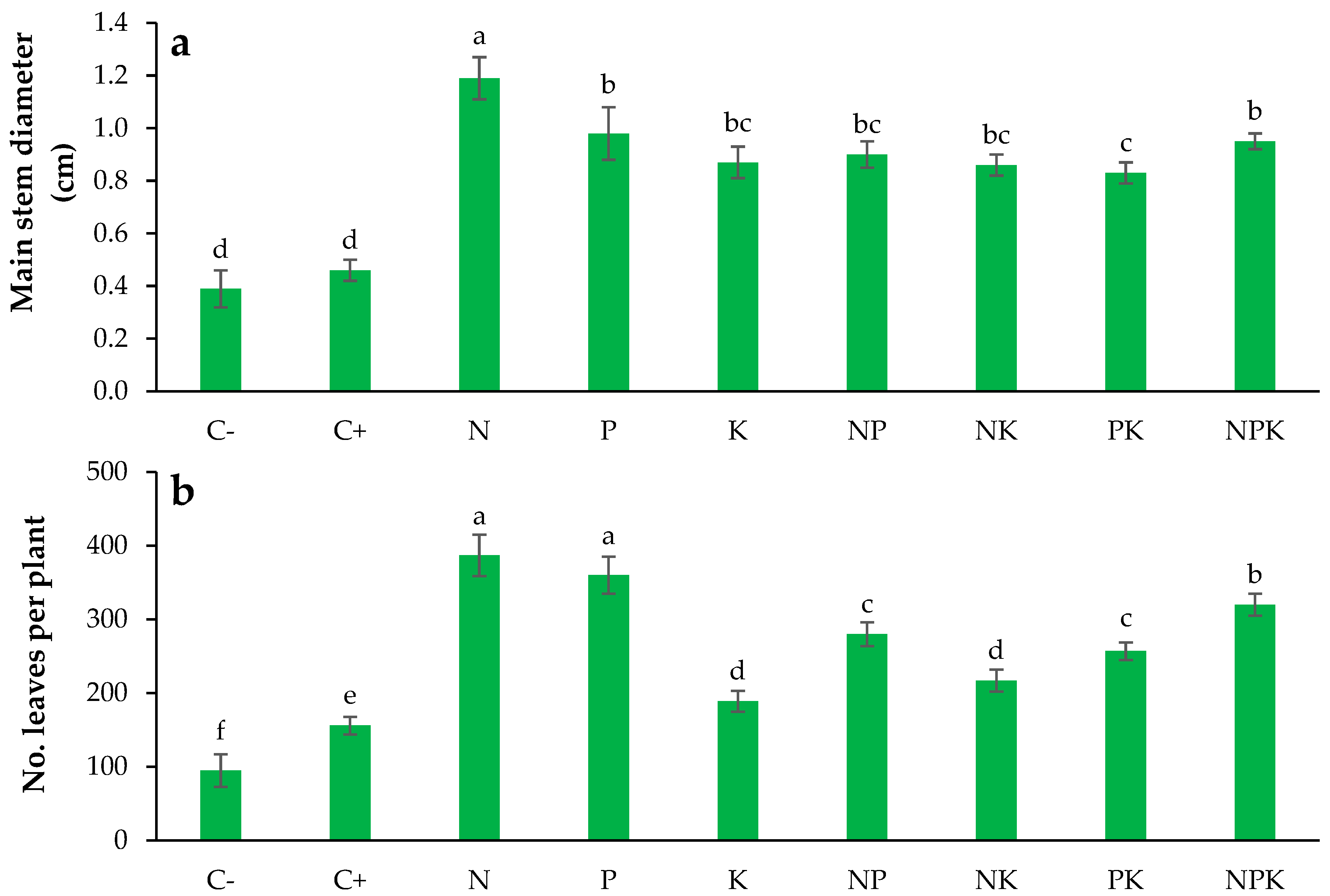
3.3. Main Stem Diameter
3.4. Leaf Number
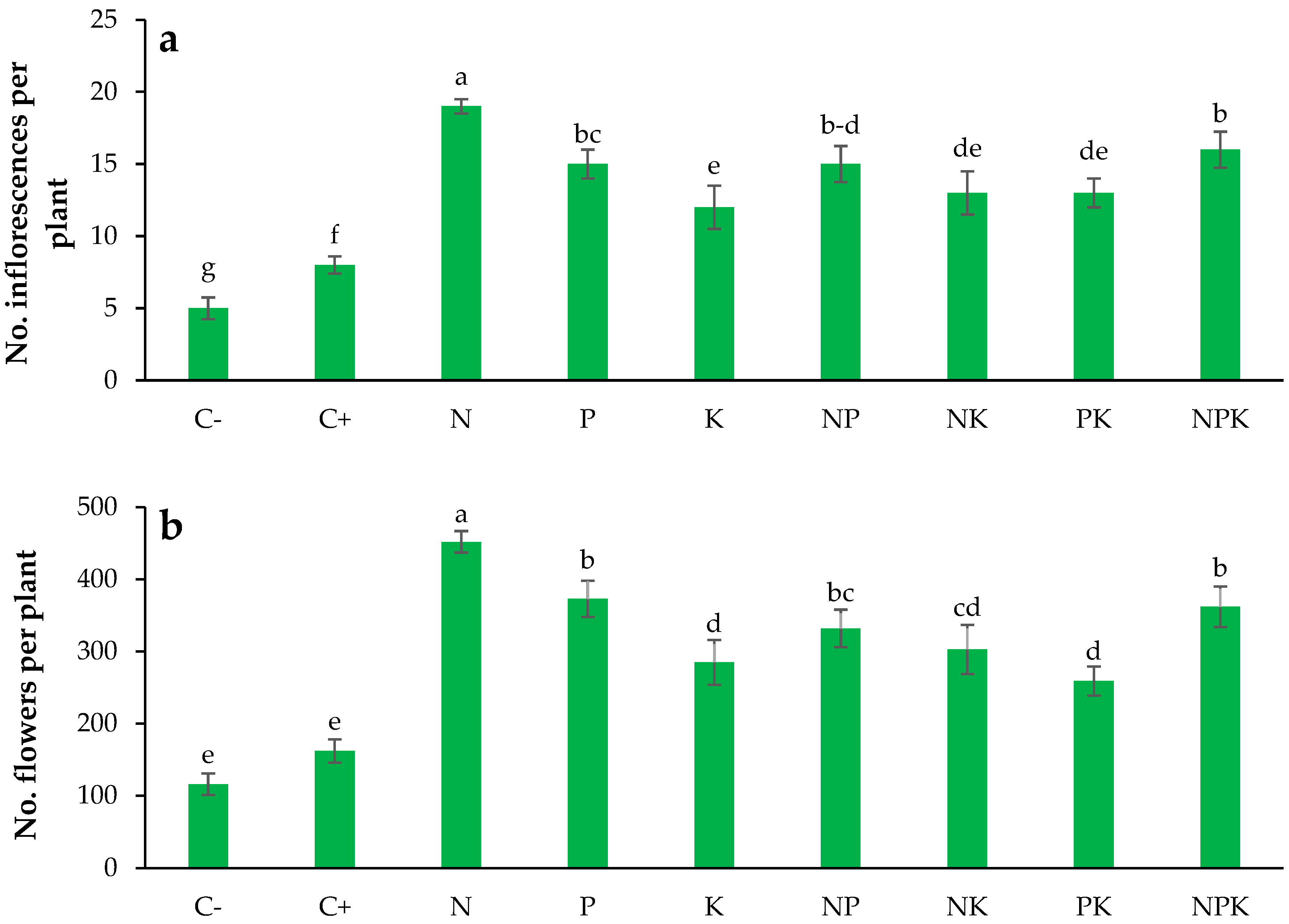
3.5. Inflorescence Number
3.6. Flower Number
3.7. Stem Dry Weight
3.8. Leaf Dry Weight
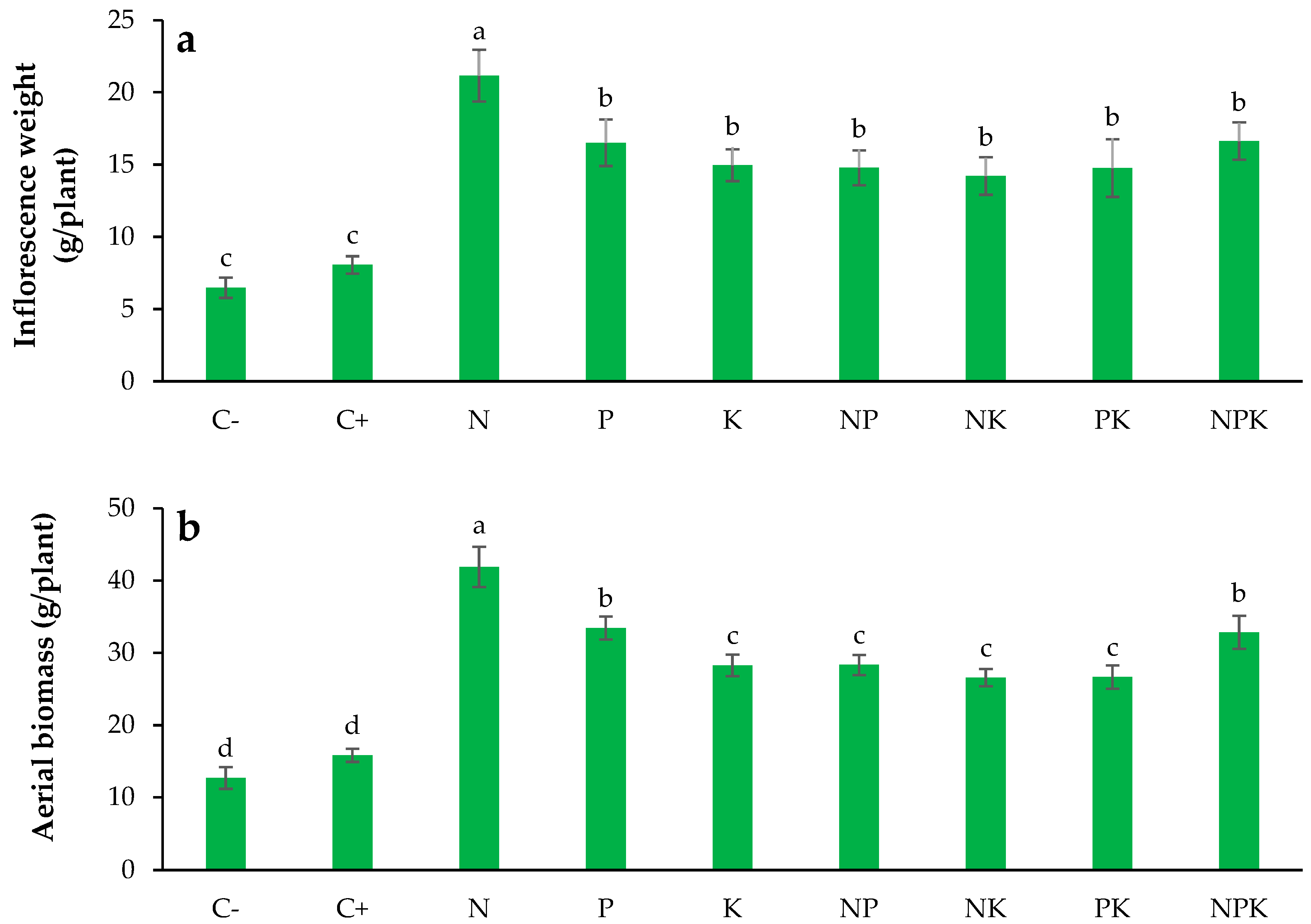
3.9. Inflorescence Dry Weight
3.10. Aerial Biomass
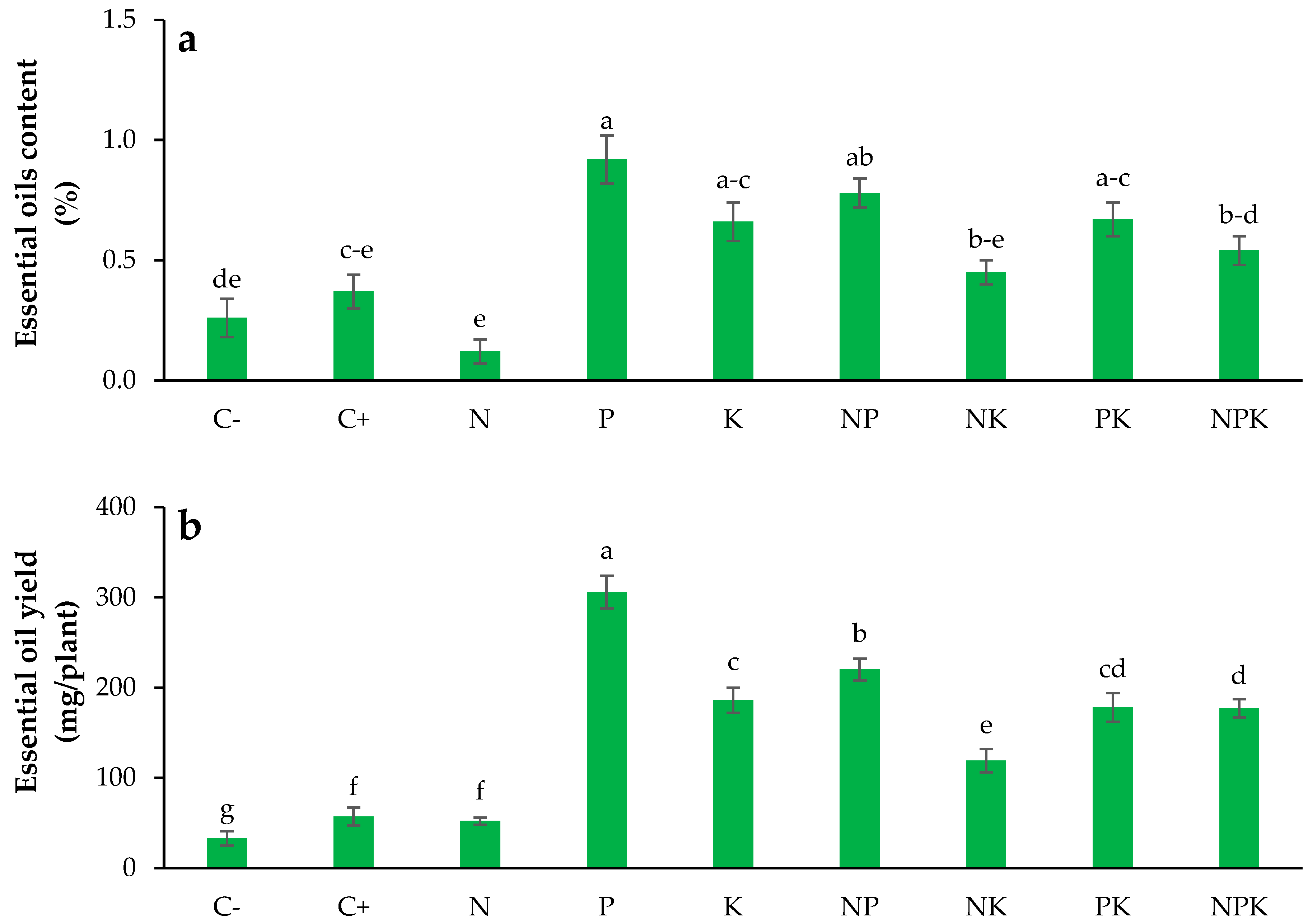
3.11. Essential Oils Content
3.12. Essential Oils Yield
3.13. Essential Oil Chemical Compositions
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassanpouraghdam, M.B.; Ghorbani, H.; Esmaeilpour, M.; Alford, M.H.; Strzemski, M.; Dresler, S. Diversity and distribution patterns of endemic medicinal and aromatic plants of Iran: Implications for conservation and habitat management. Int. J. Environ. Res. Public Health 2022, 19, 1552. [Google Scholar] [CrossRef]
- Davis, C.C.; Choisy, P. Medicinal plants meet modern biodiversity science. Curr. Biol. 2024, 24, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Tabin, S.; Gupta, R.C.; Kamili, A.N.; Parray, J.A. Medical and medicinal importance of Rheum spp. collected from different altitudes of the Kashmir Himalayan range. Cell. Mol. Biomed. Rep. 2022, 2, 187–201. [Google Scholar] [CrossRef]
- Morovati, Z.; Karimzadeh, G.; Naghavi, M.R.; Rashidi Monfared, S. Essential oil profile of areal part of Datura stramonium L. and D. innoxia from Iran. Agrotech. Ind. Crops 2023, 3, 53–59. [Google Scholar] [CrossRef]
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide research trends on medicinal plants. Int. J. Environ. Res. Public. Health 2020, 17, 3376. [Google Scholar] [CrossRef] [PubMed]
- Kidane, Y.O.; Steinbauer, M.J.; Beierkuhnlein, C. Dead end for endemic plant species? A biodiversity hotspot under pressure. Glob. Ecol. Conserv. 2019, 19, e00670. [Google Scholar] [CrossRef]
- Sasani, S.; Rashidi Monfared, S.; Mirzaei, A.R. Identification of some Echinophora platyloba miRNAs using computational methods and the effect of these miRNAs in the expression of TLN2 and ZNF521 genes in different human body organs. Cell. Mol. Biomed. Rep. 2024, 4, 43–53. [Google Scholar] [CrossRef]
