Abstract
Soil and water pollution caused by excessive use of fertilizers and resource scarcity are critical issues in modern horticulture. Although laboratory tests are reliable, they take time and use chemical reagents that must be disposed of and complex protocols. Monitoring plant nutrient status through technologies that allow continuous and rapid assessment is crucial for precise resource management. Several proximal and remote sensors that use different physico-chemical principles to monitor plant nutrient status are available nowadays. However, these technologies still have important operative and structural limitations that must be overcome. The aim of this review is to summarize the current status and latest developments in proximal and remote sensors capable of monitoring plant and soil nutrients, focusing on sensor types, principles, applications, and their strengths and weaknesses. Electrochemical proximal sensors allow continuous monitoring of nutrients in the plant sap or in the soil solution but work on a single spot basis. Instruments based on optical sensors allow immediate measurements and quick analysis, but do not work on a continuous basis. On the other hand, remote sensors, such as drone-mounted cameras and satellite systems, are based on large-area imaging and can be used to estimate crop nutrient status by processing images at different wavelengths. Finally, combining proximal and remote techniques may be needed to achieve very accurate monitoring of plant and nutrient status.
1. Introduction
Plants require a range of nutrients to effectively carry out various metabolic processes essential for producing an optimal yield [1,2]. These chemical elements are absorbed in varying quantities, depending on the plant’s specific requirements. Nutrients absorbed in large quantities are classified as macronutrients, primarily nitrogen (N), phosphorus (P), and potassium (K). Additionally, calcium (Ca), magnesium (Mg), and sulfur (S) are also considered macronutrients, although they are absorbed in slightly lower quantities [1,3]. Each nutrient plays a crucial role in the plant’s vital functions, enabling its survival and ensuring the efficient progression of its production cycle. An excess or deficiency of these elements can disrupt the plant’s internal processes, adversely affecting its overall development and productivity [4].
The primary source of nutrients for plants is the soil. Soil comprises three phases (solid, liquid, and gaseous) interacting with mineral elements [5]. The solid phase serves as a reservoir of both inorganic nutrients and organic compounds, providing essential elements such as N, P, and K. The liquid phase, also known as the soil solution, plays a crucial role in nutrient absorption by plant roots, as it contains dissolved nutrients and facilitates their movement towards the root surface [6]. The gaseous phase consists of the space between soil particles, occupied by gases such as oxygen, carbon dioxide, and N. Furthermore, the soil has a significant biological component that drives the mineralization of organic matter, making organic nutrients available through their conversion to mineral forms. Various soil characteristics can influence the availability of nutrients to plants, either positively or negatively. Key soil properties include texture, reaction of the soil solution (pH), cation exchange capacity (CEC), levels of N, P, and K, electrical conductivity, organic matter content, and total limestone content [7]. For example, soils with small particles, such as clays, have a high surface-to-volume ratio, which leads to an increase in CEC [8,9]. Soil with a higher CEC generally holds a greater reserve of mineral nutrients. However, mineral anions, such as nitrate (NO3−), Cl−, and phosphate (PO43−), are not adsorbed by the soil particles due to the negative charge on their surfaces and therefore remain dissolved in the soil solution [10]. These anions are particularly susceptible to leaching losses. On the other hand, nutrients can be immobilized in the soil. P tends to bind with elements like Ca, iron (Fe), and aluminum, forming insoluble compounds, such as Ca3(PO4)2 and FePO4, that plants cannot absorb [11]. K can be immobilized or “fixed” in the crystalline structures of clay minerals in the soil, especially in layered clays such as illite or vermiculite [12]. Even if present, K can be immobilized or “fixed” in the crystalline structures of clay minerals in the soil, compromising the absorption by roots, and vital processes such as water regulation and protein synthesis [13]. Another crucial chemical property affecting nutrient availability is the pH, or hydrogen ion concentration, of the soil solution. The pH, whether neutral, alkaline, or acidic, has a marked impact on the availability of mineral elements to plant roots. For most crops, the optimal pH range is approximately 5.5 to 6.5, where the greatest average availability of essential plant nutrients is achieved [7,14,15].
N, P, and K dynamics within plants are critical for supporting key metabolic processes and overall physiological development [16,17]. N, a major nutrient required in large quantities, is primarily absorbed by roots in the form of NO3− or ammonium (NH4+) and transported via the xylem to the aerial parts of the plant [18,19,20,21]. Within plant tissues, N is incorporated into amino acids through NO3− and NH4+ assimilation pathways and subsequently used in the synthesis of proteins, nucleic acids, chlorophyll and other pigments [22]. N is crucial for protein and nucleic acid synthesis, as well as essential for leaf growth and general plant development; its deficiency manifests itself in chlorosis of older leaves and reduced growth [23]. Plants carefully regulate N transport to balance the demand in photosynthetically active regions and redistribute it from older leaves through a recycling mechanism [24].
P, another vital macronutrient, is absorbed as PO43− ions and primarily transported via the xylem [25]. Once inside the plant, P is immediately incorporated into high-energy compounds such as adenosine triphosphate (ATP), which is needed for a wide range of biochemical processes, including photosynthesis, respiration, and nucleic acid synthesis [26,27]. P also plays a key role in cell signaling, participating in protein phosphorylation and intracellular signal transduction. Due to its limited mobility in soil, P uptake is often enhanced through symbiotic relationships with mycorrhizal fungi, improving its availability to plant roots [28].
K, although not directly involved in the synthesis of structural biomolecules, is essential for osmotic regulation and ionic balance [29]. It is absorbed as K+ ions and is highly mobile within the plant [25]. K distribution is crucial for maintaining cell turgor, regulating stomatal opening and closing, and facilitating the transport of photosynthates via the phloem [30]. Additionally, K activates a range of enzymes involved in protein synthesis and photosynthesis [31]. Its high mobility allows for efficient redistribution from older to younger leaves, ensuring optimal response to environmental stresses such as drought and heat, as well as efficient water balance management [32].
Ca is taken up mainly through the roots in the form of a Ca2+ ion present in the soil solution. Ca is normally present in the soil as salts, such as CaCO3, CaSO4, or other soluble forms [33]. Its availability depends on the pH of the soil being more readily available in neutral or slightly acidic soils. Once absorbed, the Ca moves into the xylem with the flow of water transpired [34]. Ca is a key component of pectin in cell walls and contributes to their rigidity and stability. Ca is essential for cell division and the development of young tissues [35].
Mg is found in the soil mainly as a soluble ion Mg2+ present in the solution of the soil. Mg can be derived from minerals such as dolomite (CaMg (CO3)2), magnesite (MgCO3) or silicates of Mg. In too acidic soils, Mg may become less available due to leaching [36]. Mg is a highly mobile element in the plant. After absorption, Mg moves through the phloem to the older leaves, but it can be redistributed to young tissues if necessary [37]. Mg is essential for photosynthesis, as it is at the center of the chlorophyll molecule, and it is an essential co-factor for many enzymes involved in plant metabolism. Mg is involved in key processes such as sugar and protein synthesis [38].
S is mainly found in the form of soluble sulphate (SO2−) in the solution of the soil. S is also present in organic compounds that can be mineralized by micro-organisms. S, once absorbed as SO42−, is translocated into the xylem to the growing leaves and organs. Unlike Mg, S is not very mobile inside the plant [39]. S is an essential component of sulphated amino acids (cysteine and methionine); it is fundamental for the construction of proteins. S participates in the formation of essential oils, glucosinolates and other substances involved in the defense of the plant. S is also important for the functioning of different enzymes [40].
It is of fundamental importance for farmers to efficiently determine the dose of fertilizers to apply. Although fertilizers contribute greatly to increased agricultural yields, their overuse has posed a serious threat to soil and crop quality and productivity, as well as to the environment and human health [41,42,43,44]. Continuous exposure to fertilizer overuse can lead to ecosystem imbalances and ultimately contribute to global pollution. For example, when N or P from fertilizers is not fully absorbed by plants, it can leach into groundwater or runoff into surface water, causing eutrophication [45]. Eutrophication is the over-enrichment of water bodies with nutrients, leading to overgrowth of plant biomass. As this biomass decomposes, it depletes dissolved oxygen, severely degrading water quality and altering the structure and function of aquatic ecosystems [46]. Human communities use groundwater as the primary source for drinking. However, in many areas, especially agricultural areas, NO3− levels in groundwater are high. This is mainly due to the outflow of N fertilizers from cultivated land, which also contaminates water sources. Using nitrate-contaminated groundwater or consuming plant foods with a high NO3− content can expose people to serious health risks [47].
Quantitative measurements of plant nutrients and metabolites are usually conducted in the laboratory using chemical-based approaches, such as visible and near-infrared spectroscopy [48], flame atomic absorption spectroscopy [49], mass spectrometry [50], chromatography [51], or fluorescence [52]. Although these technologies offer great selectivity, sensitivity, and accuracy, their big size, high cost, complicated pre-treatment, expensive equipment, and high skills requested make them unsuitable for field applications [53,54]. In recent decades, precision agriculture has become one of the key tools for the agronomic management of orchards, aiming to minimize input and labor while maintaining high yields and enhancing sustainability. In precision agriculture, fertilization primarily aims to ensure the sustained and optimal distribution of nutrients based on the specific needs of the crop over time [55,56]. In this context, geostatistical methods utilizing geographic information system (GIS) software for processing and analysis allow for the development of prescription maps that illustrate soil variability in terms of fertility. These maps facilitate the strategic distribution of nutrients, compensating for specific deficiencies across the field [57,58]. However, soil nutrient composition may not accurately reflect plant needs, leading to a potential overestimation of plant nutrient status and making fertilizer application less efficient. A potential solution to this issue can be plant-based fertilization, which allows for variable rate fertilizer application based on the specific nutrient requirements of individual trees [59]. This approach ensures that nutrients are applied in the right quantities, adapted to the needs of individual plants, optimizing resource use [60].
In recent years, researchers have successfully developed a variety of real-time plant-based sensors and measurement systems, such as electrochemistry [61,62,63] and optical remote sensing [64,65] to monitor nutrients and metabolites in living cells. On-tree nutrition sensors are unique devices that monitor and analyze nutrient levels in real-time in trees without interfering with their physiology to optimize their growth and development [66]. These sensors serve as tools for sustainable and precision horticulture, promoting efficient resource utilization and responsible environmental management in modern farming practices [67,68,69]. They are critical in precision horticulture because they provide real-time data on plant nutrient status, enabling targeted and efficient interventions to optimize plant health and productivity [70,71,72]. Conversely, remote sensing technologies offer a broader perspective by capturing images of entire fields, utilizing a range of optical sensors (such as multispectral and hyperspectral) to analyze spatial variability in tree and soil health, nutrient levels, and water status [73]. Thus, a combination of proximal (temporal scale) and remote (spatial scale) sensing can enhance the accuracy of information [74]. Nowadays, machine learning models and deep learning are highly useful and efficient for developing systems that can provide information and perform real-time applications. For example, these technologies have been developed to carry out real-time foliar fertilization on individual plants using unmanned aerial vehicles (UAVs) [75].
In addition to nutrient status, monitoring the status of primary and secondary metabolites, such as amino acids, carbohydrates, phenols, polyamines, and terpenoids, has recently emerged as a key for understanding the fundamentals of physiological stress and biochemistry in plants. Abiotic factors such as drought, salinity, extreme temperatures, ultraviolet radiation (UVr), and soil pH have been shown to globally impair and reduce crop yields. Plant responses to these stresses vary greatly, and abiotic factors also trigger multi-genic responses that alter the accumulation of primary and secondary metabolites in plants. Through monitoring the status of metabolites accumulated in plants, we can predict the level of stress and plant defense against different biotic and abiotic pressures [76].
The aim of this review is to summarize the current state and latest developments of sensors for plant nutrients, focusing on sensor types, principles, applications, and their strengths and weaknesses. These technologies have the potential to revolutionize how we maximize crop yields while minimizing resource wastage, supporting sustainable precision farming practices. The following list of sensors and principles on which they are based, along with their strengths and weaknesses, should spur and stimulate new investigations and improvements to overcome present limitations.
2. Proximal Sensing
Proximal sensing is a key approach in plant and soil monitoring, involving the deployment of sensors or instruments near, or in direct contact, with the plant or soil. This technique uses a variety of sensors, each based on distinct physico-chemical principles to gather precise, real-time data. The integration of soil and plant sensors plays a critical role in improving the accuracy and efficiency of nutrient monitoring in agricultural systems. By combining data from both sources, farmers and researchers can gain a comprehensive understanding of nutrient dynamics, which enhances decision-making for nutrient management and supports sustainable agricultural practices [71]. Combining data from soil and plant favors our understanding of the interaction between soil nutrient supply and plant nutrient uptake, identifying potential deficiencies or inefficiencies in nutrient use [77,78]. Soil sensors may detect adequate nutrient levels in the soil, but plant sensors can reveal whether the plants are effectively absorbing those nutrients. This integration helps diagnose issues such as nutrient lock-up, poor root health, or water stress that might hinder nutrient uptake [79]. The following sections outline the primary physico-chemical principles, and the main types of sensors commonly utilized in agricultural applications for monitoring the nutrient status of both plants and soils.
2.1. Electrochemical Sensors
Electrochemical sensors are a type of device that produces an electric current through the reaction between an electrode and an analyte [80]. The sensor is based on a combination of molecular recognition (receptor), which interacts with an analyte turning it into a product, and an electrochemical signal converter (transducer) [81,82]. These two components are built with artificially constructed layers of deposited materials (e.g., silver/silver chloride, graphene, Fe oxide), allowing these sensors to continuously monitor the operation of the system and produce a signal that can be quantitative or qualitative [80,81,82,83] (Table 1).
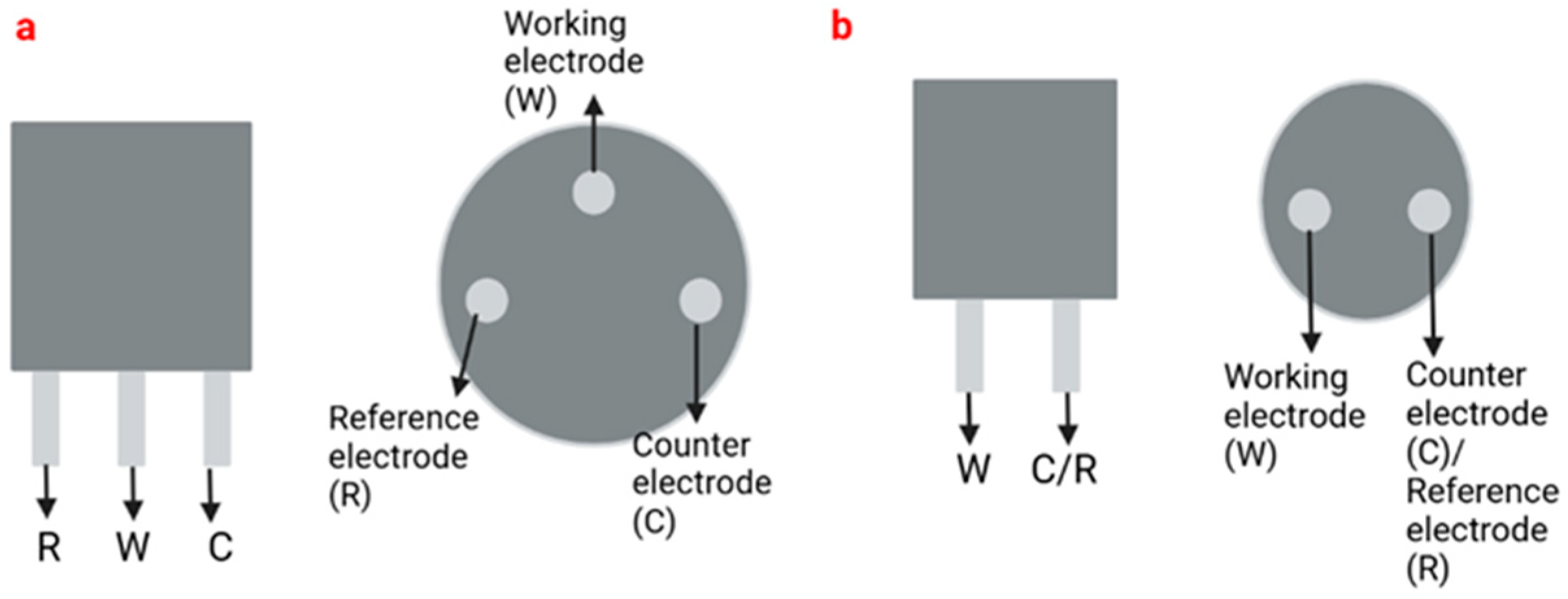
The most common electrode systems in electrochemical sensors are two-electrode (2E) and three-electrode (3E)-based systems (Figure 1). Electrochemical cells based on the 3E system contain reference, auxiliary or counter, and working electrodes, while the 2E system only contains reference and working electrodes that can be made of different materials, such as platinum (Pt), gold (Au) or carbon (C). The reference electrode is used to regulate the electrical potential of the electrode. Generally, reference electrodes have stable and reproducible electrical potential and are reversible [84,85]. The working electrodes act as transduction elements in chemical reactions, while the counter electrode establishes connections with the electrolyte solution so that the current can be applied to the working electrode. These electrodes must be conductive and chemically stable [86,87].
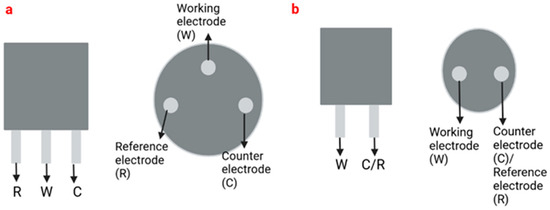
Figure 1.
Representation of a three-electrode system (a) and a two-electrode system (b).

Table 1.
Electrochemical systems used for monitoring plant nutrient status with their main characteristics and equations.
Table 1.
Electrochemical systems used for monitoring plant nutrient status with their main characteristics and equations.
| Electrochemical Systems | Analyte | Transducers | Electrical Properties Monitoring | Units | Equation | References |
|---|---|---|---|---|---|---|
| Potentiometric | Ions (N, P, K) | Ion-selective electrodes (e.g., solid-contact ion-selective electrodes) | Voltage (potential difference) | Volts (V) | Nernst equation: | [53,81,86] |
| Amperometric | Electroactive species (e.g., O2, glucose, metabolites) | Working electrode (Pt, Au or C) | Current | Amperes (A) |
Faraday’s Law: | [81,83,86] |
| Voltametric | Electroactive species (e.g., O2, glucose) | Working electrode (Pt, Au or C) | Current as a function of applied voltage | Amperes (A) or Volts (V) |
Randles–Sevcik equation for reversible reactions:
| [83,86] |
| Conductometric | Ions (e.g., N, P, K) | Conductivity cells | Conductance (or resistance) | Siemens (S) or ohms (Ω) | where G is conductance and R is resistance | [81,83,88] |
| Coulometric | Ions (e.g., N, P, K) | Working electrode (Pt, Au or C) | Total charge | Coulombs (C) | [81] | |
| Impedimetric | Ions (e.g., N, P, K), cells, biomolecules (e.g., carbohydrate, protein, DNA, hormone) | Electrodes with immobilized biomolecules | Impedance | ohms (Ω) | , where R is resistance and Z is reactance | [81,86] |
| Capacitive | Ions (e.g., N, P, K) | Capacitive plates or electrodes | Capacitance | Farads (F) | [81] |
Electrochemical sensors used to assess soil and plant nutrients, as well as plant metabolites, function by enabling electrochemical reactions that involve the transfer of electrons between the analytes (such as ions and metabolites) and the electrodes [89]. These reactions take place through several pathways that are determined by the ion or molecule involved. In oxidation–reduction (redox) reactions, substances undergo electron exchange. During the photosynthesis process, water containing nutrients useful to plants is converted into oxygen and hydrogen ions using light energy [90]. These ions are subsequently used to create glucose and generate chemical energy [91]. On the other hand, in soil, chemical processes involving elements such as Fe, manganese (Mn), and S can affect the availability of nutrients for plants. For example, in acid soils (pH < 6.0), P binds to Fe forming Fe PO43−, an insoluble compound. This means that P is locked in the soil and therefore not available for absorption by plant roots [92]. Electrochemical sensors make use of electrodes that are capable of detecting variations in ion or metabolite concentrations. This allows for the continuous measurement and quantification of these processes, providing vital and immediate information on the nutrients in plants and soil and allowing the optimization of nutrient use [93].
There are different kinds of electrochemical sensors for monitoring plant and soil nutrients and metabolites [94,95,96,97]. Each type caters to specific types of analysis. Ion-selective electrodes (ISEs) often detect NO3−, PO43−, K+, Ca2+, and NH4+ ions in soil solutions [98,99]. Amperometric biosensors are another type of electrochemical sensor used to analyze plants and soil. These sensors can detect specific chemicals in plant cells, such as glucose, sucrose, and amino acids, by utilizing enzyme reactions. Voltametric sensors can be used in various ways to identify the amount of different analytes in soil and plant samples [96,97].
