Valorisation of Sunflower Crop Residue as a Potentially New Source of Bioactive Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Sunflower Ethanol Extract and Its Ethyl Acetate Fraction
2.3. Isolation of Main Compounds from Sunflower Ethanol Extract
2.4. Evaluation of Total Phenol and Flavonoid Content
2.5. LC-DAD-ESI-MS Analysis
2.6. Evaluation of Antioxidant Activity
2.7. Evaluation of Enzyme Inhibitory Activity
2.8. Evaluation of Antimicrobial Activity
2.9. Statistical Analysis
3. Results
3.1. Main Compounds Isolated from Sunflower Ethanol Extract
3.2. Chemical Composition of Sunflower Samples
3.3. Antioxidant Activity of Sunflower Ethanol Extract, Its Ethyl Acetate Fraction and Isolated Compounds
3.4. Enzyme Inhibitory Activity of Sunflower Ethanol Extract, Its Ethyl Acetate Fraction and Isolated Compounds
3.5. Antimicrobial Activity of Sunflower Ethanol Extract, Its Ethyl Acetate Fraction and Isolated Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haque, F.; Fan, C.; Lee, Y.Y. From waste to value: Addressing the relevance of waste recovery to agricultural sector in line with circular economy. J. Clean. Prod. 2023, 415, 137873. [Google Scholar] [CrossRef]
- Rani, G.M.; Pathania, D.; Umapathi, R.; Rustagi, S.; Huh, Y.S.; Gupta, V.K.; Kaushik, A.; Chaudhary, V. Agro-waste to sustainable energy: A green strategy of converting agricultural waste to nano-enabled energy applications. Sci. Total Environ. 2023, 875, 162667. [Google Scholar]
- Chiocchio, I.; Mandrone, M.; Tacchini, M.; Guerrini, A.; Poli, F. Phytochemical profile and in vitro bioactivities of plant-based by-products in view of a potential reuse and valorization. Plants 2023, 12, 795. [Google Scholar] [CrossRef]
- Tasneem Bashir, T.B.; Zia-ur-Rehman Mashwani, Z.U.R.M.; Kulsoom Zahara, K.Z.; Shakeela Haider, S.H.; Shaista Tabassum, S.T.; Mudrikah, M. Chemistry, pharmacology and ethnomedicinal uses of Helianthus annuus (sunflower): A review. Pure App. Biol. 2015, 4, 226–235. [Google Scholar] [CrossRef]
- Gai, F.; Karamać, M.; Janiak, M.A.; Amarowicz, R.; Peiretti, P.G. Sunflower (Helianthus annuus L.) plants at various growth stages subjected to extraction—Comparison of the antioxidant activity and phenolic profile. Antioxidants 2020, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, A.A.; Yagi, S.; Abdallah, A.H.; Abdalla, M.; Sinan, K.I.; Zengin, G. Phenolic profile, antioxidant and enzyme inhibition properties of seed methanolic extract of seven new Sunflower lines: From fields to industrial applications. Process Biochem. 2021, 111, 53–61. [Google Scholar] [CrossRef]
- Seiler, G.J.; Jan, C. Chapter 1, Basic Information. In Genetics, Genomics and Breeding of Sunflower; Hu, J., Seiler, G., Kole, C., Eds.; Science Publishers: Enfield, NH, USA, 2010; pp. 1–50. [Google Scholar]