- Avijgan, M.; Mahboubi, N. Echinophora platyloba DC. as a new natural antifungal agent. Asian Pac. J. Trop. Dis. 2015, 5, 169–174. [Google Scholar] [CrossRef]
- Shahneh, F.Z.; Baradaran, B.; Majidi, J.; Babaloo, Z. Echinophora platyloba DC (Apiaceae) crude extract induces apoptosis in human prostate adenocarcinoma cells (PC 3). Biomed. J. 2014, 37, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Wszelaczyńska, E.; Pobereżny, J.; Gościnna, K.; Retmańska, K.; Kozera, W.J. Nutritional value of parsley roots depending on nitrogen and magnesium fertilization. Agriculture 2024, 14, 143. [Google Scholar] [CrossRef]
- Ismail, M.S.; Nawaz, F.; Shehzad, M.A. Contributions of Nitrogen Metabolic Enzymes in Storage Protein Assimilation and Mineral Accumulation Regulated by Nitrogen and Selenium in Triticum aestivum L. Plant Physiol. Biochem. 2025, 221, 109597. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.M.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Dang, K.; Gong, X.; Liang, H.; Guo, S.; Zhang, S.; Feng, B. Phosphorous fertilization alleviates shading stress by regulating leaf photosynthesis and the antioxidant system in mung bean (Vigna radiata L.). Plant Physiol. Biochem. 2023, 196, 111–1121. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Peng, S.; Yin, Z.; Li, X.; Xie, L.; Shen, M.; Li, D.; Gao, J. Effects of N, P, K nutrition levels on the growth, flowering attributes and functional components in Chrysanthemum morifolium. Horticulturae 2024, 10, 226. [Google Scholar] [CrossRef]
- Xiong, C.; Li, X.; Wang, X.; Wang, J.; Lambers, H.; Vance, C.P.; Shen, J.; Cheng, L. Flavonoids are involved in phosphorus-deficiency-induced cluster-root formation in white lupin. Ann. Bot. 2020, 129, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Fallah, S.; Maggi, F.; Ghanbari-Odivi, A.; Rostaei, M. Optimizing lemon balm (Melissa officinalis L.) cultivation: Effects of different manures on plant growth and essential oil production during consecutive harvests. Horticulturae 2024, 10, 1105. [Google Scholar] [CrossRef]