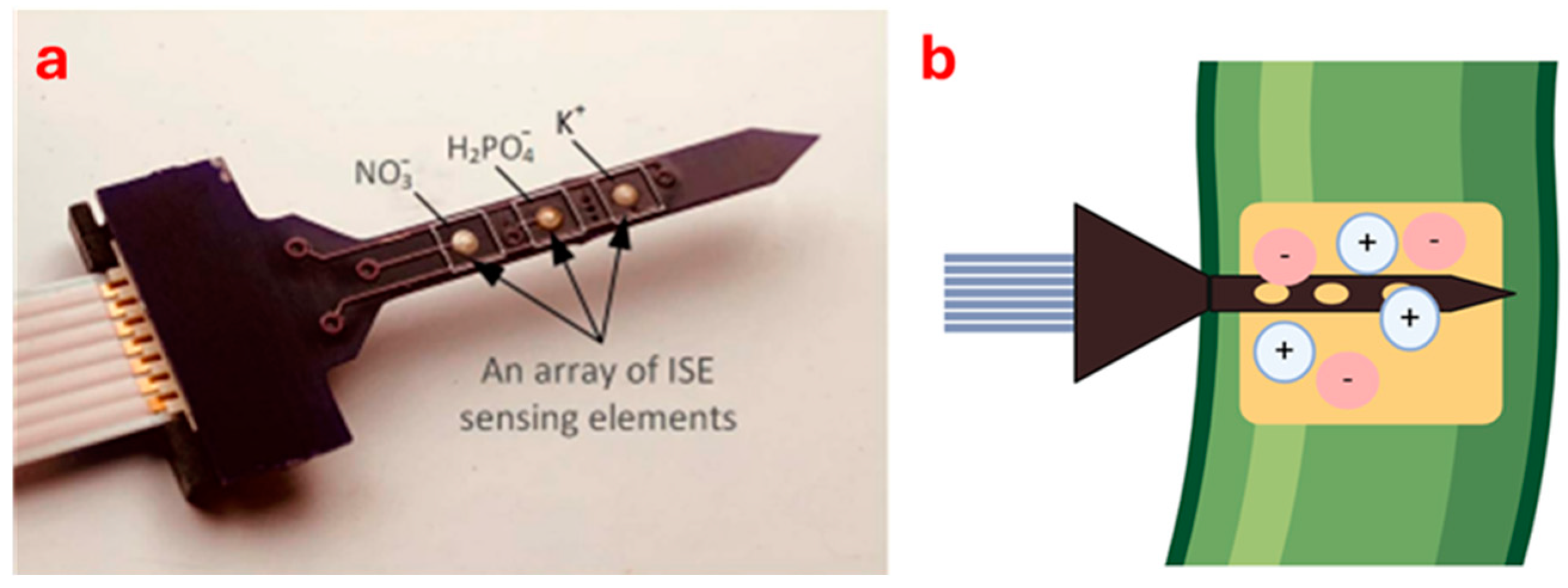
Several prototype sensors have been developed to detect ion forms in soil and plant using the principles of electrochemistry. Chen et al. [53] developed a multi-ion sensor to monitor different ions in plants and soil (Figure 2). The miniature multi-ion sensor works by using ISEs to detect specific ions like NO3−, H2PO4−, and K+. Nanomaterials are added to the surface to improve sensitivity and selectivity. An artificial neural network (ANN) processes the sensor data to reduce errors from interfering ions like chlorine (Cl−) and Ca2+, leading to more accurate readings. The sensor has been tested on maize (Zea mays L.), soil, and drainage water under laboratory conditions. The authors found a linear regression between laboratory analysis data and sensor-determined values with an R2 of 0.92 for N, 0.71 for P and 0.81 for K in the different materials tested. One of the advantages is that the sensor can detect multi-ions like NO3−, H2PO4−, and K+, reducing errors with ANN for reliable data. The main limitations are that the calibration is long (24 h) and that the K selective membrane has encountered problems with the recognition of the element itself. Further testing is necessary as the sensor has been tested only under one type of soil and climate conditions. One aspect to be observed might be how the ANN model behaves on other soil and climate conditions.
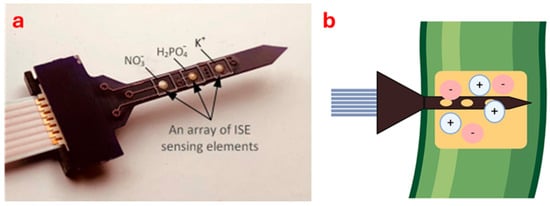
Figure 2.
Electrochemical sensor developed by Chen et al. [53] (a) and an illustration of this sensor inserted into a plant stem (b).
Another ISE sensor was developed by Baumbauer et al. [100]. The sensor detects soil NO3− ion levels by measuring the potential difference between two electrodes: the ISE and the reference electrode (RE). The ISE features a polymer membrane with a molecule that facilitates the transport of ions across a membrane and specifically binds to NO3− ions. When the NO3− concentration in the solution or soil changes, it alters the potential at the membrane–electrolyte interface. The sensor parts are printed, which could allow for low-cost production, but mass production may be affected by the fact that the sensor needs calibration before use. The sensor can be used directly in soil; in fact, it was tested on soils with very high organic matter under laboratory conditions resulting in regressions with R2 values between 0.87 and 0.99 when comparing laboratory measurements with sensor measurements. The sensor has a rapid response and low power consumption. Nevertheless, some limitations are the required calibration, interference from Ca2+ ions that limit selectivity, and reduced sensitivity with the printed electrode reference.
Hossain et al. [101] made ion-selective electrodes that are specifically designed to detect N, P, and K (NPK) ions present in soil or water samples under laboratory conditions. When the target ions from the sample interact with the selective electrode surface, it causes a change in the electrochemical properties of the electrode, typically resulting in a shift in voltage or current. This shift is proportional to the concentration of the specific ions. By measuring these changes in electrical signals, the sensor can determine the exact concentration of NPK ions in the sample, providing real-time, precise data for optimizing nutrient management in agricultural practices. This sensor features fast detection, a high sensitivity to NPK, and it is portable and perfect for on-field use. However, the sensor is limited to detecting amounts of each of these three macronutrients and may not be applicable for other nutrients or contaminants. It also requires regular calibration to maintain accuracy.
A sensor detecting NO3− ions via a redox reaction in which vitamin B12 (VB12) serves as a biocatalyst was developed by Ibrahim et al. [102]. In this prototype, the cobalt ion undergoes reversible oxidation and reduction, facilitating the electrochemical reduction of NO3− ions to nitrite ions (NO2−). This process occurs at a specific voltage potential, where electrons are transferred from the cobalt ion to NO3−, driving the reduction reaction. Graphene oxide (GO) nanosheets, integrated into the sensor, enhance electron transfer between the sensor’s electrode surface and the NO3− ions thanks to their high surface area and conductivity. This accelerated electron mobility increases the efficiency of the redox reaction, producing a measurable oxidation current that is proportional to the NO3− concentration. The combined effects of VB12’s catalytic activity and GO’s electron transport capabilities result in a highly sensitive and efficient sensor, capable of real-time NO3− detection in complex environments. The sensor was tested on sap samples of maize fertilized with different doses of N. Sensor measurements were compared with conventional spectrophotometer measurements. From the results, the authors noted that the highest amount of N in the sap samples corresponded to plants fertilized with higher N doses. Indeed, a linear regression with an R2 of 0.97 was found between sensor and conventional measures. The sensor is characterized by high sensitivity and selectivity for NO3− detection, wide dynamic range (0.2 to 2000 ppm), durability and ability to be reused with stable performance. In addition, it does not need complex sample preparation. On the other hand, it suffers from interference with other ions like Ca2+, PO43−, and K+ at high concentrations, complex fabrication process, and potential physical damage.
In general, all these sensors have been used in a lab context on herbaceous plants or soil. Potentially, these sensors can be used in the field, if their limits are overcome. Currently, these sensors are prototypes, and they are not produced on a large scale. The future goal should be to improve the possibility of producing sensors on a large scale by lowering their cost, reducing the time and complexity of calibration, allowing measurements over long periods, and reducing the interference with other ions. In addition, these sensors should be tested under variable field conditions to verify the accuracy of the sensors and any possible effect of varying climatic conditions.
2.2. NPK Probes
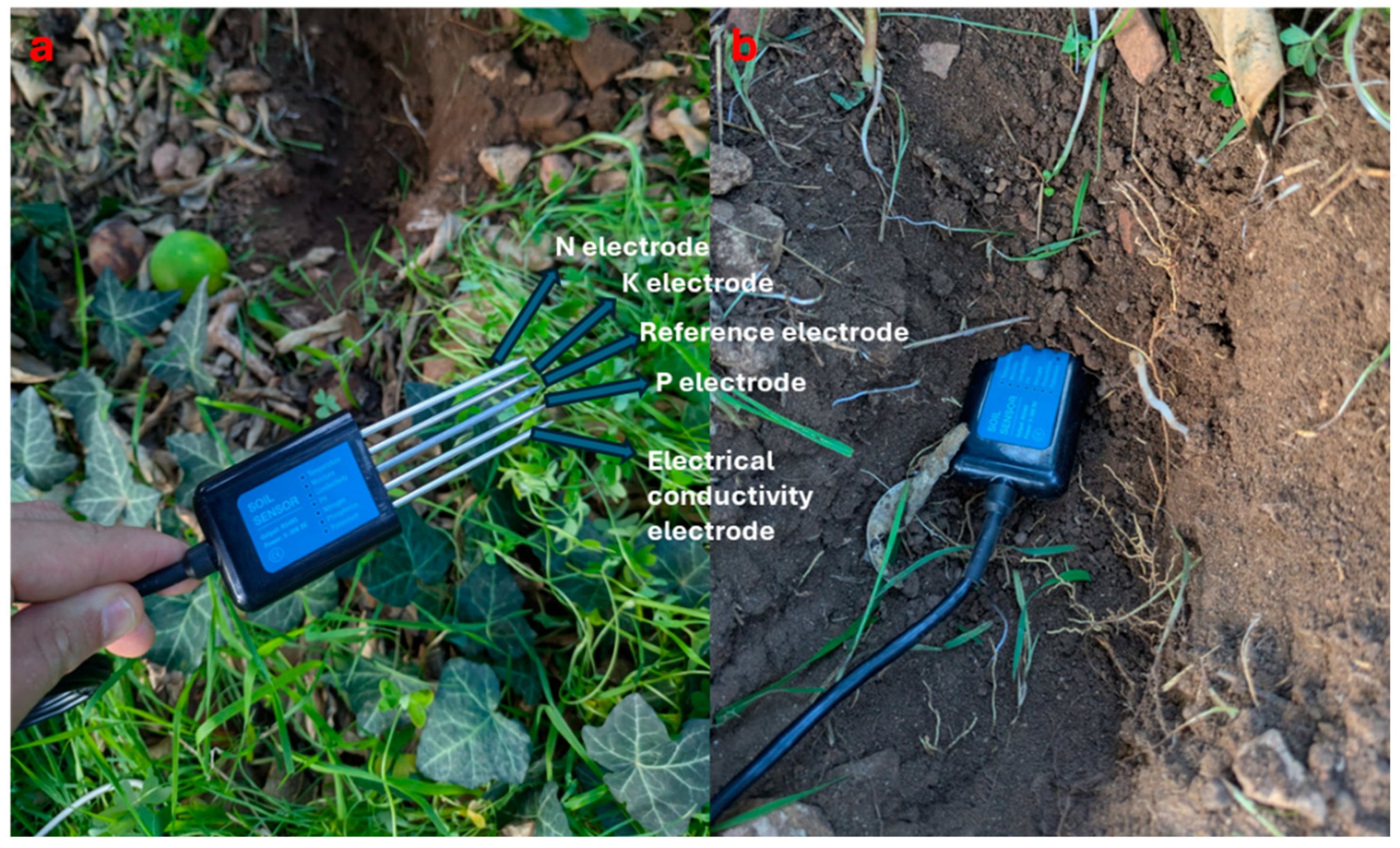
As mentioned above, it is crucial to monitor the nutrient status of the soil since it is the primary source of nutrients for the plant. One of the methods used for this purpose is to exploit the electrical properties of the soil [103]. Commercially, sensors like “NPK probes” (Figure 3) exploit the soil electrical properties to detect the N, P and K content in the soil [104] (Table 2). The probe is a conductivity meter that measures the EC to derive the concentration of the ions using an equation. It is made of stainless steel, and it can be buried in the soil for a long time as it is resistant to long-term electrolysis, alkali corrosion, and salt [105]. Usually, these probes have a range of 0–1999 mg/kg and an accuracy of ±3%. In addition, the data acquired by the probes are processed and sent by an Arduino controller equipped with a LoRa module to directly obtain the element concentrations in the soil [106]. Patrama et al. [105] found that the probes can quantify N, P, and K in the soil, and they observed that in wet soil conditions, the quantities of N, P, and K were higher than in dry soil conditions. Unfortunately, the authors did not carry out preliminary soil analyses that confirmed the real quantities of N, P, and K contained in the soil. Furthermore, they did not distinguish which form of N was measured, whether ammonium or nitrate, and this information could be useful to understand whether N is readily available for plant uptake or not. In another study, Bellosta-Diest et al. [104] found that probes are highly sensitive to EC. However, they found significant regressions between the elements determined by probes and the traditional laboratory analyses with an R2 of 0.99 for N, 0.95 for P, and 0.96 for K in a solution. When they ran the same tests in sandy soil, they found that the regressions were far from 1:1. One aspect of NPK probes that needs improvement is certainly the strong dependence of electrodes on soil adhesion to facilitate more accurate measurements. This may be more difficult in sandy soils where the compaction and adhesion between soil particles is much lower than in clay soils [107,108,109].
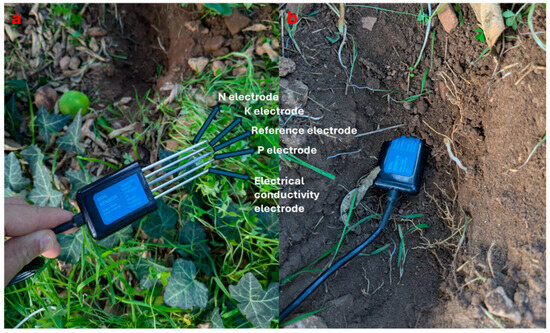
Figure 3.
NPK probe electrodes (a) and probe insertion into the soil (b).

Table 2.
Main features of the electrochemical sensors used for nutrient monitoring in the plant and soil.
2.3. Biosensors
Biosensors are analytical devices that provide information about a biological process through a transducer. Unlike purely electrochemical sensors, biosensors employ a biochemical receptor to gather information about the biological process, which is then transmitted to a transducer for signal conversion [110,111,112]. Various types of receptors are utilized in biosensors, with the most common being enzymes, antibodies, nucleic acids, plant cells, or tissues. The transducers used can also vary, including electrochemical, optical, and piezoelectric types [54,113].
In agriculture, the most extensively developed biosensors for continuous monitoring of plant nutrient status are electrochemical biosensors, while other types such as optical and DNA are particularly useful for detecting physiological stress and plant diseases [114,115]. Electrochemical biosensors are widely used in agriculture due to their high sensitivity, real-time detection capabilities, and relatively low cost. They are versatile, capable of monitoring a broad range of nutrients and environmental parameters, and easy to operate, making them ideal for integration into precision agriculture systems [116]. Additionally, their robustness in challenging environmental conditions and potential for miniaturization make them practical for on-field use, contributing to more efficient and sustainable management of agricultural resources. Electrochemical biosensors can be further classified into different categories depending on the specific electrochemical principle applied [111,117]. The classification of electrochemical biosensors into amperometric, potentiometric, conductometric, voltametric, and impedimetric types is based on different electrochemical detection mechanisms, with each category offering specific advantages for agricultural monitoring, from plant nutrition to the detection of toxic substances or pathogens.
Recently, organic electrochemical transistor (OECT) biosensors have been developed, further expanding the capabilities of electrochemical sensing in agriculture. In recent years, this type of biosensor has been highly suitable for real-time and in-field monitoring of plant nutrient status. OECT biosensors are highly sensitive to ionic variations, able to operate in humid environments, and provide real-time responses [118,119]. They are robust, long-term stable, and operate at low power, enabling precise and extended monitoring in crop systems. Furthermore, they can be easily integrated into precision agriculture systems, enhancing the efficiency of resource management [42,43]. OECT sensors consist of transistors based on an organic semiconductor material, referred to as the channel. The channel material is ion-permeable, allowing charge accumulation throughout the entire volume of the channel when in contact with an electroactive medium and a gate electrode. This creates a potential that drives ion flow from the medium into the channel [120,121,122,123]. The first OECT was developed by White et al. [124], who modulated the conductivity of a polyaniline layer by applying a gate-to-source voltage (VGS) through an electrolyte. The movement of ions between the organic semiconductor and the electrolyte, induced by the applied VGS, is the primary mechanism behind the modulation of the semiconductor’s conductivity. In the agricultural field, prototypes of OECT biosensors have been developed using polystyrene sulfonate (PEDOT:PSS) as the semiconductor. PEDOT is an anionic polymer that acts as both a dopant and stabilizer. PEDOT provides the conductive properties, while PSS protects PEDOT from environmental oxidation and gives the film greater elasticity and mechanical strength, making it slightly more resistant [125,126]. Its high conductivity, flexibility, and transparency, as well as its ability to be applied on flexible substrates, make it ideal for agricultural applications [120,121,127]. These features enable real-time, non-invasive monitoring of plant nutrient status and health in agricultural environments.
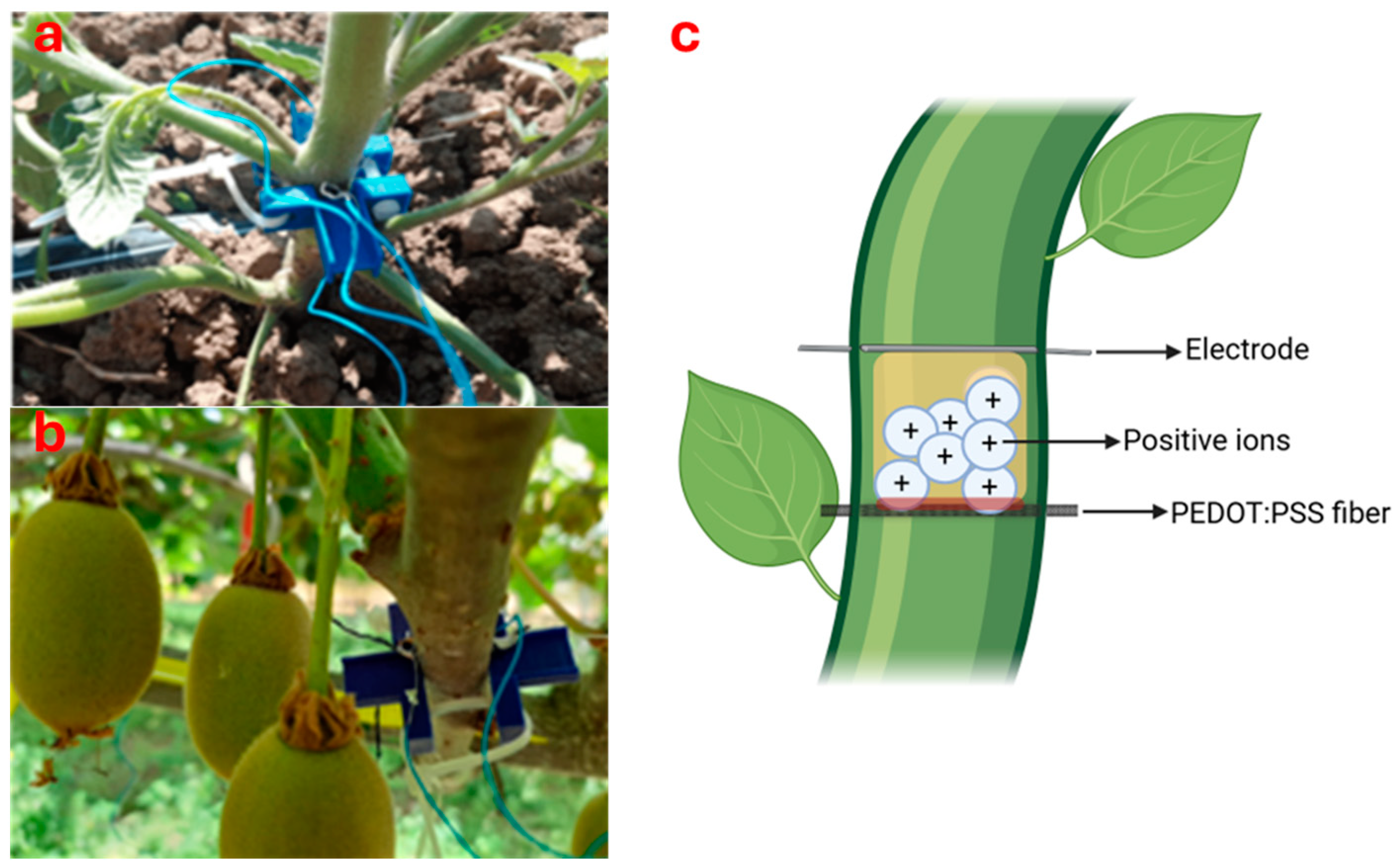
Coppedè et al. [128,129] have developed a biosensor of the OECT type called “bioristor”, which is suitable for monitoring plant nutrient status as well as plant water status since it can give information about the ionic concentration of the sap (Figure 4). This sensor consists of a natural textile fiber treated with a conductive polymer, directly integrated in the stem of the plant, and a thin silver wire that acts as a sliding electrode and is capable of recording data every 24 min. Measurements are made by applying a constant voltage through the fiber and a positive voltage at the gate (Vg); the behavior of the resulting current (IdS) is monitored over time. The sensor response parameter can be expressed as R = (IdS − IdS0)/IdS0, where R is the slope of the electrical response curve and IdS0 represents the current when Vg is zero. Vurro et al. [130] tested the Bioristor sensor on tomato plants (Solanum lycopersicum L.). In this study, the sensor was tested under drought and salt stress conditions. In the first case, the concentration of ions detected by the sensor was higher as water availability decreased. In the other one, the concentration of ions in the sap increased as the salt concentration increased. The prototype allowed monitoring the temporal variation of the total electrolyte content in plant sap, providing important information about the plant’s nutritional status. The sensor was also tested on tree species to monitor sap ion concentration in response to water stress. In kiwifruit (Actinidia chinensis Lindl.), the bioristor successfully tracked the dynamic changes in sap ion concentrations over a period of 120 days according to irrigation regimes [131]. In grapevines (Vitis vinifera L.), the sensor operated continuously for 180 days without irrigation, showing a drop in response during the summer [132]. Finally, in apple trees (Malus domestica Borkh.), the sensor response decreased after the end of the scheduled irrigation, indicating low water availability. Afterward, a sharp decrease in the R slope was recorded, and the R signal stabilized at low values, indicating the plant’s general state of water deficit [131]. A significant limitation of the sensor is that it cannot discriminate different ions in the plant sap. Indeed, the output of the sensor is the total concentration among all the ionic species together present in the plant sap.
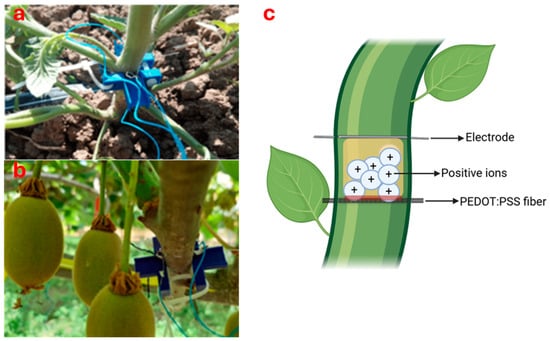
Figure 4.
Bioristor sensor developed by Coppedè et al. [122] on a tomato plant (a) [130] and on a kiwifruit vine (b) [131] with an illustration of its operating mechanism (c).