- Seiler, G.J.; Qi, L.L.; Marek, L.F. Utilization of sunflower crop wild relatives for cultivated sunflower improvement. Crop Sci. 2017, 57, 1083–1101. [Google Scholar] [CrossRef]
- Pilorgé, E. Sunflower in the global vegetable oil system: Situation, specificities and perspectives. OCL 2020, 27, 34. [Google Scholar] [CrossRef]
- Onoja, S.O.; Anaga, A.O. Evaluation of the antidiabetic and antioxidant potentials of methanolic leaf extract of Helianthus annuus L. on alloxan-induced hyperglycemic rats. Comp. Clin. Pat. 2013, 23, 1565–1573. [Google Scholar] [CrossRef]
- Onoja, S.O.; Anaga, A.O. Bioassay-guided fractionation, antihyperglycemic and antioxidant properties of the methanol leaf extract of Helianthus annuus. Int. J. Pharm. Phytochem. Res. 2015, 7, 340–346. [Google Scholar]
- Akpor, O.B.; Olaolu, T.D. Antibacterial and antioxidant potentials of leave extracts of Helianthus annuus. Potravin. Slovak J. Food Sci. 2019, 13, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Amirul, R. Literature Review: Study of Antibacterial Activity of Sunflower (Helianthus annuus L.) Extract and Its Phytochemical Profiles. JNHM 2020, 3, 29–37. [Google Scholar]
- Salwa, M. Antioxidant activity of sunflower (Helianthus annuus L.) ethanolic extract with DPPH method and determination of total phenolic and flavonoid levels. J. Nutraceuticals Herb. Med. 2021, 4, 31–42. [Google Scholar] [CrossRef]
- Sharma, S.K.; Afroz, A.L.A.M. Phytochemical screening, antimicrobial, and antioxidant properties of Helianthus annuus and Hyophila involuta: A comparative account. Not. Sci. Biol. 2024, 16, 11557. [Google Scholar] [CrossRef]
- Kovačević, V. Korišćenje Poljoprivredne Biomase za Energetske Potrebe u Srbiji; UNDP, Ministarstvo Energetike Republike Srbije: Belgrade, Serbia, 2018. [Google Scholar]
- Statistical Office of The Republic of Serbia. Statistical Yearbook of the Republic of Serbia; Statistical Office of The Republic of Serbia: Belgrade, Serbia, 2018.
- Glišić, M.; Bošković Cabrol, M.; Čobanović, N.; Starčević, M.; Samardžić, S.; Veličković, I.; Maksimović, Z. The effects of sunflower and maize crop residue extracts as a new ingredient on the quality properties of pork liver pâtés. Foods 2024, 13, 788. [Google Scholar] [CrossRef] [PubMed]
- Lukić, M.; Ćirić, A.; Božić, D.D.; Stanković, J.A.; Medarević, Đ.; Maksimović, Z. Extracts from Wheat, Maize, and Sunflower Waste as Natural Raw Materials for Cosmetics: Value-Added Products Reaching Sustainability Goals. Pharmaceutics 2024, 16, 1182. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, D.S.; Rosenbohm, C. Dry column vacuum chromatography. Synthesis 2001, 2001, 2431–2434. [Google Scholar] [CrossRef]