- Ghanbari-Odivi, A.; Fallah, S.; Carrubba, A. Optimizing hyssop (Hyssopus officinalis L.) cultivation: Effects of different manures on plant growth and essential oil yield. Horticulturae 2024, 10, 894. [Google Scholar] [CrossRef]
- Fallah, S.; Maggi, F.; Ghanbari-Odivi, A.; Rostaei, M.; Shahbazi, E. Improvement of production and quality of essential oils in multi-cut peppermint (Mentha x piperita L.) through eco-friendly fertilizers in the semi-arid highlands. Ind. Crops Prod. 2024, 216, 118801. [Google Scholar] [CrossRef]
- Bustamante, M.Á.; Michelozzi, M.; Barra Caracciolo, A.; Grenni, P.; Verbokkem, J.; Geerdink, P.; Safi, C.; Nogues, I. Effects of soil fertilization on terpenoids and other carbon-based secondary metabolites in Rosmarinus officinalis plants: A comparative ttudy. Plants 2020, 9, 830. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Anand, G.; Gupta, P.; Mandal, S. Insight into the mechanisms of enhanced production of valuable terpenoids by arbuscular mycorrhiza. Phytochem. Rev. 2017, 16, 677–692. [Google Scholar] [CrossRef]
- Liang, H.; Sun, H.; Shao, C.; Lv, B.; Cao, W.; Zhou, J.; Zhu, J.; Zhang, Y. Regulation of nitrogen and phosphorus content and terpenoid metabolite accumulation in P. ginseng by exogenous strigolactone. Curr. Plant Biol. 2024, 41, 100425. [Google Scholar] [CrossRef]
- Velikova, V.; Müller, C.; Ghirardo, A.; Rock, T.M.; Aichler, M.; Walch, A.; Schmitt-Kopplin, P.; Schnitzler, J.P. Knocking down of osoprene emission modifies the lipid matrix of thylakoid membranes and influences the chloroplast ultrastructure in poplar. Plant Physiol. 2015, 168, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Wagatsuma, T. The membrane lipid bilayer as a regulated barrier to cope with detrimental ionic conditions: Making new tolerant plant lines with altered membrane lipid bilayer. Soil. Sci. Plant Nutr. 2017, 63, 507–516. [Google Scholar] [CrossRef]