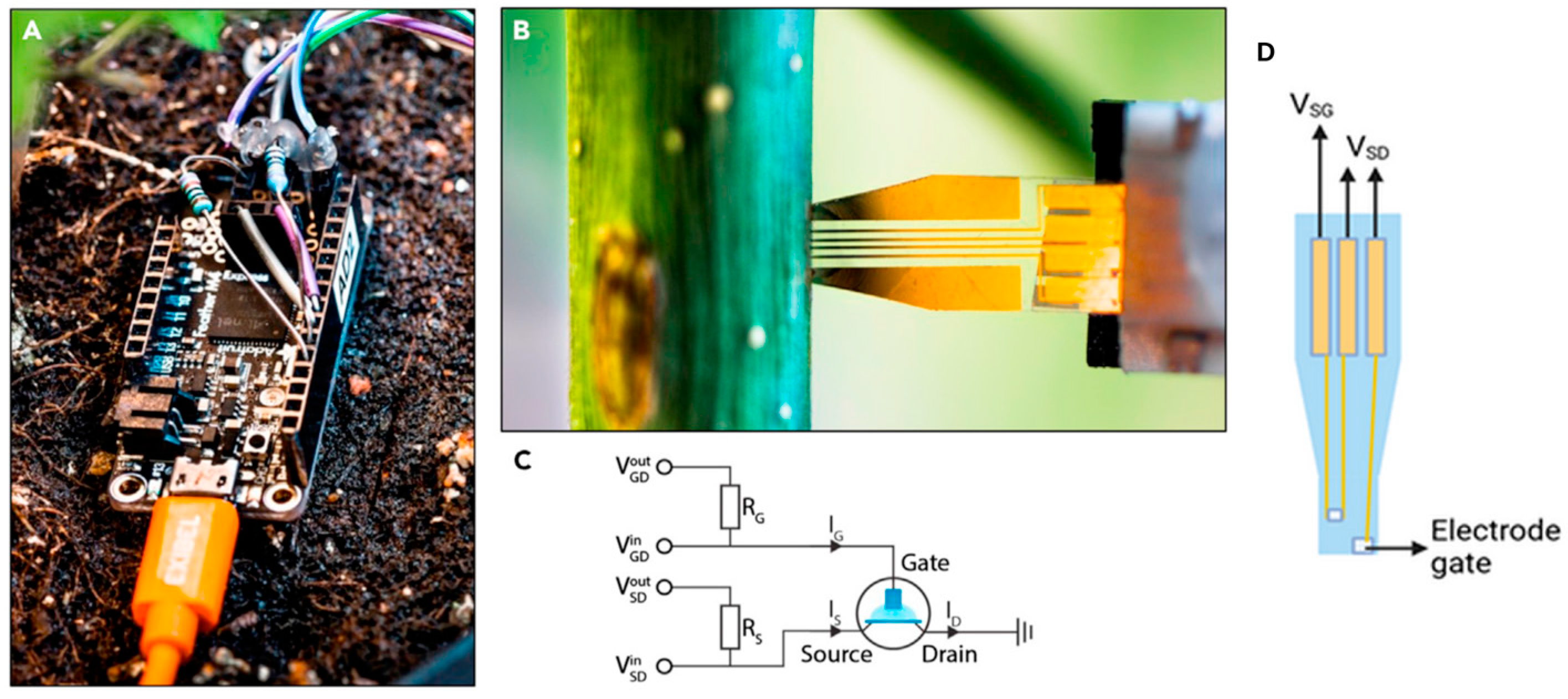
Another useful sensor for monitoring glucose and sucrose concentrations tested in the xylem of aspen trees during 48 h under laboratory conditions was developed by Diacci et al. [133] (Figure 5). Monitoring glucose levels in the xylem sap is crucial for understanding plant metabolism and overall health. Glucose is a primary product of photosynthesis, and it serves as a key energy source [134]. Tracking its levels provides insights into the plant’s energy distribution, its response to environmental stress (such as drought or nutrient deficiencies), and can assist agricultural practices to optimize growth and yield. Therefore, xylem glucose monitoring is an essential tool for assessing physiological status and enhancing plant productivity [135]. On the other hand, sucrose is the main transport carbohydrate in plants, transferring the energy produced by photosynthesis to the different parts of the plant through the phloem, but it may also be transported into the transpiration flow of the xylem during early leaf growth of certain tree species [136]. The sensor consists of a polyethylene naphthalate (PEN) substrate with a thickness of 125 µm. A Ti/Au electrode is used for the source, drain, gate electrodes, and wiring, while the channel is based on PEDOT. The gate electrode is coated with a thin film of PEDOT to enhance its capacity for efficient modulation of channel conductivity and is further treated with enzymes and Pt nanoparticles (PtNPs). The PtNPs are electrostatically deposited onto the gate, while the enzymes are immobilized using a chitosan matrix, which is applied dropwise onto the gate. When the analyte is present in the solution, an enzymatic reaction occurs at the gate, producing hydrogen peroxide (H2O2). The H2O2 is then oxidized on the PtNPs, which is associated with electron transfer, altering the effective gate potential and consequently leading to a further decrease in channel current. The analytes detectable by enzymatic electrochemical sensors are limited by the availability of enzymes capable of participating in redox reactions. Glucose oxidase is an oxidoreductase enzyme that catalyzes the oxidation of β-D-glucose into H2O2 and D-glucono-1,5-lactone. Sucrose, on the other hand, does not have a corresponding oxidoreductase enzyme. To enzymatically detect sucrose, a cascade of reactions is facilitated by incorporating three enzymes into the chitosan matrix, allowing the reactions to occur in a confined space. First, invertase hydrolyzes sucrose into fructose and α-D-glucose; next, mutarotase catalyzes the conversion of α-D-glucose into β-D-glucose, and finally, β-D-glucose reacts with glucose oxidase. One limitation consists of the fact that the sensor is limited to proving a qualitative and not quantitative analysis, because the initial concentrations of glucose or sucrose are not known in the xylem. Another limitation is the duration of the sensor in the plant, which is strongly affected by the cork formation around the sensor disturbing the measurement. Given the possible interference of sucrose in measuring glucose with a sensor, the authors decided to integrate the ability to measure both compounds into the sensor.
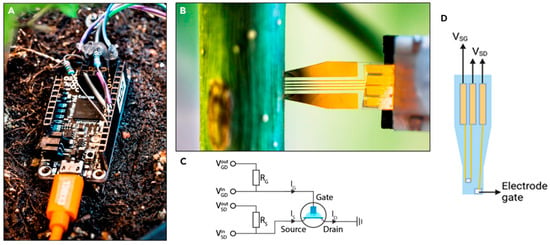
Figure 5.
OECT biosensor developed by Diacci et al. [133]. The figure shows the Arduino measurement unit (A), the biosensor installed into the tree stem (B), the Arduino unit circuit (C) and an illustration indicating the biosensor voltage source–drain (VSD) and voltage source–gate (VSG) (D).
Another OECT sensor designed to detect macronutrient concentrations in plant sap was developed by Strand et al. [137]. The ion sensor exhibited high sensitivity in terms of current (170 μA dec−1) and voltage (99 mV dec−1), along with a low detection limit (10 µM). This OECT biosensor effectively measures K concentrations in raw sap and sap-like aqueous solutions, showing a log-linear response within the physiological range of plant cations. The sensor has been tested on maize sap, chokecherry (Prunus virginiana L.) and blue spruce (Picea pungens L.) under laboratory conditions. The performance of these printed devices supports their application in high-throughput plant health monitoring for both agricultural and ecological purposes. Despite the use of ion-selective membranes for K+ ions, the sensor is not perfectly selective. The results show that the sensor response is also influenced by the presence of other ions, such as Na+, Ca2+ and Mg2+, which are present in significant concentrations in plant sap. Due to the influence of interfering ions and the fact that each type of plant has a unique sap ionic composition, the sensor requires a specific calibration curve for each installation.
Overall, OECT biosensors offer several advantages for monitoring the nutrient status of plants in open fields, such as their high sensitivity, flexibility, and ability to detect biological signals in real time, even in complex environments. They can also be integrated into flexible, low-cost substrates, ideal for large-scale use. However, they have some disadvantages such as the degradation of organic materials in adverse environmental conditions, such as extreme humidity and temperatures, and the need for constant calibration to maintain the accuracy of measurements over time [54,116,130,138,139] (Table 3).

Table 3.
Main features of biosensors used for plant nutrient monitoring.
Future trends should focus on improving the stability of organic matrices, which are easily degraded, and decreasing the time and frequency of sensor calibration. In addition, it is important to improve sensor sensitivity and selectivity without harming plants to accurately detect essential plant nutrients such as N, P and K, and micronutrients like Fe and Zn directly in the plant sap. This can be achieved by utilizing advanced nanomaterials such as carbon nanotubes, graphene, and metal–organic frameworks, which can enhance sensor performance, allowing for faster response times and lower detection limits. In addition, the development of sensors capable of detecting multi-ions should be prioritized to provide a comprehensive and complete nutrient profile in real time.
Ensuring the durability of sensors in harsh field conditions, with innovations such as protective coatings, automatic calibration mechanisms, and improved packaging, will also be crucial for long-term reliability. The main trend in sensor development includes the miniaturization of sensors and integration with IoT, enabling continuous monitoring through microscale and low-power sensors embedded in the soil or plant branches or trunk, and connected to wireless networks for remote data analysis. AI and machine learning will play a crucial role by analyzing large datasets to predict nutrient deficiencies, optimize fertilization, and enhance sensor design. The data fusion technique, which combines electrochemical data with other types of environmental sensors, will increasingly enhance our understanding of plant health. Ultimately, sustainability and affordability must be the primary focus of this development, with an emphasis on eco-friendly materials and cost-effective solutions to ensure widespread adoption, especially in small-scale farming and developing regions. These innovations will drive the future of precision plant fertilization, enhancing productivity and sustainability.
2.4. Optical Sensors
The use of optical sensors for a non-destructive determination of plant nutrient status represents an innovative approach that integrates agricultural science with information and communication technologies [141]. These tools are particularly useful in precision agriculture, where the information collected can be utilized to optimize farming practices, improve resource management efficiency, and reduce the environmental impact of agricultural activities [142,143]. The ability to continuously monitor plant conditions allows for timely interventions, contributing to maximizing crop yields and improving the quality of agricultural products [144].
Moreover, the adoption of such technologies not only enables more responsible nutrient management but also promotes greater awareness among farmers regarding soil fertility and plant health. This smart and precise approach provides the opportunity for targeted interventions while simultaneously reducing the excessive use of chemical fertilizers, which can lead to negative environmental consequences such as water eutrophication, decrease in soil microorganisms, and air pollution due to the volatilization of chemical compounds, such as ammonia [145]. Reducing environmental impact through the use of nutrient monitoring technologies is especially relevant in a context of growing concern over the sustainability of agricultural practices and the quality of water resources [146].
Optical sensors exploit the interaction between light and plant tissues, based on the principle that leaves reflect, absorb, and emit light differently depending on their physiological and biochemical properties [147]. This ability to analyze light interactions allows for the estimation of key parameters in plants, such as chlorophyll concentration, water content, and photosynthetic efficiency, which are closely related to the nutrient status of the plant [148,149,150].
The physical principles underlying the operation of proximal optical sensors include light absorbance, fluorescence, and spectral reflectance [151,152,153]. Each of these principles provides different information regarding the nutrient and physiological status of the plant. For instance, the absorption of specific wavelengths of light by chlorophyll is an indicator of N content [154], while fluorescence emitted by chlorophyll during photosynthesis can be used to assess the efficiency of energy conversion in the plant’s photosystems [155]. Spectral reflectance, on the other hand, allows for the estimation of vegetation indices, such as NDVI (Normalized Difference Vegetation Index), which correlate with biomass and plant vigor [156]. By combining data from these different detection principles, a comprehensive picture of the nutrient status and health of plants can be obtained.
2.4.1. Light Absorbance
The soil–plant analysis development (SPAD) meter (Minolta Camera Co., Ltd., Osaka, Japan) is a portable spectrophotometer designed to quickly and non-invasively assess the relative greenness of plants (Figure 6a). It is one of the most commonly used instruments based on optical principles for measuring chlorophyll concentration in leaves, which is closely related to the N nutrient status [157]. The SPAD meter operates by emitting two wavelengths of light, typically in the red (650 nm) and infrared (940 nm) regions, and measuring the amount of light absorbed by the leaf [158]. Chlorophyll strongly absorbs light in the red region (around 650 nm), while infrared light is minimally absorbed and is used as a reference. The ratio of absorbed red light to transmitted infrared light provides a value directly correlated with the chlorophyll concentration in the leaf [159,160]. This simple and effective method allows for rapid assessments of N levels in fruit trees, giving farmers the ability to make timely decisions regarding fertilization.

Figure 6.
SPAD taking measurements on an avocado (Persea americana Mill.) leaf (a) and DUALEX taking measurements on a peach (Prunus persica L.) leaf (b).
Initially developed for N management in rice (Oryza sativa L.) cultivation, this device has been widely adopted for this purpose in Japan [161]. In this study, threshold SPAD values were identified, specifically values below 40, which suggested a potential N deficiency. Unfortunately, the sensor proved useful only during specific growth stages of the rice plant. It has also been used as a diagnostic aid for N management in maize by detecting N deficiencies in the growing crop [154]. Similar N contents have been observed in tomato and cucumber (Cucumis sativus L.) leaves, comparing destructive and SPAD measurements, using different N sources and application rates [162]. SPAD was found to be more reliable when used on tomatoes rather than cucumbers. Regardless of species, the authors recommend using the instrument at the same hour and on the same leaves to reduce variability in the data during the experiment, indicating a sensitivity of the instrument to environmental conditions or sunlight. Genotypic variation in soybean (Glycine max (L.) Merr.) chlorophyll composition and content has been tested in relation to leaf tissue N content using SPAD [163]. In initial applications on fruit trees, researchers have monitored chlorophyll content to optimize fertilization and reduce excessive N use in apple, grapevine, citrus, olive (Olea europaea L.), banana (Musa acuminata L.) and pear (Pyrus communis L.) trees [164,165,166,167,168,169]. Multiple measurements on the same leaf were required to obtain a representative estimate of its chlorophyll content. Factors such as species, cultivar, rootstock, phenological stage, climate (solar radiation, air temperature and humidity), the presence of pests and diseases, nutritional deficiencies (particularly iron chlorosis), or water stress can influence the measurements and reduce the reliability of N content estimates. It was also observed that SPAD values tend to overestimate chlorophyll in thicker leaves. Since leaf blade thickness increases with age, the gradual decrease in chlorophyll content may be offset by the increased thickness of older basal leaves. Consequently, SPAD values may not accurately reflect the actual variations in chlorophyll content in leaves across different growth stages [164].
The SPAD meter allows for rapid and continuous measurements without causing damage to the plant [157,170], enabling an immediate assessment of its nutrient status. It is also well-suited for direct field measurements, eliminating the need to collect samples for laboratory analysis and providing real-time data. The SPAD meter does not provide direct information on other essential nutrients, such as P and K. Additionally, its measurements can be influenced by factors such as leaf thickness, damage, senescence, and position [170,171]. The best conditions for measurements are in broad daylight with stable light or under diffuse lighting conditions, on leaves free from dew, water or dust on the surface, on leaves representative of the plant, usually young or mature, and at non-extreme ambient temperatures.
The Dualex meter (µMetos by Pessl Instruments, Weiz, Austria) extends the capabilities of the SPAD meter by measuring not only chlorophyll concentration but also flavonoids, pigments that play a role in plant stress responses and are inversely correlated with N availability [172] (Figure 6b). The Dualex meter works by comparing light transmitted through the leaf with and without UV light excitation [173]. When exposed to UV light (280–375 nm), flavonoids in the leaves absorb the UV, and the amount of absorbed UV light can be used as an indicator of flavonoid concentration [174]. These data are combined with chlorophyll absorbance measurements, allowing Dualex to provide a more comprehensive view of the plant’s nutrient and physiological status. Flavonoid measurements, in particular, help correct for variations in leaf thickness and structure, which can affect the accuracy of chlorophyll-only readings [175]. This makes the Dualex especially useful in orchards, where leaf thickness can vary significantly between species and even within the same tree.
It gives information about the combination of chlorophyll and flavonoids, offering a more comprehensive assessment of plant status compared to sensors that measure only chlorophyll, such as the SPAD meter [176,177]. The ratio of chlorophyll to epidermal flavonoid leaf content provides the N Balance Index (NBI), a useful indicator for assessing plant N status [178,179]. Dualex has been successfully used in a variety of species, both herbaceous and tree species [177,180,181,182,183,184,185], and it has demonstrated to be effective in monitoring nutrient and physiological status of plants, providing rapid measurements both in the field and in the laboratory. Overbeck et al. [177] observed that Dualex is a useful tool for assessing flavonoid and chlorophyll content in leaves, but the results are influenced by various factors such as canopy cover, planting density, canopy structure, and cultivar. It was evident that Dualex readings were influenced by environmental factors such as sunlight. Measurements taken on the upper side of the leaves were higher than those on the lower side, due to greater sunlight exposure and higher carbohydrate production. Because the upper side of the leaves is exposed more to sunlight and therefore tends to produce more soluble carbohydrates, if they are in excess of the metabolic demand for growth, phenol synthesis is promoted [180,181,182,183,184,185].
A major disadvantage of flavonol measurements is that they are made at leaf level on single or multiple leaves; therefore, appreciable replication is required to overcome the heterogeneity associated with individual leaf measurements [172]. Measurements with flavonol meters should be taken on a regular basis or at critical stages of the crop on representative plants randomly selected from the center of representative areas of farms or within plots in research studies [186]. Measurements are made on the most recent fully expanded and well-lit leaf, between the stalk and the tip of the leaf, and midway between the margin and the mid-rib of the leaf. The best conditions for measurements are in the absence of direct sunlight, preferably with a shaded or overcast sky, stable ambient temperatures, and on dry and clean young or fully developed but not senescent leaves.
The AtLeaf (FT Green LLC, Wilmington, DE, USA) is the cheapest commercially available chlorophyll meter [187,188] (Figure 7). The AtLeaf operates, like SPAD and Dualex, on the principle of light absorbance by photosynthetic pigments, particularly chlorophyll, present in the leaves. It measures the absorption of light at 660 nm and 940 nm. Light is emitted through the leaf, and the sensor detects the amount of transmitted (non-absorbed) light. Higher chlorophyll levels in the leaf correspond to greater amounts of absorbed light and, consequently, lower transmitted light. Using the two absorbance values, the meter calculates a dimensionless numerical value, which is related to chlorophyll content.

Figure 7.
The AtLeaf sensor used to measure leaf chlorophyll content (https://www.atleaf.com/, accessed on 5 January 2025).
The main wavelengths used by the AtLeaf meter are in the red and far-red regions [189]. Chlorophyll efficiently absorbs red light during photosynthesis, and the sensor takes advantage of this property to indirectly quantify chlorophyll content, which is closely related to the plant’s nutrient status [190]. The output is presented as a value that reflects chlorophyll concentration, and these measurements can be used to assess plant health and determine the need for agronomic interventions, such as N fertilization [188].
The AtLeaf sensor has been utilized on wheat (Triticum aestivum L.), potato (Solanum tuberosum L.), corn, coconut (Cocos nucifera L.), grape, olive and citrus [189,190,191,192,193] to monitor chlorophyll content, providing insights into soil N availability and helping farmers optimize fertilizer management and to evaluate fruit tree nutrient status, allowing for the identification of nutrient stress. These studies confirmed the efficacy of the AtLeaf device due to its rapid estimation of chlorophyll content. However, it was also noted that the relationship between the AtLeaf value and chlorophyll content is species-specific, both for calculations per unit of leaf area and per unit of dry mass. This means that species-specific equations are required when converting the AtLeaf value into absolute chlorophyll content. The major practical advantages of AtLeaf, like other chlorophyll meters, as an indicator of crop N status are its ease of use, no training required and rapid measurements, with no or very little data processing [194]. On the contrary, the AtLeaf meter does not directly measure N or other nutrient contents but instead provides an indirect indication through chlorophyll levels. In some cases, plant health may be affected by factors other than chlorophyll content, such as disease or water stress, which the meter cannot detect. The accuracy of measurements may be influenced by variable ambient light conditions and dust or dirt on the leaves, as well as differences in leaf thickness [190]. The best conditions for measurements are during the day with stable but not too intense light, in regular field conditions (no strong wind or extreme weather) and on dry and clean mature or slightly young leaves.
2.4.2. Spectral Reflectance
The GreenSeeker (Trimble, Westminster, CO, USA) meter utilizes spectral reflectance to calculate vegetation indices, such as the NDVI, which is commonly used to assess plant vigor, biomass, and overall health. The GreenSeeker emits light in both the red and near-infrared (NIR) regions (i.e., 660 nm and 770 nm) and measures the proportion of light reflected by the plant [195]. Healthy, N-rich plants reflect more NIR light and absorb more red light due to their higher chlorophyll content, resulting in a higher NDVI value. By calculating NDVI or similar indices, the GreenSeeker meter can provide a real-time assessment of the nutrient status of fruit trees and guide site-specific fertilization practices [196].
Some versions of GreenSeeker also use a ‘red-edge’ band, located between the red and near-infrared regions, to improve the sensor’s sensitivity in more advanced vegetation stages or in crops with complex structure [197].
The sensor can be used to monitor stress in crops caused by drought, diseases, or nutrient deficiencies. This enables farmers to make timely decisions to correct soil or water conditions. The NDVI data obtained with the GreenSeeker can also be used to forecast crop yields [198]. Vegetation indices can detect crop conditions early, allowing farmers to estimate potential final yields. This sensor can be integrated with agronomic management software to create prescription maps that guide tractors or agricultural machinery, applying fertilizers at varying rates according to the needs of specific areas of the field [199]. It is particularly useful for monitoring large fields, where spatial variability can be significant [200].
If paired with advanced technologies such as artificial intelligence and big data analysis, the GreenSeeker (Figure 8) could be integrated into advanced monitoring systems to provide even more accurate predictions regarding crop health and nutrient management [201]. Additionally, integration with drones and agricultural robots could further extend the use of this sensor, automating real-time data collection over even larger areas [202]. However, it requires proper calibration for different tree species and environmental conditions, such as dense clouds or dust, can affect the measurements [203]. It has been successfully used to monitor plant health and predict production potential, integrating with large-scale precision agriculture systems in corn, wheat, rice, sugarcane (Saccharum officinarum L.), olive, and grape [204,205,206,207]. GreenSeeker demonstrated limited performance at late plant growth stages. Additionally, a possible sensor saturation occurred due to the increased average N concentration in the leaves. This suggests that the GreenSeeker may not be reliable when biomass or N content is high. In these studies, the reliability of the GreenSeeker in predicting leaf N content was influenced by crop variety, sampling period, and growing season. The best conditions for measurements are in full natural light or overcast, provided that the light is diffused and stable during the day, avoiding peak hours with direct sunlight, and on plants in good health, with clean leaves.