- Ahmad, I.; Yanuar, A.; Mulia, K.; Mun’im, A. Application of Ionic Liquid as a Green Solvent for Polyphenolics Content Extraction of Peperomia Pellucida (L) Kunth Herb. J. Young Pharm. 2017, 9, 486–490. [Google Scholar] [CrossRef]
- Chatatikun, M.; Chiabchalard, A. Phytochemical Screening and Free Radical Scavenging Activities of Orange Baby Carrot and Carrot (Daucus Carota Linn. ) Root Crude Extracts. J. Chem. Pharm. Res. 2013, 5, 97–102. [Google Scholar]
- Sembiring, E.; Elya, B.; Sauriasari, R.; Sauriasari, R. Phytochemical Screening, Total Flavonoid and Total Phenolic Content and Antioxidant Activity of Different Parts of Caesalpinia Bonduc (L.) Roxb. Pharmacogn. J. 2018, 10, 123–127. [Google Scholar] [CrossRef]
- Prieto, J. Procedure: Preparation of DPPH Radical, and Antioxidant Scavenging Assay. DPPH Microplate Protoc. 2012, 2012, 7–9. [Google Scholar]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for Antioxidant Assays for Food Components. Food Front. 2020, 1, 60–69, 838. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Devaki, M. The Ferric Reducing/Antioxidant Power (FRAP) Assay for Non-Enzymatic Antioxidant Capacity: Concepts, Procedures, Limitations and Applications. In Measurement of Antioxidant Activity & Capacity; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 77–106. ISBN 978-1-119-13538-8. [Google Scholar]
- Mokrani, A.; Krisa, S.; Cluzet, S.; Da Costa, G.; Temsamani, H.; Renouf, E.; Mérillon, J.-M.; Madani, K.; Mesnil, M.; Monvoisin, A.; et al. Phenolic Contents and Bioactive Potential of Peach Fruit Extracts. Food Chem. 2016, 202, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R.; Ceylan, O. A Comprehensive Study on Phytochemical Characterization of Haplophyllum myrtifolium Boiss. Endemic to Turkey and Its Inhibitory Potential against Key Enzymes Involved in Alzheimer, Skin Diseases and Type II Diabetes. Ind. Crops Prod. 2014, 53, 244–251. [Google Scholar] [CrossRef]
- Wan, L.-S.; Min, Q.-X.; Wang, Y.-L.; Yue, Y.-D.; Chen, J.-C. Xanthone Glycoside Constituents of Swertia kouitchensis with α-Glucosidase Inhibitory Activity. J. Nat. Prod. 2013, 76, 1248–1253. [Google Scholar] [CrossRef]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Antioxidant Potentials and Anticholinesterase Activities of Methanolic and Aqueous Extracts of Three Endemic Centaurea L. Species. Food Chem. Toxicol. 2013, 55, 290–296. [Google Scholar] [CrossRef]
- Moonrungsee, N.; Shimamura, T.; Kashiwagi, T.; Jakmunee, J.; Higuchi, K.; Ukeda, H. Sequential Injection Spectrophotometric System for Evaluation of Mushroom Tyrosinase-Inhibitory Activity. Talanta 2012, 101, 233–239. [Google Scholar] [CrossRef]
- Panizo, F.M.; Rodriguez, B. Some diterpenic constituents of the sunflower (Helianthus annuus L.). Anal. Quim. 1979, 75, 428–430. [Google Scholar]