- Moghaddam, M.; Taheri, P.; Ghasemi Pirbalouti, M.; Mehdizadeh, L. Chemical composition and antifungal activity of essential oil from the seed of Echinophora platyloba DC. against phytopathogens fungi by two different screening methods. LWT—Food Sci. Technol. 2015, 61, 536–542. [Google Scholar] [CrossRef]
- Sodeifian, G.; Sajadian, S.A. Investigation of essential oil extraction and antioxidant activity of Echinophora platyloba DC. using supercritical carbon dioxide. J. Supercrit. Fluids. 2017, 121, 52–62. [Google Scholar] [CrossRef]
- Abbaszadeh, B.; Layeghhaghighi, M.; Azimi, R.; Hadi, N. Improving water use efficiency through drought stress and using salicylic acid for proper production of Rosmarinus officinalis L. Ind. Crops Prod. 2020, 144, 111893. [Google Scholar] [CrossRef]
- Mahdi Navehsi, F.; Abdossi, V.; Abbaszadeh, B.; Azimi, R.; Dianat, M. Effect of gamma rays on the essential oil and biochemical characteristics of the Satureja mutica Fisch & C. A. Mey. Sci. Rep. 2024, 14, 7581. [Google Scholar] [CrossRef] [PubMed]
- Solouki, A.; Zare Mehrjerdi, M.; Aliniaeifard, S.; Azimi, R. Postharvest light and temperature elicitors improve chemical composition and level of essential oils in basil (Ocimum basilicum L.) through boosting antioxidant machinery. Postharvest Biol. Technol. 2023, 199, 112279. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/mass Spectrometry, 4.1st ed.; Allured Publishing: Carol Stream, IL, USA, 2017. [Google Scholar]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef] [PubMed]
- Gang, S.; Sharma, S.; Saraf, M.; Buck, M.; Schumacher, J. Bacterial Indole-3-Acetic Acid Influences Soil Nitrogen Acquisition in Barley and Chickpea. Plants 2021, 10, 780. [Google Scholar] [CrossRef]
- Nofal, E.M.S.; EL-Mahrouk, M.E.; EL-Sayed, B.A.; Radwan, A.M.M. Effect of NPK fertilizer and some natural extract treatments on the vegetative growth and flowering of African marigold (Tagetes erecta L. var. Dwarf Chrysanthemum). Appl. Ecol. Environ. Res. 2021, 19, 3167–3179. [Google Scholar] [CrossRef]
- Gaber, M.K. Vegetative and flowering growth of geranium as affected by mineral fertilization and ascorbic acid foliar application. Middle East. J. Appl. Sci. 2019, 9, 220–230. [Google Scholar]