Figure 8.
The Greenseeker sensor used to estimate vegetation indices (https://it.ptxtrimble.com/product/sensore-di-raccolto-portatile-greenseeker/, accessed on 5 January 2025).
2.4.3. Chlorophyll Fluorescence
The Pocket PEA (Plant Efficiency Analyzer) by Hansatech Instruments, Norfolk, UK, is based on the principle of chlorophyll fluorescence (Figure 9), which occurs when chlorophyll molecules in the leaf absorb light during photosynthesis and re-emit a portion of this energy as fluorescence [208]. This sensor measures the fluorescence emitted by chlorophyll when the plant is exposed to a brief pulse of intense light [155]. By analyzing the kinetics of fluorescence emission, the Pocket PEA can provide detailed information about the efficiency of the photosynthetic process, particularly the functionality of Photosystem II (PSII) [209]. Photosystem II is a critical component of light reactions in photosynthesis, and its efficiency is closely linked to plant nutrient status, especially N and Mg.

Figure 9.
A Pocket PEA sensor measuring chlorophyll fluorescence parameters on a tomato leaf (https://www.hansatech-instruments.com/product/pocket-pea/, accessed on 5 January 2025).
The Pocket PEA provides data on several key parameters:
- Fv/Fm: Indicates the maximum potential for photosynthesis. A high Fv/Fm value suggests good plant health and high photosynthesis efficiency [210].
- Yield: Indicates the fraction of energy used for photosynthesis. This value helps understand how effectively the plant is converting solar energy into chemical energy [211].
- qP (quenching coefficient): Measures the efficiency of light capture. High qP values indicate a healthy plant that is using available light for photosynthesis [212].
It has been used on tomato, corn, wheat, canola (Brassica napus L.), papaya (Carica papaya L.), apple, and grape to assess plant health and nutrient management practices, contributing to the improvement of grape quality and harvest, as well as to monitor N fertilization conditions and optimize applications for better final yields [213,214,215,216,217,218]. The results obtained with the Pocket PEA in analyzing photosynthetic activity through chlorophyll fluorescence kinetics were less accurate than stomatal conductance, net photosynthesis, transpiration, growth, and water use efficiency measured with a gas exchange analyzer. This was due to the limited sensitivity of the Pocket PEA to the early signs of water stress in plants. The instrument was found to be species- and variety-dependent.
The portable Pocket PEA device can be easily used in the field, providing rapid and immediate measurements without the need for complex lab analyses or operations, facilitating effective agronomic management [219]. Unlike traditional methods that require leaf sampling, it allows for non-destructive monitoring of plant health [218]. Integration with advanced technologies such as drones and agronomic management systems makes the Pocket PEA more efficient and technologically advanced [220]. The Pocket PEA is highly sensitive to early signs of nutrient stress, making it an invaluable tool for monitoring fruit tree health in orchards. Additionally, chlorophyll fluorescence measurements can be used to detect other types of stress, such as water deficit or diseases, further enhancing the utility of this device in precision agriculture [221]. Fluorescence measurements can be influenced by environmental lighting conditions and external factors, such as dust or dirt on the leaves, which may alter the results. The best conditions for measurements are homogeneous light conditions, ideally in the shade or under diffuse lighting conditions (early morning or late afternoon), on clean and dry leaves and at moderate ambient temperatures.
A major limitation of optical sensors is the lack of continuous measurement. Future trends should focus on the implementation of small leaf sensors based on optical principles that can be installed on the leaf and make continuous measurements. The sensing system could be equipped with a light source emitting a beam through the leaf clip at certain intervals to measure leaf absorbance and reflectance. Of course, the leaf clip attached on the leaf blade for a long time could form chlorotic, non-photosynthesizing rings and eventually kill the tissue; so, it would have to be periodically moved from one leaf to another to avoid this issue (Table 4).

Table 4.
Main features of optical sensors used to monitor plant nutrient status.
3. Remote Sensing
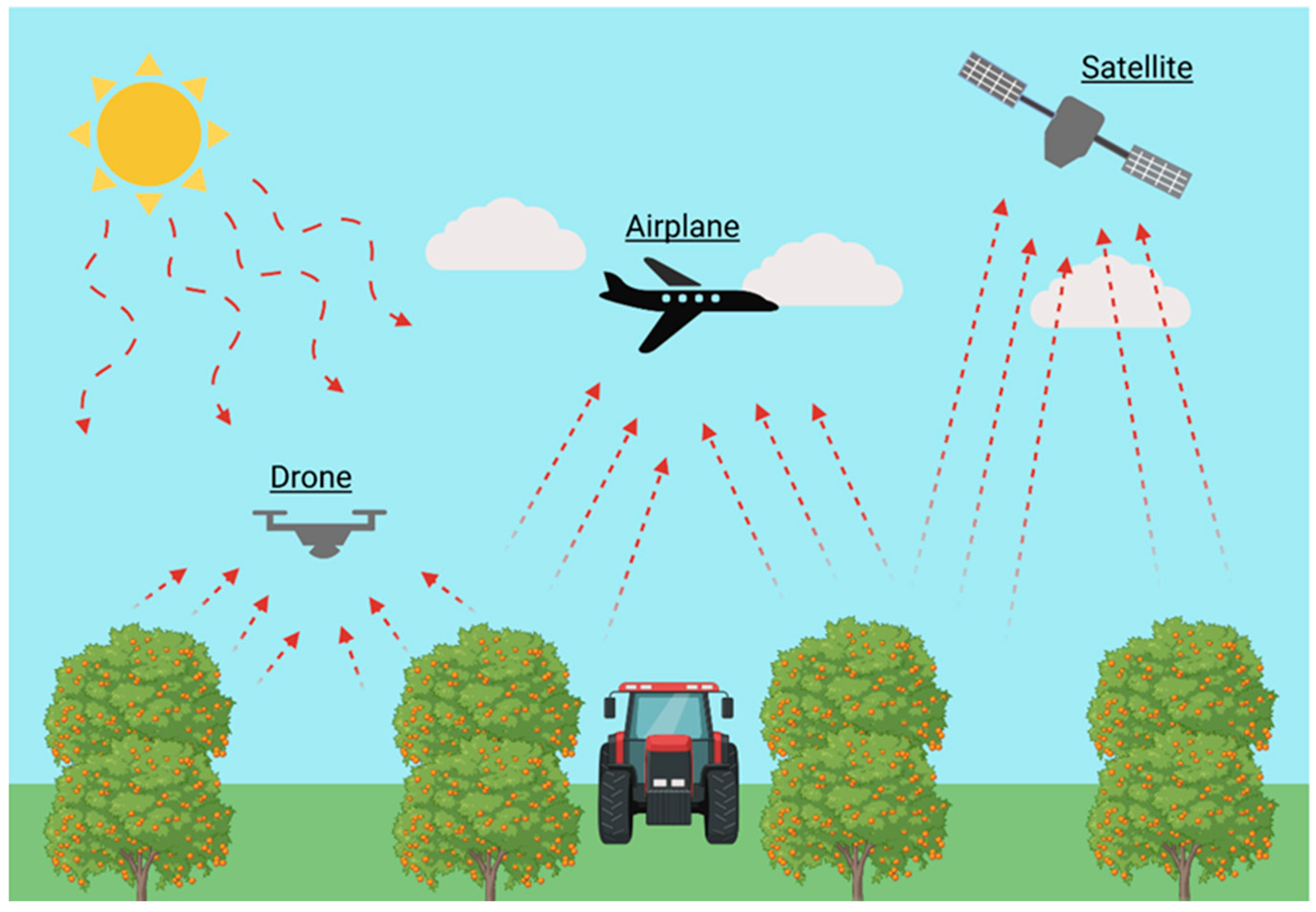
Obtaining information on both temporal and spatial variability is crucial for achieving comprehensive precision management in orchard fertilization. Proximal sensors provide reliable data over time but only offer detailed information from a limited number of points within the orchard. In contrast, remote sensing enables monitoring on a spatial scale, coping with the limitations of proximal sensing, although data are not continuously recorded [74]. Remote sensing techniques involve the use of optical sensors providing information over large areas. Since traditional methods, based on individual plant sampling and laboratory analysis, are destructive, time-consuming, and therefore applied to only a few plants of an orchard, the ability to map crop variability would allow fertilizer applications to be adjusted according to the needs of each part of the field, thus improving the efficiency of current fertilization programs [227]. In this regard, remote sensing has been proven to be a very important and useful tool (Figure 10).
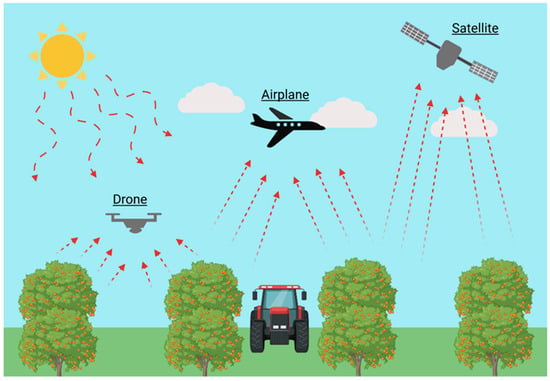
Figure 10.
Representation of different types of remote sensing based on the instruments used and the distance from which it is carried out.
Generally, systems that measure reflectance or transmittance using multispectral and hyperspectral cameras are employed. Specifically, they measure the energy reflected or transmitted by the canopy at different wavelengths, which depends on the specific molecules present. In precision agriculture, the most widely used spectral bands include ultraviolet (UV; 300–400 nm), visible (VIS; 400–700 nm), infrared (NIR; 700–1400 nm), shortwave infrared (SWIR; 1400–3000 nm), and thermal infrared (TIR; 3000–25,000 nm) [73,74]. However, for estimating nutrient concentration, only the UV, VIS, and NIR regions are typically referenced, while the SWIR and TIR bands primarily provide information related to plant water status [228]. Spectral data acquired from multispectral and hyperspectral cameras are typically normalized by calculating various vegetation indices, which combine reflectance from different spectral bands depending on the specific evaluation goal. Examples of widely used vegetation indices to assess plant nutrient status include NDVI, green normalized difference vegetation index (GNDVI), canopy chlorophyll content index (CCCI), modified chlorophyll absorption in reflectance index (MCARI), chlorophyll red edge index (CIRE), normalized difference red edge index (NDRE), etc. Another approach involves analyzing remotely acquired RGB images to assess the nutrient status by identifying visible canopy features that characterize nutrient deficiency [229,230]. This is considered a low-cost method because basic cameras can be used.
In remote sensing, the primary technologies utilized are satellites and unmanned aircraft systems (UASs) (Figure 11). Satellite imagery provides a large amount of data over vast areas at varying resolutions, with the cost of the images depending on the resolution. On the other hand, UASs generally capture high-resolution images due to their closer proximity to the ground (20–120 m above ground level). However, UAS can cover significantly smaller areas compared to satellites [231,232]. In recent years, the use of satellites to acquire images for agricultural purposes has become more affordable and user-friendly. The number of space agencies offering various national platforms has been increasing, and several of those now provide free datasets [233]. One example is the Copernicus program of the European Space Agency (ESA), which offers satellite data for security and environmental monitoring through its Sentinel series [234]. For multispectral data in agronomy, Sentinel-2 is generally referenced [235]. Additionally, the retrieval of high-resolution satellite images is becoming increasingly affordable, thanks to the development of low-cost, miniaturized satellite platforms (e.g., Planet) [74,233,236]. Remote sensing with drones and satellites can be negatively affected by adverse weather conditions such as clouds, rain, strong winds, smog, and fog, which decrease data quality, especially for optical sensors. Technical challenges like limited resolution, sensor calibration, and the short battery life of drones can further complicate measurements, particularly in heterogeneous terrains. Optimal conditions include clear skies, low humidity, weak winds, or the use of multispectral or radar sensors, which can operate even in challenging environments. Careful planning and the integration of drone and satellite data can enhance the reliability and accuracy of measurements.

Figure 11.
Drone multispectral camera (a) and in-flight drone monitoring (b).
The following paragraphs briefly describe the main remote sensing techniques using multispectral and hyperspectral techniques for assessing the nutrient status of fruit trees.
3.1. Multispectral
As mentioned earlier, optical methods are based on the study of reflection, absorption, and transmission of light incident on the canopy [237]. The electromagnetic radiation reflected by plants carries valuable information about their biophysical and physiological properties. Since different spectral bands are involved in the reflectance of various elements, vegetation indices are calculated to provide more accurate information. The most widely used vegetation index for assessing fruit tree nutrient status is the NDVI, calculated using NIR and Red reflectance according to the following relationship [238]:
To date, multispectral analysis has been primarily used for the estimation of the three main macronutrients: N, P, and K, with a predominant focus on N [239]. N is a fundamental component of chlorophyll and proteins in plant cells. For this reason, the wavelengths where N absorption peaks occur are in the blue and, more notably, the red regions, similar to chlorophyll itself [240]. Reflectance peaks for P were observed in the red and NIR regions, while for K, peaks were observed in the green, red, and NIR regions [241,242,243,244].
The use of multispectral remote sensing with UAVs has proven highly effective in assessing the nutrient status of several fruit trees. In olive, Illana Rico et al. [245] employed UAV-acquired multispectral data from five orchards in Southern Spain to predict leaf nutrient content. Using support vector regression, their models achieved robust results for N, P, and K, with R2 values of 0.76, 0.87, and 0.91 for cv. Hojiblanca, and 0.79, 0.80, and 0.80 for cv. Picual. Additionally, the authors were able to obtain high scores for predicting other nutrients such as B (B, R2 = 0.69) and Cu (Cu, R2 = 0.93). However, the study has some shortcomings related to the sampling method not being clearly explained and intra-tree variability not specified. In an olive grove, nutrient content may vary within the crown of the same tree. In addition, the relationship between laboratory data and multispectral images is not analyzed. Measurements of foliar nutrient content were directly correlated with spectral data. Noguera et al. [246] developed an efficient method for retrieving N, P, and K content using multispectral imaging and artificial neural networks, with notable R2 values for P (0.89) and K (0.93). Often, it is not only the absolute level of a nutrient that affects plant health, but also the ratio of nutrients. One aspect that needs further investigation is the N:K or N:P ratio since they could provide more accurate indications of nutritional status and potential productivity.
For citrus trees, Li et al. [247] proposed a semi-supervised twin neural network regression model with multispectral indices, achieving a high estimation accuracy for leaf N content with an R2 of 0.71. Osco et al. [248] applied a random forest algorithm to UAV-derived spectral vegetation indices, successfully predicting canopy N content (CNC) with an R2 of 0.90, further demonstrating the effectiveness of machine learning techniques in citrus nutrient monitoring. However, an analysis of uncertainty at pixel-level predictions is not provided in the study. It would have been useful to know the degree of certainty of the model predictions used, identifying areas where segmentation is most uncertain. Such an analysis could help to better understand the limitations of the models and identify areas that need more attention. This could be achieved through visualization of uncertainty maps or through “dropout” techniques that serve to reduce overfitting. Costa et al. [249] further explored nutrient levels in citrus trees using gradient boosting regression trees. Their model predicted macro- and micronutrients with an average error of less than 9% in ‘Hamlin’ and 17% in ‘Valencia’ sweet orange (Citrus sinensis Osbeck), demonstrating the efficiency of AI-based methodologies for generating orchard nutrient maps in orange orchards. The results showed good accuracy for macronutrients such as N, P, and S, with errors less than 10 percent in tests on the “Hamlin” cultivar dataset. However, computational efficiency improvement techniques, such as pruning the models or combining gradient boosting with other deep learning architectures, could be used to achieve a further decrease in errors. This combination could also help reduce processing time and improve the model’s ability to handle larger or more complex datasets.
In apple, Zhao et al. [250] used random forest regression on UAV multispectral imagery to estimate CNC, achieving R2 values ranging from 0.67 to 0.79 across different growth stages. The article describes the use of different irrigation regimes. However, how the combination of different irrigation regimes and N fertilization interact in influencing leaf N content should be investigated to provide more precise recommendations for combined water and nutrient management. The ability to establish optimal irrigation thresholds would allow implementation of targeted irrigation regimes to maximize N assimilation and avoid wastage of water and fertilizer. Sun et al. [251] used UAV multispectral data with the diagnosis and recommendation integrated system (DRIS) method to measure N, P, and K content, demonstrating that UAV imagery combined with DRIS has strong potential for guiding fertigation of apple orchards. However, they did not carry out a systematic comparison with other nutrient diagnosis methodologies or laboratory analysis as a reference standard.
Benefits from UAV-based multispectral remote sensing were observed also in grapevine. Peng et al. [252] monitored N, P, and K levels in grapevine leaves across different growth stages, applying machine learning algorithms like random forest and support vector machine models. Their results showed high prediction accuracy, particularly for N during the shoot growth period, with an R2 above 0.65, highlighting the ability of multispectral UAV imagery to support precision viticulture.
Finally, Wang et al. [253] used UAV multispectral images and random forest models to estimate CNC in walnut trees. The authors combined information of CNC and the common vegetation indices across three different phenological stages, achieving R2 values between 0.61 and 0.84. The study compares a “delineated canopy” method, which consists of plotting the outline of the canopy, with a “simulated canopy” method, which employs mathematical and geometric models to virtually represent the tree canopy. The simulated canopy method used in the study, while more efficient, is less accurate due to the inclusion of pixels not relevant to the canopy. This led to mapping N values even where there was actually no vegetation. It would be useful to explore automatic canopy segmentation techniques based on machine learning, such as convolutional neural networks (CNNs), which could offer a trade-off between accuracy and efficiency. This could reduce dependence on manual intervention and improve the accuracy of the simulated method.
Despite the limited number of studies in fruit trees, satellite multispectral imaging has proven valuable for monitoring the nutrient status of orchards, offering a valid alternative to UAV-based methods.
A study on citrus orchards utilized Landsat 8 multispectral data to estimate leaf N content (LNC). By applying various machine learning models, including partial least square regression (PLSR), support vector regression, and deep neural networks, the authors demonstrated that satellite data could effectively predict LNC, with the PLSR model outperforming others (R2 = 0.86) [254].
In conclusion, the studies mentioned above highlight the potential of multispectral data to detect spatial variability in orchard nutrient needs, thereby improving fertilizer application strategies. Both satellite and UAV-based multispectral remote sensing represent valuable tools for precision horticulture, with satellite data offering a broader approach to orchard monitoring and UAVs providing higher-resolution, site-specific analysis.
3.2. Hyperspectral Remote Sensing
Hyperspectral remote sensing, as previously mentioned, involves the simultaneous acquisition of images across numerous spectral bands [255,256]. Specifically, devices such as drones and satellites, employed for capturing these images, can record data in hundreds of distinct spectral bands. A key distinction between multispectral and hyperspectral remote sensing lies in the number of spectral bands and the resolution of the images [257]. Hyperspectral systems detect light within the 400 to 2500 nm range, including the visible, NIR, and SWIR regions, and cover hundreds of spectral bands. In contrast, multispectral systems typically capture less than ten spectral bands [258,259]. Thanks to these features, hyperspectral imaging enables the calculation of several narrow-band indices by combining spectral bands related to crop biophysical parameters, such as the leaf area index (LAI), and biochemical parameters, including chlorophyll, water, and N content [256,260]. This technology facilitates the assessment of plant nutrient status and, consequently, its overall health [261,262,263]. The color characteristics of the plant canopy can be described by the ratio of incoming electromagnetic energy to the energy reflected at each wavelength across the electromagnetic spectrum. The amount of reflected light varies depending on both the intensity and wavelength of the incoming radiation. By analyzing these variations, it is possible to identify specific spectral regions relevant to plant nutrition and health. The NIR region, ranging from 700 to 1200 nm, is commonly used and has been shown to have a strong correlation with foliar N content.
Numerous studies in the literature report the application of hyperspectral remote sensing principles to determine various chemical elements in plant tissues or soil [257,264,265,266,267,268]. Zhang et al. [269] collected hyperspectral data using a combination of aerial (drone-based) and proximal sensors to assess the nutrient content of the apple tree canopy. By applying the Convolution Calculation of the Spectral Response Function and calculating various vegetation indices, including NDVI, they found that the R2 values for both raw and simulated data across all bands exceeded 0.70. Furthermore, the correlation coefficients between each spectral index and CNC increased from 0.30 to 0.55–0.68 after data fusion. The XGBoost model was identified as the optimal model for the transformation of CNC raw data obtained from hyperspectral monitoring into numerical data (R2 = 0.759, RMSE = 0.098, RPD = 1.855). The CNC distribution derived from this inversion was closely aligned with the actual distribution. These findings provide a theoretical foundation and technical support for efficient, non-destructive monitoring of the nutrient status in apple orchards using hyperspectral systems and AI techniques.
Gómez-Casero et al. [265] evaluated the effects of different levels of N and P on olive trees. The various treatments differed according to the amount of N and P applied to the soil and through foliar fertilization. Data were collected using an ASD Handheld FieldSpec Spectroradiometer (Analytical Spectral Devices, Inc., Boulder, CO, USA). The wavelengths within the NIR region (710 to 900 nm) were selected to discriminate between N treatments, with an overall accuracy of up to 99.2%. In addition, the wavelength range from 710 to 890 nm and the NDVI were selected to discriminate K treatments with an overall accuracy of up to 94.4%. Another example is the study by Ye et al. [270], in which the authors utilized an ImSpector V10 system (Spectral Imaging Ltd., Oulu, Finland) to capture hyperspectral images (400–1000 nm) of apple tree leaves and canopies. The N content in the apple leaves was measured using the Vario EL cube (Elementar Analytical, Munich, Germany). Both the raw reflectance and first-derivative reflectance data were analyzed to correlate with leaf N content. To estimate the N levels, the authors employed partial least square regression (PLS) and multiple linear regression (MLR) analysis. The results demonstrated that both the PLS and MLR models provided reasonable predictive accuracy (PLS and MLR models based on raw reflectance: R2 = 0.773 and 0.784, respectively (p < 0.001); PLS and MLR models based on first-derivative reflectance: R2 = 0.775 and 0.774, respectively (p < 0.001)). However, the MLR model based on raw reflectance showed an advantage over the PLS and first-derivative reflectance models, as it only required four key wavelengths (505, 560, 675, and 705 nm), whereas the other models relied on either the full spectrum (132 wavelengths) or narrower bands adjacent to the selected key wavelengths.