- Nakano, M.; Fukushima, M.; Azuma, H. Isolation and chemical characterization of antimicrobial compounds from sunflower (Helianthus annuus L.). Food Hyg. Saf. Sci. (Shokuhin Eiseigaku Zasshi) 1995, 36, 22–28. [Google Scholar] [CrossRef]
- Lyu, J.H.; San Lee, G.; Kim, K.H.; Kim, H.W.; Cho, S.I.; Jeong, S.I.; Hong-Jun, K.; Young-Seung, J.; Ho-Kyoung, K.; Ruxana, T.S.; et al. ent-kaur-16-en-19-oic Acid, isolated from the roots of Aralia continentalis, induces activation of Nrf2. J. Ethnopharmacol. 2011, 137, 1442–1449. [Google Scholar] [CrossRef]
- Móricz, Á.M.; Ott, P.G.; Yüce, I.; Darcsi, A.; Béni, S.; Morlock, G.E. Effect-directed analysis via hyphenated high-performance thin-layer chromatography for bioanalytical profiling of sunflower leaves. J. Chromatogr. A. 2018, 1533, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Lin, B.Y. A new dinorxanthane and chromone from the root of Tithonia diversifolia. Chem. Pharm. Bull. 1999, 47, 428–429. [Google Scholar] [CrossRef]
- Wu Di, W.D.; Zhang Mian, Z.M.; Zhang Chao Feng, Z.C.; Wang Zheng Tao, W.Z. Chromones from the flower buds of Tussilago farfara. Biochem. Syst. Ecol. 2008, 36, 219–222. [Google Scholar]
- Fei, Y.H. Chemical constituents from seeds of Helianthus annuus. Chin. Tradit. Herb. Drugs 2014, 24, 631–634. [Google Scholar]
- Mehmood, A.; Zhao, L.; Ishaq, M.; Safdar, B.; Wang, C.; Nadeem, M. Optimization of total phenolic contents, antioxidant, and in-vitro xanthine oxidase inhibitory activity of sunflower head. CyTA-J. Food 2018, 16, 957–964. [Google Scholar] [CrossRef]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Effect of solvent type and extraction conditions on the recovery of phenolic compounds from artichoke waste. Chem. Eng. 2014, 39, 463–468. [Google Scholar]
- Fuentes-Gandara, F.; Torres, A.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Varela, R.; Martínez de la Ossa-Fernández, E.J.; Macias, F.A. Selective fractionation and isolation of allelopathic compounds from Helianthus annuus L. leaves by means of high-pressure techniques. J. Supercrit. Fluids 2019, 143, 32–41. [Google Scholar] [CrossRef]
- Kusmiati, E.B.N.; Indriati, R.; Mellova, A. Antibacterial and antioxidant activity test of crude lutein Extracted from sunflower (Helianthus annuus L.). AIP Conf. Proc. 2021, 2331, 050001. [Google Scholar]
- Pyrek, J.S. New pentacyclic diterpene acid: Trachyloban-19-oic acid from sunflower. Tetrahedron 1970, 26, 5029–5032. [Google Scholar] [CrossRef]
- Mitscher, L.A.; Rao, G.S.R.; Veysoglu, T.; Drake, S.; Haas, T. Isolation and identification of trachyloban-19-oic and (-)-kaur-16-en-19-oic acids as antimicrobial agents from the prairie sunflower, Helianthus annuus. J. Nat. Prod. 1983, 46, 745–746. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Trokowski, K.; Szłyk, E. Optimization of extraction conditions of antioxidants from sunflower shells (Helianthus annuus L.) before and after enzymatic treatment. Ind. Crops Prod. 2011, 33, 123–131. [Google Scholar]