- Khan, F.; Siddique, A.B.; Shabala, S.; Zhou, M.; Zhao, C. Phosphorus Plays Key Roles in Regulating Plants’ Physiological Responses to Abiotic Stresses. Plants 2023, 12, 2861. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Du, X.; Wang, F.; Sha, J.; Chen, Q.; Tian, G.; Zhu, Z.; Ge, S.; Jiang, Y. Effects of Potassium Levels on Plant Growth, Accumulation and Distribution of Carbon, and Nitrate Metabolism in Apple Dwarf Rootstock Seedlings. Front. Plant Sci. 2020, 11, 904. [Google Scholar] [CrossRef]
- Hu, W.; Loka, D.A.; Fitzsimons, T.R.; Zhou, Z.; Oosterhuis, D.M. Potassium deficiency limits reproductive success by altering carbohydrate and protein balances in cotton (Gossypium hirsutum L.). Environ. Exp. Bot. 2018, 145, 87–94. [Google Scholar] [CrossRef]
- Önder, S.; Tonguç, M.; Önder, D.; Erbaş, S.; Mutlucan, M. Flower color and carbohydrate metabolism changes during the floral development of Rosa damascena. S. Afr. J. Bot. 2023, 156, 234–243. [Google Scholar] [CrossRef]
- Pandian, K.; Mustaffa, M.R.A.F.; Mahalingam, G.; Paramasivam, A.; John Prince, A.; Gajendiren, M.; Rafiqi Mohammad, A.R.; Varanasi, S.T. Synergistic conservation approaches for nurturing soil, food security and human health towards sustainable development goals. J. Hazard. Mater. Adv. 2024, 16, 100479. [Google Scholar] [CrossRef]
- Jia, Z.; Giehl, R.F.H.; von Wirén, N. Nutrient–hormone relations: Driving root plasticity in plants. Molecular Plant 2022, 15, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Shah, I.H.; Jinhui, W.; Li, X.; Hameed, M.K.; Manzoor, M.A.; Li, P.; Zhang, Y.; Niu, Q.; Chang, L. Exploring the role of nitrogen and potassium in photosynthesis implications for sugar: Accumulation and translocation in horticultural crops. Sci. Hortic. 2024, 327, 112832. [Google Scholar] [CrossRef]
- Nilofer, N.; Singh, S.; Singh, A.; Kaur, P.; Siddiqui, A.; Kumar, D.; Lal, R.; Chanotiya, C. Influence of the season on the quantity and chemical composition of the essential oil and synthesis of secondary metabolites in Cymbopogon martinii (Roxb.). Wats. Agrotech. Ind. Crops 2023, 3, 170–191. [Google Scholar] [CrossRef]