In a study on monitoring nutrient levels in vines, Chancia et al. [271] collected spectral data covering the 400–2500 nm range and 100 leaf samples from Concord grapes to identify the optimal bands for nutrient regression models. Spectral data of the vines were captured using UAS equipped with push-broom hyperspectral sensors. Hyperspectral data used to determine nutrient content in the canopy were taken using a Headwall Photonics Nano-Hyperspecimaging spectrometer (272 spectral bands from 400 to 1000 nm) for visible and near-infrared range (VNIR) data and a Headwall Photonics Micro-Hyperspec M384 imaging spectrometer (170 bands from 900 to 2500 nm) for the SWIR data. Each spectrometer was mounted on a separate DJI Matrice 600 Pro UAV. The authors identified a set of biochemically consistent wavelengths (606, 641, and 1494 nm) for predicting N content, achieving an RMSE of 0.17% (using leave-one-out cross-validation) in samples with N content ranging from 2.4% to 3.6%. Further studies with larger dataset sizes are needed to confirm the relevance and consistency of these selected wavelengths for each nutrient to develop nutrient regression models that consider also the environmental, climatic, and genetic variability of vines. However, the selection of these specific wavelengths shows promise for developing stable spectral bands that can accurately assess nutrient content in grapevines from canopy-level spectral data.
An insightful study on soil nutrient monitoring using hyperspectral remote sensing was conducted by Song et al. in China [268]. The researchers used hyperspectral remote sensing images (115 bands) from the Chinese environmental satellite 1A as auxiliary variables, and dimensionality reduction was applied using Pearson’s correlation analysis and principal component analysis. The total N (TN), available P (AP), and available K (AK) in the soil were predicted for the study area based on these reduced auxiliary variables. Several models were tested, including stepwise linear regression (SLR), support vector machines (SVMs), random forest (RF), and back-propagation neural network (BPNN) models. Predictive performance was evaluated using 324 independent validation points. The BPNN model incorporating ordinary kriging (BPNNOK) for spatial variability mapping of soil nutrients demonstrated the highest predictive accuracy among all models. The results showed that the BPNN model with two hidden layers outperformed SLR, SVM, and RF models for soil TN (RMSE = 0.409 mg/kg, R2 = 0.442), soil AP (RMSE = 40.8 mg/kg, R2 = 0.429), and soil AK (RMSE = 67.5 mg/kg, R2 = 0.485). The BPNNOK achieved even better results, with an RMSE of 0.292 mg/kg and R2 of 0.685 for soil TN, an RMSE of 29.6 mg/kg and R2 of 0.693 for soil AP, and an RMSE of 49.7 mg/kg and R2 of 0.706 for soil AK. These findings highlight the strong adaptability of hyperspectral remote sensing bands in predicting soil nutrient levels.
In conclusion, hyperspectral remote sensing offers a highly detailed, non-destructive method for monitoring nutrient content in fruit trees, providing precise information through the analysis of numerous spectral bands. This technology allows for large-scale monitoring and can integrate with other data sources for a comprehensive understanding of crop nutrients and health status. However, the high cost, data complexity, sensitivity to environmental conditions, and the need for sophisticated models limit the widespread adoption of this technology, particularly in less technologically advanced agricultural settings.
4. Combination of Proximal and Remote Sensing
Proximal sensing involves sensors positioned within a few centimeters or meters of the plant canopy. These sensors operate using various techniques such as reflectance spectroscopy in the visible and near-infrared (VIS–NIR) regions, fluorescence, and thermal imaging [272,273]. For example, the chlorophyll content, which is strongly linked to N availability, can be estimated through reflectance indices at specific wavelengths [274]. The main advantage of proximal sensing lies in detailed and precise analysis of individual plants or small field sections and are non-destructive. They also save time and money since traditional laboratory analysis can be avoided through their use. This approach is particularly useful for real-time and continuous monitoring of crop health and nutrient status [275]. However, the method is difficult to implement, as it requires significant labor and time for large-scale applications [272].
Remote sensing, on the other hand, involves sensors positioned on platforms farther from the ground, such as satellites, airplanes, or drones. These systems use spectral information from VIS–NIR, and thermal bands to assess plant nutrient status across large areas. Indices such as NDVI and NDRE are widely used to estimate N content, chlorophyll levels, and water stress at regional scales [276].
The primary strength of remote sensing is its ability to monitor extensive areas efficiently, making it ideal for large-scale crop management. However, the lack of continuous measurements and the influence of weather conditions are severe limitations [277,278,279,280].
The integration of proximal and remote sensing offers a comprehensive approach to evaluating plant nutritional status [281]. Proximal sensing provides detailed insights at the plant level, while remote sensing enables large-scale monitoring. By combining these methods, precision agriculture can optimize resource use, improve crop management, and enhance sustainability.
The combination of both techniques is very useful to obtain more accurate data on the nutrient status of plants or soil. The technique would consist of installing proximal sensors or measuring hand tools in specific areas of the field to integrate the proximal data with those obtained by remote sensing and have a representative figure for the whole field. Also, for monitoring plant water status, only a few studies have tested the combination of remote and proximal sensors [74]. Unlike for monitoring plant and soil nutrient status, for plant water status, several proximal sensors have been developed [74,282]. Thus, the need to develop additional nutrient sensors that are cost-effective, easy to install and at the same time allow for reliable measurement remains a primary objective.
There are very few studies that have combined proximal and remote sensing technologies for monitoring plant and soil nutrient status. An example is the study by Bausch et al. [283] in which the authors compared different vegetation indices calculated from two different systems, the QuickBird satellite and a mobile ground-based radiometric system, for the assessment of N maize content. The crop area was divided into 12 zones with different N applications. In addition, the authors carried out measurements with the SPAD meter on all samples to calculate the N sufficiency index. They found that both systems showed a good correlation between N sufficiency index and the Normalized Green-Blue Normalized Difference Vegetation Index (NGNDVI) with R2 values ranging from 0.91 to 0.95 during V12 and V15 maize growth stages.
Fiorentini et al. [284] analyzed how different combinations of soil management (untilled-NT vs. conventional-CT) and N fertilization levels (0, 90, and 180 kg N ha−1) influence the nutrient status and development of durum wheat using vegetation indices and proximal detection tools. Chlorophyll and N content were measured using both the SPAD meter (proximal sensing) and multispectral images acquired by drones (remote sensing). In addition to finding that NT performed better than CT, the authors found a linear relationship between the two monitoring methods with an R2 of 0.72.
Another study combining proximal and remote sensing data was carried out by Darra et al. [285]. The aim in this case was to assess the correlation between outputs of different detection methods for the calculation of NDVI in vineyards. Remote sensing data were acquired from the satellite Sentinel 2, while proximal data were acquired with a sensor called Crop Circle ACS-470, an optical sensor very similar to the Greenseeker described in this review. The authors revealed the usefulness of the remote-sensing-derived vegetation index and variables, in particular the NDVI and the fraction of photosynthetically absorbed active radiation in monitoring vineyard conditions. They found a high correlation coefficient of 0.87 between the NDVI calculated with Crop Circle ACS-470 and Fraction of Absorbed Photosynthetically Active Radiation calculated with Sentinel 2.
Further studies testing the combination of proximal and remote sensing are needed. Most studies in the literature combine optical proximal sensors with remote sensing, which is based on optical principles. It would be interesting to carry out studies combining proximal sensors based on other physical–chemical principles, such as electrochemical sensors or biosensors, with remote sensing to assess the reliability of data or whether there are correlations between the two. Traditionally, remote sensor calibration is achieved through sampling and analysis in the laboratory, but this requires a great deal of time and resources. Previously cited studies in this manuscript have shown the efficiency of using vegetation indices from remote sensing calibrated through optical devices (e.g., SPAD) [283,284]. The possibility of using proximal sensors to replace the traditional calibration method presents itself as a very viable alternative because it allows the acquisition of a larger amount of data in real time, on a larger number of plants.
5. Conclusions
Effective resource management is essential to prevent soil and water pollution, as well as economic losses from excessive fertilizer use. Continuous and rapid monitoring of plant nutrient status remains a critical area for improvement. The physico-chemical and optical sensors discussed in this work offer a promising foundation for developing practical, farmer-friendly tools. Continuous proximal sensors enable real-time crop monitoring, and their integration with remote sensing can provide comprehensive nutrient status data for orchards and fields.
However, certain limitations persist. Electrochemical sensors, while precise in detecting and quantifying ions, require frequent calibration and are challenging to manufacture at the commercial scale. Biosensors also offer accurate nutrient monitoring but are hindered by calibration issues and degradation under adverse conditions. Proximal optical sensors, relying on leaf reflectance, absorbance, and fluorescence, face challenges such as ambient light variability, dirt, and species-dependent leaf properties. Remote sensing supports large-scale monitoring but is limited by its discontinuous nature, high cost, and sensitivity to environmental conditions.
Overcoming these limitations through technological advancements will enable the development of integrated systems that combine proximal and remote sensing, enhancing their efficacy and accuracy in assessing plant nutrient status. Indeed, a combination of time- and space-scale monitoring would provide precise answers to the critical questions of when and where to apply a certain amount of fertilizer.
Author Contributions
Conceptualization, P.T.B.F. and R.L.B.; methodology, P.T.B.F.; investigation, P.T.B.F.; resources, P.T.B.F., R.L.B., A.C., R.M. and R.F.; data curation, P.T.B.F., R.M., A.C. and R.F.; writing—original draft preparation, P.T.B.F., A.C., R.M., R.F. and R.L.B.; writing—review and editing, P.T.B.F., A.C, R.M., R.F. and R.L.B.; visualization, P.T.B.F., A.C. and R.M.; supervision, R.M. and R.L.B.; funding acquisition, R.L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the following projects: SUSTAINABLE project, funded by the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie-RISE Grant Agreement No. 101007702 (https://www.projectsustainable.eu, accessed on 5 January 2025), Ecosistema dell’innovazione Sicilian MicronanoTech Research and Innovation Center (SAMOTHRACE) and Fondo Finalizzato alla Ricerca di Ateneo FFR_D13_008811, funded by Ministero dell’Università e della Ricerca (MUR), Grant Agreement No. ECS_00000022.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The Authors declare no conflicts of interest.
References
- Fageria, N.K.; Moreira, A. The Role of Mineral Nutrition on Root Growth of Crop Plants. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 110, pp. 251–331. [Google Scholar]
- Pandey, R. Mineral Nutrition of Plants. In Plant Biology and Biotechnology: Volume I: Plant Diversity, Organization, Function and Improvement; Bahadur, B., Venkat Rajam, M., Sahijram, L., Krishnamurthy, K.V., Eds.; Springer: New Delhi, India, 2015; pp. 499–538. ISBN 978-81-322-2286-6. [Google Scholar]
- Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M.; Dubey, N.K. Role of Macronutrients in Plant Growth and Acclimation: Recent Advances and Future Prospective. In Improvement of Crops in the Era of Climatic Changes: Volume 2; Ahmad, P., Wani, M.R., Azooz, M.M., Phan Tran, L.-S., Eds.; Springer: New York, NY, USA, 2014; pp. 197–216. ISBN 978-1-4614-8824-8. [Google Scholar]
- Maathuis, F.J.M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Aslam, M.A.; Hayat, R.; Nasim, W.; Akmal, M.; Mubeen, M.; Ahmad, S. Nutrient Dynamics and the Role of Modeling. In Building Climate Resilience in Agriculture; Springer: Cham, Switzerland, 2022; pp. 297–316. [Google Scholar]
- Jungk, A.O. Dynamics of Nutrient Movement at the Soil-Root Interface. In Plant Roots; CRC Press: Boca Raton, FL, USA, 2002; pp. 919–1016. ISBN 978-0-429-22185-9. [Google Scholar]
- Binkley, D.; Vitousek, P. Soil Nutrient Availability. In Plant Physiological Ecology: Field Methods and Instrumentation; Pearcy, R.W., Ehleringer, J.R., Mooney, H.A., Rundel, P.W., Eds.; Springer: Dordrecht, The Netherlands, 1989; pp. 75–96. ISBN 978-94-009-2221-1. [Google Scholar]
- Murray, H.H. Chapter 2 Structure and Composition of the Clay Minerals and Their Physical and Chemical Properties. In Developments in Clay Science; Murray, H.H., Ed.; Applied Clay Mineralogy; Elsevier: Amsterdam, The Netherlands, 2006; Volume 2, pp. 7–31. [Google Scholar]
- Goldberg, S. Interaction of Aluminum and Iron Oxides and Clay Minerals and Their Effect on Soil Physical Properties: A Review. Commun. Soil Sci. Plant Anal. 1989, 20, 1181–1207. [Google Scholar] [CrossRef]
- Gao, X.; Li, S.; Liu, X.; Hu, F.; Tian, R.; Li, H. The Effects of NO3− and Cl− on Negatively Charged Clay Aggregation. Soil Tillage Res. 2019, 186, 242–248. [Google Scholar] [CrossRef]
- Hafsteinsdóttir, E.G.; Camenzuli, D.; Rocavert, A.L.; Walworth, J.; Gore, D.B. Chemical Immobilization of Metals and Metalloids by Phosphates. Appl. Geochem. 2015, 59, 47–62. [Google Scholar] [CrossRef]
- Bouabid, R.; Badraoui, M.; Bloom, P.R. Potassium Fixation and Charge Characteristics of Soil Clays. Soil Sci. Soc. Am. J. 1991, 55, 1493–1498. [Google Scholar] [CrossRef]
- Sparks, D.L.; Huang, P.M. Physical Chemistry of Soil Potassium. In Potassium in Agriculture; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1985; pp. 201–276. ISBN 978-0-89118-247-4. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Nutrient Availability. Encycl. Soils Environ. 2005, 3, 63–71. [Google Scholar]
- Cakmak, I.; Brown, P.; Colmenero-Flores, J.M.; Husted, S.; Kutman, B.Y.; Nikolic, M.; Rengel, Z.; Schmidt, S.B.; Zhao, F.-J. Micronutrients. In Marschner’s Mineral Nutrition of Plants; Elsevier: Amsterdam, The Netherlands, 2023; pp. 283–385. [Google Scholar]
- Sinha, D.; Tandon, P.K. An Overview of Nitrogen, Phosphorus and Potassium: Key Players of Nutrition Process in Plants. In Sustainable Solutions for Elemental Deficiency and Excess in Crop Plants; Springer: Singapore, 2020; pp. 85–117. [Google Scholar]
- Amtmann, A.; Hammond, J.P.; Armengaud, P.; White, P.J. Nutrient sensing and signalling in plants: Potassium and phosphorus. Adv. Bot. Res. 2005, 43, 209–257. [Google Scholar] [CrossRef]
- Duan, Y.; Yang, H.; Yang, H.; Wei, Z.; Che, J.; Wu, W.; Lyu, L.; Li, W. Physiological and Morphological Responses of Blackberry Seedlings to Different Nitrogen Forms. Plants 2023, 12, 1480. [Google Scholar] [CrossRef] [PubMed]
- Krapp, A. Plant nitrogen assimilation and its regulation: A complex puzzle with missing pieces. Curr. Opin. Plant Biol. 2015, 25, 115–122. [Google Scholar] [CrossRef]
- Yoneyama, T.; Ito, O.; Engelaar, W.M.H.G. Uptake, metabolism, and distribution of nitrogen in crop plants traced by enriched and natural 15N: Progress over the last 30 years. Phytochem. Rev. 2003, 2, 121–132. [Google Scholar] [CrossRef]
- Raven, J.A.; Smith, F.A. Nitrogen Assimilation and Transport in Vascular Land Plants in Relation to Intracellular pH Regulation. New Phytol. 1976, 76, 415–431. [Google Scholar] [CrossRef]
- Ohyama, T. Nitrogen as a Major Essential Element of Plants. Nitr. Assim. Plants 2010, 37, 2–17. [Google Scholar]
- Wang, Q.; Li, S.; Li, J.; Huang, D. The Utilization and Roles of Nitrogen in Plants. Forests 2024, 15, 1191. [Google Scholar] [CrossRef]
- Havé, M.; Marmagne, A.; Chardon, F.; Masclaux-Daubresse, C. Nitrogen Remobilization during Leaf Senescence: Lessons from Arabidopsis to Crops. J. Exp. Bot. 2017, 68, 2513–2529. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.-F.; Wu, W.-H. Potassium and Phosphorus Transport and Signaling in Plants. J. Integr. Plant Biol. 2021, 63, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Rychter, A.M.; Rao, I.M. Role of phosphorus in photosynthetic carbon metabolism. In Handbook of Photosynthesis; Pessarakli, M., Ed.; Taylor and Francis Group, LLC: Tucson, AZ, USA, 2005; pp. 123–148. ISBN 978-131-537-213-6. [Google Scholar]
- Raven, J.A. RNA function and phosphorus use by photosynthetic organisms. Front. Plant Sci. 2013, 4, 61258. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Phosphorus Nutrition: Interactions between Pathways of Phosphorus Uptake in Arbuscular Mycorrhizal Roots Have Important Implications for Understanding and Manipulating Plant Phosphorus Acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef]
- Pandey, G.K.; Mahiwal, S. Role of Potassium in Plants; SpringerBriefs in Plant Science; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-45952-9. [Google Scholar]
- Sardans, J.; Peñuelas, J. Potassium control of plant functions: Ecological and agricultural implications. Plants 2021, 10, 419. [Google Scholar] [CrossRef]
- Rawat, J.; Pandey, N.; Saxena, J. Role of Potassium in Plant Photosynthesis, Transport, Growth and Yield. In Role of Potassium in Abiotic Stress; Iqbal, N., Umar, S., Eds.; Springer Nature: Singapore, 2022; pp. 1–14. ISBN 978-981-164461-0. [Google Scholar] [CrossRef]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in Agriculture—Status and Perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef]
- Wallace, A.; Mueller, R.T. Calcium uptake and distribution in plants. J. Plant Nut. 1980, 2, 247–256. [Google Scholar] [CrossRef]
- Hanger, B.C. Movement of calcium in plants. Commun. Soil Sci. Plant Anal. 1979, 10, 171–193. [Google Scholar] [CrossRef]
- Jones, R.G.W.; Lunt, O.R. The function of calcium in plants. Bot. Rev. 1967, 33, 407–426. [Google Scholar] [CrossRef]
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef]
- Hermans, C.; Conn, S.J.; Chen, J.; Xiao, Q.; Verbruggen, N. Update on Magnesium Homeostasis Mechanisms in Plants. Metallomics 2013, 5, 1170–1183. [Google Scholar] [CrossRef] [PubMed]