- Rodríguez, M.; Nolasco, S.; Izquierdo, N.; Mascheroni, R.; Madrigal, M.S.; Flores, D.C.; Ramos, A.Q. Microwave-assisted extraction of antioxidant compounds from sunflower hulls. Heat Mass Transf. 2019, 55, 3017–3027. [Google Scholar] [CrossRef]
- De’Nobili, M.; Bernhardt, D.C.; Basanta, M.F.; Rojas, A.M. Sunflower (Helianthus annuus L.) seed hull waste: Composition, antioxidant activity, and filler performance in pectin-based film composites. Front. Nut. 2021, 8, 777214. [Google Scholar] [CrossRef]
- Hallouch, O.; Ibourki, M.; Devkota, K.P.; Gharby, S. Proximate Composition, Antioxidant Activity, Lipids and Elemental Profiling of Argan, Almond, Sesame, Nigella, Soybean and Sunflower Oil Press Cakes Reveal a Great Potential of Valorization. Waste Biomass. Valor. 2024; under review. [Google Scholar]
- Ye, F.; Liang, Q.; Li, H.; Zhao, G. Solvent effects on phenolic content, composition, and antioxidant activity of extracts from florets of sunflower (Helianthus annuus L.). Ind. Crops Prod. 2015, 76, 574–581. [Google Scholar] [CrossRef]
- Khan, M.A.R.; Islam, M.A.; Biswas, K.; Al-Amin, M.Y.; Ahammed, M.S.; Manik, M.I.N.; Islam, K.M.M.; Kader, M.A.; Khurshid Alam, A.H.M.; Zaman, S.; et al. Compounds from the Petroleum Ether Extract of Wedelia chinensis with Cytotoxic, Anticholinesterase, Antioxidant, and Antimicrobial Activities. Molecules 2023, 28, 793. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Lee, E.J.; Kim, J.S.; Kang, S.S.; Lee, J.H.; Min, B.S.; Choi, J.S. Cholinesterase and BACE1 inhibitory diterpenoids from Aralia cordata. Arch. Pharmacol. Res. 2009, 32, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Hashem, F.A. Phenolic compounds of Erucaria microcarpa Boiss. and their effect as scavengers for singlet oxygen. J. Herbs. Spices Med. Plants 2007, 12, 27–41. [Google Scholar] [CrossRef]
- Lee, S.; Do, S.G.; Kim, S.Y.; Kim, J.; Jin, Y.; Lee, C.H. Mass spectrometry-based metabolite profiling and antioxidant activity of Aloe vera (Aloe barbadensis Miller) in different growth stages. J. Agricult. Food Chem. 2012, 60, 11222–11228. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Uchida, S.; Watanabe, K.; Mimaki, Y. Chromones from the tubers of Eranthis cilicica and their antioxidant activity. Phytochemistry 2009, 70, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Lucini, L.; Pellizzoni, M.; Pellegrino, R.; Molinari, G.P.; Colla, G. Phytochemical constituents and in vitro radical scavenging activity of different Aloe species. Food Chem. 2015, 170, 501–507. [Google Scholar] [CrossRef]
- Jantaharn, P.; Mongkolthanaruk, W.; Senawong, T.; Jogloy, S.; McCloskey, S. Bioactive compounds from organic extracts of Helianthus tuberosus L. flowers. Ind. Crops Prod. 2018, 119, 57–63. [Google Scholar] [CrossRef]