| Air Temperature (°C) | Daylight Length | Relative Humidity | Precipitation | |||
|---|---|---|---|---|---|---|
| Min. | Max. | Mean | (h) | (%) | (mm) | |
| January | −4.7 | 9 | 0.8 | 231 | 53 | 17.4 |
| February | −2 | 12.6 | 5.3 | 188 | 52 | 43.6 |
| March | −1.1 | 19.2 | 9 | 291 | 31 | 2.3 |
| April | 2.5 | 18.5 | 10.5 | 223 | 49 | 50.1 |
| May | 6 | 23.5 | 14.8 | 268 | 49 | 36.7 |
| June | 9.2 | 32.1 | 20.7 | 350 | 21 | 0 |
| July | 13.2 | 34.3 | 23.8 | 343 | 16 | 0 |
| August | 11.7 | 34.5 | 23.1 | 343 | 19 | 0 |
| September | 7.7 | 30.8 | 19.3 | 318 | 22 | 0 |
| October | 3.4 | 22.2 | 12.8 | 224 | 43 | 21.7 |
| November | −0.1 | 13 | 6.5 | 165 | 63 | 70.7 |
| December | −5.4 | 12.2 | 3.4 | 252 | 51 | 20.8 |
| Treatment | Description |
|---|---|
| Nitrogen (N) | 250 mg N/plant, urea + 500 mL water |
| Phosphorus (P) | 250 mg P/plant, triple superphosphate + 500 mL water |
| Potassium (K) | 250 mg K/plant, potassium sulfate + 500 mL water |
| Nitrogen + Phosphorus (NP) | 250 mg N/plant, urea + 250 mg P/plant, triple superphosphate + 500 mL water |
| Nitrogen + Potassium (NK) | 250 mg N/plant, urea + 250 mg K/plant, potassium sulfate + 500 mL water |
| Phosphorus + Potassium (PK) | 250 mg P/plant, triple superphosphate + 250 mg K/plant, potassium sulfate + 500 mL water |
| Nitrogen + Phosphorus + Potassium (NPK) | 250 mg N/plant, urea + 250 mg P/plant, triple superphosphate + 250 mg K/plant, potassium sulfate + 500 mL water |
| Positive control) (C+) | 500 mL water and no fertilizer |
| Negative control (C−) | No fertilizer and no water |
| Source of Variation | df | Plant Height | Branche Number | Main Stem Diameter | Leaf Number | Inflorescences Number | Flowers Number |
|---|---|---|---|---|---|---|---|
| Treatment | 8 | 429 ** | 111 ** | 0.25 ** | 35,589 ** | 79 ** | 40,692 ** |
| Error | 27 | 4.4 | 7.65 | 0.01 | 476 | 1.89 | 1253 |
| CV (%) | 7.3 | 19.3 | 10.7 | 8.7 | 10.7 | 12.6 |