- Gerendás, J.; Führs, H. The Significance of Magnesium for Crop Quality. Plants Soil 2013, 368, 101–128. [Google Scholar] [CrossRef]
- Li, Q.; Gao, Y.; Yang, A. Sulfur Homeostasis in Plants. Int. J. Mol. Sci. 2020, 21, 8926. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.F.; Smith, I.K.; Madison, J.T. Sulfur Metabolism in Plants. In Sulfur in Agriculture; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1986; pp. 57–121. ISBN 978-0-89118-220-7. [Google Scholar]
- Govindasamy, P.; Muthusamy, S.K.; Bagavathiannan, M.; Mowrer, J.; Jagannadham, P.T.K.; Maity, A.; Halli, H.M.; K., S.G.; Vadivel, R.; K., D.T.; et al. Nitrogen Use Efficiency—A Key to Enhance Crop Productivity under a Changing Climate. Front. Plant Sci. 2023, 14, 1121073. [Google Scholar] [CrossRef]
- Yu, X.; Keitel, C.; Zhang, Y.; Wangeci, A.N.; Dijkstra, F.A. Global Meta-Analysis of Nitrogen Fertilizer Use Efficiency in Rice, Wheat and Maize. Agric. Ecosyst. Environ. 2022, 338, 108089. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.T.; Shi, W.; Kronzucker, H.J. Nitrogen Transformations in Modern Agriculture and the Role of Biological Nitrification Inhibition. Nat. Plants 2017, 3, 17074. [Google Scholar] [CrossRef] [PubMed]
- de Vries, W. Impacts of Nitrogen Emissions on Ecosystems and Human Health: A Mini Review. Curr. Opin. Environ. Sci. Health 2021, 21, 100249. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, X.; Wei, X.; Kai, Z.; Xu, Y. Excessive Application of Chemical Fertilizer and Organophosphorus Pesticides Induced Total Phosphorus Loss from Planting Causing Surface Water Eutrophication. Sci. Rep. 2021, 11, 23015. [Google Scholar] [CrossRef]
- Khan, M.N.; Mohammad, F. Eutrophication: Challenges and Solutions. In Eutrophication: Causes, Consequences and Control: Volume 2; Ansari, A.A., Gill, S.S., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–15. ISBN 978-94-007-7814-6. [Google Scholar] [CrossRef]
- Ahmed, M.; Rauf, M.; Mukhtar, Z.; Saeed, N.A. Excessive Use of Nitrogenous Fertilizers: An Unawareness Causing Serious Threats to Environment and Human Health. Environ. Sci. Pollut. Res. 2017, 24, 26983–26987. [Google Scholar] [CrossRef] [PubMed]
- Zahir, S.A.D.M.; Jamlos, M.F.; Omar, A.F.; Jamlos, M.A.; Mamat, R.; Muncan, J.; Tsenkova, R. Review—Plant Nutritional Status Analysis Employing the Visible and Near-Infrared Spectroscopy Spectral Sensor. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 304, 123273. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.R.; Gomes Neto, J.A.; Nóbrega, J.A.; Jones, B.T. Determination of Macro- and Micronutrients in Plant Leaves by High-Resolution Continuum Source Flame Atomic Absorption Spectrometry Combining Instrumental and Sample Preparation Strategies. Spectrochim. Acta B At. Spectrosc. 2010, 65, 316–320. [Google Scholar] [CrossRef]
- Manickam, S.; Rajagopalan, V.R.; Kambale, R.; Rajasekaran, R.; Kanagarajan, S.; Muthurajan, R. Plant Metabolomics: Current Initiatives and Future Prospects. Curr. Issues Mol. Biol. 2023, 45, 8894–8906. [Google Scholar] [CrossRef] [PubMed]
- Lilay, G.H.; Persson, D.P.; Castro, P.H.; Liao, F.; Alexander, R.D.; Aarts, M.G.M.; Assunção, A.G.L. Arabidopsis bZIP19 and bZIP23 Act as Zinc Sensors to Control Plant Zinc Status. Nat. Plants 2021, 7, 137–143. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Bąba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Dimitrova, S.; Piszcz, U.; Bielecki, K.; Karmowska, K.; et al. Chlorophyll Fluorescence as a Tool for Nutrient Status Identification in Rapeseed Plants. Photosynth. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Z.; Zhu, Y.; Castellano, M.J.; Dong, L. Miniature Multi-Ion Sensor Integrated with Artificial Neural Network. IEEE Sens. J. 2021, 21, 25606–25615. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Lee, K.H. Electrochemical Sensors for Sustainable Precision Agriculture—A Review. Front. Chem. 2022, 10, 848320. [Google Scholar] [CrossRef]
- Vizzari, M.; Santaga, F.; Benincasa, P. Sentinel 2-Based Nitrogen VRT Fertilization in Wheat: Comparison between Traditional and Simple Precision Practices. Agronomy 2019, 9, 278. [Google Scholar] [CrossRef]
- Radočaj, D.; Jurišić, M.; Gašparović, M. The Role of Remote Sensing Data and Methods in a Modern Approach to Fertilization in Precision Agriculture. Remote Sens. 2022, 14, 778. [Google Scholar] [CrossRef]
- Dong, W.; Wu, T.; Luo, J.; Sun, Y.; Xia, L. Land Parcel-Based Digital Soil Mapping of Soil Nutrient Properties in an Alluvial-Diluvial Plain Agricultural Area in China. Geoderma 2019, 340, 234–248. [Google Scholar] [CrossRef]
- Jurišić, M.; Radočaj, D.; Plaščak, I.; Rapčan, I. A Comparison of Precise Fertilization Prescription Rates to a Conventional Approach Based on the Open Source GIS Software. Poljoprivreda 2021, 27, 52–59. [Google Scholar] [CrossRef]
- Roma, E.; Laudicina, V.A.; Vallone, M.; Catania, P. Application of Precision Agriculture for the Sustainable Management of Fertilization in Olive Groves. Agronomy 2023, 13, 324. [Google Scholar] [CrossRef]
- Tomkiewicz, D.; Piskier, T. A Plant-Based Sensing Method for Nutrition Stress Monitoring. Precis. Agric. 2012, 13, 370–383. [Google Scholar] [CrossRef]
- Lin, J.; Wang, M.; Zhang, M.; Zhang, Y.; Chen, L. Electrochemical Sensors for Soil Nutrient Detection: Opportunity and Challenge. In Computer and Computing Technologies in Agriculture, Volume II; Li, D., Ed.; Springer: Boston, MA, USA, 2008; pp. 1349–1353. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Min, J.; Song, Y.; Tu, J.; Mukasa, D.; Ye, C.; Xu, C.; Heflin, N.; McCune, J.S.; et al. A Wearable Electrochemical Biosensor for the Monitoring of Metabolites and Nutrients. Nat. Biomed. Eng. 2022, 6, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-Y.; Lee, J.-W.; Park, D.J.; Lee, J.-Y.; Myung, N.V.; Kwon, S.H.; Lee, K.H. Highly Stable Potentiometric Sensor with Reduced Graphene Oxide Aerogel as a Solid Contact for Detection of Nitrate and Calcium Ions. J. Electroanal. Chem. 2021, 897, 115553. [Google Scholar] [CrossRef]
- Terentev, A.; Dolzhenko, V. Can Metabolomic Approaches Become a Tool for Improving Early Plant Disease Detection and Diagnosis with Modern Remote Sensing Methods? A Review. Sensors 2023, 23, 5366. [Google Scholar] [CrossRef]
- Feng, D.; Xu, W.; He, Z.; Zhao, W.; Yang, M. Advances in Plant Nutrition Diagnosis Based on Remote Sensing and Computer Application. Neural Comput. Appl. 2020, 32, 16833–16842. [Google Scholar] [CrossRef]
- Sadoine, M.; De Michele, R.; Župunski, M.; Grossmann, G.; Castro-Rodríguez, V. Monitoring Nutrients in Plants with Genetically Encoded Sensors: Achievements and Perspectives. Plant Physiol. 2023, 193, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Rumjit, N.P.; Roy, S. Smart Agriculture and Nanotechnology: Technology, Challenges, and New Perspective. Adv. Agrochem. 2023, 3, 115–125. [Google Scholar] [CrossRef]
- Dhanaraju, M.; Chenniappan, P.; Ramalingam, K.; Pazhanivelan, S.; Kaliaperumal, R. Smart Farming: Internet of Things (IoT)-Based Sustainable Agriculture. Agriculture 2022, 12, 1745. [Google Scholar] [CrossRef]
- Karunathilake, E.M.B.M.; Le, A.T.; Heo, S.; Chung, Y.S.; Mansoor, S. The Path to Smart Farming: Innovations and Opportunities in Precision Agriculture. Agriculture 2023, 13, 1593. [Google Scholar] [CrossRef]
- Soussi, A.; Zero, E.; Sacile, R.; Trinchero, D.; Fossa, M. Smart Sensors and Smart Data for Precision Agriculture: A Review. Sensors 2024, 24, 2647. [Google Scholar] [CrossRef]
- Yin, H.; Cao, Y.; Marelli, B.; Zeng, X.; Mason, A.J.; Cao, C. Soil Sensors and Plant Wearables for Smart and Precision Agriculture. Adv. Mater. 2021, 33, 2007764. [Google Scholar] [CrossRef] [PubMed]
- Sanaeifar, A.; Yang, C.; Min, A.; Jones, C.R.; Michaels, T.E.; Krueger, Q.J.; Barnes, R.; Velte, T.J. Noninvasive Early Detection of Nutrient Deficiencies in Greenhouse-Grown Industrial Hemp Using Hyperspectral Imaging. Remote Sens. 2024, 16, 187. [Google Scholar] [CrossRef]
- Roma, E.; Catania, P. Precision Oliviculture: Research Topics, Challenges, and Opportunities—A Review. Remote Sens. 2022, 14, 1668. [Google Scholar] [CrossRef]
- Carella, A.; Bulacio Fischer, P.T.; Massenti, R.; Lo Bianco, R. Continuous Plant-Based and Remote Sensing for Determination of Fruit Tree Water Status. Horticulturae 2024, 10, 516. [Google Scholar] [CrossRef]
- Ghanimi, H.M.; Suguna, R.; Jeyaraj, J.P.G.; Sreekanth, K.; Rangasamy, R.; Sengan, S. Smart Fertilizing Using IoT Multi-Sensor and Variable Rate Sprayer Integrated UAV. Scalable Comput. Pract. Exp. 2024, 25, 3766–3777. [Google Scholar] [CrossRef]
- Salam, U.; Ullah, S.; Tang, Z.-H.; Elateeq, A.A.; Khan, Y.; Khan, J.; Khan, A.; Ali, S. Plant Metabolomics: An Overview of the Role of Primary and Secondary Metabolites against Different Environmental Stress Factors. Life 2023, 13, 706. [Google Scholar] [CrossRef]
- Djaman, K.; Irmak, S.; Martin, D.L.; Ferguson, R.B.; Bernards, M.L. Plant Nutrient Uptake and Soil Nutrient Dynamics under Full and Limited Irrigation and Rainfed Maize Production. Agron. J. 2013, 105, 527–538. [Google Scholar] [CrossRef]
- El-Ramady, H.R.; Alshaal, T.A.; Amer, M.; Domokos-Szabolcsy, É.; Elhawat, N.; Prokisch, J.; Fári, M. Soil Quality and Plant Nutrition. In Sustainable Agriculture Reviews 14: Agroecology and Global Change; Ozier-Lafontaine, H., Lesueur-Jannoyer, M., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 345–447. ISBN 978-3-319-06016-3. [Google Scholar] [CrossRef]
- Kashyap, B.; Kumar, R. Sensing Methodologies in Agriculture for Soil Moisture and Nutrient Monitoring. IEEE Access 2021, 9, 14095–14121. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Chen, C.-L.; Shih, Y.-J. Electrochemical Sensors. In Handbook of Nanosensors: Materials and Technological Applications; Ali, G.A.M., Chong, K.F., Makhlouf, A.S.H., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 1–33. ISBN 978-3-031-16338-8. [Google Scholar] [CrossRef]
- Simoes, F.R.; Xavier, M.G. 6—Electrochemical Sensors. In Micro and Nano Technologies, Nanoscience and Its Applications; da Roz, A.L., Ferreira, M., de Lima Leite, F., Oliveira, O.N., Eds.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 155–178. [Google Scholar]
- Brinda, K.N.; Yhobu, Z.; Nagaraju, D.H.; Budagumpi, S. Working Principle and Sensing Mechanism of Electrochemical Sensors. In 2D Materials-Based Electrochemical Sensors; Rout, C.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 9–44. ISBN 978-0-443-15293-1. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Ota, K.I.; Kreysa, G.; Savinell, R.F. (Eds.) Encyclopedia of Applied Electrochemistry, 1st ed.; Springer: New York, NY, USA, 2013; ISBN 978-1-4419-6997-2. [Google Scholar] [CrossRef]
- Papavinasam, S. 3-Electrochemical Polarization Techniques for Corrosion Monitoring. In Techniques for Corrosion Monitoring, 2nd ed.; Yang, L., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 45–77. ISBN 9780081030035. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Banakar, M.; Hamidi, M.; Khurshid, Z.; Zafar, M.S.; Sapkota, J.; Azizian, R.; Rokaya, D. Electrochemical Biosensors for Pathogen Detection: An Updated Review. Biosensors 2022, 12, 927. [Google Scholar] [CrossRef]
- Barhoum, A.; Hamimed, S.; Slimi, H.; Othmani, A.; Abdel-Haleem, F.M.; Bechelany, M. Modern Designs of Electrochemical Sensor Platforms for Environmental Analyses: Principles, Nanofabrication Opportunities, and Challenges. Trends Environ. Anal. Chem. 2023, 38, e00199. [Google Scholar] [CrossRef]
- Gandhi, M.; Amreen, K. Electrochemical Profiling of Plants. Electrochem 2022, 3, 434–450. [Google Scholar] [CrossRef]
- Rabinowitch, E.; U.S. Atomic Energy Commission. Photosynthesis; U.S. Atomic Energy Commission: Washington, DC, USA, 1949. [Google Scholar]
- Eberhard, S.; Finazzi, G.; Wollman, F.-A. The Dynamics of Photosynthesis. Annu. Rev. Genet. 2008, 42, 463–515. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A Critical Review on Soil Chemical Processes That Control How Soil pH Affects Phosphorus Availability to Plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Chugh, B.; Thakur, S.; Singh, A.K.; Joany, R.M.; Rajendran, S.; Nguyen, T.A. Electrochemical Sensors for Agricultural Application. In Nanosensors for Smart Agriculture; Denizli, A., Nguyen, T.A., Rajendran, S., Yasin, G., Nadda, A.K., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2022; pp. 147–164. ISBN 978-0-12-824554-5. [Google Scholar] [CrossRef]
- Nadporozhskaya, M.; Kovsh, N.; Paolesse, R.; Lvova, L. Recent advances in chemical sensors for soil analysis: A review. Chemosensors 2022, 10, 35. [Google Scholar] [CrossRef]
- Caron, W.-O.; Lamhamedi, M.S.; Viens, J.; Messaddeq, Y. Practical Application of Electrochemical Nitrate Sensor under Laboratory and Forest Nursery Conditions. Sensors 2016, 16, 1190. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, N.P.; Venugopalan, S.; Senthilkumar, N.; Santhosh, P.; Kavita, B.; Prabu, H.G. Voltammetric determination of nitroaromatic and nitramine explosives contamination in soil. Talanta 2006, 69, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Burton, L.; Jayachandran, K.; Bhansali, S. Review—The “Real-Time” Revolution for In Situ Soil Nutrient Sensing. J. Electrochem. Soc. 2020, 167, 037569. [Google Scholar] [CrossRef]
- Mishra, P.; Mapara, S.; Vyas, P. Testing/monitoring of soil chemical level using wireless sensor network technology. Int. J. Appl. Innov. Eng. Manag. 2015, 4, 114–117. [Google Scholar]
- Kundu, M.; Krishnan, P.; Chobhe, K.A.; Manjaiah, K.M.; Pant, R.P.; Chawla, G. Fabrication of Electrochemical Nanosensor for Detection of Nitrate Content in Soil Extract. J. Soil Sci. Plant Nutr. 2022, 22, 2777–2792. [Google Scholar] [CrossRef]
- Baumbauer, C.L.; Goodrich, P.J.; Payne, M.E.; Anthony, T.; Beckstoffer, C.; Toor, A.; Silver, W.; Arias, A.C. Printed Potentiometric Nitrate Sensors for Use in Soil. Sensors 2022, 22, 4095. [Google Scholar] [CrossRef]
- Hossain, M.I.; Khaleque, M.A.; Ali, M.R.; Bacchu, M.S.; Hossain, M.S.; Shahed, S.M.F.; Saad Aly, M.A.; Khan, M.Z.H. Development of Electrochemical Sensors for Quick Detection of Environmental (Soil and Water) NPK Ions. RSC Adv. 2024, 14, 9137–9158. [Google Scholar] [CrossRef]
- Ibrahim, H.; Yin, S.; Moru, S.; Zhu, Y.; Castellano, M.J.; Dong, L. In Planta Nitrate Sensor Using a Photosensitive Epoxy Bioresin. ACS Appl. Mater. Interfaces 2022, 14, 25949–25961. [Google Scholar] [CrossRef] [PubMed]
- Murad, M.O. Obtaining Chemical Properties through Soil Electrical Resistivity. J. Civ. Eng. Res. 2012, 2, 120–128. [Google Scholar] [CrossRef]
- Bellosta-Diest, A.; Campo-Bescós, M.Á.; Zapatería-Miranda, J.; Casalí, J.; Arregui, L.M. Evaluation of Nitrate Soil Probes for a More Sustainable Agriculture. Sensors 2022, 22, 9288. [Google Scholar] [CrossRef]
- Pratama, H.; Yunan, A.; Candra, R.A. Design and Build a Soil Nutrient Measurement Tool for Citrus Plants Using NPK Soil Sensors Based on the Internet of Things. Brill. Res. Artif. Intell. 2021, 1, 67–74. [Google Scholar] [CrossRef]
- Kumar, L.; Srivani, M.; Nishath, M.; Akhil, T.; Naveen, A.; Kumar, K. Monitoring of Soil Nutrients Using Soil NPK Sensor and Arduino. Ecol. Environ. Conserv. 2024, 30, 239–246. [Google Scholar] [CrossRef]
- Huang, J.; Hartemink, A.E. Soil and Environmental Issues in Sandy Soils. Earth-Sci. Rev. 2020, 208, 103295. [Google Scholar] [CrossRef]
- Riaz, M.; Marschner, P. Sandy Soil Amended with Clay Soil: Effect of Clay Soil Properties on Soil Respiration, Microbial Biomass, and Water Extractable Organic C. J. Soil Sci. Plant Nutr. 2020, 20, 2465–2470. [Google Scholar] [CrossRef]
- Qi, F.; Kunihiko, E.; Guodong, C. Soil Water and Chemical Characteristics of Sandy Soils and Their Significance to Land Reclamation. J. Arid Environ. 2002, 51, 35–54. [Google Scholar] [CrossRef]
- Shinwari, M.W.; Zhitomirsky, D.; Deen, I.A.; Selvaganapathy, P.R.; Deen, M.J.; Landheer, D. Microfabricated Reference Electrodes and Their Biosensing Applications. Sensors 2010, 10, 1679–1715. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.; Krishnan, P.; Kotnala, R.K.; Sumana, G. Recent developments in biosensors to combat agricultural challenges and their future prospects. Trends Food Sci. Technol. 2019, 88, 157–178. [Google Scholar] [CrossRef]
- Deswal, S. Biosensors and Their Application in Environmental Monitoring. J. Technol. 2024, 12, 875–888. [Google Scholar]
- Sadanandom, A.; Napier, R.M. Biosensors in Plants. Curr. Opin. Plant Biol. 2010, 13, 736–743. [Google Scholar] [CrossRef]
- Madufor, N.J.K.; Perold, W.J.; Opara, U.L. Detection of Plant Diseases Using Biosensors: A Review. Acta Hortic. 2018, 1201, 83–90. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, M.; Yang, Q.; Wei, H.; Xia, A.; Wang, L.; Ben, Y.; Zhou, Q.; Yang, Z.; Huang, X. A Living Plant Cell-Based Biosensor for Real-Time Monitoring Invisible Damage of Plant Cells under Heavy Metal Stress. Sci. Total Environ. 2019, 697, 134097. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical Biosensors: Recommended Definitions and Classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Pudake, R.N.; Jain, U.; Kole, C. (Eds.) Biosensors in Agriculture: Recent Trends and Future Perspectives; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Hamed, S.; Ibba, P.; Petrelli, M.; Ciocca, M.; Lugli, P.; Petti, L. Transistor-Based Plant Sensors for Agriculture 4.0 Measurements. In Proceedings of the 2021 IEEE International Workshop on Metrology for Agriculture and Forestry (MetroAgriFor), Trento-Bolzano, Italy, 3–5 November 2021; pp. 69–74. [Google Scholar]
- Lo Presti, D.; Di Tocco, J.; Massaroni, C.; Cimini, S.; De Gara, L.; Singh, S.; Raucci, A.; Manganiello, G.; Woo, S.L.; Schena, E.; et al. Current Understanding, Challenges and Perspective on Portable Systems Applied to Plant Monitoring and Precision Agriculture. Biosens. Bioelectron. 2023, 222, 115005. [Google Scholar] [CrossRef] [PubMed]
- Elli, G.; Hamed, S.; Petrelli, M.; Ibba, P.; Ciocca, M.; Lugli, P.; Petti, L. Field-Effect Transistor-Based Biosensors for Environmental and Agricultural Monitoring. Sensors 2022, 22, 4178. [Google Scholar] [CrossRef] [PubMed]
- Marks, A.; Griggs, S.; Gasparini, N.; Moser, M. Organic Electrochemical Transistors: An Emerging Technology for Biosensing. Adv. Mater. Interfaces 2022, 9, 2102039. [Google Scholar] [CrossRef]
- Coppedè, N.; Vurro, F.; Manfredi, R.; Janni, M.; Zappettini, A.; Gentile, F. Introducing State Variables in Organic Electrochemical Transistors with Application to Biophysical Systems. IEEE Sens. J. 2019, 19, 11753–11758. [Google Scholar] [CrossRef]
- Niu, Y.; Qin, Z.; Zhang, Y.; Chen, C.; Liu, S.; Chen, H. Expanding the potential of biosensors: A review on organic field effect transistor (OFET) and organic electrochemical transistor (OECT) biosensors. Mater. Futures 2023, 2, 042401. [Google Scholar] [CrossRef]
- White, H.S.; Kittlesen, G.P.; Wrighton, M.S. Chemical Derivatization of an Array of Three Gold Microelectrodes with Polypyrrole: Fabrication of a Molecule-Based Transistor. J. Am. Chem. Soc. 1984, 106, 5375–5377. [Google Scholar] [CrossRef]
- Hu, F.; Xue, Y.; Xu, J.; Lu, B. PEDOT-Based Conducting Polymer Actuators. Front. Robot. AI 2019, 6, 114. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, H.; Fu, Q. Recent Progress on PEDOT:PSS-Based Polymer Blends and Composites for Flexible Electronics and Thermoelectric Devices. Mater. Chem. Front. 2020, 4, 3130–3152. [Google Scholar] [CrossRef]