- Abrão, F.; De Araújo Costa, L.D.; Alves, J.M.; Senedese, J.M.; De Castro, P.T.; Ambrósio, S.R.; Cássio, R.; Veneziani, S.; Kenupp Bastos, J.; Crispim Tavares, D.; et al. Copaifera langsdorffii oleoresin and its isolated compounds: Antibacterial effect and antiproliferative activity in cancer cell lines. BMC Comp. Altern. Med. 2019, 15, 443. [Google Scholar] [CrossRef]
- Arciniegas, A.; Pérez-Castorena, A.L.; Meléndez-Aguirre, M.; Ávila, J.G.; García-Bores, A.M.; Villaseñor, J.L.; Romo de Vivar, A. Chemical composition and antimicrobial activity of Ageratina deltoidea. Chem. Biodiver. 2018, 15, e1700529. [Google Scholar] [CrossRef]
- Soares, A.C.F.; Matos, P.M.; Silva, K.F.D.; Martins, C.H.; Veneziani, R.C.; Ambrósio, S.R.; Herbert, J.D.; Raquel, A.S.; Heleno, V.C. Antimicrobial potential of natural and semi-synthetic ent-kaurane and ent-pimarane diterpenes against clinically isolated gram-positive multidrug-resistant bacteria. J. Braz. Chem. Soc. 2019, 30, 333–341. [Google Scholar] [CrossRef]
- Çiçek, S.S.; Wenzel-Storjohann, A.; Girreser, U.; Tasdemir, D. Biological activities of two major copaiba diterpenoids and their semi-synthetic derivatives. Rev. Bras. Farmacogn. 2020, 30, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Zgoda-Pols, J.R.; Freyer, A.J.; Killmer, L.B.; Porter, J.R. Antimicrobial diterpenes from the stem bark of Mitrephora celebica. Fitoteraia 2002, 73, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Boeck, P.; Sá, M.M.; Souza, B.S.D.; Cercená, R.; Escalante, A.M.; Zachino, S.A.; Valdir Cechinel, F.; Yunes, R.A. A simple synthesis of kaurenoic esters and other derivatives and evaluation of their antifungal activity. J. Braz. Chem. Soc. 2005, 16, 1360–1366. [Google Scholar] [CrossRef]
- Polyiam, P.; Thukhammee, W. A Comparison of Phenolic, Flavonoid, and Amino Acid Compositions and In Vitro Antioxidant and Neuroprotective Activities in Thai Plant Protein Extracts. Molecules 2024, 29, 2990. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.F.D.; Pereira, R.G.; Boaventura, M.A.; Macias, F.A.; Lima, G.D.S.; Coelho, A.; Jose, M.G.; Molinillob, A.C.; Takahashi, J.A. Structure-activity relationship study of diterpenes for treatment of Alzheimer’s Disease. Química Nova 2017, 40, 1045–1050. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Alimboyoguen, A.B.; Urban, S.; Raga, D.D. A bioactive diterpene from Smallanthus sonchifolius. Nat. Prod. Commun. 2008, 3, 1934578X0800301016. [Google Scholar] [CrossRef]
| Parameter/Compound | EES * | EFS |
|---|---|---|
| TPC (mg GAE/g) | 15.83 ± 0.30 a | 19.20 ± 0.83 b |
| TFC (mg QE/g) | 8.98 ± 0.34 a | 15.29 ± 1.67 b |
| Chlorogenic acid (mg/g) | 2.40 ± 0.06 a | 1.00 ± 0.01 b |
| Ent-kaur-16-en-19-oic acid (mg/g) | 59.36 ± 1.09 a | 245.51 ± 2.00 b |