| Source of Variation | df | Stem Dry Weight | Leaf Dry Weight | Inflorescence Dry Weight | Aerial Biomass | Essential Oils Content | Essential Oils Yield |
|---|---|---|---|---|---|---|---|
| Treatment | 8 | 12.1 ** | 31.9 ** | 79.1 ** | 317 ** | 0.26 ** | 32,438 ** |
| Error | 27 | 0.75 | 1.43 | 4.05 | 7.18 | 0.05 | 31.6 |
| CV (%) | 15.1 | 15.5 | 14.2 | 9.7 | 23.9 | 3.8 |
| Compound | Formula | RIa | RIb | C− | C+ | N | P | K | NP | NK | PK | NPK |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α-Pinene | C10H16 | 943 | 939 | 1.20 | 1.16 | 1.46 | 1.8 | 1.92 | 1.80 | 1.69 | 2.04 | 1.25 |
| β-Pinene | C10H16 | 975 | 979 | 2.04 | 3.19 | 5.33 | 6.10 | 7.70 | 8.78 | 6.51 | 6.61 | 4.47 |
| Myrcene | C10H16 | 996 | 991 | 0.75 | 0.81 | 2.09 | 1.05 | 1.11 | 1.19 | 1.60 | 1.04 | 0.87 |
| p-Cymene | C10H14 | 1028 | 1025 | 1.84 | 2.72 | 1.21 | 2.48 | 2.06 | 1.12 | 1.64 | 3.00 | 1.51 |
| Limonene | C10H16 | 1035 | 1029 | 3.68 | 2.61 | 5.82 | 3.37 | 2.95 | 3.65 | 4.38 | 2.25 | 3.89 |
| (E)-β-Ocimene | C10H16 | 1050 | 1044 | 27.81 | 26.64 | 21.32 | 30.10 | 26.97 | 24.69 | 24.14 | 29.25 | 26.85 |
| Terpinolene | C10H16 | 1080 | 1088 | 0.70 | 0.88 | 0.75 | 0.97 | 0.74 | 0.70 | 0.75 | 0.79 | 0.98 |
| Linalool | C10H18O | 1100 | 1096 | 1.96 | 7.11 | 6.60 | 4.60 | 3.90 | 3.35 | 5.25 | 4.45 | 3.99 |
| (3Z)-Hexenyl-2-methyl butanoate | C11H20O2 | 1235 | 1224 | 0.57 | 0.44 | 0.77 | 0.53 | 0.46 | 0.47 | 0.61 | 0.44 | 0.53 |
| (3Z)-Hexenyl-3-methyl butanoate | C11H20O2 | 1237 | 1233 | 0.32 | 1.46 | 0.96 | 0.86 | 0.66 | 0.45 | 0.81 | 0.87 | 0.70 |
| Neral | C10H16O | 1242 | 1238 | 0.30 | 0.96 | 2.06 | 2.03 | 1.35 | 1.62 | 1.70 | 1.07 | 2.63 |
| Geraniol | C10H18O | 1256 | 1253 | - | 0.96 | 3.10 | 2.02 | 1.34 | 1.10 | 2.22 | 1.59 | 2.09 |
| Geranial | C10H16O | 1273 | 1267 | 1.42 | 2.78 | 4.20 | 3.90 | 3.16 | 3.95 | 3.68 | 2.36 | 4.87 |
| Limonen-10-ol | C10H16O | 1295 | 1288 | 1.36 | 1.70 | 2.66 | 2.34 | 1.67 | 1.59 | 2.16 | 1.71 | 2.56 |
| Methyl eugenol | C11H14O2 | 1412 | 1403 | 0.86 | 1.22 | 0.85 | 1.21 | 1.63 | 2.38 | 1.24 | 0.90 | 1.30 |