- Amato, D.; Montanaro, G.; Vurro, F.; Coppedé, N.; Briglia, N.; Petrozza, A.; Janni, M.; Zappettini, A.; Cellini, F.; Nuzzo, V. Towards In Vivo Monitoring of Ions Accumulation in Trees: Response of an in Planta Organic Electrochemical Transistor Based Sensor to Water Flux Density, Light and Vapor Pressure Deficit Variation. Appl. Sci. 2021, 11, 4729. [Google Scholar] [CrossRef]
- Coppedè, N.; Janni, M.; Bettelli, M.; Maida, C.L.; Gentile, F.; Villani, M.; Ruotolo, R.; Iannotta, S.; Marmiroli, N.; Marmiroli, M.; et al. An in vivo biosensing, biomimetic electrochemical transistor with applications in plant science and precision farming. Sci. Rep. 2017, 7, 16195. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.; Vurro, F.; Janni, M.; Manfredi, R.; Cellini, F.; Petrozza, A.; Zappettini, A.; Coppedè, N. A Biomimetic, Biocompatible OECT Sensor for the Real-Time Measurement of Concentration and Saturation of Ions in Plant Sap. Adv. Electron. Mater. 2022, 8, 2200092. [Google Scholar] [CrossRef]
- Vurro, F.; Manfredi, R.; Bettelli, M.; Bocci, G.; Cologni, A.L.; Cornali, S.; Reggiani, R.; Marchetti, E.; Coppedè, N.; Caselli, S.; et al. In Vivo Sensing to Monitor Tomato Plants in Field Conditions and Optimize Crop Water Management. Precis. Agric. 2023, 24, 2479–2499. [Google Scholar] [CrossRef]
- Vurro, F.; Marchetti, E.; Bettelli, M.; Manfrini, L.; Finco, A.; Sportolaro, C.; Coppedè, N.; Palermo, N.; Tommasini, M.G.; Zappettini, A.; et al. Application of the OECT-Based In Vivo Biosensor Bioristor in Fruit Tree Monitoring to Improve Agricultural Sustainability. Chemosensors 2023, 11, 374. [Google Scholar] [CrossRef]
- Finco, A.; Bentivoglio, D.; Chiaraluce, G.; Alberi, M.; Chiarelli, E.; Maino, A.; Mantovani, F.; Montuschi, M.; Raptis, K.G.C.; Semenza, F.; et al. Combining Precision Viticulture Technologies and Economic Indices to Sustainable Water Use Management. Water 2022, 14, 1493. [Google Scholar] [CrossRef]
- Diacci, C.; Abedi, T.; Lee, J.W.; Gabrielsson, E.O.; Berggren, M.; Simon, D.T.; Niittylä, T.; Stavrinidou, E. Diurnal in Vivo Xylem Sap Glucose and Sucrose Monitoring Using Implantable Organic Electrochemical Transistor Sensors. iScience 2021, 24, 101966. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M. Photosynthesis. In The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Geiger, D. Plant Glucose Transporter Structure and Function. Pflug. Arch. Eur. J. Physiol. 2020, 472, 1111–1128. [Google Scholar] [CrossRef]
- Mayrhofer, S.; Heizmann, U.; Magel, E.; Eiblmeier, M.; Müller, A.; Rennenberg, H.; Hampp, R.; Schnitzler, J.-P.; Kreuzwieser, J. Carbon Balance in Leaves of Young Poplar Trees. Plant Biol. 2004, 6, 730–739. [Google Scholar] [CrossRef]
- Strand, E.; Bihar, E.; Gleason, S.; Han, S.; Schreiber, S.; Renny, M.; Malliaras, G.; Mcleod, R.; Whiting, G. Printed Organic Electrochemical Transistors for Detecting Nutrients in Whole Plant Sap. Adv. Electron. Mater. 2022, 8, 2100853. [Google Scholar] [CrossRef]
- Nawaz, A.; Liu, Q.; Leong, W.L.; Fairfull-Smith, K.E.; Sonar, P. Organic Electrochemical Transistors for In Vivo Bioelectronics. Adv. Mater. 2021, 33, 2101874. [Google Scholar] [CrossRef]
- Ajayan, J.; Mohankumar, P.; Mathew, R.; Thoutam, L.R.; Kaushik, B.K.; Nirmal, D. Organic Electrochemical Transistors (OECTs): Advancements and Exciting Prospects for Future Biosensing Applications. IEEE Trans. Electron Devices 2023, 70, 3401–3412. [Google Scholar] [CrossRef]
- Bihar, E.; Strand, E.J.; Crichton, C.A.; Renny, M.N.; Bonter, I.; Tran, T.; Atreya, M.; Gestos, A.; Haseloff, J.; McLeod, R.R.; et al. Self-healable stretchable printed electronic cryogels for in-vivo plant monitoring. npj Flex. Electron. 2023, 7, 48. [Google Scholar] [CrossRef]
- Li, D.; Zhang, P.; Chen, T.; Qin, W. Recent Development and Challenges in Spectroscopy and Machine Vision Technologies for Crop Nitrogen Diagnosis: A Review. Remote Sens. 2020, 12, 2578. [Google Scholar] [CrossRef]
- Sishodia, R.P.; Ray, R.L.; Singh, S.K. Applications of Remote Sensing in Precision Agriculture: A Review. Remote Sens. 2020, 12, 3136. [Google Scholar] [CrossRef]
- Loures, L.; Chamizo, A.; Ferreira, P.; Loures, A.; Castanho, R.; Panagopoulos, T. Assessing the Effectiveness of Precision Agriculture Management Systems in Mediterranean Small Farms. Sustainability 2020, 12, 3765. [Google Scholar] [CrossRef]
- Martos, V.; Ahmad, A.; Cartujo, P.; Ordoñez, J. Ensuring Agricultural Sustainability through Remote Sensing in the Era of Agriculture 5.0. Appl. Sci. 2021, 11, 5911. [Google Scholar] [CrossRef]
- Muhie, S.H. Novel Approaches and Practices to Sustainable Agriculture. J. Agric. Food Res. 2022, 10, 100446. [Google Scholar] [CrossRef]
- Massa, D.; Magán, J.J.; Montesano, F.F.; Tzortzakis, N. Minimizing Water and Nutrient Losses from Soilless Cropping in Southern Europe. Agric. Water Manag. 2020, 241, 106395. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Jin, X.; Bian, L.; Ge, Y. High-Throughput Phenotyping of Plant Leaf Morphological, Physiological, and Biochemical Traits on Multiple Scales Using Optical Sensing. Crop J. 2023, 11, 1303–1318. [Google Scholar] [CrossRef]
- Calzadilla, P.I.; Carvalho, F.E.L.; Gomez, R.; Lima Neto, M.C.; Signorelli, S. Assessing Photosynthesis in Plant Systems: A Cornerstone to Aid in the Selection of Resistant and Productive Crops. Environ. Exp. Bot. 2022, 201, 104950. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Yao, Y.; Li, M.; Liu, L. Modern Imaging Techniques in Plant Nutrition Analysis: A Review. Comput. Electron. Agric. 2020, 174, 105459. [Google Scholar] [CrossRef]
- Urban, L.; Aarrouf, J.; Bidel, L.P. Assessing the effects of water deficit on photosynthesis using parameters derived from measurements of leaf gas exchange and of chlorophyll a fluorescence. Front. Plant Sci. 2017, 8, 2068. [Google Scholar] [CrossRef]
- Hallik, L.; Šarauskis, E.; Kazlauskas, M.; Bručienė, I.; Mozgeris, G.; Steponavičius, D.; Tõrra, T. Proximal Sensing Sensors for Monitoring Crop Growth. In Information and Communication Technologies for Agriculture—Theme I: Sensors; Bochtis, D.D., Lampridi, M., Petropoulos, G.P., Ampatzidis, Y., Pardalos, P., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 43–97. ISBN 978-3-030-84144-7. [Google Scholar]
- Rajak, P.; Ganguly, A.; Adhikary, S.; Bhattacharya, S. Internet of Things and Smart Sensors in Agriculture: Scopes and Challenges. J. Agric. Food Res. 2023, 14, 100776. [Google Scholar] [CrossRef]
- Azhar, A.; Merdekawati, E.; Pramudia, A.; Cahyo, A.N.; Ehara, H.; Dahliani, L. Ambient Air Temperatures and Solar Radiation Affect OJIP Fluorescence Transients of Coffee Plants in an Agroforestry System. Agrosyst. Geosci. Environ. 2023, 6, e20340. [Google Scholar] [CrossRef]
- Bullock, D.G.; Anderson, D.S. Evaluation of the Minolta SPAD-502 Chlorophyll Meter for Nitrogen Management in Corn. J. Plant Nutr. 1998, 21, 741–755. [Google Scholar] [CrossRef]
- Vlaović, J.; Balen, J.; Grgić, K.; Žagar, D.; Galić, V.; Šimić, D. An Overview of Chlorophyll Fluorescence Measurement Process, Meters and Methods. In Proceedings of the 2020 International Conference on Smart Systems and Technologies (SST), Osijek, Croatia, 14–16 October 2020; pp. 245–250. [Google Scholar]
- Laveglia, S.; Altieri, G.; Genovese, F.; Matera, A.; Di Renzo, G.C. Advances in Sustainable Crop Management: Integrating Precision Agriculture and Proximal Sensing. AgriEngineering 2024, 6, 3084–3120. [Google Scholar] [CrossRef]
- Ali, M.M.; Al-Ani, A.; Eamus, D.; Tan, D.K.Y. Leaf Nitrogen Determination Using Non-Destructive Techniques–A Review. J. Plant Nutr. 2017, 40, 928–953. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, P.; Liu, S.; Wang, C.; Liu, J. Evaluation of the Methods for Estimating Leaf Chlorophyll Content with SPAD Chlorophyll Meters. Remote Sens. 2022, 14, 5144. [Google Scholar] [CrossRef]
- Gitelson, A.; Solovchenko, A.; Viña, A. Foliar Absorption Coefficient Derived from Reflectance Spectra: A Gauge of the Efficiency of in Situ Light-Capture by Different Pigment Groups. J. Plant Physiol. 2020, 254, 153277. [Google Scholar] [CrossRef] [PubMed]
- Ates, F.; Kaya, O. The Relationship Between Iron and Nitrogen Concentrations Based on Kjeldahl Method and SPAD-502 Readings in Grapevine (Vitis vinifera L. cv. ‘Sultana Seedless’). Erwerbs-Obstbau 2021, 63, 53–59. [Google Scholar] [CrossRef]
- Turner, F.T.; Jund, M.F. Chlorophyll Meter to Predict Nitrogen Topdress Requirement for Semidwarf Rice. Agron. J. 1991, 83, 926–928. [Google Scholar] [CrossRef]
- Güler, S.; Büyük, G. Relationships Among Chlorophyll-Meter Reading Value, Leaf N, and Yield of Cucumber and Tomatoes. Acta Hortic. 2007, 729, 307–311. [Google Scholar] [CrossRef]
- Fritschi, F.B.; Ray, J.D. Soybean Leaf Nitrogen, Chlorophyll Content, and Chlorophyll a/b Ratio. Photosynthetica 2007, 45, 92–98. [Google Scholar] [CrossRef]
- Glenn, D.M.; Tabb, A. Evaluation of Five Methods to Measure Normalized Difference Vegetation Index (NDVI) in Apple and Citrus. Int. J. Fruit Sci. 2019, 19, 191–210. [Google Scholar] [CrossRef]
- Taskos, D.G.; Koundouras, S.; Stamatiadis, S.; Zioziou, E.; Nikolaou, N.; Karakioulakis, K.; Theodorou, N. Using Active Canopy Sensors and Chlorophyll Meters to Estimate Grapevine Nitrogen Status and Productivity. Precis. Agric. 2015, 16, 77–98. [Google Scholar] [CrossRef]
- Wang, K.; Li, W.; Deng, L.; Lyu, Q.; Zheng, Y.; Yi, S.; Xie, R.; Ma, Y.; He, S. Rapid Detection of Chlorophyll Content and Distribution in Citrus Orchards Based on Low-Altitude Remote Sensing and Bio-Sensors. Int. J. Agric. Biol. Eng. 2018, 11, 164–169. [Google Scholar] [CrossRef]
- Boussadia, O.; Steppe, K.; Zgallai, H.; Ben El Hadj, S.; Braham, M.; Lemeur, R.; Van Labeke, M.C. Nondestructive Determination of Nitrogen and Chlorophyll Content in Olive Tree Leaves and the Relation with Photosynthesis and Fluorescence Parameters. Photosynthetica 2011, 49, 149–153. [Google Scholar] [CrossRef]
- Arantes, A.d.M.; Donato, S.L.R.; de Siqueira, D.L.; Amorim, E.P.; Rodrigues Filho, V.A. Chlorophyll Index for Real-Time Prediction of Nutritional Status of “Prata” Banana. Rev. Bras. Eng. Agríc. Ambient. 2016, 20, 99–106. [Google Scholar] [CrossRef]
- Neto, C.B.; Carranca, C.; Clemente, J.; de Varennes, A. Assessing the Nitrogen Nutritional Status of Young Non-Bearing ‘Rocha’ Pear Trees Grown in a Mediterranean Region by Using a Chlorophyll Meter. J. Plant Nutr. 2011, 34, 627–639. [Google Scholar] [CrossRef]
- Shibaeva, T.G.; Mamaev, A.V.; Sherudilo, E.G. Evaluation of a SPAD-502 Plus Chlorophyll Meter to Estimate Chlorophyll Content in Leaves with Interveinal Chlorosis. Russ. J. Plant Physiol. 2020, 67, 690–696. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, J.; Han, G.D. Environmental Influences on SPAD Values in Prunus Mume Trees: A Comparative Study of Leaf Position and Photosynthetic Efficiency Across Different Light Conditions. J. Environ. Sci. Int. 2024, 33, 501–509. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Ghozlen, N.B.; Milhade, C.; Obert, M.; Debuisson, S.; Moigne, M.L. Nondestructive Diagnostic Test for Nitrogen Nutrition of Grapevine (Vitis vinifera L.) Based on Dualex Leaf-Clip Measurements in the Field. J. Agric. Food Chem. 2015, 63, 3669–3680. [Google Scholar] [CrossRef] [PubMed]
- Goulas, Y.; Cerovic, Z.G.; Cartelat, A.; Moya, I. Dualex: A New Instrument for Field Measurements of Epidermal Ultraviolet Absorbance by Chlorophyll Fluorescence. Appl. Opt. 2004, 43, 4488. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R.; Nenadis, N.; Neugart, S.; Robson, M.; Agati, G.; Vepsäläinen, J.; Zipoli, G.; Nybakken, L.; Winkler, B.; Jansen, M.A.K. Assessing the Response of Plant Flavonoids to UV Radiation: An Overview of Appropriate Techniques. Phytochem. Rev. 2015, 14, 273–297. [Google Scholar] [CrossRef]
- Samborski, S.M.; Tremblay, N.; Fallon, E. Strategies to Make Use of Plant Sensors-Based Diagnostic Information for Nitrogen Recommendations. Agron. J. 2009, 101, 800–816. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, X.; Ma, Y.; Zhang, R.; Cao, Q.; Zhu, Y.; Cao, W.; Tian, Y. A Comparative Assessment of Measures of Leaf Nitrogen in Rice Using Two Leaf-Clip Meters. Sensors 2020, 20, 175. [Google Scholar] [CrossRef] [PubMed]
- Overbeck, V.; Schmitz, M.; Tartachnyk, I.; Blanke, M. Identification of Light Availability in Different Sweet Cherry Orchards under Cover by Using Non-Destructive Measurements with a Dualex™. Eur. J. Agron. 2018, 93, 50–56. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Cerovic, Z.G. Sensing Crop Nitrogen Status with Fluorescence Indicators. A Review. Agron. Sustain. Dev. 2012, 32, 451–464. [Google Scholar] [CrossRef]
- Cartelat, A.; Cerovic, Z.G.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.-L.; Barbottin, A.; Jeuffroy, M.-H.; Gate, P.; Agati, G.; et al. Optically Assessed Contents of Leaf Polyphenolics and Chlorophyll as Indicators of Nitrogen Deficiency in Wheat (Triticum aestivum L.). Field Crops Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Bélec, C. Evaluation of the Dualex for the Assessment of Corn Nitrogen Status. J. Plant Nutr. 2007, 30, 1355–1369. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Bélec, C. Performance of Dualex in Spring Wheat for Crop Nitrogen Status Assessment, Yield Prediction and Estimation of Soil Nitrate Content. J. Plant Nutr. 2009, 33, 57–70. [Google Scholar] [CrossRef]
- Ronga, D.; Rizza, F.; Badeck, F.-W.; Milc, J.; Laviano, L.; Montevecchi, G.; Pecchioni, N.; Francia, E. Physiological Responses to Chilling in Cultivars of Processing Tomato Released and Cultivated over the Past Decades in Southern Europe. Sci. Hortic. 2018, 231, 118–125. [Google Scholar] [CrossRef]
- Friedel, M.; Hendgen, M.; Stoll, M.; Löhnertz, O. Performance of Reflectance Indices and of a Handheld Device for Estimating In-Field the Nitrogen Status of Grapevine Leaves. Aust. J. Grape Wine Res. 2020, 26, 110–120. [Google Scholar] [CrossRef]
- Oustric, J.; Morillon, R.; Luro, F.; Herbette, S.; Lourkisti, R.; Giannettini, J.; Berti, L.; Santini, J. Tetraploid Carrizo Citrange Rootstock (Citrus sinensis Osb. × Poncirus trifoliata L. Raf.) Enhances Natural Chilling Stress Tolerance of Common Clementine (Citrus clementina Hort. Ex Tan). J. Plant Physiol. 2017, 214, 108–115. [Google Scholar] [CrossRef]
- Román Ecija, M.; Olivares-García, C.; Rivas, J.C.; Velasco-Amo, M.P.; Navas Cortés, J.A.; Landa, B.B. Assessment of Physiological Traits of Olive Trees Infected by Xylella fastidiosa Subspecies. In Proceedings of the 9th Meeting of the IOBC-WPRS Working Group “Integrated Protection of Olive Crops”, Lisboa, Portugal, 26–29 October 2021. [Google Scholar] [CrossRef]
- Muñoz-Huerta, R.F.; Guevara-Gonzalez, R.G.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. A Review of Methods for Sensing the Nitrogen Status in Plants: Advantages, Disadvantages and Recent Advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef] [PubMed]
- de Souza, R.; Peña-Fleitas, M.T.; Thompson, R.B.; Gallardo, M.; Grasso, R.; Padilla, F.M. The Use of Chlorophyll Meters to Assess Crop N Status and Derivation of Sufficiency Values for Sweet Pepper. Sensors 2019, 19, 2949. [Google Scholar] [CrossRef] [PubMed]
- Padilla, F.M.; Gallardo, M.; Peña-Fleitas, M.T.; De Souza, R.; Thompson, R.B. Proximal Optical Sensors for Nitrogen Management of Vegetable Crops: A Review. Sensors 2018, 18, 2083. [Google Scholar] [CrossRef]
- Zhu, J.; Tremblay, N.; Liang, Y. Comparing SPAD and atLEAF Values for Chlorophyll Assessment in Crop Species. Can. J. Soil Sci. 2012, 92, 645–648. [Google Scholar] [CrossRef]
- Novichonok, E.V.; Novichonok, A.O.; Kurbatova, J.A.; Markovskaya, E.F. Use of the atLEAF+ Chlorophyll Meter for a Nondestructive Estimate of Chlorophyll Content. Photosynthetica 2016, 54, 130–137. [Google Scholar] [CrossRef]
- da Silva, T.M.M.; Costa, B.R.S.; Oldoni, H.; Mitsuyuki, M.C.; Bassoi, L.H. Calibration of Chlorophyll Hand-Held Meter Based on Vineyard NDVI Zones for Estimation of Leaf N Content. Cienc. E Agrotecnologia 2022, 46, e006222. [Google Scholar] [CrossRef]
- Hebbar, K.B.; Subramanian, P.; Sheena, T.L.; Shwetha, K.; Sugatha, P.; Arivalagan, M.; Varaprasad, P.V. Chlorophyll and Nitrogen Determination in Coconut Using a Non-Destructive Method. J. Plant Nutr. 2016, 39, 1610–1619. [Google Scholar] [CrossRef]
- Kamarianakis, Z.; Panagiotakis, S. Design and Implementation of a Low-Cost Chlorophyll Content Meter. Sensors 2023, 23, 2699. [Google Scholar] [CrossRef] [PubMed]
- Gianquinto, G.; Goffart, J.P.; Olivier, M.; Guarda, G.; Colauzzi, M.; Dalla Costa, L.; Delle Vedove, G.; Vos, J.; Mackerron, D.K.L. The Use of Hand-Held Chlorophyll Meters as a Tool to Assess the Nitrogen Status and to Guide Nitrogen Fertilization of Potato Crop. Potato Res. 2004, 47, 35–80. [Google Scholar] [CrossRef]
- Bracke, J.; Elsen, A.; Adriaenssens, S.; Vandendriessche, H.; Van Labeke, M.-C. Utility of Proximal Plant Sensors to Support Nitrogen Fertilization in Chrysanthemum. Sci. Hortic. 2019, 256, 108544. [Google Scholar] [CrossRef]
- Gorai, T.; Yadav, P.K.; Choudhary, G.L.; Kumar, A. Site-Specific Crop Nutrient Management for Precision Agriculture—A Review. Curr. J. Appl. Sci. Technol. 2021, 40, 37–52. [Google Scholar] [CrossRef]
- Wang, X.; Miao, Y.; Guan, Y.; Xia, T.; Lu, J.; Mulla, D.J. An Evaluation of Two Active Canopy Sensor Systems for Non-Destructive Estimation of Spring Maize Biomass. In Proceedings of the 2016 Fifth International Conference on Agro-Geoinformatics (Agro-Geoinformatics), Tianjin, China, 18–20 July 2016; pp. 1–6. [Google Scholar]
- Maresma, A.; Chamberlain, L.; Tagarakis, A.; Kharel, T.; Godwin, G.; Czymmek, K.J.; Shields, E.; Ketterings, Q.M. Accuracy of NDVI-Derived Corn Yield Predictions Is Impacted by Time of Sensing. Comput. Electron. Agric. 2020, 169, 105236. [Google Scholar] [CrossRef]
- Van Loon, J.; Speratti, A.B.; Gabarra, L.; Govaerts, B. Precision for Smallholder Farmers: A Small-Scale-Tailored Variable Rate Fertilizer Application Kit. Agriculture 2018, 8, 48. [Google Scholar] [CrossRef]
- Govaerts, B.; Verhulst, N.; Sayre, K.D.; De Corte, P.; Goudeseune, B.; Lichter, K.; Crossa, J.; Deckers, J.; Dendooven, L. Evaluating Spatial within Plot Crop Variability for Different Management Practices with an Optical Sensor. Plant Soil 2007, 299, 29–42. [Google Scholar] [CrossRef]
- Ennaji, O.; Vergütz, L.; El Allali, A. Machine Learning in Nutrient Management: A Review. Artif. Intell. Agric. 2023, 9, 1–11. [Google Scholar] [CrossRef]
- Zsebő, S.; Bede, L.; Kukorelli, G.; Kulmány, I.M.; Milics, G.; Stencinger, D.; Teschner, G.; Varga, Z.; Vona, V.; Kovács, A.J. Yield Prediction Using NDVI Values from GreenSeeker and MicaSense Cameras at Different Stages of Winter Wheat Phenology. Drones 2024, 8, 88. [Google Scholar] [CrossRef]
- Mirzakhaninafchi, H.; Singh, M.; Bector, V.; Gupta, O.P.; Singh, R. Design and Development of a Variable Rate Applicator for Real-Time Application of Fertilizer. Sustainability 2021, 13, 8694. [Google Scholar] [CrossRef]
- Freeman, K.W.; Girma, K.; Arnall, D.B.; Mullen, R.W.; Martin, K.L.; Teal, R.K.; Raun, W.R. By-Plant Prediction of Corn Forage Biomass and Nitrogen Uptake at Various Growth Stages Using Remote Sensing and Plant Height. Agron. J. 2007, 99, 530–536. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Shen, J.; Yu, W.; Yuan, F.; Cheng, S.; Huang, S.; Wang, H.; Yang, W.; Liu, F. Improving In-Season Estimation of Rice Yield Potential and Responsiveness to Topdressing Nitrogen Application with Crop Circle Active Crop Canopy Sensor. Precis. Agric. 2016, 17, 136–154. [Google Scholar] [CrossRef]