| 6Ac-7OH-Dimethylchromone (mg/g) | 3.99 ± 0.05 a | 16.79 ± 0.11 b |
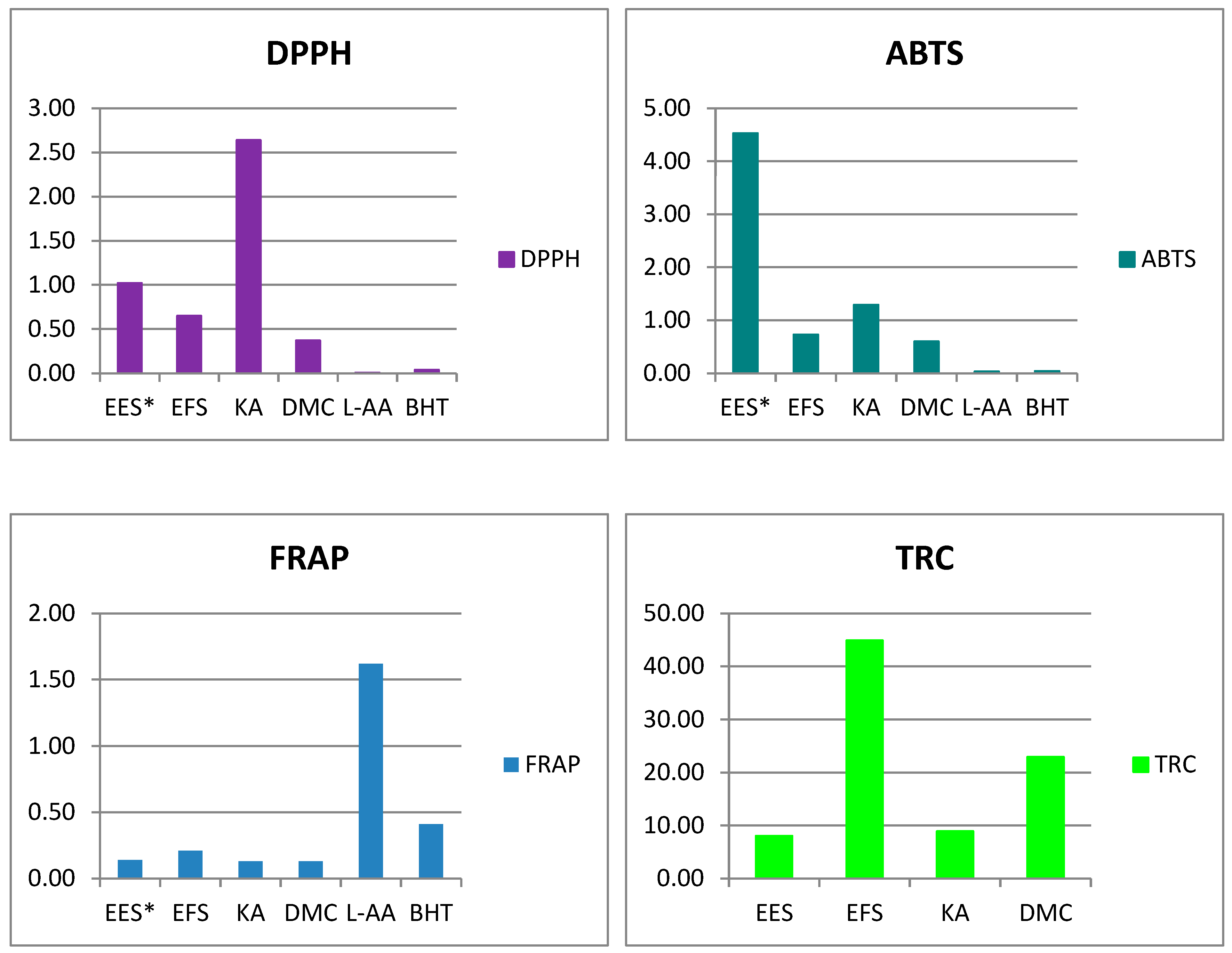
| Sample/Control | DPPH IC50 (mg/mL) | ABTS IC50 (mg/mL) | FRAP µmol Fe2+/g | TRC mg L-AAE/g |
|---|---|---|---|---|
| EES * | 1.02 ± 0.01 b | 4.53 ± 1.20 a | 0.14 ± 0.01 d | 8.03 ± 0.54 c |
| EFS | 0.65 ± 0.02 c | 0.73 ± 0.03 b,c | 0.21 ± 0.01 c | 44.88 ± 2.16 a |
| KA | 2.64 ± 0.19 a | 1.29 ± 0.13 b | 0.13 ± 0.03 d | 8.93 ± 0.80 c |
| DMC | 0.37 ± 0.00 d | 0.60 ± 0.01 b,c | 0.13 ± 0.03 d | 22.92 ± 1.47 b |
| L-AA | 0.006 ± 0.000 e | 0.03 ± 0.00 c | 1.62 ± 0.00 a | nt |
| BHT | 0.038 ± 0.000 e | 0.04 ± 0.00 c | 0.41 ± 0.03 b | nt |
| Sample/Control | α-AMY | α-GLU | AChE | BChE | TyR |
|---|---|---|---|---|---|
| IC50 (mg/mL) | |||||
| EES | >50 | 48.79 ± 3.98 a | >50 | >50 | >50 |
| EFS | 48.57 ± 5.52 a | 20.62 ± 2.58 b | >50 | 34.54 ± 1.54 a | 40.69 ± 8.77 a |
| KA | / | 0.04 ± 0.00 c | 1.44 ± 0.45 b | 1.63 ± 0.13 c | 0.26 ± 0.08 b |
| DMC | 0.92 ± 0.08 b | 0.05 ± 0.00 c | 1.20 ± 0.09 b | 1.37 ± 0.22 c | 0.61 ± 0.03 b |
| Acarbose | 0.40 ± 0.04 b | 0.37 ± 0.01 c | nt | nt | nt |
| Galantamine | nt | nt | 9.66 ± 0.92 a | 9.87 ± 1.26 b | nt |
| Kojic acid | nt | nt | nt | nt | 0.34 ± 0.07 b |
| Sample | S. aureus | E. faecalis | B. subtilis | E. coli | K. pneumoniae | S. abony | P. aeruginosa | C. albicans |
|---|---|---|---|---|---|---|---|---|
| MIC * (µg/mL) | ||||||||
| EES | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 | >1000 |
| EFS | >500 | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| KA | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
| DMC | >250 | >250 | >250 | >250 | >250 | >250 | >250 | >250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veličković, I.; Samardžić, S.; Milenković, M.T.; Petković, M.; Maksimović, Z. Valorisation of Sunflower Crop Residue as a Potentially New Source of Bioactive Compounds. Horticulturae 2025, 11, 206. https://doi.org/10.3390/horticulturae11020206
Veličković I, Samardžić S, Milenković MT, Petković M, Maksimović Z. Valorisation of Sunflower Crop Residue as a Potentially New Source of Bioactive Compounds. Horticulturae. 2025; 11(2):206. https://doi.org/10.3390/horticulturae11020206
Chicago/Turabian StyleVeličković, Ivona, Stevan Samardžić, Marina T. Milenković, Miloš Petković, and Zoran Maksimović. 2025. "Valorisation of Sunflower Crop Residue as a Potentially New Source of Bioactive Compounds" Horticulturae 11, no. 2: 206. https://doi.org/10.3390/horticulturae11020206
APA StyleVeličković, I., Samardžić, S., Milenković, M. T., Petković, M., & Maksimović, Z. (2025). Valorisation of Sunflower Crop Residue as a Potentially New Source of Bioactive Compounds. Horticulturae, 11(2), 206. https://doi.org/10.3390/horticulturae11020206