| y-Decalactone | C10H18O2 | 1470 | 1465 | 1.19 | 2.37 | 1.67 | 1.85 | 3.76 | 5.91 | 2.72 | 1.62 | 1.75 |
| Myristicin | C11H12O3 | 1510 | 1517 | 0.36 | 0.62 | 0.51 | 0.47 | 0.70 | 0.95 | 0.61 | 0.46 | 0.59 |
| Kessane | C15H26O | 1536 | 1529 | 1.06 | 0.55 | 0.53 | 0.55 | 1.12 | 1.70 | 0.83 | 0.55 | 0.65 |
| (3Z)-hexenyl benzoate | C13H16O2 | 1560 | 1565 | 0.39 | 1.96 | 0.60 | 0.68 | 0.75 | 0.93 | 0.68 | 0.57 | 0.86 |
| Spathulenol | C15H24O | 1582 | 1577 | 1.70 | 5.81 | 4.14 | 4.80 | 5.34 | 6.21 | 4.74 | 4.47 | 4.32 |
| (E)-Sesquilavandulol | C15H26O | 1643 | 1631 | 42.00 | 20.76 | 22.43 | 20.91 | 17.17 | 14.08 | 19.80 | 20.25 | 19.61 |
| α-Muurolol | C15H26O | 1650 | 1644 | 0.33 | - | 0.55 | - | 0.66 | 0.80 | 0.60 | 0.51 | - |
| y-Dodecalactone | C12H22O2 | 1674 | 1676 | 0.95 | - | 0.72 | 1.01 | 1.38 | 1.87 | 1.05 | 0.89 | 0.95 |
| Total identified (%) | 92.79 | 86.71 | 90.33 | 93.63 | 88.50 | 89.29 | 89.41 | 87.69 | 87.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadi, S.; Fallah, S.; Maggi, F. Effects of Macronutrients on the Growth, Essential Oil Production, and Quality of Echinophora platyloba (DC.) in Natural Ecosystems. Horticulturae 2025, 11, 185. https://doi.org/10.3390/horticulturae11020185
Mohammadi S, Fallah S, Maggi F. Effects of Macronutrients on the Growth, Essential Oil Production, and Quality of Echinophora platyloba (DC.) in Natural Ecosystems. Horticulturae. 2025; 11(2):185. https://doi.org/10.3390/horticulturae11020185
Chicago/Turabian StyleMohammadi, Somayeh, Sina Fallah, and Filippo Maggi. 2025. "Effects of Macronutrients on the Growth, Essential Oil Production, and Quality of Echinophora platyloba (DC.) in Natural Ecosystems" Horticulturae 11, no. 2: 185. https://doi.org/10.3390/horticulturae11020185
APA StyleMohammadi, S., Fallah, S., & Maggi, F. (2025). Effects of Macronutrients on the Growth, Essential Oil Production, and Quality of Echinophora platyloba (DC.) in Natural Ecosystems. Horticulturae, 11(2), 185. https://doi.org/10.3390/horticulturae11020185