- Walker, H.V.; Jones, J.E.; Swarts, N.D.; Rodemann, T.; Kerslake, F.; Dambergs, R.G. Predicting Grapevine Canopy Nitrogen Status Using Proximal Sensors and Near-Infrared Reflectance Spectroscopy. J. Plant Nutr. Soil Sci. 2021, 184, 204–304. [Google Scholar] [CrossRef]
- Poudyal, C.; Sandhu, H.; Ampatzidis, Y.; Odero, D.C.; Arbelo, O.C.; Cherry, R.H.; Costa, L.F. Prediction of Morpho-Physiological Traits in Sugarcane Using Aerial Imagery and Machine Learning. Smart Agric. Technol. 2023, 3, 100104. [Google Scholar] [CrossRef]
- Mao, L.; Song, Q.; Li, X.; Zheng, H.; Zhu, X.G. Would Reducing Chlorophyll Content Result in a Higher Photosynthesis Nitrogen Use Efficiency in Crops? Food Energy Secur. 2024, 13, e576. [Google Scholar] [CrossRef]
- Hamdani, S.; Wang, H.; Zheng, G.; Perveen, S.; Qu, M.; Khan, N.; Khan, W.; Jiang, J.; Li, M.; Liu, X.; et al. Genome-Wide Association Study Identifies Variation of Glucosidase Being Linked to Natural Variation of the Maximal Quantum Yield of Photosystem II. Physiol. Plant. 2019, 166, 105–119. [Google Scholar] [CrossRef]
- Giorio, P.; Nuzzo, V.; Guida, G.; Albrizio, R. Black Leaf-Clips of a Commercial Fluorometer Increased Leaf Temperature during Dark Adaptation under High Solar Radiation. Photosynthetica 2012, 50, 467–471. [Google Scholar] [CrossRef]
- Giorio, P. Black Leaf-Clips Increased Minimum Fluorescence Emission in Clipped Leaves Exposed to High Solar Radiation during Dark Adaptation. Photosynthetica 2011, 49, 371–379. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, Q.; Qi, Z.; Ding, J.; Song, X.; Xia, X. Stress-Induced Delay of the I-P Rise of the Fast Chlorophyll a Fluorescence Transient in Tomato. Sci. Hortic. 2024, 326, 112741. [Google Scholar] [CrossRef]
- Zarei, Z.; Heidari, H.; Honarmand, S.J.; Bafkar, A. Improving Grain Yield and Water Use Efficiency in Maize by Wick Irrigation. Irrig. Sci. 2024, 42, 785–800. [Google Scholar] [CrossRef]
- Khadem Moghadam, N.; Motesharezadeh, B.; Maali-Amiri, R.; Asgari Lajayer, B.; Astatkie, T. Effects of Potassium and Zinc on Physiology and Chlorophyll Fluorescence of Two Cultivars of Canola Grown under Salinity Stress. Arab J. Geosci. 2020, 13, 771. [Google Scholar] [CrossRef]
- de Lima, R.S.N.; Figueiredo, F.A.M.M.d.A.; Martins, A.O.; Deus, B.C.d.S.d.; Ferraz, T.M.; Gomes, M.d.M.d.A.; de Sousa, E.F.; Glenn, D.M.; Campostrini, E. Partial Rootzone Drying (PRD) and Regulated Deficit Irrigation (RDI) Effects on Stomatal Conductance, Growth, Photosynthetic Capacity, and Water-Use Efficiency of Papaya. Sci. Hortic. 2015, 183, 13–22. [Google Scholar] [CrossRef]
- Zha, Q.; Xi, X.; He, Y.; Jiang, A. Transcriptomic Analysis of the Leaves of Two Grapevine Cultivars under High-Temperature Stress. Sci. Hortic. 2020, 265, 109265. [Google Scholar] [CrossRef]
- Spyroglou, I.; Rybka, K.; Maldonado Rodriguez, R.; Stefański, P.; Valasevich, N.M. Quantitative Estimation of Water Status in Field-Grown Wheat Using Beta Mixed Regression Modelling Based on Fast Chlorophyll Fluorescence Transients: A Method for Drought Tolerance Estimation. J. Agron. Crop Sci. 2021, 207, 589–605. [Google Scholar] [CrossRef]
- Mirzaee, M.; Rees, D.; Colgan, R.J.; Tully, M.S. Diagnosing Bitter Pit in Apple During Storage by Chlorophyll Fluorescence as a Non-Destructive Tool. Acta Hortic. 2015, 1079, 235–242. [Google Scholar] [CrossRef]
- Keutgen, A.J.; Keutgen, N.; Wszelaczyńska, E.; Pobereżny, J.; Milczarek, D.; Tatarowska, B.; Flis, B. Evaluation of Photosynthetic and Yield Traits in Ten Potato Clones and Cultivars Under Farming Conditions in Poland. Potato Res. 2020, 63, 75–95. [Google Scholar] [CrossRef]
- Coswosk, G.G.; Gonçalves, V.M.L.; de Lima, V.J.; de Souza, G.A.R.; Teixeira do Amaral Junior, A.; Pereira, M.G.; de Oliveira, E.C.; Leite, J.T.; Kamphorst, S.H.; de Oliveira, U.A.; et al. Utilizing Visible Band Vegetation Indices from Unmanned Aerial Vehicle Images for Maize Phenotyping. Remote Sens. 2024, 16, 3015. [Google Scholar] [CrossRef]
- Ran, X.; Wang, X.; Gao, X.; Liang, H.; Liu, B.; Huang, X. Effects of Salt Stress on the Photosynthetic Physiology and Mineral Ion Absorption and Distribution in White Willow (Salix alba L.). PLoS ONE 2021, 16, e0260086. [Google Scholar] [CrossRef]
- Rostami, M.; Koocheki, A.R.; Mahallati, M.N.; Kafi, M. Evaluation of chlorophyll meter (SPAD) data for prediction of nitrogen status in corn (Zea mays L.). Am.-Eurasian J. Agric. Sci. 2008, 3, 79–85. [Google Scholar]
- Casa, R.; Castaldi, F.; Pascucci, S.; Pignatti, S. Chlorophyll estimation in field crops: An assessment of handheld leaf meters and spectral reflectance measurements. J. Agric. Sci. 2015, 153, 876–890. [Google Scholar] [CrossRef]
- Habibullah, M.; Mohebian, M.R.; Soolanayakanahally, R.; Wahid, K.A.; Dinh, A. A Cost-Effective and Portable Optical Sensor System to Estimate Leaf Nitrogen and Water Contents in Crops. Sensors 2020, 20, 1449. [Google Scholar] [CrossRef]
- Ninama, A.R.; Ram, K.V.; Solanki, B.P.; Choudhary, R. Greenseeker—Modern Tool for Nitrogen Management. Int. J. Res. Agron. 2024, 7, 124–129. [Google Scholar] [CrossRef]
- Yin, Z.; Meng, F.; Song, H.; He, X.; Xu, X.; Yu, D. Mapping Quantitative Trait Loci Associated with Chlorophyll a Fluorescence Parameters in Soybean (Glycine max (L.) Merr.). Planta 2010, 231, 875–885. [Google Scholar] [CrossRef]
- Saberioon, M.M.; Amin, M.S.M.; Gholizadeh, A.; Ezri, M.H. A Review of Optical Methods for Assessing Nitrogen Contents during Rice Growth. Appl. Eng. Agric. 2014, 30, 657–669. [Google Scholar]
- Kubiak, K.; Kotlarz, J.; Spiralski, M.; Szymański, J. Nitrogen Fertilization Assessment in Maize (Zea mays L.) Using Hyperspectral UV/VIS/NIR Data. Remote Sens. Lett. 2023, 14, 1251–1259. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, T.; Yang, C.; Song, H.; Jiang, Z.; Zhou, G.; Zhang, D.; Feng, H.; Xie, J. Segmenting Purple Rape-seed Leaves in the Field from UAV RGB Imagery Using Deep Learning as an Auxiliary Means for Nitrogen Stress Detection. Remote Sens. 2020, 12, 1403. [Google Scholar] [CrossRef]
- Sudhakar, M.; Priya, R.M. Computer Vision Based Machine Learning and Deep Learning Approaches for Identification of Nutrient Deficiency in Crops: A Survey. Nat. Environ. Pollut. Technol. 2023, 22, 1387–1399. [Google Scholar]
- Matese, A.; Toscano, P.; Di Gennaro, S.F.; Genesio, L.; Vaccari, F.P.; Primicerio, J.; Belli, C.; Zaldei, A.; Bianconi, R.; Gioli, B. Intercomparison of UAV, Aircraft and Satellite Remote Sensing Platforms for Precision Viticulture. Remote Sens. 2015, 7, 2971–2990. [Google Scholar] [CrossRef]
- Christiansen, M.P.; Laursen, M.S.; Jørgensen, R.N.; Skovsen, S.; Gislum, R. Designing and Testing a UAV Mapping System for Agricultural Field Surveying. Sensors 2017, 17, 2703. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A. Remote Sensing for Sustainable Agricultural Management. In Sustaining Global Food Security: The Nexus of Science and Policy; Csiro Publishing: Clayton, Australia, 2019; p. 369. [Google Scholar]
- Copernicus. Homepage|Copernicus. Available online: https://www.copernicus.eu/en (accessed on 29 November 2024).
- Segarra, J.; Buchaillot, M.L.; Araus, J.L.; Kefauver, S.C. Remote Sensing for Precision Agriculture: Sentinel-2 Improved Features and Applications. Agronomy 2020, 10, 641. [Google Scholar] [CrossRef]
- Huang, S.; Tang, L.; Hupy, J.P.; Wang, Y.; Shao, G. A Commentary Review on the Use of Normalized Difference Vegetation Index (NDVI) in the Era of Popular Remote Sensing. J. For. Res. 2021, 32, 1–6. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.; Yao, F.; Wang, P.; Guo, W.; Li, L.; Yang, L. Advances in Estimation Methods of Vegetation Water Content Based on Optical Remote Sensing Techniques. Sci. China Technol. Sci. 2010, 53, 1159–1167. [Google Scholar] [CrossRef]
- Rouse, J.W., Jr.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation; NASA/GSFC: Greenbelt, MD, USA, 1973.
- Kokaly, R.F.; Asner, G.P.; Ollinger, S.V.; Martin, M.E.; Wessman, C.A. Characterizing Canopy Biochemistry from Imaging Spectroscopy and Its Application to Ecosystem Studies. Remote Sens. Environ. 2009, 113, S78–S91. [Google Scholar] [CrossRef]
- Watt, M.S.; Pearse, G.D.; Dash, J.P.; Melia, N.; Leonardo, E.M.C. Application of Remote Sensing Technologies to Identify Impacts of Nutritional Deficiencies on Forests. ISPRS J. Photogramm. Remote Sens. 2019, 149, 226–241. [Google Scholar] [CrossRef]
- Siedliska, A.; Baranowski, P.; Pastuszka-Woźniak, J.; Zubik, M.; Krzyszczak, J. Identification of Plant Leaf Phosphorus Content at Different Growth Stages Based on Hyperspectral Reflectance. BMC Plant Biol. 2021, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Severtson, D.; Callow, N.; Flower, K.; Neuhaus, A.; Olejnik, M.; Nansen, C. Unmanned Aerial Vehicle Canopy Reflectance Data Detects Potassium Deficiency and Green Peach Aphid Susceptibility in Canola. Precis. Agric 2016, 17, 659–677. [Google Scholar] [CrossRef]
- de Oliveira, M.R.R.; Queiroz, T.R.G.; Teixeira, A.d.S.; Moreira, L.C.J.; Leão, R.A.d.O. Reflectance Spectrometry Applied to the Analysis of Nitrogen and Potassium Deficiency in Cotton. Rev. Ciênc. Agron. 2020, 51, e20196705. [Google Scholar] [CrossRef]
- Mazur, P.; Gozdowski, D.; Stępień, W.; Wójcik-Gront, E. Does Drone Data Allow the Assessment of Phosphorus and Potassium in Soil Based on Field Experiments with Winter Rye? Agronomy 2023, 13, 446. [Google Scholar] [CrossRef]
- Illana Rico, S.; Cano Marchal, P.; Martínez Gila, D.; Gámez García, J. Multiple Instance Regression for the Estimation of Leaf Nutrient Content in Olive Trees Using Multispectral Data Taken with UAVs. Biosyst. Eng. 2024, 242, 91–99. [Google Scholar] [CrossRef]
- Noguera, M.; Aquino, A.; Ponce, J.M.; Cordeiro, A.; Silvestre, J.; Arias-Calderón, R.; Marcelo, M.d.E.; Jordão, P.; Andújar, J.M. Nutritional Status Assessment of Olive Crops by Means of the Analysis and Modelling of Multispectral Images Taken with UAVs. Biosyst. Eng. 2021, 211, 1–18. [Google Scholar] [CrossRef]
- Li, Y.; Du, C.; Zhang, T.; Ge, Y.; Liu, W.; Yuan, S.; Liu, X. Estimation of citrus leaves’ nitrogen content by multispectral unmanned aerial vehicle remote sensing based on semi-supervised twin neural network regression. J. Appl. Remote Sens. 2024, 18, 034506. [Google Scholar] [CrossRef]
- Osco, L.P.; Nogueira, K.; Marques Ramos, A.P.; Faita Pinheiro, M.M.; Furuya, D.E.G.; Gonçalves, W.N.; de Castro Jorge, L.A.; Marcato Junior, J.; dos Santos, J.A. Semantic Segmentation of Citrus-Orchard Using Deep Neural Networks and Multispectral UAV-Based Imagery. Precis. Agric 2021, 22, 1171–1188. [Google Scholar] [CrossRef]
- Costa, L.; Kunwar, S.; Ampatzidis, Y.; Albrecht, U. Determining Leaf Nutrient Concentrations in Citrus Trees Using UAV Imagery and Machine Learning. Precis. Agric 2022, 23, 854–875. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Z.; Zhao, F.; Liu, J.; Li, Z.; Wang, X.; Gao, Y. An Estimation of the Leaf Nitrogen Content of Apple Tree Canopies Based on Multispectral Unmanned Aerial Vehicle Imagery and Machine Learning Methods. Agronomy 2024, 14, 552. [Google Scholar] [CrossRef]
- Sun, G.; Hu, T.; Chen, S.; Sun, J.; Zhang, J.; Ye, R.; Zhang, S.; Liu, J. Using UAV-Based Multispectral Remote Sensing Imagery Combined with DRIS Method to Diagnose Leaf Nitrogen Nutrition Status in a Fertigated Apple Orchard. Precis. Agric 2023, 24, 2522–2548. [Google Scholar] [CrossRef]
- Peng, X.; Chen, D.; Zhou, Z.; Zhang, Z.; Xu, C.; Zha, Q.; Wang, F.; Hu, X. Prediction of the Nitrogen, Phosphorus and Potassium Contents in Grape Leaves at Different Growth Stages Based on UAV Multispectral Remote Sensing. Remote Sens. 2022, 14, 2659. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, C.; Ma, Y.; Chen, X.; Lu, B.; Song, Y.; Zhang, Z.; Zhang, R. Estimation of Nitrogen Concentration in Walnut Canopies in Southern Xinjiang Based on UAV Multispectral Images. Agronomy 2023, 13, 1604. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Wu, T. Monitoring of Leaf Nitrogen Content in a Citrus Orchard by Landsat 8 OLI Imagery. In Proceedings of the Twelfth International Conference on Signal Processing Systems; SPIE: Bellingham, WA, USA, 2021; Volume 11719, pp. 23–36. [Google Scholar]
- Shukla, A.; Kot, R. An Overview of Hyperspectral Remote Sensing and Its Applications in Various Disciplines. IRA-Int. J. Appl. Sci. 2016, 5, 85. [Google Scholar] [CrossRef]
- Teke, M.; Deveci, H.S.; Haliloğlu, O.; Gürbüz, S.Z.; Sakarya, U. A Short Survey of Hyperspectral Remote Sensing Applications in Agriculture. In Proceedings of the 2013 6th International Conference on Recent Advances in Space Technologies (RAST), Istanbul, Turkey, 12–14 June 2013; pp. 171–176. [Google Scholar]
- Mariotto, I.; Thenkabail, P.S.; Huete, A.; Slonecker, E.T.; Platonov, A. Hyperspectral versus multispectral crop-productivity modeling and type discrimination for the HyspIRI mission. Remote Sens. Environ. 2013, 139, 291–305. [Google Scholar] [CrossRef]
- Adam, E.; Mutanga, O.; Rugege, D. Multispectral and hyperspectral remote sensing for identification and mapping of wetland vegetation: A review. Wetl. Ecol. Manag. 2010, 18, 281–296. [Google Scholar] [CrossRef]
- Govender, M.; Chetty, K.; Naiken, V.; Bulcock, H. A comparison of satellite hyperspectral and multispectral remote sensing imagery for improved classification and mapping of vegetation. Water SA 2008, 34, 147–154. [Google Scholar] [CrossRef]
- Sahoo, R.N.; Ray, S.S.; Manjunath, K.R. Hyperspectral Remote Sensing of Agriculture. Curr. Sci. 2015, 108, 848–859. [Google Scholar]
- Nigam, R.; Tripathy, R.; Dutta, S.; Bhagia, N.; Nagori, R.; Chandrasekar, K.; Kot, R.; Bhattacharya, B.K.; Ustin, S. Crop Type Discrimination and Health Assessment Using Hyperspectral Imaging. Curr. Sci. 2019, 116, 1108–1123. [Google Scholar] [CrossRef]
- Nguyen, H.D.D.; Pan, V.; Pham, C.; Valdez, R.; Doan, K.; Nansen, C. Night-based hyperspectral imaging to study association of horticultural crop leaf reflectance and nutrient status. Comput. Electron. Agric. 2020, 173, 105458. [Google Scholar] [CrossRef]
- Usha, K.; Singh, B. Potential Applications of Remote Sensing in Horticulture—A Review. Sci. Hortic. 2013, 153, 71–83. [Google Scholar] [CrossRef]
- Liu, Y.; Lyu, Q.; He, S.; Yi, S.; Liu, X.; Xie, R.; Zheng, Y.; Deng, L. Prediction of Nitrogen and Phosphorus Contents in Citrus Leaves Based on Hyperspectral Imaging. Int. J. Agric. Biol. Eng. 2015, 8, 80–88. [Google Scholar] [CrossRef]
- Gomez-Casero, M.T.; Lopez-Granados, F.; Pena-Barragan, J.M.; Jurado-Exposito, M.; Garcia-Torres, L. Assessing nitrogen and potassium deficiencies in olive orchards through discriminant analysis of hyperspectral data. J. Am. Soc. Hortic. Sci. 2007, 132, 611–618. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Liu, F. Application of hyperspectral remote sensing in mineral identification and mapping. In Proceedings of the 2012 2nd International Conference on Computer Science and Network Technology, Changchun, China, 29–31 December 2012; pp. 103–106. [Google Scholar]
- Bajwa, S.G.; Tian, L.F. Soil Fertility Characterization in Agricultural Fields Using Hyperspectral Remote Sensing. Trans. ASAE 2005, 48, 2399–2406. [Google Scholar] [CrossRef]
- Song, Y.-Q.; Zhao, X.; Su, H.-Y.; Li, B.; Hu, Y.-M.; Cui, X.-S. Predicting Spatial Variations in Soil Nutrients with Hyperspectral Remote Sensing at Regional Scale. Sensors 2018, 18, 3086. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, X.; Li, M.; Xue, Y.; Qin, A.; Gao, G.; Wang, M.; Jiang, Y. Utilization of the Fusion of Ground-Space Remote Sensing Data for Canopy Nitrogen Content Inversion in Apple Orchards. Horticulturae 2023, 9, 1085. [Google Scholar] [CrossRef]
- Ye, X.; Abe, S.; Zhang, S. Estimation and Mapping of Nitrogen Content in Apple Trees at Leaf and Canopy Levels Using Hyperspectral Imaging. Precis. Agric 2020, 21, 198–225. [Google Scholar] [CrossRef]
- Chancia, R.; Bates, T.; Vanden Heuvel, J.; van Aardt, J. Assessing Grapevine Nutrient Status from Unmanned Aerial System (UAS) Hyperspectral Imagery. Remote Sens. 2021, 13, 4489. [Google Scholar] [CrossRef]
- Deery, D.; Jimenez-Berni, J.; Jones, H.; Sirault, X.; Furbank, R. Proximal Remote Sensing Buggies and Potential Applications for Field-Based Phenotyping. Agronomy 2014, 4, 349–379. [Google Scholar] [CrossRef]
- Pallottino, F.; Antonucci, F.; Costa, C.; Bisaglia, C.; Figorilli, S.; Menesatti, P. Optoelectronic Proximal Sensing Vehicle-Mounted Technologies in Precision Agriculture: A Review. Comput. Electron. Agric. 2019, 162, 859–873. [Google Scholar] [CrossRef]
- Dechant, B.; Cuntz, M.; Vohland, M.; Schulz, E.; Doktor, D. Estimation of Photosynthesis Traits from Leaf Reflectance Spectra: Correlation to Nitrogen Content as the Dominant Mechanism. Remote Sens. Environ. 2017, 196, 279–292. [Google Scholar] [CrossRef]
- Tripodi, P.; Massa, D.; Venezia, A.; Cardi, T. Sensing Technologies for Precision Phenotyping in Vegetable Crops: Current Status and Future Challenges. Agronomy 2018, 8, 57. [Google Scholar] [CrossRef]
- Xi, R.; Gu, Y.; Zhang, X.; Ren, Z. Nitrogen Monitoring and Inversion Algorithms of Fruit Trees Based on Spectral Remote Sensing: A Deep Review. Front. Plant Sci. 2024, 15, 1489151. [Google Scholar] [CrossRef]
- Ozdogan, M.; Yang, Y.; Allez, G.; Cervantes, C. Remote Sensing of Irrigated Agriculture: Opportunities and Challenges. Remote Sens. 2010, 2, 2274–2304. [Google Scholar] [CrossRef]
- Atzberger, C. Advances in Remote Sensing of Agriculture: Context Description, Existing Operational Monitoring Systems and Major Information Needs. Remote Sens. 2013, 5, 949–981. [Google Scholar] [CrossRef]
- Khanal, S.; Kc, K.; Fulton, J.P.; Shearer, S.; Ozkan, E. Remote Sensing in Agriculture—Accomplishments, Limitations, and Opportunities. Remote Sens. 2020, 12, 3783. [Google Scholar] [CrossRef]
- Omia, E.; Bae, H.; Park, E.; Kim, M.S.; Baek, I.; Kabenge, I.; Cho, B.-K. Remote Sensing in Field Crop Monitoring: A Comprehensive Review of Sensor Systems, Data Analyses and Recent Advances. Remote Sens. 2023, 15, 354. [Google Scholar] [CrossRef]
- Verma, B.; Porwal, M.; Jha, A.K.; Vyshnavi, R.G.; Rajpoot, A.; Nagar, A.K. Enhancing Precision Agriculture and Environmental Monitoring Using Proximal Remote Sensing. J. Exp. Agric. Int. 2023, 45, 162–176. [Google Scholar] [CrossRef]
- Alexopoulos, A.; Koutras, K.; Ali, S.B.; Puccio, S.; Carella, A.; Ottaviano, R.; Kalogeras, A. Complementary Use of Ground-Based Proximal Sensing and Airborne/Spaceborne Remote Sensing Techniques in Precision Agriculture: A Systematic Review. Agronomy 2023, 13, 1942. [Google Scholar] [CrossRef]
- Bausch, W.C.; Khosla, R. QuickBird Satellite versus Ground-Based Multi-Spectral Data for Estimating Nitrogen Status of Irrigated Maize. Precis. Agric 2010, 11, 274–290. [Google Scholar] [CrossRef]
- Fiorentini, M.; Zenobi, S.; Orsini, R. Remote and Proximal Sensing Applications for Durum Wheat Nutritional Status Detection in Mediterranean Area. Agriculture 2021, 11, 39. [Google Scholar] [CrossRef]
- Darra, N.; Psomiadis, E.; Kasimati, A.; Anastasiou, A.; Anastasiou, E.; Fountas, S. Remote and Proximal Sensing-Derived Spectral Indices and Biophysical Variables for Spatial Variation Determination in Vineyards. Agronomy 2021, 11, 741. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).